Abstract
Quality of Life (QoL) is decreased in multiple sclerosis (MS), but studies about the impact of sleep disorders (SD) on health-related quality of Life (HRQoL) are lacking. From our original cohort, a cross-sectional polysomnographic (PSG) study in consecutive MS patients, we retrospectively analysed the previously unpublished data of the Nottingham Health Profile (NHP). Those MS patients suffering from sleep disorders (n = 49) showed significantly lower HRQoL compared to MS patients without sleep disorders (n = 17). Subsequently, we classified the patients into four subgroups: insomnia (n = 17), restless-legs syndrome, periodic limb movement disorder and SD due to leg pain (n = 24), obstructive sleep apnea (n = 8) and patients without sleep disorder (n = 17). OSA and insomnia patients showed significantly higher NHP values and decreased HRQoL not only for the sleep subscale but also for the “energy” and “emotional” area of the NHP. In addition, OSA patients also showed increased NHP values in the “physical abilities” area. Interestingly, we did not find a correlation between the objective PSG parameters and the subjective sleep items of the NHP. However, this study demonstrates that sleep disorders can reduce HRQoL in MS patients and should be considered as an important confounder in all studies investigating HRQoL in MS.
Keywords: restless legs syndrome, sleep disorders, multiple sclerosis, clinical neurophysiology, polysomnography, insomnia, pain, depression, health, quality of life
1. Introduction
Recently, we published the results of a cross-sectional polysomnographic (PSG) study in consecutive multiple sclerosis (MS) patients [1]. Of 66 patients who underwent PSG, 49 suffered from a sleep disorder (SD); seven of these suffered from more than one SD. In these cases we classified only the more severe SD. In our study, SDs were significantly related to fatigue; and a follow-up investigation showed that a consequent treatment of sleep disorders may improve fatigue in a subset of patients [2]. The improvement of MS fatigue after medical treatment of SD was seen in another follow up study as well [3]. With regards to the relationship between health related Quality of Life (HRQoL) and SD in MS patients, there are only a few studies: Neau et al. [4], as well as Sarraf et al. [5], classified MS patients into good sleepers and poor sleepers using the Pittsburgh Sleep Quality Index [6] (PSQI) (PSQI ≤ 5 vs. > 5). In their studies, poor sleepers showed a reduced HRQoL using the MS-QOL-54 [7]. To our knowledge, there is only one study investigating the relationship between SDs confirmed by PSG and HRQoL in MS: Trojan et al. demonstrated a decreased mental but not physical HRQoL in MS patients with SD [8] using the Short Form Health Survey (SF-36) [9].
To date, there is no study investigating the HRQoL in MS patients with the Nottingham Health Profile (NHP) [10]. The NHP is a valid and reliable indicator of subjective health status in physical, social and emotional areas [10]. The NHP consists of two parts (part 1 and 2). Only part 1 is weighted and is composed of six subscales (sleep, physical mobility, energy, pain, emotional reactions and social isolation); the maximum of any subscale is 100. As a result, the maximum of the NHP total score is 600 (the higher the NHP values, the lower the HRQoL). The weighting of the 38 statements reflects the symptom severity and represents rather severe problems in order to avoid picking up a large number of false positives [10]. In the literature mine rescue workers show a very low global mean NHP score of 8.8; fit elderly persons show a mean global NHP score of 12.4; whereas pregnant women at 37 weeks, fracture victims and chronically ill elderly patients (mean global NHP 127.0/129.6/156.4) show increased NHP values, and especially high values were obtained in patients with osteoarthrosis (mean global NHP 271.3) [10]. Verwimp et al. investigated 75 OSA patients [11] and found an increased global NHP median (218). In their study the negative perception in the “physical abilities” domain was effectively related to an objective low level of physical activity measured by actigraphy.
In our previous cross-sectional trial we also collected NHP data, which had not been analysed and published before. The aim of this study is to describe these data and to investigate the relationship between SD and HRQoL in MS.
2. Results
2.1. Patients
We classified the 66 patients (21 men and 45 women aged 20–66 years) into four subgroups: no sleep disorder (NSD) (n = 17), insomnia (n = 17) (INS), periodic limb movement disorder (PLMD), restless legs syndrome (RLS) or SD due to leg pain (PLMD/RLS) (n = 24), and untreated obstructive sleep apnea (OSA) (n = 8). Expanded Disability Status Scale (EDSS) [12] values ranged from zero to eight.
2.1.1. HRQoL in MS Patients with Sleep Disorders Compared to Patients without Sleep Disorders
Table 1 shows the NHP values in patients without SD compared with those patients suffering from SD (all SD together). MS patients suffering from SD showed significantly increased NHP values, indicating poorer HRQoL using the Mann–Whitney-U-test.
Table 1.
NHP values in patients with and without sleep disorders.
| NHP Global Score and Subscales | Average and Range | All Patients | Patients without Sleep Disorders | Patients with Sleep Disorders | Differences between the Two Subgroups |
|---|---|---|---|---|---|
| NHP-Total | Mean (±standard deviation) | 146.1 (±119.8) | 67.3 (±60.0) | 175.2 (±123.6) | p = 0.001 |
| Min–Max | 0.0–78.7 | 0.0–188.6 | 0.0–413.7 | ||
| 25–75 | 0.0–32.6 | 21.8–120.5 | 61.3–273.3 | ||
| median | 126.4 | 34.8 | 175.5 | ||
| Physical abilities | Mean (±standard deviation) | 20.9 (±21.5) | 10.2 (±14.7) | 24.8 (±22.4) | p = 0.010 |
| Min–Max | 0.0–78.7 | 0.0–54.5 | 0.0–78.7 | ||
| 25–75 | 0.0–32.6 | 0.0–22.0 | 10.8–36.5 | ||
| median | 12.7 | 0.0 | 21.7 | ||
| Social isolation | Mean (±standard deviation) | 11.7 (±19.6) | 3.6 (±8.0) | 14.7 (±21.8) | p = 0.048 |
| Min–Max | 0.0–80.6 | 0.0–22.5 | 0.0–80.6 | ||
| 25–75 | 0.0–20.1 | 0.0–0.0 | 0.0–22.5 | ||
| median | 0.0 | 0.0 | 0.0 | ||
| Sleep | Mean (±standard deviation) | 29.3 (±29.5) | 10.6 (±15.1) | 36.2 (±30.6) | p = 0.001 |
| Min–Max | 0.0–100.0 | 0.0–50.4 | 0.0–100.0 | ||
| 25–75 | 0.0–16.1 | 0.0–12.6 | 12.6–72.7 | ||
| median | 50.4 | 0.0 | 28.7 | ||
| Pain | Mean (±standard deviation) | 15.8 (±24.8) | 3.8 (±9.8) | 20.2 (±27.2) | p = 0.009 |
| Min–Max | 0.0–100.0 | 0.0–32.3 | 0.0–100.0 | ||
| 25–75 | 0.0–0.0 | 0.0–0.0 | 0.0–30.6 | ||
| median | 26.0 | 0.0 | 9.9 | ||
| Energy | Mean (±standard deviation) | 48.9 (±40.9) | 29.9 (±35.5) | 55.8 (±40.9) | p = 0.016 |
| Min–Max | 0.0–100.0 | 0.0–100.0 | 0.0–100.0 | ||
| 25–75 | 0.0–60.8 | 0.0–62.0 | 24.0–100.0 | ||
| median | 100.0 | 0.0 | 62.0 | ||
| Emotional | Mean (±standard deviation) | 19.6 (±18.8) | 9.3 (±12.2) | 23.5 (±19.5) | p = 0.006 |
| Min–Max | 0.0–69.0 | 0.0–41.4 | 0.0–69.0 | ||
| 25–75 | 0.0–30.9 | 0.0–18.6 | 5.3–41.4 | ||
| median | 16.8 | 0.0 | 21.0 |
2.1.2. Comparison of the Global NHP Values (Global HRQoL) in the Four Subgroups
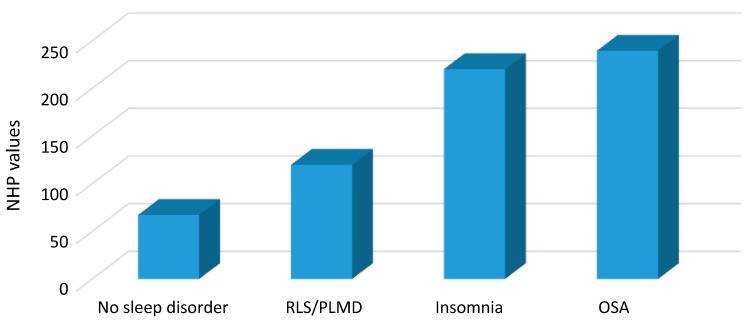
The comparison of the global NHP (including all six subscales) in the four subgroups showed significantly lower NHP values in the NSD and PLMD/RLS patients compared to OSA and insomnia patients; whereas there were no significant differences between NSD and PLMD/RLS patients neither between OSA and insomnia patients (see Figure 1 and Table 2). This suggests that NSD and PLMD/RLS patients have a better global HRQoL compared to OSA or insomnia patients.
Figure 1.
Nottingham Health Profile total values in the four subgroups.
Table 2.
NHP total score and NHP items in the different subgroups.
| NHP Values | Average and Range | All Patients | NSD | INS | OSA | PLM | Differences between the Two Subgroups |
|---|---|---|---|---|---|---|---|
| Total | Mean (SD) | 146.1 | 67.3 | 220.3 | 239.6 | 119.9 | NSD
vs. OSA p = 0.003 NSD vs. INS p < 0.0001 INS vs. PLM p = 0.002 OSA vs. PLM p = 0.042 NSD vs. PLM p = 0.210 OSA vs. INS p = 0.804 |
| Standard deviation | 119.8 | 60.0 | 88.2 | 136.2 | 123.7 | ||
| Min–Max | 0.0–78.7 | 0.0–188.6 | 60.7–369.9 | 24.7–413.7 | 0.0–408.61 | ||
| 25–75 | 0.0–32.6 | 21.8–120.5 | 147.0–276.0 | 180.8–393.3 | 29.7–174.4 | ||
| Median | 126.4 | 34.8 | 212.3 | 194.9 | 75.8 |
2.1.3. Comparison of the NHP Subscales in the Four Subgroups
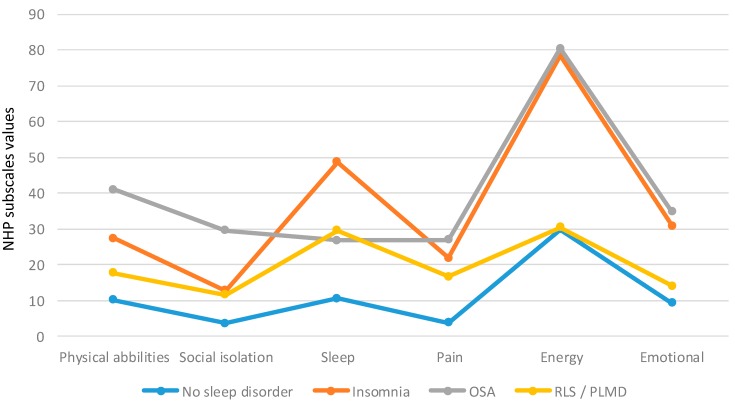
Figure 2 displays the NHP values in the different subgroups. The patients without sleep disorders showed the lowest NHP values in all items. The insomnia subgroup showed the highest values in the “sleep” item. Attention should also be paid to the high values concerning “energy” (and to a lower extent regarding “emotions”) in the insomnia and OSA subgroup. Please take into account the high values regarding “physical abilities” in the OSA subgroup.
Figure 2.
Subscales of the NHP in the different sleep disorders (mean values).
Kruskal–Wallis-Test
We performed the non-parametric Kruskal–Wallis-Test for comparing the four subgroups with different sample sizes. The Kruskal–Wallis-Test showed significant differences between the four subgroups for all items except for “social isolation”, meaning that this item seemed not to be different in the four subgroups—therefore, this item was not included in the further analysis (see Table 3).
Table 3.
Kruskal–Wallis-Test.
| NHP Total | Physical Abilities | Social Isolation | Sleep | Pain | Energy | Emotional |
|---|---|---|---|---|---|---|
| p < 0.0001 | p = 0.007 | p = 0.054 | p = 0.001 | p = 0.034 | p < 0.0001 | p < 0.0001 |
Mann–Whitney-U-Test
We subsequently analysed the NHP subscales (except for “social isolation”): a Mann–Whitney-U-test was performed in order to analyse the differences between two specific subgroups (OSA–PLM/OSA–INS/OSA–NSD/PLM–INS/PLM–NSD/INS–NSD). Five items (physical abilities, sleep, pain, energy, emotional) remained in the further analysis (see Table 3).
In four subscales (physical abilities, sleep, energy, emotional) we found significantly lower NHP values in NSD patients compared to insomnia and OSA patients (in the pain area there was only a significant difference between NSD patients and insomnia patients—but not between NSD patients and OSA patients) (see Table 4). This means that the HRQoL in these specific areas was higher in MS patients without comorbid sleep disorders compared to MS patients suffering from comorbid OSA or insomnia.
Table 4.
NHP subscale values in all patients and in the four subgroups.
| Subscales | Average and Range | All Patients | NSD | INS | OSA | PLM | Differences between the Two Subgroups |
|---|---|---|---|---|---|---|---|
| Physical abilities | Mean (Standard deviation) | 20.9 (21.5) | 10.2 (14.7) | 27.4 (20.8) | 41.0 (24.3) | 17.7 (20.8) | NSD
vs. OSA p = 0.003 NSD vs. INS p = 0.009 INS vs. PLM p = 0.138 OSA vs. PLM p = 0.032 NSD vs. PLM p = 0.211 OSA vs. INS p = 0.260 |
| Min–Max | 0.0–78.7 | 0.0–54.5 | 0.0–77.3 | 10.8–78.2 | 0.0–78.7 | ||
| 25–75 | 0.0–32.6 | 0.0–21.9 | 10.8–42.6 | 21.7–67.2 | 0.0–25.8 | ||
| Median | 12.7 | 0.0 | 22.0 | 32.6 | 11.2 | ||
| Social isolation | Mean (Standard deviation) | 11.7 (19.6) | 3.6 (8.0) | 12.8 (19.0) | 29.5 (31.0) | 11.6 (19.4) | For this subgroup no Mann–Whitney-U-Test was performed (see Table 2) |
| Min–Max | 0.0–80.6 | 0.0–22.5 | 0.0–64.7 | 0.0–80.6 | 0.0–63.9 | ||
| 25–75 | 0.0–20.1 | 0.0–0.0 | 0.0–22.5 | 0.0–63.9 | 0.0–20.2 | ||
| Median | 0.0 | 0.0 | 0.0 | 20.1 | 0.0 | ||
| Sleep | Mean (Standard deviation) | 29.3 (29.5) | 10.6 (15.2) | 48.7 (26.7) | 26.8 (27.4) | 29.5 (32.4) | NSD
vs. OSA p = 0.087 NSD vs. INS p < 0.0001 INS vs. PLM p = 0.048 OSA vs. PLM p = 0.980 NSD vs. PLM p = 0.063 OSA vs. INS p = 0.075 |
| Min–Max | 0.0–100.0 | 0.0–50.4 | 0.0–77.6 | 0.0–77.6 | 0.0–100 | ||
| 25–75 | 0.0–16.1 | 0.0–12.6 | 25.2–75.2 | 12.6–50.4 | 0.0–50.4 | ||
| Median | 50.4 | 0.0 | 50.4 | 12.6 | 14.3 | ||
| Pain | Mean (Standard deviation) | 15.8 (24.8) | 3.8 (9.8) | 21.9 (23.9) | 27.0 (31.4) | 16.6 (28.9) | NSD
vs. OSA p = 0.114 NSD vs. INS p = 0.012 INS vs. PLM p = 0.221 OSA vs. PLM p = 0.469 NSD vs. PLM p = 0.117 OSA vs. INS p = 0.804 |
| Min–Max | 0.0–100.0 | 0.0–32.7 | 0.0–69.8 | 0.0–80.2 | 0.0–100.0 | ||
| 25–75 | 0.0–0.0 | 0.0–0.0 | 0.0–40.1 | 0.0–56.9 | 0.0–18.6 | ||
| Median | 26.0 | 0.0 | 15.8 | 26.0 | 0.0 | ||
| Energy | Mean (Standard deviation) | 48.9 (40.9) | 29.9 (35.5) | 78.5 (28.7) | 80.5 (38.0) | 30.5 (35.4) | NSD
vs. OSA p = 0.007 NSD vs. INS p < 0.0001 INS vs. PLM p < 0.0001 OSA vs. PLM p = 0.013 NSD vs. PLM p = 0.790 OSA vs. INS p = 0.710 |
| Min–Max | 0.0–100.0 | 0.0–100.0 | 24.0–100.0 | 0.0–100.0 | 0.0–100.0 | ||
| 25–75 | 0.0–60.8 | 0.0–62.0 | 60.8–100.0 | 63.2–100.0 | 0.0–61.4 | ||
| Median | 100.0 | 0.0 | 100.0 | 100.0 | 24.0 | ||
| Emotional | Mean (Standard deviation) | 19.6 (18.8) | 9.3 (12.2) | 30.9 (19.8) | 34.8 (16.8) | 14.1 (15.9) | NSD
vs. OSA p = 0.001 NSD vs. INS p = 0.001 INS vs. PLM p = 0.007 OSA vs. PLM p = 0.008 NSD vs. PLM p = 0.392 OSA vs. INS p = 0.619 |
| Min–Max | 0.0–69.0 | 0.0–41.4 | 0.0–69.0 | 13.6–55.9 | 0.0–48.5 | ||
| 25–75 | 0.0–30.9 | 0.0–18.6 | 14.1–47.1 | 17.0–52.0 | 0.0–22.7 | ||
| Median | 16.8 | 0.0 | 30.9 | 30.9 | 10.9 |
In sum, the differences between the OSA and insomnia subgroups were very small and not significant. Similarly, the differences between NSD and the PLMD/RLS patients were negligible. Significant clinical relevant differences were found comparing NSD and the PLMD/RLS patients to OSA and insomnia patients.
The comparison between PLMD/RLS patients and insomnia patients showed significantly increased NHP values in the “sleep” subscale and highly significant increased NHP values in the “energy” and “emotional” subscale. That means that insomnia patients showed a reduced HRQoL in these areas compared to PLMD/RLS patients.
When comparing PLMD/RLS patients with OSA patients, there were significantly higher NHP values in OSA patients (decreased HRQoL) in the “physical abilities”, “energy” and “emotional” subscales.
2.1.4. Comparison of the Objective (PSG) Sleep Parameters and the NHP Sleep Items
The sleep subscales consist of five items: “I sleep badly at night”, “I lie awake for most of the night”, “It takes me a long time to get to sleep”, “I’m waking up in the early hours of the morning”, “I take pills to help me sleep”. Except for the last item (“I take pills to help me sleep”), we compared the other four items with PSG parameters using the Mann–Whitney-U-test: Table 5 shows the results:
Table 5.
Comparison of polysomnographic data and NHP sleep items.
| Items | Average and Range | Sleep Efficiency | Awakenings | Arousal-Index | Sleep Latency | Wake after Sleep Onset |
|---|---|---|---|---|---|---|
| I sleep badly at night YES | Mean (±standard deviation) | 73.6 (±12.6) | 25.5 (±7.7) | 18.5 (±9.6) | ||
| Min–Max | 50–94 | 9–41 | 3.9–43.9 | |||
| 25–75 | 63.7–83.5 | 20–30.5 | 12.2–22.9 | |||
| median | 74.8 | 26.0 | 16.5 | |||
| I sleep badly at night NO | Mean (±standard deviation) | 76.7 (±16.2) | 27.5 (±14.2) | 20.5 (±10.0) | ||
| Min–Max | 8–93 | 8–72 | 1.1–47.1 | |||
| 25–75 | 73.0–87.1 | 17.8–33.3 | 14.4–24.9 | |||
| median | 80.45 | 26.0 | 21.7 | |||
| Differences between YES and NO | p = 0.148 | p = 0.860 | p = 0.255 | |||
| I lie awake for most of the night YES | Mean (±standard deviation) | 76.2 (±15.0) | ||||
| Min–Max | 8–93 | |||||
| 25–75 | 69.8–87.0 | |||||
| median | 79.7 | |||||
| I lie awake for most of the night NO | Mean (±standard deviation) | 71.1 (±13.5) | ||||
| Min–Max | 50–94 | |||||
| 25–75 | 60.0–80.7 | |||||
| median | 69.6 | |||||
| Differences between YES and NO | p = 0.175 | |||||
| It takes me a long time to get to sleep YES | Mean (±standard deviation) | 38.5 (±39.8) | ||||
| Min–Max | 2–198 | |||||
| 25–75 | 15.3–49.5 | |||||
| median | 29.0 | |||||
| It takes me a long time to get to sleep NO | Mean (±standard deviation) | 26.4 (±31.1) | ||||
| Min–Max | 0–190 | |||||
| 25–75 | 11.0–32.0 | |||||
| median | 21.0 | |||||
| Differences between YES and NO | p = 0.08 | |||||
| I’m waking up in the early hours of the morning YES | Mean (±standard deviation) | 88.4 (±59.0) | ||||
| Min–Max | 27–258 | |||||
| 25–75 | 43.0–73.0 | |||||
| median | 73.0 | |||||
| I’m waking up in the early hours of the morning NO | Mean (±standard deviation) | 69.8 (±41.4) | ||||
| Min–Max | 20–173 | |||||
| 25–75 | 43.3–88.8 | |||||
| median | 52.5 | |||||
| Differences between YES and NO | p = 0.336 | |||||
When we compared “It takes me a long time to get to sleep” to the sleep latency measured by PSG, there was no significant correlation between this subjective (NHP) and objective (PSG) measurement of sleep latency. Furthermore, we did not find any correlation between “I sleep badly at night” and sleep efficiency measured by PSG. Similarly the item “I’m waking up in the early hours of the morning” did not correlate with wake-after-sleep-onset in the PSG nor arousal-index or awakenings.
2.2. Correlation between NHP Values and Other Questionnaires
Table 6 shows the non-parametric correlations (Spearman–Rho) between NHP and other self-assessed questionnaires (Modified Fatigue Impact Scale (MFIS) [13]; Beck Depression Inventory (BDI) [14]; Pittsburgh Sleep Quality Index (PSQI) [6]).
Table 6.
Non parametric correlations (Spearman-Rho) between NHP and other questionnaires (Beck Depression Inventory and Pittsburgh Sleep Quality Index).
| NHP and MFIS | NHP and BDI | NHP and PSQI |
|---|---|---|
| p < 0.0001 | p < 0.0001 | p < 0.0001 |
| r = 0.737 | r = 0.836 | r = 0.612 |
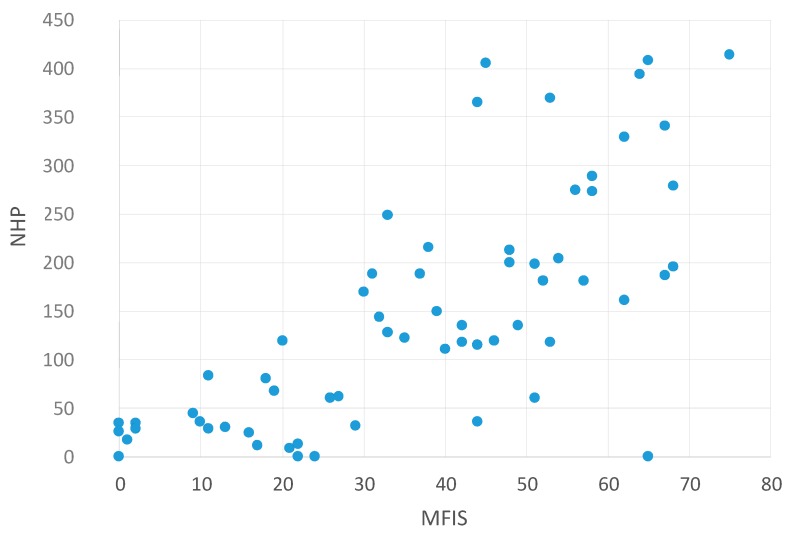
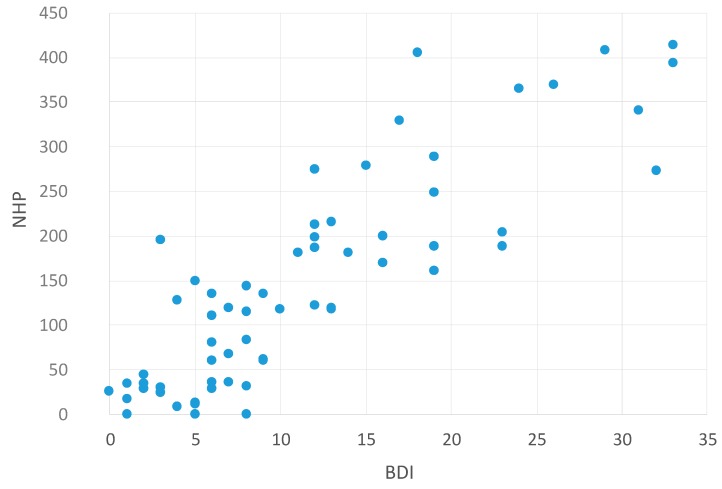
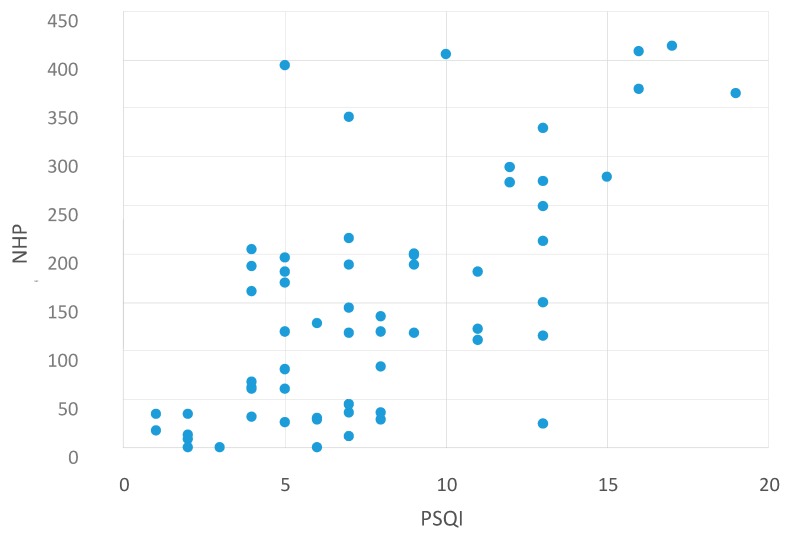
The scatter plots visualize these findings. There was a significant correlation between NHP values and MFIS values—meaning that higher fatigue values are associated with reduced HRQoL (Figure 3). In addition, higher NHP values (reduced HRQoL) were also associated with higher depression values (BDI, Figure 4) and higher PSQI values (low sleep quality, Figure 5). This indicates that reduced HRQoL is associated with depression, fatigue, and bad sleep quality.
Figure 3.
Correlation between NHP and MFIS values. Abbreviations: NHP = Nottingham Health Profile; MFIS = Modified Fatigue Impact Scale [13].
Figure 4.
Correlation between NHP and BDI values. Abbreviations: NHP = Nottingham Health Profile; BDI = Beck Depression Inventory [14].
Figure 5.
Correlation between NHP and PSQI values. Abbreviations: NHP = Nottingham Health Profile; PSQI = Pittsburgh Sleep Quality Index [6].
Correlation between NHP Values and the MFIS Subscales
Furthermore, we investigated the correlation between the NHP global score and the three subscales of the MFIS (cognition, psychosocial and physical): a significant correlation was found between the global HRQoL (NHP values) and psychosocial aspects of fatigue, as well cognitive fatigue and physical fatigue (see Table 7):
Table 7.
Non-parametric correlations (Spearman–Rho) between NHP and the fatigue subscales.
| NHP and Cognitive MFIS-Subscale | NHP and Physical MFIS-Subscale | NHP and Psychosocial MFIS-Subscale |
|---|---|---|
| p < 0.0001 | p < 0.0001 | p < 0.0001 |
| r = 0.635 | r = 0.726 | r = 0.548 |
Abbreviations: NHP = Nottingham Health Profile; MFIS = Modified Fatigue Impact Scale.
3. Discussion
Our study demonstrates poor HRQoL in MS patients suffering from sleep disorders confirmed by PSG—especially from OSA and insomnia. The global NHP median was marginally lower in MS patients suffering from OSA (194.9) compared with OSA patients in the general population (218.0) [11]. Increased NHP values indicate severe health problems, and the mean global NHP score in MS patients with comorbid insomnia or OSA was higher than the mean scores described in the literature in pregnant women at 37 weeks, fracture victims and chronically ill elderly patients and almost as high as in patients with osteoarthrosis, whereas MS patients without sleep disorders show only moderately increased NHP values. OSA and insomnia can significantly reduce HRQoL in MS patients. MS patients suffering from PLMD, RLS or sleep disorders due to leg pain show a decreased HRQoL as well—although to a lesser extent.
The impairment of HRQoL in OSA and insomnia patients (besides the sleep problems) was more pronounced in the “energy” and “emotional” area. OSA patients are severely affected in the “physical abilities” as well (as described previously by Verwimp et al. in OSA patients without MS [11]).
We cannot explain the lack of a correlation between the (objective) PSG parameter and the (subjective) sleep problems measured in the sleep subscale. This could be due to the fact that we investigated this relationship in different SDs (OSA, insomnia, PLMD/RLS).
The decreased HRQoL in OSA and insomnia patients in the “energy” and “emotional” area argue for consecutive daytime symptoms due to the sleep disorders (and “physical abilities” in OSA patients as well). Here, it is difficult to explain what exactly drives these daytime symptoms. As recently reported [15], there is an overlap between fatigue, pain, depression, and sleep disorders. Moreover, OSA can lead to depression and continuous positive airway (CPAP) therapy can improve depression [16]. Insomnia has been found to be a clinical predictor of subsequent depression [17] and increased PSQI values are significantly associated with fatigue in MS patients [18]. To date, evidence-based therapies of MS-related fatigue are lacking [19,20], and patients without MS suffering from sleep disorders show equally high values on the fatigue scales (MFIS and FSS) [21].
Sleep disorders can lead to fatigue [21] and depression [16] and CPAP therapy can subsequently improve these symptoms in patients with sleep apnea [15,22]. This suggests that reduced HRQoL, fatigue and depression can be common features of sleep disorders. Due to the close and complex relationship between fatigue, depression and sleep disorders in MS, and the overlap of the used questionnaires [15], we cannot state if sleep disorders lead to depression and subsequently to decreased HRQoL in the “energy” and “emotional” area—or vice versa, if sleep disorders lead to reduced daytime functioning and, subsequently, to depression.
Our findings underscore that sleep disorders should be considered an important confounder in all future studies investigating HRQoL in MS patients.
4. Experimental Section
4.1. Literature Search
A literature search was performed until May 2015 in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) with the following keywords: “multiple sclerosis AND Nottingham Health Profile” and “multiple sclerosis AND quality of life”. After reading the abstracts, only relevant articles were read. Moreover, the references of these articles were read and hand-searched for potentially relevant studies or articles as well.
4.2. Patients
We classified the 66 patients (21 men and 45 women aged 20–66 years) into four subgroups: no sleep disorder (NSD) (n = 17), insomnia (n = 17), periodic limb movement disorder (PLMD), restless legs syndrome (RLS) or SD due to leg pain (PLMD/RLS) (n = 24), and untreated obstructive sleep apnea (OSA) (n = 8).
Expanded Disability Status Scale (EDSS) [12] values ranged from zero to eight. For more demographic details please see the original article [1]. All patients completed the NHP [10], MFIS [13], BDI [14], and the PSQI [6] in a German validation [23,24,25,26].
The original study was approved by the local ethics committees (Charité University Medicine Berlin, Berlin, Germany and Ernst Moritz Arndt University Medicine, Greifwald, Germany, project identification code BB 03/08; 31 January 2008), and all participants gave written informed consent prior to the assessment.
4.3. Data Collection
Data collection and extraction from the questionnaires (NHP) was performed by the corresponding author (CV). The PSG data extraction from the original study and the extraction of all questionnaires were performed by the corresponding author as well [1].
4.4. Polysomnography and Scoring Criteria
As described in our original article [1], we performed PSG using a mobile polysomnographic device worn on the body, which has been validated in three different sleep centers [27] (Somnocheck 2R&K, Weinmann Medical Technology; software: Somnolab; analysis software: Artisana, Hamburg, Germany) without a video or audio signal, but otherwise with full recording facilities as in a sleep laboratory.
Measurements were made over a period of 8 h: C3/C4-EEG electrodes to the contralateral mastoid electrode, ground electrode, electrooculogram on the ipsilateral mastoid electrode, bipolar chin electromyogram (EMG) of the muscle mentalis or muscle submentalis (according to biosignals testing and anatomical conditions), nasal airflow, thoracic breathing, abdominal breathing, position sensor, snoring signal, pulse oxymetry, pulse, electrocardiogram, bipolar 2-point EMG electrodes on both anterior tibial muscles. Prior to each measurement, an impedance test and a biosignal test were performed. A sleep specialist who was blinded to the clinical situation and the questionnaires analysed PSGs. Visual classification of sleep stages took place manually in accordance with Rechtschaffen and Kales [28]. Respiratory events were manually classified using the diagnostic guidelines of the Task Force of the American Academy of Sleep Medicine [29]. Periodic leg movements were pre-classified by the equipment’s software and manually corrected using the Coleman criteria [30]. We also investigated the hypnogram: sleep efficiency, sleep onset latency, sleep stages, wake-time after sleep onset, number of waking events, number of changes in sleep stages, arousal index, periodic leg movement (PLM) index, PLM index in rapid-eye-movement (REM) sleep and non-REM sleep, PLM arousal index in REM sleep and non-REM sleep, respiratory disturbance index (RDI), blood oxygen desaturation, as well as chin EMG tonus, all respiratory events depending on position, arousal and sleep stage, and further standard polysomnographic parameters. Due to the first-night effect (patient is not yet familiar with the polysomnographic device), no pathological findings were assessed from the first-night hypnogram. On the first night, only PLMs and respiratory and cardiac events were considered. Following classification of the PSGs, sleep histories were obtained (CV), and a sleep diagnosis was made according to the International Classification of Sleep Disorders second edition (ICSD-2) [31]. To avoid false conclusions with respect to mild sleep disorders as possible causes of tiredness, mild insomnias, nocturia, mild PLMDs and sleep-related breathing disorders with RDI below 10 per hour were not considered relevant sleep disorders. We classified as relevant sleep disorders only sleep disorders with disturbed hypnogram, which are able to cause consecutive daytime sleepiness.
4.5. Statistical Analyses
The results were expressed as mean, standard deviation, and range. Patients were classified into four subgroups by the presence of a sleep disorder. Following an exploratory analysis of the data the non-parametric Kruskal–Wallis-Test and subsequently the Mann–Whitney-U-Test for pairwise comparisons were performed. Non-parametric correlations (Spearman–Rho) were carried out.
Statistical significance was established at p < 0.05. Due to the exploratory nature of the study, all tests were performed as exploratory data analyses, such that no adjustments for multiple testing have been made. Analysis was performed with SPSS software (IBM© SPSS© Statistics, Version 21, ©Copyright 1989, 2010 SPSS Inc. an IBM Company, Chicago, IL, USA).
5. Conclusions
Sleep disorders can decrease HRQoL in MS patients—especially in the “energy” and “emotional” areas. In OSA patients, the “physical abilities” area can be negatively impacted as well. Future studies should investigate the impact of the treatment of sleep disorders on HRQoL in MS patients.
Acknowledgments
The authors thank Gosia Sullivan for reviewing the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (Exc 257 to FP).
Author Contributions
Christian Veauthier performed PSG and classified all PSG, collected the NHP data, designed the study and prepared the manuscript. Gunnar Gaede performed some of the PSG and collected data. Helena Radbruch recruited patients and collected data. Klaus-Dieter Wernecke analysed the data and performed a statistic analysis. Friedemann Paul gave critical advice and revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Veauthier C., Radbruch H., Gaede G., Pfueller C.F., Dörr J., Bellmann-Strobl J., Wernecke K.D., Zipp F., Paul F, Sieb J.P. Fatigue in multiple sclerosis is closely related to sleep disorders: A polysomnographic cross-sectional study. Mult. Scler. 2011;17:613–622. doi: 10.1177/1352458510393772. [DOI] [PubMed] [Google Scholar]
- 2.Veauthier C., Gaede G., Radbruch H., Gottschalk S., Wernecke K.D., Paul F. Treatment of sleep disorders may improve fatigue in multiple sclerosis. Clin. Neurol. Neurosurg. 2013;115:1826–1830. doi: 10.1016/j.clineuro.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Côté I., Trojan D.A., Kaminska M., Cardoso M., Benedetti A., Weiss D., Robinson A., Bar-Or A., Lapierre Y., Kimoff R.J. Impact of sleep disorder treatment on fatigue in multiple sclerosis. Mult. Scler. 2013;19:480–489. doi: 10.1177/1352458512455958. [DOI] [PubMed] [Google Scholar]
- 4.Neau J.P., Paquereau J., Auche V., Mathis S., Godeneche G., Ciron J., Moinot N., Bouche G. Sleep disorders and multiple sclerosis: A clinical and polysomnography study. Eur. Neurol. 2012;68:8–15. doi: 10.1159/000335076. [DOI] [PubMed] [Google Scholar]
- 5.Sarraf P., Azizi S., Moghaddasi A.N., Sahraian M.A., Tafakhori A., Ghajarzadeh M. Relationship between sleep quality and quality of life in patients with multiple sclerosis. Int. J. Prev. Med. 2014;5:1582–1586. [PMC free article] [PubMed] [Google Scholar]
- 6.Buysse D.J., Reynolds C.F., 3rd., Monk T.H., Berman S.R., Kupfer D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 7.Vickrey B.G., Hays R.D., Harooni R., Myers L.W., Ellison G.W. A health-related quality of life measure for multiple sclerosis. Qual. Life Res. 1995;4:187–206. doi: 10.1007/BF02260859. [DOI] [PubMed] [Google Scholar]
- 8.Trojan D.A., Kaminska M., Bar-Or A., Benedetti A., Lapierre Y., da Costa D., Robinson A., Cardoso M., Schwartzman K., Kimoff R.J. Polysomnographic measures of disturbed sleep are associated with reduced quality of life in multiple sclerosis. Neurol. Sci. 2012;316:158–163. doi: 10.1016/j.jns.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Brazier J.E., Harper R., Jones N.M., O’Cathain A., Thomas K.J., Usherwood T., Westlake L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt S.M., McEwen J., McKenna S.P. Measuring health status: A new tool for clinicians and epidemiologists. J. R. Coll. Gen. Pract. 1985;35:185–188. [PMC free article] [PubMed] [Google Scholar]
- 11.Verwimp J., Ameye L., Bruyneel M. Correlation between sleep parameters, physical activity and quality of life in somnolent moderate to severe obstructive sleep apnea adult patients. Sleep Breath. 2013;17:1039–1046. doi: 10.1007/s11325-012-0796-x. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 13.Multiple Sclerosis Council for Clinical Practice Guidelines . Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis. Paralyzed Veterans of America; Washington, DC, USA: 1998. [Google Scholar]
- 14.Beck A.T., Steer R.A. Internal consistencies of the original and revised Beck Depression Inventory. J. Clin. Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::AID-JCLP2270400615>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Veauthier C., Paul F. Sleep disorders in multiple sclerosis and their relationship to fatigue. Sleep Med. 2014;15:5–14. doi: 10.1016/j.sleep.2013.08.791. [DOI] [PubMed] [Google Scholar]
- 16.El-Sherbini A.M., Bediwy A.S., El-Mitwalli A. Association between obstructive sleep apnea (OSA) and depression and the effect of continuous positive airway pressure (CPAP) treatment. Neuropsychiatr. Dis. Treat. 2011;7:715–721. doi: 10.2147/NDT.S26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baglioni C., Riemann D. Is chronic insomnia a precursor to major depression? Epidemiological and biological findings. Curr. Psychiatry Rep. 2012;14:511–518. doi: 10.1007/s11920-012-0308-5. [DOI] [PubMed] [Google Scholar]
- 18.Veauthier C., Paul F. Fatigue in multiple sclerosis: Which patient should be referred to a sleep specialist? Mult. Scler. 2012;18:248–249. doi: 10.1177/1352458511411229. [DOI] [PubMed] [Google Scholar]
- 19.Paul F., Veauthier C. Fatigue in multiple sclerosis: A diagnostic and therapeutic challenge. Expert Opin. Pharmacother. 2012;13:791–793. doi: 10.1517/14656566.2012.667075. [DOI] [PubMed] [Google Scholar]
- 20.Weinges-Evers N., Brandt A.U., Bock M., Pfueller C.F., Dörr J., Bellmann-Strobl J., Scherer P., Urbanek C., Boers C., Ohlraun S., et al. Correlation of self-assessed fatigue and alertness in multiple sclerosis. Mult. Scler. 2010;16:1134–1140. doi: 10.1177/1352458510374202. [DOI] [PubMed] [Google Scholar]
- 21.Veauthier C. Younger age, female sex, and high number of awakenings and arousals predict fatigue in patients with sleep disorders: A retrospective polysomnographic observational study. Neuropsychiatr. Dis. Treat. 2013;9:1483–1494. doi: 10.2147/NDT.S50763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chotinaiwattarakul W., O’Brien L.M., Fan L., Chervin R.D. Fatigue, tiredness, and lack of energy improve with treatment for OSA. J. Clin. Sleep Med. 2009;5:222–227. [PMC free article] [PubMed] [Google Scholar]
- 23.Kohlmann T., Bullinger M., Kirchberger-Blumstein I. German version of the Nottingham Health Profile (NHP): Translation and psychometric validation. Soz. Praventivmed. 1997;42:175–185. doi: 10.1007/BF01300568. (In German) [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann C., Hohlfeld R. “Fatigue” in multiple sclerosis. Nervenarzt. 1999;70:566–574. doi: 10.1007/s001150050482. (In German) [DOI] [PubMed] [Google Scholar]
- 25.Kühner C., Bürger C., Keller F., Hautzinger M. Reliability and validity of the Revised Beck Depression Inventory (BDI-II). Results from German samples. Nervenarzt. 2007;78:651–656. doi: 10.1007/s00115-006-2098-7. (In German) [DOI] [PubMed] [Google Scholar]
- 26.Backhaus J., Junghanns K., Broocks A., Riemann D., Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002;53:737–740. doi: 10.1016/S0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 27.Canisius S., Roth H., Ploch T., Loh A., Astrid S., Werner C. Validation of Signal Quality in a New Ambulatory Polysomnography System. European Respiratory Society; Stockholm, Sweden: 2007. p. 3523. [Google Scholar]
- 28.Rechtschaffen A., Kales A. A Manual of Standardized Terminology Techniques and Scoring System for Sleep Stages of Human Subjects. Brain Information Service, Brain Research Institute (National Institutes of Health, US); Los Angeles, CA, USA: 1968. Number 204. [Google Scholar]
- 29.American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 30.Coleman R.M., Guilleminault C. Sleeping and Waking Disorders: Indications and Techniques. Addison-Wesley; New York, NY, USA: 1982. Periodic movement in sleep (nocturnal myoclonus) and restless legs syndrome; pp. 265–295. [Google Scholar]
- 31.International Classification of Sleep Disorders. 2nd ed. AASM; Westchester, IL, USA: 2005. American Academy of Sleep Medicine (AASM) [Google Scholar]