Abstract
The impact of shifts in the spectral quality of light on morphogenesis, flowering, and photoperiodic gene expression during exposure to light quality of night interruption (NI) was investigated in Dendranthema grandiflorum. The circadian rhythms of plants grown in a closed walk-in growth chamber were interrupted at night for a total of 4 h, using light-emitting diodes with an intensity of 10 μmol·m−2·s−1 PPF. The light quality of the NI was shifted from one wavelength to another after the first 2 h. Light treatments consisting of all possible pairings of blue (B), red (R), far-red (Fr), and white (W) light were tested. Plants in the NI treatment groups exposed to Fr light grew larger than plants in other treatment groups. Of plants in NI treatment groups, those in the NI-WB treatment grew the least. In addition, the impact of shifts in the light quality of NI on leaf expansion was greater in treatment groups exposed to a combination of either B and R or R and W light, regardless of their order of supply. Flowering was observed in the NI-RB, NI-FrR, NI-BFr, NI-FrB, NI-WB, NI-FrW, NI-WFr, NI-WR, and SD (short-day) treatments, and was especially promoted in the NI-BFr and NI-FrB treatments. In a combined shift treatment of B and R or B and W light, the NI concluded with B light (NI-RB and NI-WB) treatment induced flowering. The transcriptional factors phyA, cry1 and FTL (FLOWERING LOCUS T) were positively affected, while phyB and AFT were negatively affected. In conclusion, morphogenesis, flowering, and transcriptional factors were all significantly affected either positively or negatively by shifts in the light quality of NI. The light quality of the first 2 h of NI affected neither morphogenesis nor flowering, while the light quality of the last 2 h of NI significantly affected both morphogenesis and flowering.
Keywords: anthesis, blue LED (light-emitting diode), chrysanthemum, flowering, light quality, photomorphogenesis, photoreceptor
1. Introduction
Light signals regulate plant growth and development by sustaining environmental conditions [1]. To enhance growth and development, 24 h day/night plants develop an endogenous circadian clock [2]. This circadian clock is used to anticipate environmental changes and coordinate physiological and behavioral changes with environmental shifts [3]. Most flowering plants are severely dependent on light intensity patterns similar to their natural photoperiodic conditions to trigger normal biological functions [4,5,6,7]. However, photoperiodic conditions can be manipulated artificially to induce early flowering for commercial horticulture [8]. Manipulation of the photoperiod can also reduce production costs by reducing production times and improving the overall quality of the crop [9]. The photoperiod regulates growth and flowering in photoperiodic plants [10,11,12].
The use of artificial lighting at night (night interruption, NI) can regulate the flowering of photoperiodic species [13,14]. The NI breaks a long dark period to deliver photoperiodic lighting and thereby create modified long day (LD) conditions [15]. It has been reported that Cymbidium aloifolium photosynthesizes during 4 h NIs of photon fluence as low as 3–5 μmol·m−2·s−1 suggesting that the increased growth and earlier flowering times that occur in response to NI be attributed to an increase in net photosynthesis during this NI period [16]. Therefore, even NIs of low intensity are economical and effective means of controlling flowering in some species. The duration of the NI used in many studies [16,17,18,19,20,21,22,23,24,25] investigating flowering control was ≥4 h. However, a short (15 min) interruption of the long-night phase effectively controlled flowering in chrysanthemum plants that were grown under B light during their photoperiod [24].
It is well known that the effect of light quality on flowering regulation varies among plant species [24]. Many long-day plants (LDPs) flower most rapidly when the artificial light they are exposed to contains far-red (Fr) wavelengths, particularly when the artificial light is applied at the end of the photoperiod [26,27]. In SDPs, a night break with red (R) light effectively inhibits flowering, while flowering is promoted by subsequent exposure to Fr light [28]. This suggests that R/Fr reversible phytochromes may be involved in this response. The phytochrome photoreceptors mediate light quality perception, stem elongation, and flowering in photoperiodic plants [29]. Recent molecular and genetic investigations [30,31] have revealed that phytochromes are essential for photoperiodic flowering in rice (a facultative SDP). Loss-of-function of all phytochrome rice genes (in the se5 mutant or phyA phyB phyC triple mutant) resulted in a deficient photoperiodic response and early flowering in short-day (SD) and long-day (LD) [30,31].
Flowering control by NIs presumably depends on the intensity, duration, and light quality of NI, as well as the plant species under consideration. Numerous papers have investigated the overall inhibitory effect of NIs on flowering when the NIs was timed to occur during the middle of the dark period. Although the effects of the duration and light quality of NIs were studied by Higuchi et al. [24] and Craig and Runkle [29], the effects of shifts in light quality of the NI have yet to be documented. Interestingly, Higuchi et al. [24] reported that in chrysanthemum grown with white light during photoperiod, flowering was observed under the SD interrupted in the middle of the dark period by a low intensity of blue (B) or far-red (Fr), while no flowering was observed under the same SD interrupted by red (R). Similar flowering responses were observed in our previous studies with chrysanthemum [32]. Therefore, we hypothesized that shifts in the light quality of low-intensity NI during the 4 h NI would affect flowering, either synergistically or antagonistically, and the light quality of the last 2 h of NI would have greater impact on flowering than the first 2 h of NI, as shown in a classical study on seed germination being affected by R and Fr shifting. In this study, we investigated the effect of shifts in the light quality of low-intensity NIs on not only flowering but also morphogenesis of Dendranthema grandiflorum “Gaya Yellow” (a qualitative SDP). In addition, the study examined the processes mediated by photoreceptors, with the goal of devising potential applications in floricultural crop production.
2. Results
2.1. Morphogenesis
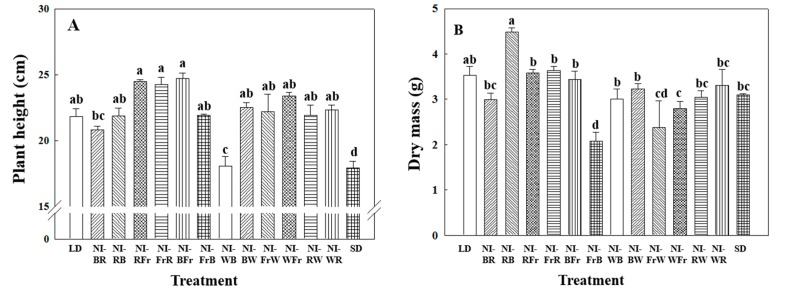
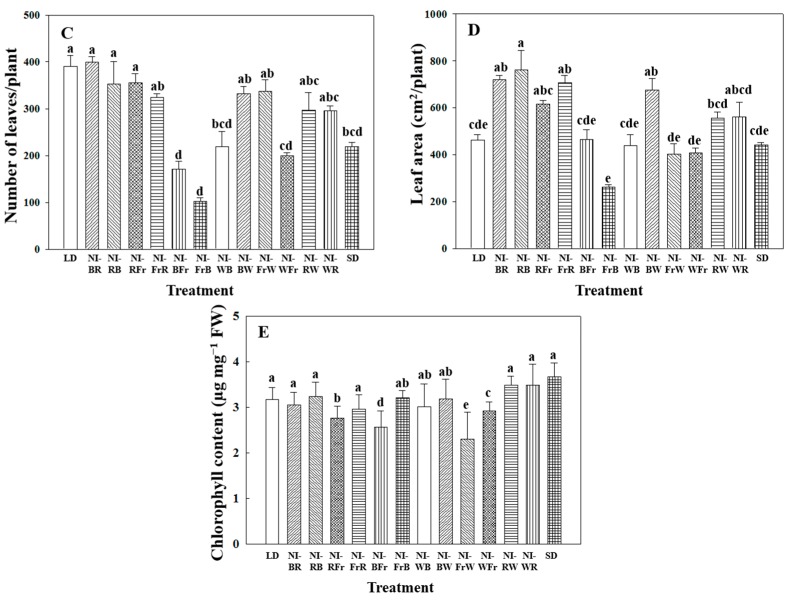
Compared to the SD control, plant height increased in all NI treatments except the NI-WB treatment (Figure 1A). The height increases were greatest in NI treatment groups subjected to Fr light, regardless of the order of the two light qualities used. Dry masses were similar across all NI treatments to that of the SD control, with the exception of the LD, NI-RB, NI-FrB, and NR-FrW, treatments (Figure 1B). Compared to the SD control group, dry mass increased by 45% in the NI-RB treatment, and decreased by 33% in the NI-FrB treatment. The number of leaves per plant was significantly lower in the NI-BFr and NI-FrB treatments, compared to the SD control (Figure 1C). Leaf area was significantly higher in the NI-BR and NI-RB treatments, and lower in the NI-FrB treatments (Figure 1D), suggesting that leaf expansion was the result of the combined effect of the first and last light quality of NI. For example, the combination of the B and R light promotes leaf expansion, while the combination of the Fr and B suppresses it. Overall, the chlorophyll content was lower in the NI of Fr light treatment plants than that of plants in other treatment groups, regardless of the light quality of NI order used (Figure 1E). Chlorophyll content was 37% lower in the NI-FrW treatment compared to the SD control.
Figure 1.
Effects of the shifts in the light quality of night interruption (NI) on (A) plant height; (B) dry mass; (C) Number of leaves per plant; (D) leaf area; and (E) chlorophyll content in Dendranthema grandiflorum “Gaya Yellow” (Please refer to Figure 1 for details of light quality of NI). Vertical bars indicate ± S.E of the means for n = 3. Means accompanied by different letters are significantly different (p < 0.05) according to the Tukey’s studentized range test.
2.2. Flowering
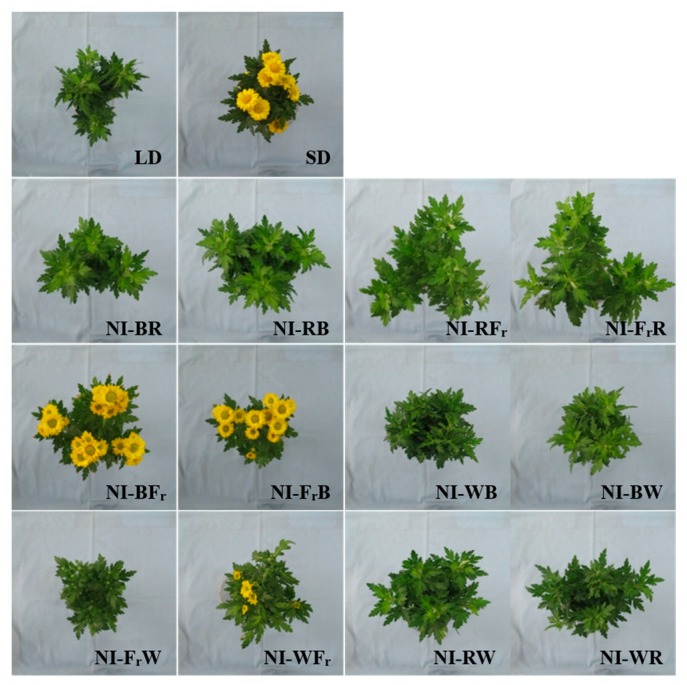
Flowering was induced by the NI-RB, NI-FrR, NI-BFr, NI-FrB, NI-WB, NI-FrW, NI-WFr, NI-WR, and SD treatments, and the DVB was shortened in the NI-BFr and NI-FrB treatments (Figure 2 and Table 1). The DVB increased in the NI-RB, NI-FrR, NI-FrW, and NI-WR treatments (Table 1). The results also showed that number of flowers per plant increased by 32% in the NI-BFr treatment, compared to the SD control (Table 1). The number of flowers per plant was the least in the NI-RB (Table 1).
Figure 2.
Effects of the shifts in the light quality of night interruption (NI) provided at 10 μmol·m−2·s−1 PPF (photosynthetic photon flux) on the flowering of Dendranthema grandiflorum “Gaya Yellow” measured at 49 days after treatment: (A) side view and (B) top view.
Table 1.
Effects of shifting the light quality of night interruption (NI) provided at 10 μmol·m−2·s−1 PPF (photosynthetic photon flux), on the flowering characteristics of Dendranthema grandiflorum “Gaya Yellow”, measured at 49 days after treatment.
| Treatment | DVB y (Day) | Number of Flowers/Plant |
|---|---|---|
| LD | – z | – |
| NI-BR | – | – |
| NI-RB | 44.0 | 0.3 e w |
| NI-RFr | – | – |
| NI-FrR | 44.0 | – |
| NI-BFr | 14.8 | 27.3 a w |
| NI-FrB | 12.2 | 20.0 b w |
| NI-WB | 26.4 | 9.3 d w |
| NI-BW | – | – |
| NI-FrW | 33.7 | 12.3 c w |
| NI-WFr | 17.6 | 15.0 c w |
| NI-RW | – | – |
| NI-WR | 41.6 | 5.3 e w |
| SD | 13.6 | 20.6 b w |
y, Days from treatment initiation to visible flower bud, or days to visible buds; z, No flowering; w, Mean separation within columns by Tukey’s studentized range test at a 5% significance level.
2.3. Photoreceptor Gene Expression Analysis
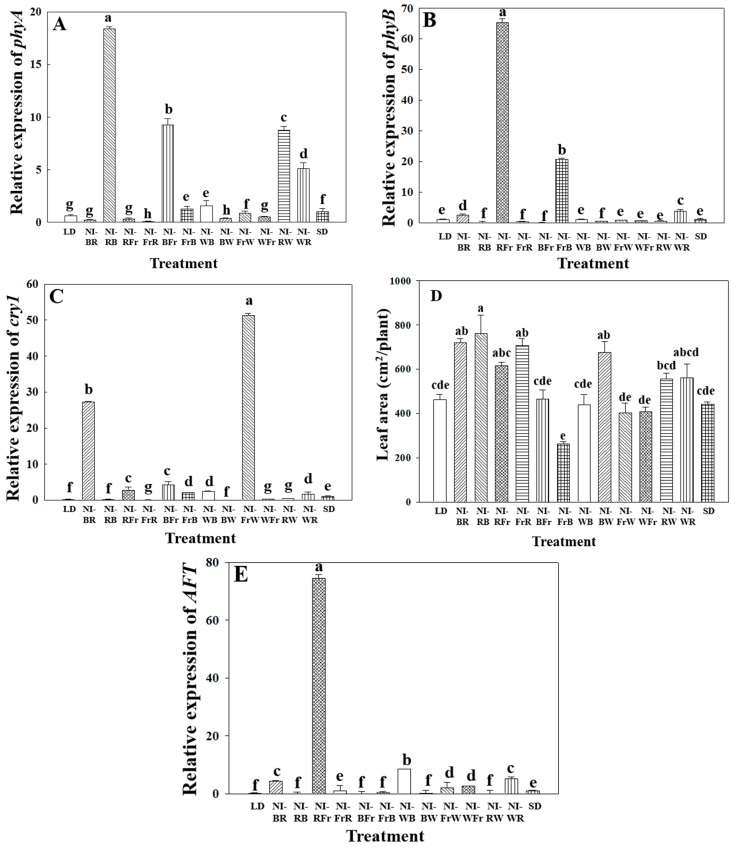
The results of the real-time PCR analysis indicated that phyA was highly expressed in the NI-RB, NI-BFr, NI-RW, and NI-WR treatments (Figure 3A). The relative expression rate of phyB was higher under the NI-RFr and NI-FrB treatments (Figure 3B). Higher expression rates of cry1 were observed in the NI-BR and NI-FrW treatments, compared to the SD control (Figure 3C). The FTL and AFT genes were highly expressed in the NI-RFr as compared to the SD control (Figure 3D,E).
Figure 3.
Relative gene expression of (A) phyA (B) phyB; (C) cry1; (D) FTL; and (E) AFT of Dendranthema grandiflorum “Gaya Yellow”. Vertical bars indicate ± S.E of the means for n = 3. Means accompanied by different letters are significantly different (p < 0.05) according to the Tukey’s studentized range test.
3. Discussion
3.1. Morphogenesis
Compared to the SD control, plant height decreased in the NI-WB treatment (Figure 1A). The successful use of exposure to B light (NI-WB) during the photoperiod to suppress plant elongation has previously been documented in chrysanthemum. Folta and Spalding [33] found that the inhibition response was associated with the involvement of cryptochromes and phototropins, and demonstrated that the inhibition process was a de-etiolated response. In this study, the suppression of vertical plant growth observed in the NI-WB treatment group was likely due to the use of B light in the second half of the NI. Moreover, this finding suggests that the B and W light receptors have a synergistic effect on the inhibition of vertical plant growth in chrysanthemum. The Fr light induced increase of the plant height, irrespective of the order of the two light qualities used (Figure 1A). The results are in agreement with those of Liao et al. [23] who suggested the enhancement of shoot extension by Fr light during the photoperiod. Shoot elongation is generally controlled by the endogenous gibberellins. The Fr light possesses the ability to induce the gibberellin biosynthesis resulting in increased shoot elongation [23].
The decrease of dry mass observed in the NI-FrB, NI-FrW, and NI-WFr treatment groups (Figure 1B) might be due to the effect of the Fr, as evidenced by the existence of the plant’s shade avoidance response, which is often accompanied by reductions in leaf area, shoot biomass, and the size of harvestable organs, a likely consequence of the reallocation of resources towards reproductive structures [34,35,36]. Compared to the SD control group, dry mass was significantly higher by 45% in the NI-RB treatment (Figure 1B) indicating that the treatment promoted photosynthesis, even at low light intensities. Photon fluxes lower than the compensation point still can help the plant growth by increasing the daily net photosynthesis, and the accumulative effect of this over the whole culture period could be considerable. Although the reason for this is not clear, similarly, significantly increased dry masses by the NI at 3–7 μmol·m−2·s−1 were reported in Cymbidium [16]. Also the NI with low photon fluxes promoted plant growth and flowering in Cyclamen (by 20 μmol·m−2·s−1 photon flux) [37], and Pansy and Hibiscus (by 40–50 μmol·m−2·s−1· photon flux) [38]. Daily light integral in this study was only 2.2% higher in the NI-RB (6.62 mol·m−2·day) than the SD control (6.48 mol·m−2·day). Therefore, in this study, the increase in the dry mass of plants in the NI-RB treatment group could be attributable to the synergistic effects of the yet unknown reasons of the R and B on vegetative growth characteristics such as leaf area which had a significant 73% increase in the NI-RB over the SD control.
The number of leaves per plant was significantly lower in the NI-BFr and NI-FrB treatments as compared to the SD control; this was likely due to the termination of vegetative growth caused by early flowering (Figure 1C). Constitutively, the number of leaves was dependent on changes in the number of days to flowering, especially when flowering was initiated early [12]. Decrease in chlorophyll content in the NI-FrW treatment as compared to the SD control in this study suggests that the Fr fluorescence consisted of low-intensity light as compared to the higher levels of incident light reflected from the leaves in this spectral region [39].
3.2. Flowering
The DVB (days from treatment initiation to visible flower bud) was higher in the NI-RB, NI-FrR, NI-FrW, and NI-WR treatments than the SD control (Table 1). This suggests that B, R, W, and Fr light receptors had an antagonistic effect on the promotion of flowering in chrysanthemum plants. The shortened DVBs in the NI-BFr and NI-FrB treatments (Table 1) suggest that in chrysanthemum (a SDP), flowering was promoted by the synergistic effects of B and Fr light. In a combined shifting treatment of B and R light or B and W light, the NI ending with B light (NI-RB and NI-WB) induced flowering. Thus, it is probable that the B light receptor can enhance floral-inducer activity, resulting in a higher energy requirement for the promotion of flowering. Previously, it was reported that Fr and B light promote flowering, whereas R light often inhibits it [12]. However, in both the combined R and Fr light treatment (NI-RFr and NI-FrR), and the Fr and W (NI-FrW and NI-WFr) light treatment, flowering was not affected by changes in the light quality of the NI. These findings suggest that the Pr/Pfr ratio might have a bigger impact on flowering than the effects of changes in the light quality of the NI. In many SDPs, the NI is effective only when the supplied dose of light is sufficient to fully photoconvert all Pr (the phytochrome that absorbs R light) [40]. A subsequent exposure to Fr light, which photoconverts the pigment back to its physiologically inactive Pr form, restores the flowering response.
In this study, taller plants due to Fr light produced more flowers per plant, and this could be explained by two reasons. The increase in the number of flowers per plant in the NI-BFr light (Table 1) might be due firstly to high light energy induction and evasion of darkness in the shoot with longer internodes, which is known as the shade avoidance response. The reactions in response to shade avoidance are all initiated by a single environmental signal: the reduction in the ratio of R to Fr radiation (i.e., R:Fr) that occurs within crowded plant communities [41]. This shade avoidance response is characterized by increased hypocotyl, stem, and petiole elongation; a more erect leaf position; increased apical dominance; and early flowering [42,43]. When grown in a close proximity to neighboring plants, many species grow elongated stems and smaller leaves, a behavior referred to as the shade avoidance response [44]. This response increases their ability to access sunlight by growing taller than other plants, and thus constitutes a competitive advantage [44]. This increase in the height growth could results in an increase in penetration of light energy through the canopy; leading to the increased production of photosynthetic assimilates during the photoperiod, due to increased stem growth and the development of a larger number of axillary buds. The second reason for the increased number of flowers in the NI-BFr light could be due to B light-related photoreceptors playing important roles toward inducing the flowering responses [45,46]. This finding on the increase in the number of flowers per plant in the NI-BFr light suggests that the B and Fr light, when given in this order, has a synergistic effect on the increase of the number of flowers per plant.
3.3. Photoreceptor Gene Expression Analysis
The phyA mediates the promotion of flowering by Fr light [47,48]. In contrast, phyB acts in a partially redundant manner with phytochrome D (phyD) and phytochrome E (phyE), mediating the inhibition of flowering by R light [34,49,50,51,52]. However, the function of phyB in floral initiation may be more complex than a simple floral inhibitor. The cry1 and cry2 act redundantly to mediate the promotion of flowering by B light [48,53]. Photoperiodic floral initiation is regulated by a systemic flowering inducer (florigen) and inhibitor (antiflorigen) such as the FTL and AFT genes, respectively, which are produced in the leaves [54].
The flowering that occurred in the NI-FrB treatment, even when the phyB gene (which is thought to be a flowering inhibitor gene) was expressed may be caused by high levels of expression of the flowering promotor genes phyA and cry1 (Figure 3A–C). The phyB-mediated antiflorigen production system plays a predominant role in the obligate photoperiodic flowering response in chrysanthemum, allowing the strict maintenance of the vegetative state under non-inductive photoperiod conditions [54]. In the NI-RW treatment, even though a high level of phyA gene expression was induced, the plants did not flower (Figure 3A). Similarly, the lack of flowering was observed in the NI-BR treatment, in spite of high levels of cry1 and FTL expression (Figure 3C,D). In this treatment, it is thought that although the first 2 h of NI with B light promoted flowering by stimulating the expression of the cry1 and FTL genes, flowering was suppressed by exposure to R light during the last 2 h of the NI and/or interactions among the flowering-related genes. The absence of flowering that was observed even when the FTL gene was highly expressed in the NI-RFr treatment may be due to the high expression levels of the phyB and AFT genes observed in that treatment (Figure 3B,D,E). Based on the results in the NI-BR and NI-RFr treatment, it is assumed that the R light has relatively large, while the Fr light has relatively small, effect on flowering in chrysanthemum. The flowering-suppressing AFT gene was most highly expressed in the NI-RFr treatment, in which flowering was not observed as expected, and slightly expressed in the NI-WB treatment, in which plants did flower. It is assumed that R light has more effect on flowering in chrysanthemum than W light.
The overall expression patterns of photomorphogenic genes observed in this study are different from those observed in previous studies, as described in the previous paragraph. These differences might be due to the use of different chrysanthemum genotypes, differences in the experimental environment, or differences in sample collection time, the plant part from which the samples were collected, and the specific wavelengths of light produced by the LEDs used in each study. It seems contradictory to have similarly low levels of most photoreceptor genes in both inductive (SD) and noninductive (LD) treatments. The real reason for low expressions of most photoreceptor genes in both LD and SD treatments relative to the NI treatment is not clearly explainable. It could be that the expression level of these genes changes over time, presumably the greatest at the early stage of flowering induction and gradually decreasing afterward. Thus, it might be necessary to test whether ≤4 h of NI treatment with specific wavelengths and at considerably lower PPFs than currently employed can be used to suppress flowering in chrysanthemum, a qualitative SDP. The practical applicability of the light quality shifting of NI treatments in commercial crop production systems needs to be investigated in cases where the goal is the prevention of physiological disorders caused by light contamination during the dark period. In potted plants, consumers usually prefer smaller plant height. Therefore, B light has potential practical applicability for potted floricultural crops. We recommended that use in combination of B and Fr light (NI-BFr and NI-FrB) promote flowering and get many flowers, while use in combination of Fr and W light (NI-FrW) prolong flowering. In addition, the optimization of NI treatments in terms of intensity, duration, wavelength, and time of application also requires further study.
4. Experimental Section
4.1. Plant Material and Growth Conditions
Spray-type chrysanthemum (Dendranthema grandiflorum “Gaya Yellow” (a qualitative short-day plant, SDP)) cuttings, provided by the Flower Research Institute of the Gyeongsangnam-do Agricultural Research and Extension Services, Republic of Korea, were stuck in 50-cell plug trays containing a commercial medium (Tosilee Medium, Shinan Grow Co., Jinju, Korea), then placed on a glasshouse bench for rooting. Rooted cuttings were transferred to a closed walk-in growth chamber 7700 cm × 2500 cm × 2695 cm for 12 days at 20 ± 1 °C, 60% ± 10% RH, and 140 μmol·m−2·s−1 PPF provided by fluorescent lamps (F48T12-CW-VHO, Philips Co., Ltd., Eindhoven, The Netherlands), for acclimatization. This growth chamber was specially designed to blow in the conditioned air horizontally into the growing spaces through numerous uniformly distributed holes. The CO2 concentration was maintained at atmospheric levels (350 ± 50 μmol·mol−1) by supplementation from a compressed gas tank. The critical day length required for flowering in the SDP used in this study was about 14 h; therefore, a 10 h period of darkness was sufficient to initiate flowering. During the acclimatization period, plants were grown under a 16 h photoperiod (a long day condition, LD) to suppress flower initiation. After 12 days of acclimatization, the plants (approximately 13.3 cm in height) were transferred to the photoperiodic light treatments. The plants were fertilized once a day with a multipurpose greenhouse nutrient solution (in mg·L−1 Ca(NO3)2·4H2O 737.0, KNO3 343.4, KH2PO4 163.2, K2SO4 43.5, MgSO4·H2O 246.0, NH4NO3 80.0, Fe-EDTA 15.0, H3BO3 1.40, NaMoO4·2H2O 0.12, MnSO4·4H2O 2.10, and ZnSO4·7H2O 0.44 (electrical conductivity 1.5 mS·cm−1) throughout the experiment.
4.2. Photoperiodic Light Treatments
The plants were grown under 180 μmol·m−2·s−1 PPF provided by white (W) light-emitting diodes (LEDs) (MEF50120, More Electronics Co., Ltd., Changwon, Korea), under light treatment regimens of either a long day (LD, 16 h light/8 h dark), short-day (SD, 10 h light/14 h dark), or SD with a night interruption (NI) for a total of 4 h (from 11:00 p.m. to 3:00 a.m.), using LEDs at an intensity of 10 μmol·m−2·s−1 PPF. The photoperiod was started at 8:00 a.m. every day in all treatments. The light quality of the night interruption (NI) was shifted from one state to another after the first 2 h. Light quality combinations included all possible paired arrangements of blue (B, 450 nm), red (R, 660 nm), far-red (Fr, 730 nm), and white (W, 400–750 nm, with 28% B, 37% R, and 15% Fr light) light, as follows: from B to R (NI-BR), from R to B (NI-RB), from R to Fr (NI-RFr), from Fr to R (NI-FrR), from B to Fr (NI-BFr), from Fr to B (NI-FrB), from W to B (NI-WB), from B to W (NI-BW), from Fr to W (NI-FrW), from W to Fr (NI-WFr), from R to W (NI-RW), and from W to R (NI-WR) (Figure 4). All NI treatments were provided to plants separately, by subdividing the plant growth chamber with black cloth to prevent the spatial overlap of different light treatments. The LD and uninterrupted SD conditions were used as controls.
Figure 4.
Representative image of the shifts in the light quality of night interruption (NI) by light emitting diodes (LEDs) during 4 h night interruptions (NI) of the 10 h short-day (SD) treatments in Dendranthema grandiflorum “Gaya Yellow”: NI-BR, blue to red; NI-RB, red to blue; NI-RFr, red to far-red; NI-FrR, far-red to red; NI-BFr, blue to far-red; NI-FrB, far-red to blue; NI-WB, white to blue; NI-BW, blue to white; NI-FrW, far-red to white; NI-WFr, white to far-red; NI-RW, red to white; and NI-WR, white to red. The LD indicates the 16 h long day treatment.
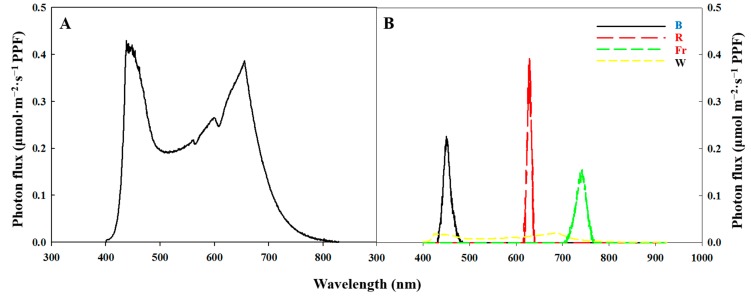
The spectral distributions of all light treatments were scanned using a spectroradiometer (USB 2000 Fiber Optic Spectrometer, Ocean Optics Inc., Dunedin, FL, USA; wavelength detection range of 200 to 1000 nm) 25 cm from the bench top, at an interval of 1 nm (Figure 5). In each light treatment, the spectral distribution and the average value of maximum absolute irradiance were measured at three locations on the plant growing bench.
Figure 5.
Spectral distribution of light used in a closed walk-in growth chamber: white light emitting diodes (LEDs) used as daily light (A) and different LEDs (B, blue; R, red; Fr, far-red; and W, white) used as light of night interruption (NI) (B).
4.3. Data Collection and Analysis
The cultivation experiment with all the treatments was repeated twice and, since the results of both experiments showed same trends, only results of the second experiment were presented in this manuscript. At 49 days after the initiation of the photoperiodic treatments, the plant height, number of leaves per plant, chlorophyll content, fresh and dry masses of the shoot and root, percentage of flowering plants, days from treatment initiation to visible buds (DVB), number of flowers and flower buds per plant (mentioned as “number of flowers hereafter”), and the expression of important photoreceptor genes were either measured or assessed.
For the estimation of chlorophyll content, 10 mg of fresh leaf sample was taken from the young fully developed leaves from each biological replicate, and extracted using 80% ice-cold acetone. After centrifugation at 3000 rpm, the absorbance of the supernatant was measured using a spectrophotometer (Biochrom Libra S22, Biochrom Co., Ltd., Holliston, MA, USA) at 663 and 645 nm. Calculation of total chlorophyll was done according to the method described by Dere et al. [55]. The dry masses of the shoots and roots were determined after drying the samples in an oven (Model FO-450M, Jeio Technology Co., Ltd., Seoul, Korea) at 75 °C for 3 days.
4.4. Total RNA Isolation, cDNA Synthesis, and Real-Time Polymerase Chain Reaction (PCR) of Selected Genes
For total RNA extraction, the most recently mature leaf was used which was collected in the morning (9:00 a.m.) at 30 days after the initiation of the photoperiodic light treatments in all treatments when the plants in the SD treatment already started to bloom, those in the inductive NI treatments had flower buds formed, and those in the LD treatment were still vegetative. Total RNA was extracted using an RNA isolation kit according to the manufacturer’s instructions (Promega, Madison, WI, USA). The sample collection time (9:00 a.m.) was selected because photosynthetic rates are high, and all plant processes including gene expression are very active at this point of time. One microgram of DNase-treated RNA was reverse transcribed using a reverse transcription kit (Promega, Madison, WI, USA) to synthesize first-strand cDNA, which was used as a template for the polymerase chain reaction (PCR). Real-time PCR was performed using a Rotor-Gene Q 2plex HRM Platform (Rotor-Gene Q 2plex HRM Platform, Westburg, The Netherland) and SYBR green from a Qiagen qPCR kit (Qiagen OneStep RT-PCR Kit, Westburg, The Netherland) as a reference dye. Independent real-time PCR reactions with equal amounts of cDNA were performed using the phytochrome A (phyA), phytochrome B (phyB), cryptochrome 1 (cry1), Anti-florigenic FT/TFL1 family protein (AFT), and FLOWERING LOCUS T (FTL) gene primers (the primer sequences are shown in Table 2). Actin was used as an internal control, as it is commonly used to normalize molecular expression studies because of its high level of conservation as an endogenous housekeeping gene. The real-time PCR conditions were as follows: initial denaturation for 5 min at 95 °C, followed by 25 cycles consisting of 20 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C, then 10 min at 72 °C for final extension. To calculate the relative expression levels (treatment/control, SD), the individual expression values of treatment plants were divided by the mean value for SDPs at each sampling date.
Table 2.
List of primers used to quantify gene expression levels.
| Gene | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| Ch-phyA | EU915082 | 5′-GACAGTGTCAGGCTTCAACAAG-3′ | 5′-ACCACCAGTGTGTGTTATCCTG-3′ |
| Ch-phyB | NM_127435 | 5′-GTGCTAGGGAGATTACGCTTTC-3′ | 5′-CCAGCTTCTGAGACTGAACAGA-3′ |
| Ch- cry1 | NM_116961 | 5′-CGTAAGGGATCACCGAGTAAAG-3′ | 5′-CTTTTAGGTGGGAGTTGTGGAG-3′ |
| Ch- FTL | AB839767 | 5′-ACAACGGACTCCTCATTTGG-3′ | 5′-CGCGAAACTACGAGTGTTGA-3′ |
| Ch- AFT | AB839766 | 5′-AGAACACCTCCATTGGATCG-3′ | 5′-CTGGAACTAGGTGGCCTCAC-3′ |
| Ch- Actin | AB205087 | 5′-CGTTTGGATCTTGCTGGTCG-3′ | 5′-CAGGACATCTGAAACGCTCA-3′ |
4.5. Statistical Analysis
A randomized complete block design with 3 replicates of 2 plants each was used in this experiment. The data collected during the experiment were analyzed for statistical significance using the SAS (Statistical Analysis System, V. 9.1, Cary, NC, USA) program. Differences among the treatment means were assessed using Tukey’s studentized range test at p < 0.05. Graphing was performed with Sigma Plot 10.0 (Systat Software, Inc., San Jose, CA, USA).
5. Conclusions
Plant height was the smallest in the NI-WB treatment among all NI treatments. The impact of changing the light quality at low intensity NI treatments on leaf expansion was greater in treatments using a combination of B and R or R and W light, regardless of their order of use. Flowering was observed in the NI-RB, NI-FrR, NI-BFr, NI-FrB, NI-WB, NI-FrW, NI-WFr, NI-WR, and SD treatments, and was especially promoted in the NI-BFr and NI-FrB treatments. In a combined shifting treatment of either B and R light or B and W light, the NI concluding with B light (NI-RB and NI-WB) exposure induced flowering. Thus, it is likely that the B light receptor enhances the activity of floral inducers, resulting in a higher energy requirement for the promotion of flowering. The transcriptional factors phyA, cry1, and FTL were positively affected, while phyB and AFT were negatively affected. The expression of morphogenesis, flowering, and transcriptional factors was significantly affected either positively or negatively by changes in the light quality of the NI. Statistically, the light quality of the first 2 h of NI exposure affected neither morphogenesis nor flowering, while the light quality of the last 2 h of NI exposure significantly affected both morphogenesis and flowering, as we hypothesized. Further studies are required to investigate the effects of B light supplementation on flowering promotion, photoreceptor gene expression, and protein production in other cultivars of chrysanthemum.
Acknowledgments
This work was supported by the BK21 Plus program, at Gyeongsang National University, Republic of Korea. The authors are thankful to Mr. Prabhakaran Soundararajan for assistance with the PCR analysis.
Abbreviations
AFT (Anti-florigenic FT/TFL1 family protein); B (blue); cry1 (cryptochrome 1); DVB (days after treatment initiation to visible flower bud or days to visible buds); FTL (FLOWERING LOCUS T); Fr (far-red); LD (long day); LDP (long day plant); LEDs (light emitting diodes); NI (night interruption); PCR (polymerase chain reaction); phyA (phytochrome A); phyB (phytochrome B); phyC (phytochrome C); phyD (phytochrome D); phyE (phytochrome E); R (red); SD (short-day); SDP (short-day plant); W (white).
Author Contributions
Yoo Gyeong Park and Byoung Ryong Jeong designed the experiment. Yoo Gyeong Park and Sowbiya Muneer performed the experiments and wrote the manuscript. Byoung Ryong Jeong proofread and finalized the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Casal J.J., Fankhauser C., Coupland G., Blázquez M.A. Signalling for developmental plasticity. Trends Plant Sci. 2004;9:309–314. doi: 10.1016/j.tplants.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Kutschera U., Briggs W.R. Seedling development in buckwheat and the discovery of the photomorphogenic shade-avoidance response. Plant Biol. 2013;15:931–940. doi: 10.1111/plb.12077. [DOI] [PubMed] [Google Scholar]
- 3.Lumsden P.J., Millar A.J. Biological Rhythms and Photoperiodism in Plants. 1st ed. Bios Scientific Publishers; Oxford, UK: 1998. [Google Scholar]
- 4.Somers D.E., Devlin P.F., Kay S.A. Phytochromes and cryptochromes in the entertainment of the Arabidopsis circadian clock. Science. 1998;282:1488–1490. doi: 10.1126/science.282.5393.1488. [DOI] [PubMed] [Google Scholar]
- 5.Somers D.E., Kim W.Y., Geng R. The F-Box protein ZEITLUPE confers dosage-dependent control on the circadian clock, photomorphogenesis, and flowering time. Plant Cell. 2004;16:769–782. doi: 10.1105/tpc.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim W.Y., Hicks K.A., Somers D.E. Independent roles for EARLY FLOWERING 3 and ZEITLUPE in the control of circadian timing, hypocotyl length, and flowering time. Plant Physiol. 2005;139:1557–1569. doi: 10.1104/pp.105.067173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara S., Wang L., Han L., Suh S.S., Salome P.A., McClung C.R., Somers D.E. Post-translational regulation of the Arabidopsis circadian clock through selective proteolysis and phosphorylation of pseudo-response regulator proteins. J. Biol. Chem. 2008;34:23073–23083. doi: 10.1074/jbc.M803471200. [DOI] [PubMed] [Google Scholar]
- 8.Warner R.M., Erwin J.E. Effect of photoperiod and daily light integral on flowering of five Hibiscus sp. Sci. Hortic. 2003;97:341–351. doi: 10.1016/S0304-4238(02)00157-7. [DOI] [Google Scholar]
- 9.Runkle E.S., Heins R.D. Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J. Am. Soc. Hortic. Sci. 2001;126:275–282. [Google Scholar]
- 10.Casal J.J., Deregibus V.A., Sanchez R.A. Variations in tiller dynamics and morphology in Lolium multiflorum Lam. vegetative and reproductive plants as affected by differences in red/far-red irradiation. Ann. Bot. 1985;56:553–559. [Google Scholar]
- 11.Leopold A.C. Photoperiodism in plants. Q. Rev. Biol. 1951;26:247–263. doi: 10.1086/398234. [DOI] [PubMed] [Google Scholar]
- 12.Lin C. Photoreception and regulation of flowering time. Plant Physiol. 2000;123:139–150. doi: 10.1104/pp.123.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard M.G., Runkle E.S. Intermittent light from a rotating high-pressure sodium lamp promotes flowering of long-day plants. HortScience. 2010;45:236–241. [Google Scholar]
- 14.Yamada A.T., Tanigawa T., Suyama T., Matsuno T., Kunitake T. Night break treatment using different light sources promotes or delays growth and flowering of Eustoma grandiflorum (Raf.) Shinn. J. Jpn. Soc. Hortic. Sci. 2008;77:69–74. doi: 10.2503/jjshs1.77.69. [DOI] [Google Scholar]
- 15.Vince-Prue D., Canham A.E. Horticultural significance of photomorphogenesis. In: Shropshire W., Mohr H., editors. Encyclopedia of Plant Physiology. Springer-Verlag; Berlin, Germany: 1983. pp. 518–544. [Google Scholar]
- 16.Kim Y.J., Lee H.J., Kim K.S. Night interruption promotes vegetative growth and flowering of Cymbidium. Sci. Hortic. 2011;130:887–893. doi: 10.1016/j.scienta.2011.08.031. [DOI] [Google Scholar]
- 17.Runkle E.S., Heins R.D., Cameron A.C., Carlson W.H. Flowering of herbaceous perennials under various night interruption and cyclic lighting treatments. HortScience. 1998;33:672–677. [Google Scholar]
- 18.Ishikawa R., Tamaki S., Yokoi S., Inagaki N., Shinomura T., Takano M., Shimamoto K. Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell. 2005;17:3326–3336. doi: 10.1105/tpc.105.037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto N., Kumagai T., Koornneef M. Flowering responses to light-breaks in photomorphogenic mutants of Arabidopsis thaliana, a long-day plant. Physiol. Plant. 2006;83:209–215. doi: 10.1111/j.1399-3054.1991.tb02144.x. [DOI] [Google Scholar]
- 20.Oh W., Rhie Y.H., Park J.H., Runkle E.S., Kim K.S. Flowering of cyclamen is accelerated by an increase in temperature, photoperiod and daily light integral. J. Hortic. Sci. Biotechnol. 2008;83:559–562. [Google Scholar]
- 21.Kim Y.J., Lee H.J., Kim K.S. Carbohydrate changes in Cymbidium “Red Fire” in response to night interruption. Sci. Hortic. 2013;162:82–89. doi: 10.1016/j.scienta.2013.07.045. [DOI] [Google Scholar]
- 22.Park Y.J., Kim Y.J., Kim K.S. Vegetative growth and flowering of Dianthus, Zinnia, and Pelargonium as affected by night interruption at different timings. Hortic. Environ. Biotechnol. 2013;54:236–242. doi: 10.1007/s13580-013-0012-3. [DOI] [Google Scholar]
- 23.Liao Y., Suzuki K., Yu W., Zhuang D., Takai Y., Ogasawara R., Shimazu T., Fukui H. Night-break effect of LED light with different wavelengths on shoot elongation of Chrysanthemum morifolium Ramat “Jimba” and “Iwa no hakusen”. Environ. Control Biol. 2014;52:51–55. doi: 10.2525/ecb.52.51. [DOI] [Google Scholar]
- 24.Higuchi Y., Sumitomo K., Oda A., Shimizu H., Hisamatsu T. Day light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J. Plant Physiol. 2012;169:1789–1796. doi: 10.1016/j.jplph.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Meng Q., Runkle E.S. Low-intensity blue light in night-interruption lighting does not influence flowering of herbaceous ornamentals. Sci. Hortic. 2015;186:230–238. doi: 10.1016/j.scienta.2015.01.038. [DOI] [Google Scholar]
- 26.Downs R.J., Thomas J.F. Phytochrome regulation of flowering in the long-day plant Hyoscyamus niger. Plant Physiol. 1982;70:898–900. doi: 10.1104/pp.70.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane H.C., Cathey H.M., Evans L.T. Dependence of flowering in several long-day plants on spectral composition of light extending photoperiod. Am. J. Bot. 1965;52:1006–1014. doi: 10.2307/2440130. [DOI] [Google Scholar]
- 28.Cathey H.M., Borthwick H.A. Photoreversibility of floral initiation in chrysanthemum. Bot. Gaz. 1957;119:71–76. doi: 10.1086/335964. [DOI] [Google Scholar]
- 29.Craig D.S., Runkle E.S. Using LEDs to quantify the effect of the red to far-red ratio of night interruption lighting on flowering of photoperiodic crops. Acta Hortic. 2012;956:179–185. [Google Scholar]
- 30.Izawa T., Oikawa T., Sugiyama N., Tanisaka T., Yano M., Shimamoto K. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 2002;16:2006–2020. doi: 10.1101/gad.999202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takano M., Inagaki N., Xie X., Kiyota S., Baba-Kasai A., Tanabata T., Shinomura T. Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc. Natl. Acad. Sci. USA. 2009;106:14705–14710. doi: 10.1073/pnas.0907378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park Y.G., Muneer S., Jeong B.R. Quality and positioning of night interruption light affects morphogenesis and flowering in chrysanthemum. Plant Growth Regul. 2015 manuscript in preparation. [Google Scholar]
- 33.Folta K.M., Spalding E.P. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 2001;26:471–478. doi: 10.1046/j.1365-313x.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- 34.Devlin P.F., Patel S.R., Whitelam G.C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1488. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keiller D., Smith H. Control of carbon portioning by light quality mediated by phytochrome. Plant Sci. 1989;63:25–29. doi: 10.1016/0168-9452(89)90097-6. [DOI] [Google Scholar]
- 36.Robson P.R.H., Whitelam G.C., Smith H. Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol. 1993;102:1179–1184. doi: 10.1104/pp.102.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang K.J., Oh W., Shin J.H., Kim K.S. Night interruption and cyclic lighting promote flowering of Cyclamen persicum under low temperature regime. Hortic. Environ. Biotechnol. 2008;49:72–77. [Google Scholar]
- 38.Runkle E.S., Heins R.D. Photocontrol of flowering and extension growth in the long-day plant pansy. J. Am. Soc. Hortic. Sci. 2003;128:479–485. [Google Scholar]
- 39.Smorenburg K., Courreges-Lacoste G.B., Berger M., Buschman C., del Bello U., Langsdorf G., Lichtenthaler H.K., Sioris C., Stoll M., Visser H. Remote sensing of solar induced fluorescence of vegetation. Int. Soc. Opt. Photonics. 2002;4542:178–190. [Google Scholar]
- 40.Purohit S.S., Ranjan R. Phytochrome and flowering. In: Purohit S.S., Ranjan R., editors. Flowering. 1st ed. Agrobios; Jodhpur, India: 2002. pp. 52–61. [Google Scholar]
- 41.Smith H., Whitelam G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997;20:840–844. doi: 10.1046/j.1365-3040.1997.d01-104.x. [DOI] [Google Scholar]
- 42.Franklin K.A. Shade avoidance. New Phytol. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 43.Vandenbussche F., Pierik R., Millenaar F.F., Voesenek L.A., van Der Straeten D. Reaching out of the shade. Curr. Opin. Plant Biol. 2005;8:462–468. doi: 10.1016/j.pbi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Hersch M., Lorrain S., Wit M., Trevisan M., Ljung K., Bergmann S., Fankhause C. Light intensity modulates the regulatory network of the shade avoidance responses in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2014;111:6515–6520. doi: 10.1073/pnas.1320355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fankhauser C., Ulm R. Light-regulated interactions with SPA proteins underlie cryptochrome-mediated gene expression. Genes Dev. 2011;2:1004–1009. doi: 10.1101/gad.2053911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirose F., Shinomura T., Tanabata T., Shimada H., Takano M. Involvement of rice cryptochromes in de-etiolation responses and flowering. Plant Cell Physiol. 2006;47:915–925. doi: 10.1093/pcp/pcj064. [DOI] [PubMed] [Google Scholar]
- 47.Johnson E., Bradley M., Harberd N.P., Whitelam G.C. Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of day length extensions) Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mockler T., Yang H., Yu W., Parikh D., Cheng Y., Dolan S., Lin C. Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc. Natl. Acad. Sci. USA. 2003;100:2140–2145. doi: 10.1073/pnas.0437826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devlin P.F., Robson P.R., Patel S.R., Goosey L., Sharrock R.A., Whitelam G.C. Phytochrome D acts in the shade avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin K.A., Praekelt U., Stoddart W.M., Billingham O.E., Halliday K.J., Whitelam G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003;131:1340–1346. doi: 10.1104/pp.102.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weller J.L., Beauchamp N., Kerckhoffs L.H.J., Platten J.D., Reid J.B. Interaction of phytochromes A and B in the control of de-etiolation and flowering in pea. Plant J. 2001;26:283–294. doi: 10.1046/j.1365-313X.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 52.Mockler T.C., Guo H., Yang H., Duong H., Lin C. Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development. 1999;126:2073–2082. doi: 10.1242/dev.126.10.2073. [DOI] [PubMed] [Google Scholar]
- 53.Guo H., Yang H., Mockler T.C., Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- 54.Higuchi Y., Narumi T., Oda A., Nakano Y., Sumitomo K., Fukai S., Hisamatsu T. The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in chrysanthemums. Proc. Natl. Acad. Sci. USA. 2013;110:17137–17142. doi: 10.1073/pnas.1307617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dere S., Gunes T., Sivaci R. Spectrophotometric determination of chlorophyll—A, B and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998;22:13–17. [Google Scholar]