Abstract
Background
Studies have demonstrated a higher rate of nodal metastases in melanoma of childhood, but there is controversy about the overall prognosis relative to adults. We describe a large single-institution experience with pediatric melanoma and assess prognostic characteristics.
Methods
Retrospective review identified 126 patients diagnosed with melanoma at <21 years of age and referred for treatment from 1986 to 2011. Atypical lesions were excluded. Clinicopathologic characteristics were correlated with sentinel lymph node (SLN) status and outcomes.
Results
SLN biopsy was positive in 18 of 62 cases (29 %). Increasing Breslow thickness correlated with a positive SLN (p < 0.05). After a median follow-up of 5 years, there were 27 recurrences and 20 deaths. Positive SLN patients had significantly worse recurrence-free survival (RFS, p < 0.05) and significantly worse melanomaspecific survival (MSS, p = 0.05) compared with negative SLN patients. The 5-year RFS and MSS for positive SLN patients were 59.5 and 77.8 %, compared with 93.7 and 96.8 % for negative SLN patients. Recurrences and melanoma-related deaths were often seen beyond 5 years. No deaths have occurred in patients <12 years, but 9.1 % of patients 12–17 years and 17.2 % of patients 18–20 years died from melanoma (p = 0.291).
Conclusions
Children with melanoma have higher rates of SLN metastases (29 %) than adults with comparable melanomas. Despite the higher incidence of nodal metastases, survival is equal to or better than what is reported for adults. However, long-term follow-up is necessary in this population since recurrences and deaths are often seen beyond 5 years.
Melanoma in children and adolescents is uncommon, accounting for 1–4 % of all melanomas and 3 % of pediatric cancers.1–5 Similar to the increase in incidence of melanoma in adults, which is rising 2–5 % per year with 76,250 new cases estimated to be diagnosed in 2012, the incidence of pediatric melanoma is also increasing at a rate of 1–4 % per year.1–4,6–12 The upper limit of age that defines the pediatric patient population varies in published reports, but is generally considered to be 21 years.7,11,12
Previous studies have reported that pediatric melanoma may be associated with thicker tumors and higher rates of lymph node metastases when compared with melanomas in adults.4,7,8,11–22 Despite the higher rate of nodal disease in pediatric patients, SEER-based and case-matched studies on pediatric melanoma have shown survival rates similar to those seen in adults with melanoma.6,13 These observations suggest that the biology of melanoma in children may be different from melanoma in adults. There are other reports that have specifically examined survival in different pediatric age groups and have reported inconsistent results.13,14,21–26
Although higher rates of lymph node metastases have been reported in pediatric melanoma patients, the prognostic value of sentinel lymph node biopsy (SLNB) has not been extensively studied in this population. The few studies on SLNB for pediatric melanoma have reported varying results, with some studies reporting no recurrences or deaths in positive SLNB patients while one study showed that the overall survival (OS) rate of 94.1 % seen in pediatric melanoma patients dropped to 79 % in positive SLNB patients.18,19
As more children are diagnosed with this disease, a better understanding of the natural history of pediatric melanoma and the ability to formulate effective treatment algorithms gain importance. However, the inconsistent and varying outcomes reported make it difficult to assess the clinical behavior of melanoma in the pediatric population. Undoubtedly, some of this variation is accounted for by the inclusion of atypical lesions along with cases of unequivocal melanoma. Herein, we present a large single-institution experience with pediatric melanoma, considering only those cases felt to represent unequivocal melanoma, to evaluate prognostic factors and identify unique clinical features of this disease.
METHODS
After obtaining Institutional Review Board approval, a retrospective review was conducted identifying all pediatric patients referred to Moffitt Cancer Center for treatment of melanoma from 1986 to 2011. We defined pediatric melanoma by presentation at <21 years of age. Only patients with an unequivocal diagnosis of invasive melanoma based on review by a Moffitt dermatopathologist were included. Patients diagnosed with melanoma in situ, atypical Spitz nevus, atypical melanocytic proliferation, or any other diagnostically controversial melanocytic proliferation were excluded. Cases were included if they fulfilled the classic histopathologic diagnostic criteria for melanoma, including but not limited to large size, poor circumscription, asymmetry, cellular growth without evidence of maturation, evidence of increased cellular proliferation, pagetoid involvement of epidermis, and cellular pleomorphism. Diagnostically challenging cases where an unequivocal diagnosis of melanoma could not be reached were specifically excluded, as were cases that required ancillary studies such as FISH or CGH. Demographic, clinical, pathology, and outcome data were reviewed.
Data on primary tumor characteristics were assessed, and evaluation of lymph nodes obtained through SLNB or completion lymph node dissection (CLND) consisted of serial sectioning and pathology assessment using hematoxylin and eosin stained sections. S-100 and Melan-A immunohistochemistry were used in the evaluation of all SLNs and in CLND specimens when indicated.
Patients were stratified by age groups <12 years, 12–17 years, and 18–20 years. An age of 12 years was chosen to represent a cutoff between prepubescent children and adolescent patients based on review of U.S. puberty timing data.27 Survival analysis was performed stratified by SLN status and by the specified age groups.
The Kaplan–Meier product limit approach was used to calculate survival probabilities for recurrence-free survival (RFS) and melanoma-specific survival (MSS). Log-rank test was used to compare the survival distributions of different groups. Univariate and multivariate Cox proportional hazard regression models were used to model the effects of independent variables on the hazard rate. Pearson's Chi-square test or Fisher exact test was used to test the independence between categorical variables. Wilcoxon rank-sum test was used to compare the distributions of a continuous variable (e.g., age and Breslow thickness) at different levels of a categorical variable (SLN test result). A p value ≤0.05 was considered significant in all tests. The analysis was conducted in Statistical Analysis System (SAS) software, version 9.2 (Cary, NC).
RESULTS
Patients
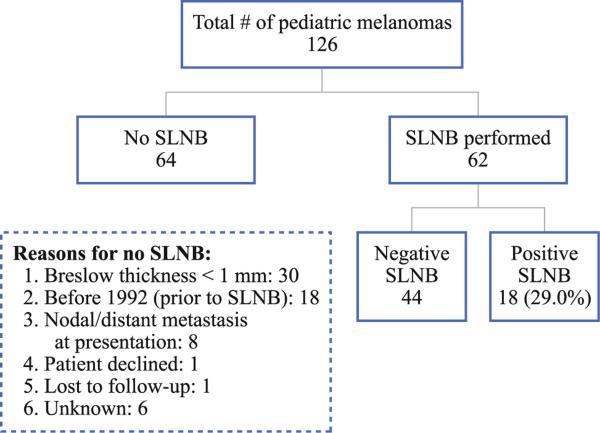
From 1986 to 2011, 126 pediatric patients were referred for treatment of a histologically confirmed melanoma. SLNB was performed in 62 cases, with 18 cases (29 %) having a positive SLN and 44 cases (71 %) having a negative SLNB. In 64 cases, SLNB was not performed. The reasons for no SLNB are shown in Fig. 1, with the most common being a Breslow thickness of <1 mm. Patient characteristics for all 126 patients and for SLNB patients are shown in Table 1. The median age for all patients was 18 years and was 17 years in the SLNB group. The majority of patients were female (57.9 % overall and 54.8 % in the SLNB group). Whites made up 94.4 %, while Hispanics made up 3.2 % of the study population. Median follow-up was 60 months for all patients and 73 months for SLNB patients.
FIG. 1.
Breakdown of pediatric melanoma patients. Sentinel lymph node (SLN) biopsy (SLNB) was performed in 62 cases with 18 cases (29 %) having a positive SLN. A SLNB was not performed in 64 cases and the reasons why not are listed
TABLE 1.
Patient characteristics
| All patients (N = 126) | SLNB performed (N = 62) | |
|---|---|---|
| Age (years) | ||
| Median | 18 | 17 |
| Range | 2–20 | 2–20 |
| Gender, n (%) | ||
| Male | 53 (42.1) | 28 (45.2) |
| Female | 73 (57.9) | 34 (54.8) |
| Race, n (%) | ||
| White | 119 (94.4) | 58 (93.5) |
| Hispanic | 4 (3.2) | 3 (4.8) |
| Native American | 1 (0.8) | |
| Mixed | 1 (0.8) | 1 (1.6) |
| Unknown | 1 (0.8) | |
| AJCC stage at presentation | ||
| I–II | 119 | 62 |
| III | 3 | 0 |
| IV | 4 | 0 |
| Follow-up (months) | ||
| Median | 60 | 73 |
| Range | 1–315 | 1–225 |
SLNB sentinel lymph node biopsy, AJCC American Joint Committee on Cancer
Characteristics of Sentinel Lymph Node Biopsy Patients
Clinicopathologic characteristics of the SLNB group are shown in Table 2. Superficial spreading melanoma was the most common histologic type (46.8 %), while the head/neck was the most common primary site (38.7 %). Median tumor thickness was 1.6 mm (range 0.42–10.4 mm). Patients with a positive SLN had significantly thicker melanomas (median 2.55 mm, range 1.05–6.2 mm) compared with negative SLN patients (median 1.3 mm, range 0.42–10.4 mm; p < 0.05). Mitotic rate (MR) ≥1/mm2 was seen in 25 patients who had a SLNB (67.6 %) with 12 positive SLN cases (85.7 %) and 13 negative SLN cases (56.5 %) having a MR ≥1/mm2. There were 17 ulcerated cases (35.4 %) with 8 positive SLN cases (57.1 %) and 9 negative SLN cases (26.5 %) having ulceration. Regression was seen in only 2 cases (5.0 %), with 1 of 11 evaluable cases (9.1 %) in the positive SLNB group compared with 1 of 29 cases (3.4 %) in the negative SLNB group. Lymphovascular invasion was seen in only 1 case and this patient had a positive SLNB. Univariate analysis showed that only Breslow thickness significantly predicted SLN metastases (p < 0.05). Rates of ulceration, MR ≥1/mm2, regression, VGP, and lymphovascular invasion did not differ significantly between positive and negative SLN patients.
TABLE 2.
Characteristics of sentinel lymph node biopsy patients
| SLNB performed (N = 62) | Positive SLNB (N = 18) | Negative SLNB (N= 44) | p value | |
|---|---|---|---|---|
| Age group | ||||
| <12 years | 2 | 1 | 1 | |
| 12–17 years | 32 | 9 | 23 | |
| 18–20 years | 28 | 8 | 20 | |
| Gender, n (%) | NS | |||
| Male | 28 (45.2) | 9 (50) | 19 (43.2) | |
| Female | 34 (54.8) | 9 (50) | 25 (56.8) | |
| Histologic type, n (%) | NS | |||
| Superficial spreading | 29 (46.8) | 6 (33.3) | 23 (52.3) | |
| Nodular | 14 (22.6) | 5 (27.8) | 9 (20.5) | |
| Spitzoid | 3 (4.8) | 1 (5.6) | 2 (4.5) | |
| Other | 4 (6.5) | 1 (5.6) | 3 (6.8) | |
| Not specified | 12 (19.4) | 5 (27.8) | 7 (15.9) | |
| Location, n (%) | NS | |||
| Head and neck | 24 (38.7) | 5 (27.8) | 19 (43.2) | |
| Trunk | 18 (29) | 6 (33.3) | 12 (27.3) | |
| Extremities | 20 (32.2) | 7 (38.9) | 13 (29.5) | |
| Breslow thickness (mm) | ||||
| Median | 1.60 | 2.55 | 1.30 | <0.05* |
| Range | 0.42–10.4 | 1.05–6.2 | 0.42–10.4 | |
| Mitotic rate, n (%)a | NS | |||
| ≥1/mm2 | 25 (67.6) | 12 (85.7) | 13 (56.5) | |
| <1/mm2 | 12 (32.4) | 2 (14.3) | 10 (43.5) | |
| Ulceration, n (%)a | 17 (35.4) | 8 (57.1) | 9 (26.5) | NS |
| Regression, n (%)a | 2 (5.0) | 1 (9.1) | 1 (3.4) | NS |
| VGP, n (%)a | 37 (94.9) | 11 (100) | 26 (92.9) | NS |
| LVI, n (%)a | 1 (2.6) | 1 (8.3) | 0 | NS |
SLNB sentinel lymph node biopsy, VGP vertical growth phase, LVI lymphovascular invasion, NS not significant
Significant on univariate analysis for Breslow thickness versus SLNB status
Data unavailable or missing for mitotic rate in 25 cases, for ulceration in 14 cases, for regression in 22 cases, for VGP in 23 cases, and for LVI in 23 cases
CLND was performed in 14 of 18 positive SLN cases with additional nodal metastases found in 2 cases (14.3 %). For the 4 positive SLN patients who did not have a CLND, 2 cases were lost to follow-up, and 2 patients declined CLND after discussion.
Recurrence and Survival
After excluding the 4 patients presenting with stage IV disease, 122 patients remained evaluable for recurrence. Recurrences occurred in 27 of 122 patients (22.1 %), including 13 of 62 SLNB patients (21 %) who developed a recurrence (Table 3). Of note, local recurrences were rare and seen in only 2.5 % of patients (3.2 % of SLNB patients). Regional recurrences occurred in 11.5 % of patients, but only in 6.5 % of the SLNB group of which 1 patient had a negative SLNB but developed a nodal recurrence after 7.7 years (false-negative SLNB).
TABLE 3.
Recurrence and survival
| All patientsa (N = 122/126) | SLNB performed (N = 62) | Positive SLNB (N = 18) | Negative SLNB (N = 44) | Presented as stage IV (N = 40) | |
|---|---|---|---|---|---|
| Total recurrences, n (%) | 27 (22.1) | 13 (21) | 8 (44.4) | 5 (11.4) | NA |
| First site of recurrence, n (%) | |||||
| Local | 3 (2.5) | 2 (3.2) | 1 (5.6) | 1 (2.3) | |
| Regional | 14 (11.5) | 4 (6.5) | 3 (16.7) | 1 (2.3) | |
| Distant | 8 (6.6) | 5 (8.1) | 3 (16.7) | 2 (4.5) | |
| Unknown | 2 (1.6) | 2 (3.2) | 1 (5.6) | 1 (2.3) | |
| Alive, n (%) | 106 (84.1) | 53 (85.5) | 13 (72.2) | 40 (90.9) | 1 (25) |
| Died of disease, n (%) | 19 (15.1) | 9 (14.5) | 5 (27.8) | 4 (9.1) | 3 (75) |
| Died of other causes, n (%) | 1 (0.8) | 0 | 0 | 0 | 0 |
SLNB sentinel lymph node biopsy, NA not applicable
In evaluating recurrences, the 4 patients who presented with stage IV disease were excluded, leaving 122 patients. For the purposes of analyzing survival, the 4 patients who presented with stage IV disease were included, giving a total of 126 patients evaluated
For the survival analysis, the 4 patients presenting with stage IV disease were included giving a total of 126 evaluable patients (Table 3). Overall, there were 20 deaths (15.9 %), with 19 melanoma-related deaths and 1 death due to a motor vehicle accident. A total of 106 patients (84.1 %) were alive at last follow-up, after a median follow-up of 60 months.
In the SLNB group, 85.5 % of patients were alive at last follow-up and 14.5 % of patients were dead from melanoma. However, for the 4 patients who presented with stage IV disease, only 1 patient (25 %) was alive at last follow-up. This was a 20-year-old patient with extensive intra-abdominal disease from an unknown primary melanoma who had a complete response to biochemotherapy and has no evidence of disease 3 years after completion of therapy.
Univariate analysis showed that Breslow thickness, stage at presentation, and SLN status correlated with RFS, while gender, Breslow thickness, and stage at presentation correlated with MSS. Multivariate analysis demonstrated that a positive SLN significantly predicted worse RFS (hazard ratio [HR] 3.91, 95 % confidence interval [95 % CI] 1.33–11.54; p < 0.05); however, a positive SLN was not a significant predictor of MSS (HR 3.45, 95 % CI 0.92–12.88, p = 0.07) when the entire patient population was considered.
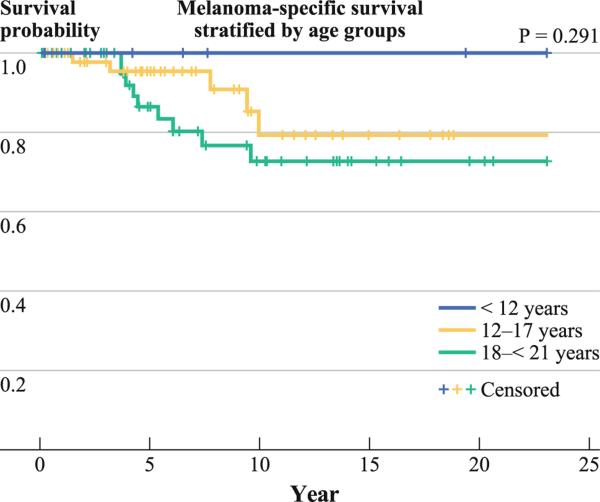
Melanoma-Specific Survival Stratified by Age Group
Only patients with clinically localized disease at presentation were included for this analysis (Fig. 2). There were 6 patients <12 years old, 55 patients 12–17 years old, and 58 patients 18–20 years old. No deaths were seen in patients <12 years old at a median follow-up of 85 months. In contrast, melanoma-specific mortality rates were 9.1 % in the 12–17 year age group and 17.2 % in the 18–20 year age group after a median follow-up of 62 months in each group. The difference in survival between the 3 age groups was not statistically significant (p = 0.291), likely because of the low number of patients in the <12 year age group.
FIG. 2.
Melanoma-specific survival in pediatric melanoma patients who presented with clinically localized disease as stratified by age groups
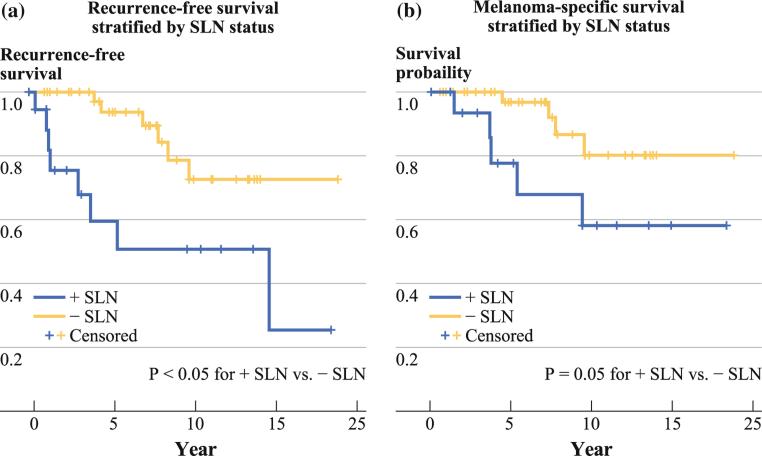
Survival Stratified by Sentinel Lymph Node Status
For negative SLN patients, 5-year RFS was 93.7 % (95 % CI 85.3–100 %) compared with 59.5 % (95 % CI 33.7–85.3 %) in positive SLN patients (Fig. 3a). The 5-year MSS in positive SLN patients was 77.8 % (95 % CI 55.5–100 %), while for negative SLN patients, it was 96.8 % (95 % CI 90.6–100 %, Fig. 3b). Both RFS and MSS were significantly worse for positive SLN patients (p < 0.05 for RFS, p = 0.05 for MSS).
FIG. 3.
Survival for pediatric melanoma patients as stratified by sentinel lymph node (SLN) status. a Recurrence-free survival was significantly worse for positive SLN patients compared with negative SLN patients (p < 0.05). b Melanoma-specific survival was significantly worse for positive SLN patients compared with negative SLN patients (p = 0.05)
Of note, several recurrences and melanoma-related deaths occurred well beyond 5 years after diagnosis in both positive and negative SLN patients. Recurrences were seen in 13 SLNB patients with dates of recurrences known in 11 cases. Of 11 recurrences, 5 (45.5 %) occurred beyond 5 years with 2 patients having a positive SLNB (recurrences at 5.1 and 14.5 years) and 3 patients having a negative SLNB (recurrences at 6.7 years, 7.7 years, and 8.3 years). There were 3 patients in the 12–17 year age group and 2 patients were in the 18–20 year age group. Of the 9 melanoma-related deaths, 5 (55.6 %) in the SLNB group were beyond 5 years with 2 deaths in positive SLN patients (deaths at 5.1 and 9.3 years) and 3 deaths in negative SLN patients (deaths at 7.3, 7.8, and 9 years).
DISCUSSION
Studies have suggested that the biology of pediatric melanoma may be different from melanoma in adults.6,10–14,18,22–25 Some of this variability is likely due to the inclusion of biologically ambiguous lesions such as atypical Spitz nevi in many series of pediatric melanoma cases. Therefore, it is important to understand the clinical behavior of “true” pediatric melanoma. This series represents one of the largest single-institution studies to date and demonstrates the clinical characteristics of melanoma in the pediatric population.
Previous studies have reported that compared with adults, pediatric melanoma patients exhibit higher rates of lymph node metastases ranging from 25 to 40 %.14,15,17,19,21,22 Our results are consistent with these reports, as we observed a positive SLN rate of 29 %. However, primary tumor characteristics predictive of SLN metastases have not been extensively studied in pediatric melanoma patients. Moore-Olufemi et al.14 evaluated 109 patients with melanoma who were ≤17 years of age and demonstrated that younger age, tumor thickness, and lack of radial growth were associated with nodal metastases. Paradela et al.22 showed that Breslow thickness and age ≤10 years correlated with a positive SLN. Our results are consistent with these reports in part, as Breslow thickness did correlate with a positive SLN.
The prognostic value of SLNB for pediatric melanomas was supported in our study by the fact that pediatric patients with a positive SLN had significantly worse RFS and MSS. Moore-Olufemi et al. also demonstrated that for pediatric melanoma patients, lymph node status was significantly correlated with OS, but in contrast Paradela et al. did not show a significant association between a positive SLNB and OS.14,22 Of note, Paradela et al.22 included mucosal melanomas, and the discrepancy between these 2 aforementioned studies regarding nodal status and survival may in part be due to different inclusion criteria. Our results are also in contrast to those reported by Roaten et al.18 where recurrences and deaths were not seen in their 8 positive SLN patients, although the median follow-up time was only 35 months. However, our study is consistent with Howman-Giles et al. who found an overall 5-year OS of 94.1 % in pediatric melanoma patients, but this was only 79 % in positive SLNB patients. Furthermore, the OS of 79 % for positive SLN patients at 60 months seen in the study by Howman-Giles et al.19 is nearly the same as the 77.8 % 5-year MSS of positive SLN patients in our study.
This study also supports the observation that pediatric melanomas have clinical characteristics that may differ from melanomas in adults. No deaths were seen in prepubescent patients, and despite the higher incidence of nodal metastases, the 5-year MSS for pediatric patients with a positive SLN (stage IIIA/IIIB) was 77.8 and 96.8 % for patients with a negative SLN (stage I/II). These results are consistent with or better than the survival rates seen for the corresponding stages in the American Joint Committee on Cancer (AJCC) seventh edition staging system, which predominantly included adults and reported 5-year survival rates of 53.3–97.2 % for stage I/II and 54–78 % for stage IIIA/IIIB.28 Furthermore, melanoma-specific mortality increased to 9.1 % in patients 12–17 years old and to 17.2 % in patients 18–20 years old. Previous studies have attempted to determine if survival rates were different among pediatric melanoma patients of varying ages. Lewis et al. found that mortality rates of melanoma patients who were 15–19 years old were 8–18 times higher when compared with mortality rates in younger age groups.23 A study by Ferrari et al.24 reported that pediatric melanoma patients who were ≥10 years of age had significantly worse OS compared with patients <10 years of age. In contrast, Lange et al.25 found in a large study that used the U.S. National Cancer Data Base that OS was significantly worse in pediatric melanoma patients who were 1–9 years of age. Several other studies on pediatric melanoma have shown no statistically significant difference in survival between different pediatric age groups.13,14,22,26 Although our study demonstrated a trend toward worse MSS in older pediatric age groups, we also were not able to demonstrate a significant difference in survival between different age groups. It is likely that with the small number of patients in the <12-year age group, we did not have the ability to find statistically significant differences if they exist.
It is possible that some of the conflicting data in the literature concerning the impact of pediatric age and SLN status on survival may relate to case definitions. Diagnostically challenging pediatric melanocytic tumors (so-called melanocytic tumors of uncertain malignant potential, atypical melanocytic proliferations, etc.) with or without SLN involvement may have been included in some of the series showing better survival. The true biologic significance of these tumors is a matter of debate, and they were deliberately excluded from the present study to obtain an undiluted view of the behavior of unequivocal childhood melanoma.
Of note, we observed a number of recurrences and melanoma-related deaths beyond 5 years. Specifically in SLNB patients, 45.5 % of recurrences and 55.6 % of melanoma-related deaths were after 5 years. Interestingly, the majority of recurrences and melanoma-related deaths (60 %) after 5 years were in negative SLN patients. These results are in contrast to Ferrari et al.24 who after a median follow-up of 122 months reported 13 recurrences in 33 pediatric melanoma patients occurring from 2 to 52 months after diagnosis, although recurrences were not stratified by lymph node status. The latest time of relapse in the study by Ferrari et al. was 4.3 years.
There are several limitations to the current study. First, this was a retrospective review, and second the study evaluated pediatric patients referred over a 25-year period. Clinical practice patterns and treatment algorithms as well as methods of pathologic evaluation have changed over this period of time. Another limitation is that there is no defined age for the onset of puberty and an age of 12 years was chosen based on a review of puberty timing data.27 However, various ages ranging from 10 to 13 years have been used in the literature to separate prepubescent children from adolescents. Children <12 years represented only 5.6 % of patients in this study, and there was only 1 patient who was <3 years of age. Therefore, congenital etiologies for melanoma could not be assessed in this study.
In summary, as the incidence of pediatric melanoma continues to rise, it is important to gain an understanding of the unique clinical characteristics of melanoma in this patient population. Lymph node metastases are seen at a higher rate and SLNB has a prognostic role for pediatric melanoma patients. Consistent with the recommendations of the AJCC Melanoma Staging Committee, we feel pediatric patients with clinically localized melanomas ≥1 mm thickness (and selectively with thinner high-risk lesions) should be offered SLNB.28,29 This study also provides additional clinical evidence for a different biology in pediatric melanoma compared with adult disease. Although lymph node metastases are seen at a higher rate, survival is comparable to or better than what has been reported for adults with melanoma. It is important to note that a significant number of recurrences and melanoma-related deaths are seen in pediatric melanoma patients more than 5 years after initial diagnosis, and long-term follow-up is necessary in this population. Additional studies using larger population bases, such as multi-institution studies, are warranted to further evaluate the unique biology of pediatric melanoma and to better establish treatment parameters and potentially improve outcomes.
Footnotes
DISCLOSURES VKS: Consultant/Advisory Board: Merck, Navidea (Neoprobe); JSZ: Consultant/Scientific Advisory Board for Delcath Systems, Inc. and a Consultant to IGEA; JLM: Consultant: Glaxo Smith Kline, Consultant: Durect Corporation.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.de Vries E, Steliarova-Foucher E, Spatz A, Ardanaz E, Egger-mont AM, Coebergh JW. Skin cancer incidence and survival in European children and adolescents (1978–1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2170–82. doi: 10.1016/j.ejca.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Ceballos PI, Ruiz-Maldonado R, Mihm MC., Jr. Melanoma in children. N Engl J Med. 1995;332:656–62. doi: 10.1056/NEJM199503093321007. [DOI] [PubMed] [Google Scholar]
- 5.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112:416–32. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 6.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–41. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 7.Pappo AS. Melanoma in children and adolescents. Eur J Cancer. 2003;39:2651–61. doi: 10.1016/j.ejca.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Jen M, Murphy M, Grant-Kels JM. Childhood melanoma. Clin Dermatol. 2009;27:529–36. doi: 10.1016/j.clindermatol.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Hamre MR, Chuba P, Bakhshi S, Thomas R, Severson RK. Cutaneous melanoma in childhood and adolescence. Pediatr Hematol Oncol. 2002;19:309–17. doi: 10.1080/08880010290057327. [DOI] [PubMed] [Google Scholar]
- 10.Downard CD, Rapkin LB, Gow KW. Melanoma in children and adolescents. Surg Oncol. 2007;16:215–20. doi: 10.1016/j.suronc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Mills O, Messina JL. Pediatric melanoma: a review. Cancer Control. 2009;16:225–33. doi: 10.1177/107327480901600304. [DOI] [PubMed] [Google Scholar]
- 12.Paradela S, Fonseca E, Prieto VG. Melanoma in children. Arch Pathol Lab Med. 2011;135:307–16. doi: 10.5858/2009-0503-RA.1. [DOI] [PubMed] [Google Scholar]
- 13.Livestro DP, Kaine EM, Michaelson JS, Mihm MC, Haluska FG, Muzikansky A, et al. Melanoma in the young: differences and similarities with adult melanoma: a case-matched controlled analysis. Cancer. 2007;110:614–24. doi: 10.1002/cncr.22818. [DOI] [PubMed] [Google Scholar]
- 14.Moore-Olufemi S, Herzog C, Warneke C, Gershenwald JE, Mansfield P, Ross M, et al. Outcomes in pediatric melanoma: comparing prepubertal to adolescent pediatric patients. Ann Surg. 2011;253:1211–5. doi: 10.1097/SLA.0b013e318217e852. [DOI] [PubMed] [Google Scholar]
- 15.Aldrink JH, Selim MA, Diesen DL, Johnson J, Pruitt SK, Tyler DS, et al. Pediatric melanoma: a single-institution experience of 150 patients. J Pediatr Surg. 2009;44:1514–21. doi: 10.1016/j.jpedsurg.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Butter A, Hui T, Chapdelaine J, Beaunoyer M, Flageole H, Bouchard S. Melanoma in children and the use of sentinel lymph node biopsy. J Pediatr Surg. 2005;40:797–800. doi: 10.1016/j.jpedsurg.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Roaten JB, Partrick DA, Pearlman N, Gonzalez RJ, Gonzalez R, McCarter MD. Sentinel lymph node biopsy for melanoma and other melanocytic tumors in adolescents. J Pediatr Surg. 2005;40:232–5. doi: 10.1016/j.jpedsurg.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Roaten JB, Partrick DA, Bensard D, Pearlman N, Gonzalez R, Fitzpatrick J, et al. Survival in sentinel lymph node-positive pediatric melanoma. J Pediatr Surg. 2005;40:988–92. doi: 10.1016/j.jpedsurg.2005.03.014. discussion 92. [DOI] [PubMed] [Google Scholar]
- 19.Howman-Giles R, Shaw HM, Scolyer RA, Murali R, Wilmott J, McCarthy SW, et al. Sentinel lymph node biopsy in pediatric and adolescent cutaneous melanoma patients. Ann Surg Oncol. 2010;17:138–43. doi: 10.1245/s10434-009-0657-4. [DOI] [PubMed] [Google Scholar]
- 20.Chao C, Martin RC, 2nd, Ross MI, Reintgen DS, Edwards MJ, Noyes RD, et al. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol. 2004;11:259–64. doi: 10.1245/aso.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Kirkwood JM, Jukic DM, Averbook BJ, Sender LS. Melanoma in pediatric, adolescent, and young adult patients. Semin Oncol. 2009;36:419–31. doi: 10.1053/j.seminoncol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paradela S, Fonseca E, Pita-Fernandez S, Kantrow SM, Diwan AH, Herzog C, et al. Prognostic factors for melanoma in children and adolescents: a clinicopathologic, single-center study of 137 patients. Cancer. 2010;116:4334–44. doi: 10.1002/cncr.25222. [DOI] [PubMed] [Google Scholar]
- 23.Lewis KG. Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol Surg. 2008;34:152–9. doi: 10.1111/j.1524-4725.2007.34032.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari A, Bono A, Baldi M, Collini P, Casanova M, Pennacchioli E, et al. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics. 2005;115:649–54. doi: 10.1542/peds.2004-0471. [DOI] [PubMed] [Google Scholar]
- 25.Lange JR, Palis BE, Chang DC, Soong SJ, Balch CM. Melanoma in children and teenagers: an analysis of patients from the National Cancer Data Base. J Clin Oncol. 2007;25:1363–8. doi: 10.1200/JCO.2006.08.8310. [DOI] [PubMed] [Google Scholar]
- 26.Daryanani D, Plukker JT, Nap RE, Kuiper H, Hoekstra HJ. Adolescent melanoma: risk factors and long term survival. Eur J Surg Oncol. 2006;32:218–23. doi: 10.1016/j.ejso.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sørensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–91. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 28.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan GQ, Messina JL, Sondak VK, Zager JS. Sentinel lymph node biopsy for melanoma: indications and rationale. Cancer Control. 2009;16:234–9. doi: 10.1177/107327480901600305. [DOI] [PubMed] [Google Scholar]