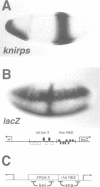
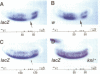
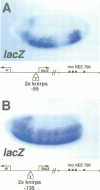
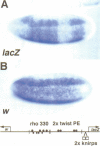
Abstract
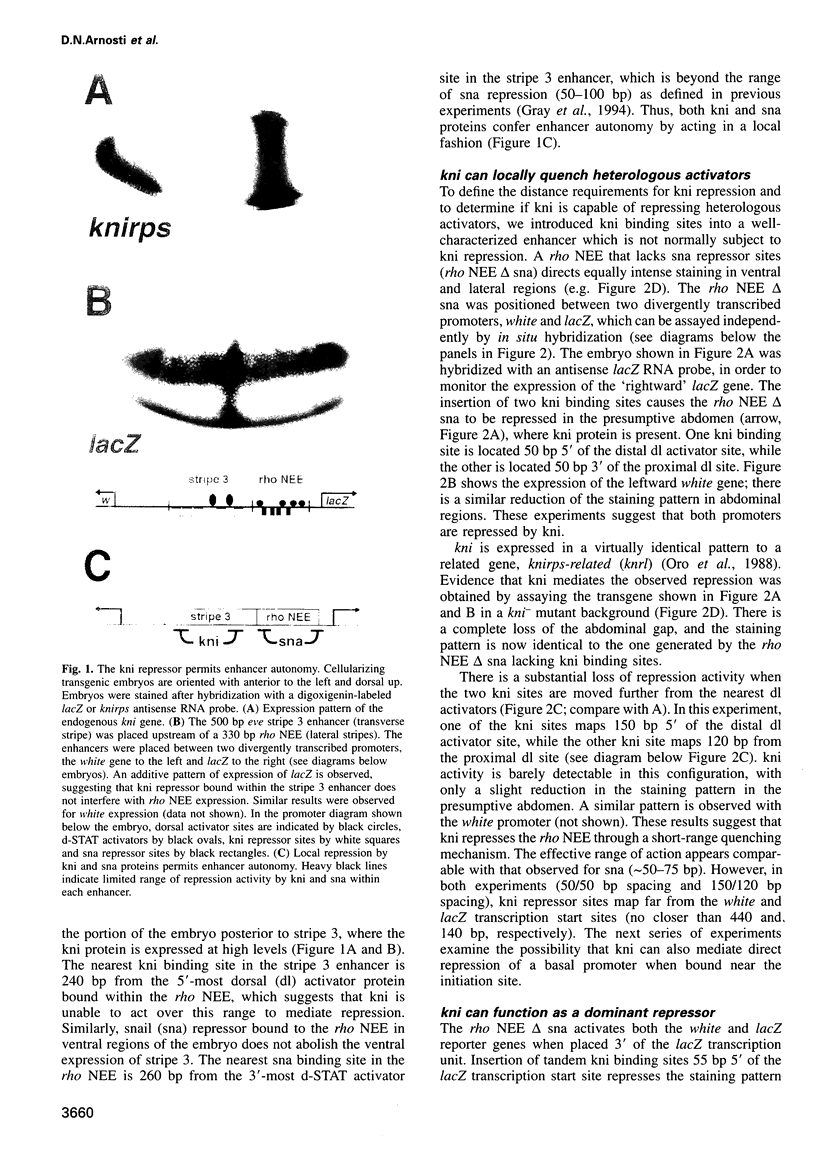
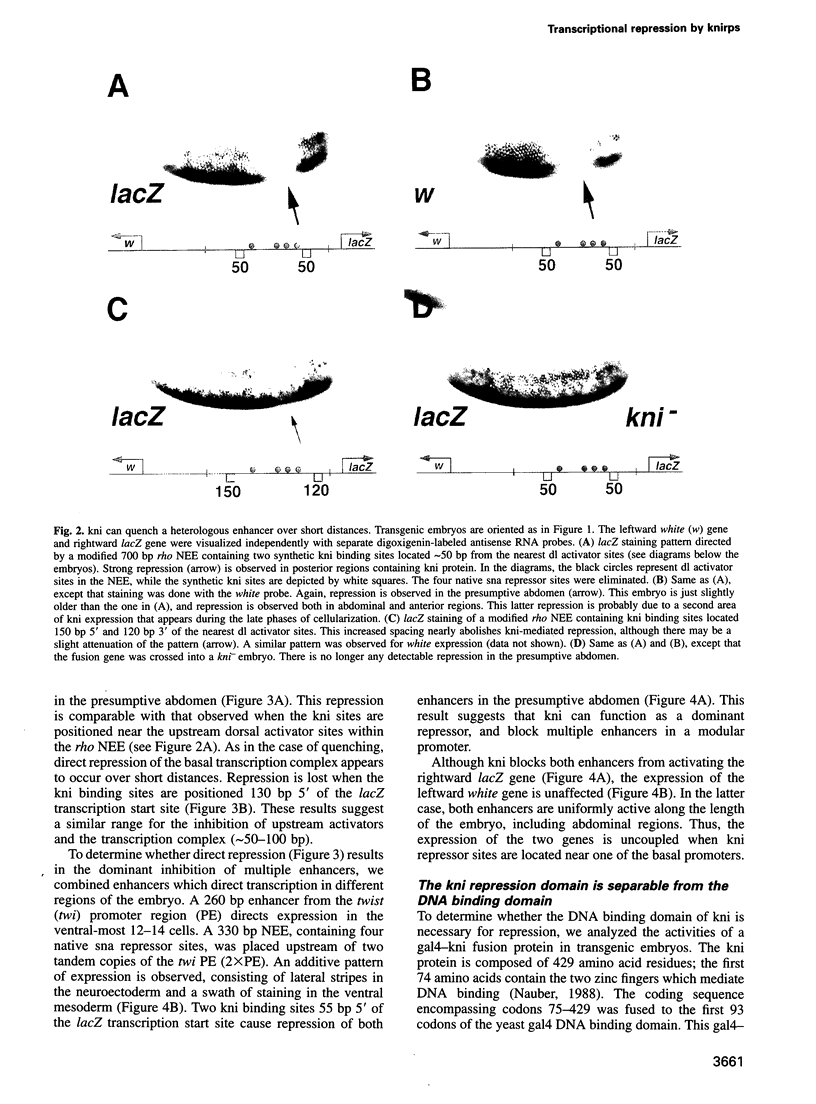
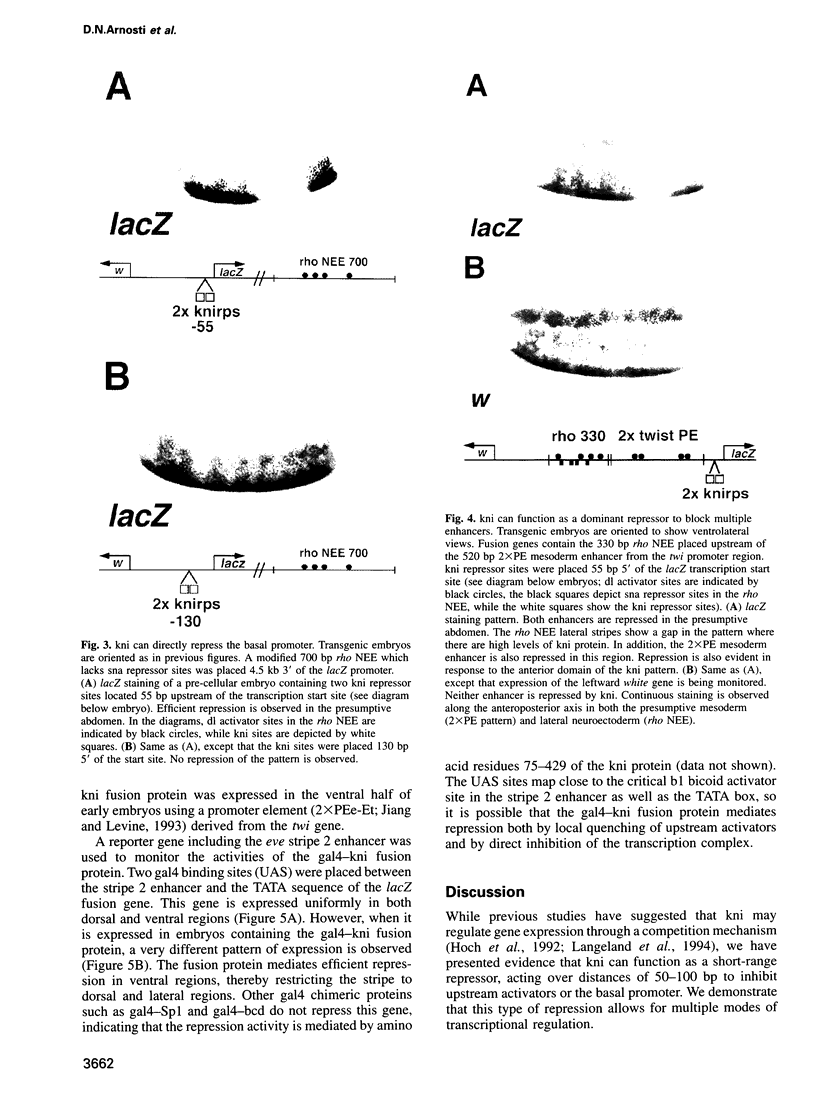
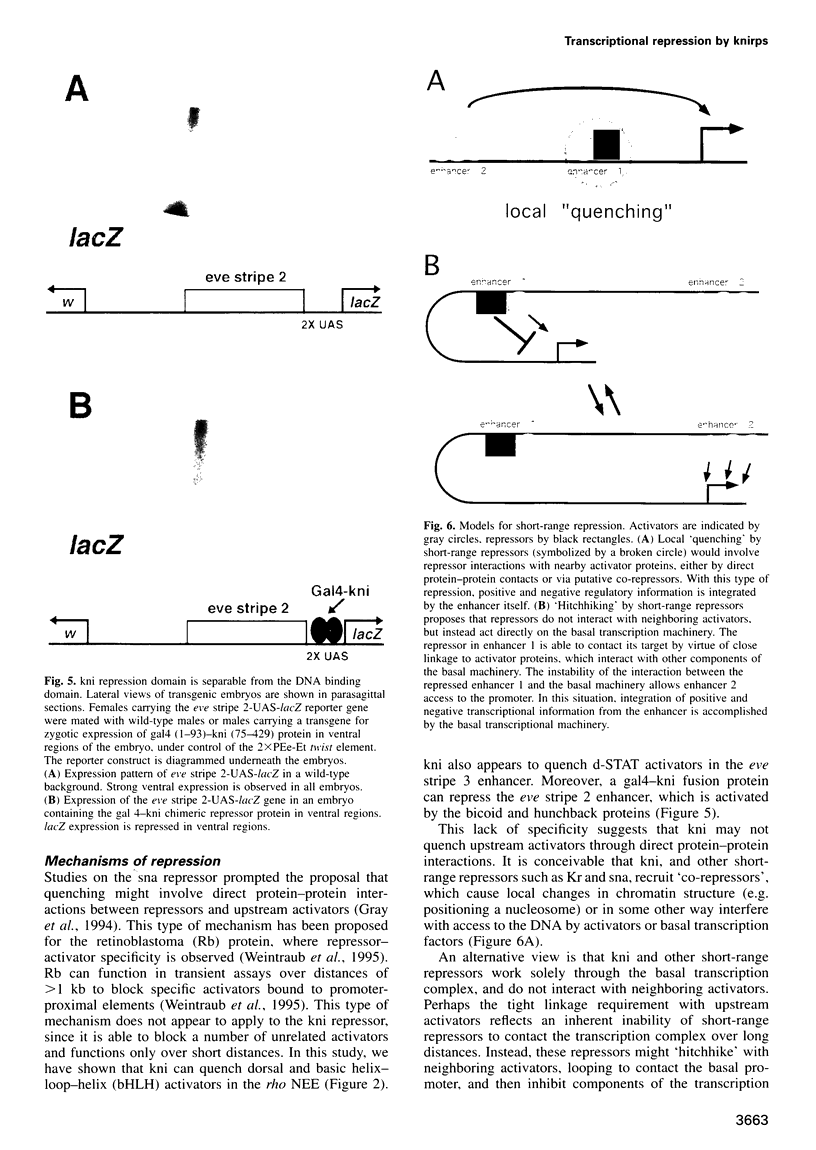
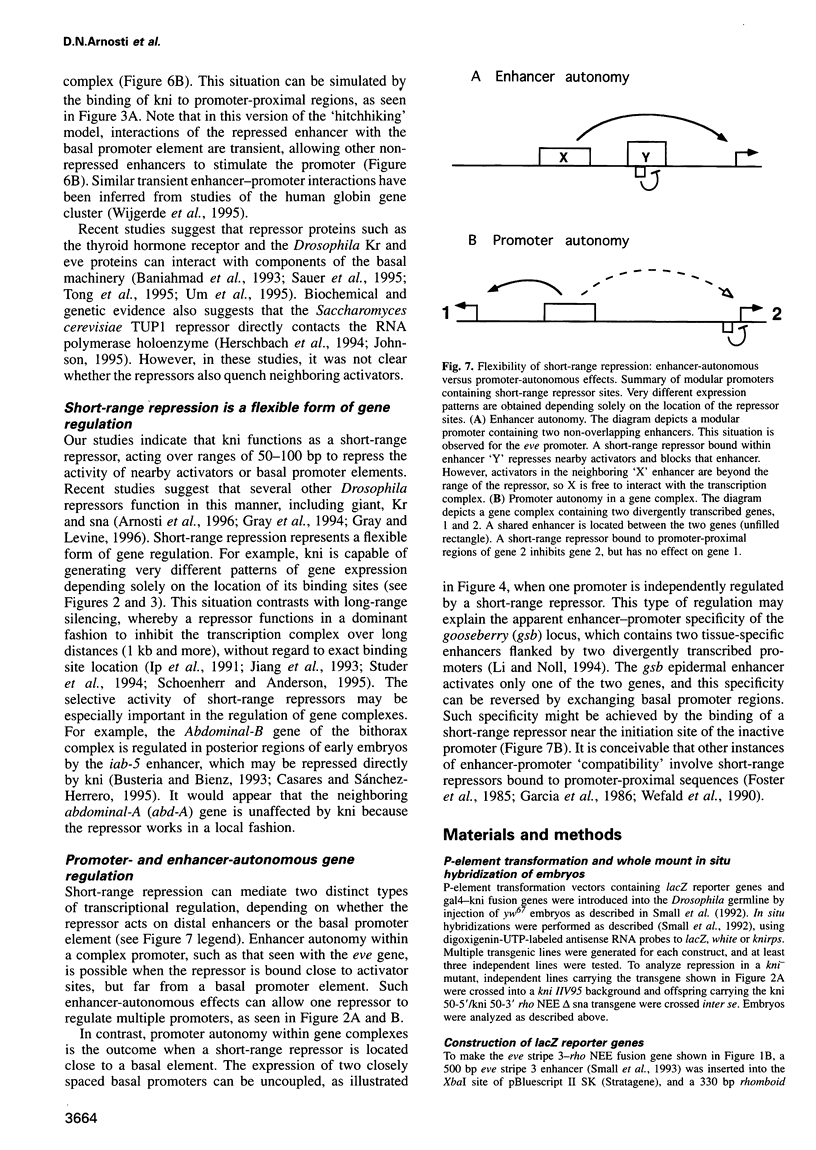
Transcriptional repression is essential for establishing localized patterns of gene expression during Drosophila embryogenesis. Several mechanisms of repression have been proposed, including competition, quenching and direct repression of the transcription complex. Previous studies suggest that the knirps orphan receptor (kni) may repress transcription via competition, and exclude the binding of the bicoid (bcd) activator to an overlapping site in a target promoter. Here we present evidence that kni can quench, or locally inhibit, upstream activators within a heterologous enhancer in transgenic embryos. The range of kni repression is approximately 50-100 bp, so that neighboring enhancers in a modular promoter are free to interact with the transcription complex (enhancer autonomy). However, kni can also repress the transcription complex when bound in promoter-proximal regions. In this position, kni functions as a dominant repressor and blocks multiple enhancers in a modular promoter. Our studies suggest that short-range repression represents a flexible form of gene regulation, exhibiting enhancer- or promoter-specific effects depending on the location of repressor binding sites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnosti D. N., Barolo S., Levine M., Small S. The eve stripe 2 enhancer employs multiple modes of transcriptional synergy. Development. 1996 Jan;122(1):205–214. doi: 10.1242/dev.122.1.205. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Ha I., Reinberg D., Tsai S., Tsai M. J., O'Malley B. W. Interaction of human thyroid hormone receptor beta with transcription factor TFIIB may mediate target gene derepression and activation by thyroid hormone. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8832–8836. doi: 10.1073/pnas.90.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busturia A., Bienz M. Silencers in abdominal-B, a homeotic Drosophila gene. EMBO J. 1993 Apr;12(4):1415–1425. doi: 10.1002/j.1460-2075.1993.tb05785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares F., Sánchez-Herrero E. Regulation of the infraabdominal regions of the bithorax complex of Drosophila by gap genes. Development. 1995 Jun;121(6):1855–1866. doi: 10.1242/dev.121.6.1855. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Evans R. M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995 Oct 5;377(6548):454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Foster J., Stafford J., Queen C. An immunoglobulin promoter displays cell-type specificity independently of the enhancer. 1985 May 30-Jun 5Nature. 315(6018):423–425. doi: 10.1038/315423a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Bich-Thuy L. T., Stafford J., Queen C. Synergism between immunoglobulin enhancers and promoters. Nature. 1986 Jul 24;322(6077):383–385. doi: 10.1038/322383a0. [DOI] [PubMed] [Google Scholar]
- Gerwin N., La Rosée A., Sauer F., Halbritter H. P., Neumann M., Jäckle H., Nauber U. Functional and conserved domains of the Drosophila transcription factor encoded by the segmentation gene knirps. Mol Cell Biol. 1994 Dec;14(12):7899–7908. doi: 10.1128/mcb.14.12.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S., Levine M. Short-range transcriptional repressors mediate both quenching and direct repression within complex loci in Drosophila. Genes Dev. 1996 Mar 15;10(6):700–710. doi: 10.1101/gad.10.6.700. [DOI] [PubMed] [Google Scholar]
- Gray S., Szymanski P., Levine M. Short-range repression permits multiple enhancers to function autonomously within a complex promoter. Genes Dev. 1994 Aug 1;8(15):1829–1838. doi: 10.1101/gad.8.15.1829. [DOI] [PubMed] [Google Scholar]
- Herschbach B. M., Arnaud M. B., Johnson A. D. Transcriptional repression directed by the yeast alpha 2 protein in vitro. Nature. 1994 Jul 28;370(6487):309–311. doi: 10.1038/370309a0. [DOI] [PubMed] [Google Scholar]
- Hoch M., Gerwin N., Taubert H., Jäckle H. Competition for overlapping sites in the regulatory region of the Drosophila gene Krüppel. Science. 1992 Apr 3;256(5053):94–97. doi: 10.1126/science.1348871. [DOI] [PubMed] [Google Scholar]
- Hörlein A. J., När A. M., Heinzel T., Torchia J., Gloss B., Kurokawa R., Ryan A., Kamei Y., Söderström M., Glass C. K. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995 Oct 5;377(6548):397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Kraut R., Levine M., Rushlow C. A. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell. 1991 Jan 25;64(2):439–446. doi: 10.1016/0092-8674(91)90651-e. [DOI] [PubMed] [Google Scholar]
- Jiang J., Cai H., Zhou Q., Levine M. Conversion of a dorsal-dependent silencer into an enhancer: evidence for dorsal corepressors. EMBO J. 1993 Aug;12(8):3201–3209. doi: 10.1002/j.1460-2075.1993.tb05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Levine M. Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the dorsal gradient morphogen. Cell. 1993 Mar 12;72(5):741–752. doi: 10.1016/0092-8674(93)90402-c. [DOI] [PubMed] [Google Scholar]
- Johnson A. D. The price of repression. Cell. 1995 Jun 2;81(5):655–658. doi: 10.1016/0092-8674(95)90524-3. [DOI] [PubMed] [Google Scholar]
- Kurokawa R., Söderström M., Hörlein A., Halachmi S., Brown M., Rosenfeld M. G., Glass C. K. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995 Oct 5;377(6548):451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- Langeland J. A., Attai S. F., Vorwerk K., Carroll S. B. Positioning adjacent pair-rule stripes in the posterior Drosophila embryo. Development. 1994 Oct;120(10):2945–2955. doi: 10.1242/dev.120.10.2945. [DOI] [PubMed] [Google Scholar]
- Li X., Noll M. Compatibility between enhancers and promoters determines the transcriptional specificity of gooseberry and gooseberry neuro in the Drosophila embryo. EMBO J. 1994 Jan 15;13(2):400–406. doi: 10.1002/j.1460-2075.1994.tb06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauber U., Pankratz M. J., Kienlin A., Seifert E., Klemm U., Jäckle H. Abdominal segmentation of the Drosophila embryo requires a hormone receptor-like protein encoded by the gap gene knirps. Nature. 1988 Dec 1;336(6198):489–492. doi: 10.1038/336489a0. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Oro A. E., Ong E. S., Margolis J. S., Posakony J. W., McKeown M., Evans R. M. The Drosophila gene knirps-related is a member of the steroid-receptor gene superfamily. Nature. 1988 Dec 1;336(6198):493–496. doi: 10.1038/336493a0. [DOI] [PubMed] [Google Scholar]
- Pankratz M. J., Hoch M., Seifert E., Jäckle H. Krüppel requirement for knirps enhancement reflects overlapping gap gene activities in the Drosophila embryo. Nature. 1989 Sep 28;341(6240):337–340. doi: 10.1038/341337a0. [DOI] [PubMed] [Google Scholar]
- Rothe M., Nauber U., Jäckle H. Three hormone receptor-like Drosophila genes encode an identical DNA-binding finger. EMBO J. 1989 Oct;8(10):3087–3094. doi: 10.1002/j.1460-2075.1989.tb08460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F., Fondell J. D., Ohkuma Y., Roeder R. G., Jäckle H. Control of transcription by Krüppel through interactions with TFIIB and TFIIE beta. Nature. 1995 May 11;375(6527):162–164. doi: 10.1038/375162a0. [DOI] [PubMed] [Google Scholar]
- Schoenherr C. J., Anderson D. J. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995 Mar 3;267(5202):1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- Seipel K., Georgiev O., Schaffner W. Different activation domains stimulate transcription from remote ('enhancer') and proximal ('promoter') positions. EMBO J. 1992 Dec;11(13):4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S., Arnosti D. N., Levine M. Spacing ensures autonomous expression of different stripe enhancers in the even-skipped promoter. Development. 1993 Nov;119(3):762–772. [PubMed] [Google Scholar]
- Small S., Blair A., Levine M. Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J. 1992 Nov;11(11):4047–4057. doi: 10.1002/j.1460-2075.1992.tb05498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S., Blair A., Levine M. Regulation of two pair-rule stripes by a single enhancer in the Drosophila embryo. Dev Biol. 1996 May 1;175(2):314–324. doi: 10.1006/dbio.1996.0117. [DOI] [PubMed] [Google Scholar]
- Studer M., Pöpperl H., Marshall H., Kuroiwa A., Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994 Sep 16;265(5179):1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Tong G. X., Tanen M. R., Bagchi M. K. Ligand modulates the interaction of thyroid hormone receptor beta with the basal transcription machinery. J Biol Chem. 1995 May 5;270(18):10601–10611. doi: 10.1074/jbc.270.18.10601. [DOI] [PubMed] [Google Scholar]
- Um M., Li C., Manley J. L. The transcriptional repressor even-skipped interacts directly with TATA-binding protein. Mol Cell Biol. 1995 Sep;15(9):5007–5016. doi: 10.1128/mcb.15.9.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Weintraub S. J., Chow K. N., Luo R. X., Zhang S. H., He S., Dean D. C. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995 Jun 29;375(6534):812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Jr, Crews S. T. CNS midline enhancers of the Drosophila slit and Toll genes. Mech Dev. 1993 Mar;40(3):141–154. doi: 10.1016/0925-4773(93)90072-6. [DOI] [PubMed] [Google Scholar]
- Wijgerde M., Grosveld F., Fraser P. Transcription complex stability and chromatin dynamics in vivo. Nature. 1995 Sep 21;377(6546):209–213. doi: 10.1038/377209a0. [DOI] [PubMed] [Google Scholar]
- Yan R., Small S., Desplan C., Dearolf C. R., Darnell J. E., Jr Identification of a Stat gene that functions in Drosophila development. Cell. 1996 Feb 9;84(3):421–430. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]