Abstract
Introduction:
The fixation of cytological smears using ethanol is the gold standard. But, there exists a quench to search a new alternative for it due to it being expensive, carcinogenic and not freely available. Honey has various properties, like dehydrant, anti-bacterial and antioxidant. The use of honey as a preservative in funerary practices is well documented. A thorough search in the literature did not reveal any matter for the utility of honey as a fixative in cytological smear, but its use in histopathology is well recognized.
Aims:
To analyze the efficacy of cytological smears fixed in ethanol and 20% unprocessed honey and to compare the efficacy between the two fixatives.
Materials and Methods:
The study group comprised of 30 normal healthy individuals who willingly gave written consent. Prior to the collection of buccal cells, subjects were asked to rinse their mouth with water. Buccal cells were collected using a wooden ice cream stick. Two smears were collected from each subject. One smear was fixed in ethanol and the other was fixed in unprocessed 20% honey. The slides were washed in tap water for about 30 s, following which they were subjected to the conventional Papanicolaou staining procedure. The slides thus fixed were evaluated separately for ethanol and honey. The cytoplasmic and nuclear details were scored for 50 cells in each slide. Data were statistically analyzed using the chi-square test and P < 0.05 was considered statistically significant.
Results:
Ninety percent of the ethanol-fixed (EF) smears were adequately fixed as compared with the honey-fixed (HF) smears, which were 80% adequate. The P-value obtained was 0.47 and the data were statistically insignificant.
Conclusion:
Both EF and HF smears were at par with each other, and honey can be safely used as a substitute to ethanol.
Keywords: Cytological fixatives; ethanol, honey, papanicolaou stain
Introduction
Oral cytology has come a long way from its primitive Papanicolaou (PAP) days. With major strides in its eventful development, it has reached beyond bars.[1] In the present era, cytopathology is a well-accepted and valid diagnostic tool. Undoubtedly, diagnostic accuracy and reliability here depend greatly on the quality of collection, fixation, staining and interpretation. Inadequacy in any of these steps will adversely affect the standards of efficient diagnostic cytology.
Fixative also plays a pivotal role in cytopathological diagnosis. Ever since the introduction of fixatives, there has always been an enthusiasm and quest for an ideal cytological fixative. Ethanol, a fixative and dehydrant, plays an important role in the processing of cytological samples. The routine fixative used is 95% ethanol, and is proven for its efficiency. Ethanol is expensive and subject to pilferage thus decreasing its availability. Methanol is used alternatively, the efficacy of which is yet to be documented. The need of the hour is hence an efficient alternative.[2]
“Innovation is change that unlocks new values” – the value of honey as a preservative is well documented for ages. One such attempt is being made by us to use honey as a fixative in cytological smears. The quantum of studies accepts honey as a fixative and as an alternative substitute to formalin in histopathology.[3] Our study on the use in cytopathology would be the first in the English language research literature.
Aims and Objectives
To analyze the efficacy of cytological smears fixed in ethanol and 20% unprocessed honey.
To compare the efficacy between the two fixatives on staining.
Materials and Methods
The study group comprised of 30 healthy individuals aged 20-30 years. After obtaining written consent, two oral smears were obtained from each individual by gently scraping the buccal mucosa using a wooden spatula following rinsing of their mouth with water. One slide was fixed in ethanol (100% alcohol) and another slide was fixed in 20% unprocessed honey (after repeated standardization in increasing concentration, finally 20 mL honey + 80 mL distilled water was considered). All slides were fixed for a minimum of 15 min. The slides were washed in tap water for about 30 s, following which they were subjected to staining of both smears simultaneously by PAP stain. An oral pathologist examined the randomized mix of 60 slides blindly and allotted the score. In each slide, 50 cells were scored at high power (400×).
Evaluation criteria
The slides were scored for the parameters and criteria described in Table 1.
Table 1.
Evaluation criteria - Slides were scored for the following parameters
Final criteria
The total scores for honey and alcohol were added for each of the components and graded into:
Adequate: 40-100 cells.
Inadequate: <40 cells.
The data were statistically compared using the chi-square test/Fisher's exact test. A P-value <0.05 was considered as significant.
Results
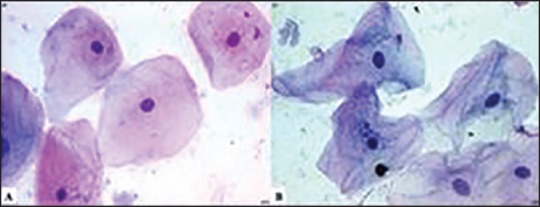
In the present study, of the 60 slides studied, 97% of the honey-fixed (HF) slides showed acceptable nuclear and cytoplasmic staining as compared with 90% and 93% of ethanol-fixed (EF) slides [Table 2]. On statistical comparison, the P-value was 0.61 for nuclear staining and 0.99 for cytoplasmic staining; hence, there was no statistically significant difference between the two fixatives.
Table 2.
Comparison of nuclear and cytoplasmic staining in ethanol-fixed and honey-fixed slides
On analysis of nuclear and cytoplasmic staining, 3% of HF and 10% of EF slides showed unacceptable nuclear staining. Further, 3% of HF and 7% of EF slides showed unacceptable cytoplasmic staining.
On analysis of the total cell morphology, HF slides were slightly better than EF slides, with 97% and 93%, respectively [Table 2]. Three percent of HF and 7% of EF slides showed unpreserved cellular morphology, ascribed to the disintegration of cell membrane and cell shrinkage. The P-value was 0.99 for both fixatives, and it was statistically insignificant.
On analysis for clarity and uniformity of staining, the EF (83% and 90%, respectively) slides were much better than the HF (67% and 87%, respectively) slides. In addition, 13% of the HF slides were not uniformly stained [Table 2].
Final analysis of the scores revealed that 90% of EF and 80% of HF slides were adequate [Table 3] for diagnosis, with a P-value of 0.47 for both fixatives. Hence, there was no statistically significant difference between the two fixatives for adequacy in diagnosis.
Table 3.
Comparison of scores for adequacy of the PAP-stained slides fixed in ethanol and honey

Discussion
Alcohol plays a significant role in cytological fixation. Good fixative is necessary for preservation of cellular details, enabling accurate cytological assessment and diagnosis. The routine fixative used is 95% ethanol, which is an efficient fixative, but is subject to pilferage, is expensive, evaporates easily and is not freely available. Among the fixatives, honey has an age old use for embalming dead bodies.[2]
Honey is a sweet, sticky, yellowish-brown fluid made by bees from nectar collected from flowers. Honey is defined as the nectar and saccharine exudation of plants, which gathered, modified and stored as honey in honeycombs by honey bees, Apis melifera.[4]
Honey is as old as the written history - dating back to 2100 B.C. - Sumerian and Babylonian cuneiform writings. Honey was man's first and most reliable source of sweetener. History knows examples of things preserved in honey for decades and even centuries. Use of honey in funerary practices in many different cultures is well documented. Burmese priests have a custom of preserving their chief abbots in coffins full of honey. Alexander the Great, as Statius records - his remains shall be preserved in honey. Herod I, King of Judea (40-4 B.C.) - executed his beautiful wife Marianne's body and preserved it in honey for 7 years. Arabs still preserve meat in honey and mummification in honey by Egyptians is very well known.[5] All these facts made us wonder whether we could broaden the horizon of use of honey as a fixative in this modern world of “GO GREEN.”
Honey is produced from many floral sources and its content varies with its origin. Honey contains lysozymes (hydrolytic enzymes active at acid pH against several bacterial species), several minerals, trace elements such as potassium, sodium, chlorine, calcium, magnesium, magnesium, iron, manganese, copper, magnesium, sulfur and silicon (as SiO2) and chromium, lithium, nickel, lead, tin, zinc, osmium, beryllium, vanadium, zirconium, silver, barium, gallium, bismuth, gold, germanium and strontium. Vitamins such as B1 (Thiamine), Riboflavin, Niacin, B6 (Pyridoxine), Pantothenic acid and B12 and C (Ascorbic acid) are also found in honey.[6,7]
The antimicrobial activity of honey is due to its inhibitory effect on growth of around 60 species of bacteria, which includes aerobes, anaerobes and gram-positive and gram-negative organisms, but its efficiency as a fixative is concealed.[8]
Honey is thought to preserve the cells by preventing autolysis and putrefaction. Thus, studies have shown that honey has a good preventive putrefactive property mainly because honey contains seven tetracycline derivatives, fatty acids, lipids, amylases and ascorbic acid and hydrogen peroxide.[7,9] Therefore, honey is used as an agent for preventing autolysis and putrefaction. The aim of the present study is to compare honey with ethanol as a cytological fixative.
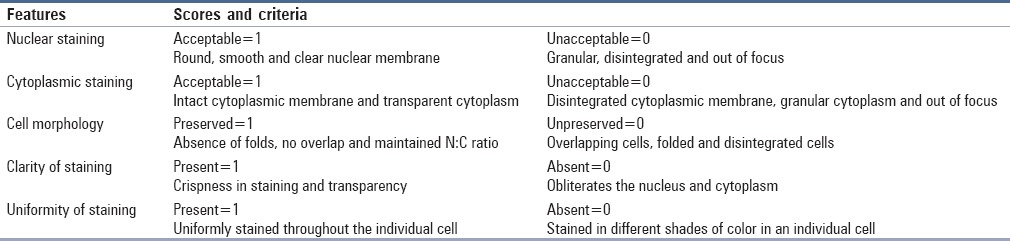
In the present study, the maximum number of HF slides showed acceptable nuclear and cytoplasmic staining as compared with EF slides. However, on statistical comparison, there was no statistically significant difference between the two fixatives. Three percent of HF and 10% of EF slides showed unacceptable nuclear staining. This was mainly because the nuclei were stained eosinophilic in EF and lightly stained in HF smears. Three percent of HF and 7% of EF slides showed unacceptable cytoplasmic staining attributed to loss of cytoplasmic transparency, which is usually seen in PAP-stained slides [Figure 1].
Figure 1.
(a) Honey-fixed smear showing good nuclear and cytoplasmic details (Pap, ×400). (b) Honey-fixed smear showing eosinophilic stained nucleus (Pap, ×400)
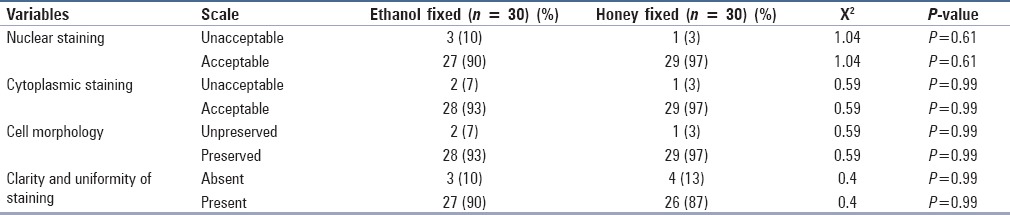
When cell morphology was analyzed in terms of size and shape of the cell, the HF slides were slightly better than the EF slides. However, 3% of the HF and 7% of the EF slides showed unpreserved cellular morphology ascribed to the disintegration of the cell membrane and cell shrinkage. Thus, the data were statistically insignificant [Figure 2].
Figure 2.
(a) Honey-fixed smear shows well-preserved cell morphology (Pap, ×400). (b) Ethanol-fixed smear showing folds and shrinkage (Pap, ×400)
On analysis for clarity and uniformity of staining, the EF slides were much better than the HF slides. Further, 33% of the HF slides were not clear because of the presence of small eosinophilic granules in the cytoplasm, which were not seen in the EF slides; may be due to the use of unprocessed honey. In addition, 13% of the HF slides were not uniformly stained [Figure 3].
Figure 3.
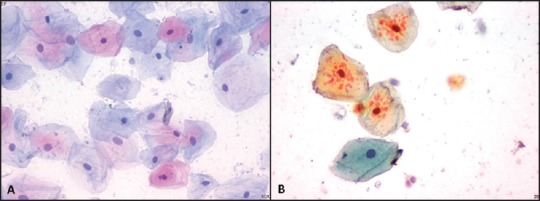
(a) Ethanol-fixed smear showing good clarity and uniformity (Pap, ×400). (b) Honey-fixed smear showing eosinophilic granules in the cytoplasm of few cells obliterating the clarity (Pap, ×400)
Final analysis of all the scores revealed that 90% of the EF and 80% of the HF slides were adequate for analysis. However, there was no statistically significant difference between the two fixatives for adequacy in diagnosis [Figure 4]. In addition, the background seen in the HF smears was much clear as compared with the EF smears, whereas the clarity and uniformity of staining was better in the EF slides. In the present study, most of the cells showed well-defined nuclear chromatin, nuclear membrane and intact cytoplasm in both EF and HF smears.
Figure 4.
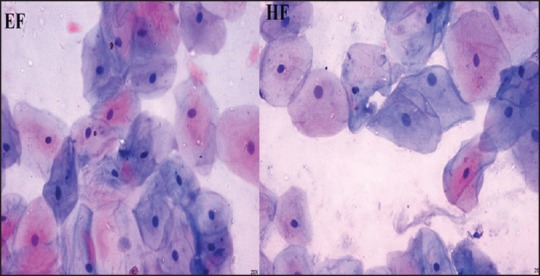
Ethanol-fixed and honey-fixed smears showing adequacy for diagnosis (Pap, ×400)
The present study was found to be the first study in the English language literature where honey was used as a cytological fixative. However, few studies have shown honey as a tissue fixative and can be used as an alternative to formalin. The results obtained in the present study were similar to the results of Ozkan et al.[3] and Al-Maaini and Bryant et al.[10] In addition, Ozkan et al. showed that honey fixation was similar to alcoholic formalin fixation in both histochemical and immuno-histochemical studies.[3]
Although ethanol and its various concentrations have been widely used in histopathology laboratories, it has many well-known disadvantages. However, honey has many advantages and disadvantages over ethanol as drawn from the present study, which includes viscosity of honey, the fact that diluted honey has to be mixed with antifungals and easy maintenance of honey, which can be stored in an air tight box. Therefore, it is revealed that any cytological smears in which preservation of cellular details is necessary can be adequately and efficiently assessed with fixation in 20% unprocessed honey, which is at par with and as good as ethanol.
Conclusion
Honey is as efficient as ethanol in cytological fixation. The component in honey that is efficiently serving as a fixative is a matter of further research. In the absence of alcohol or as a substitute to it, honey can be used as a successfully alternative. Honey can thus be a cheaper, pleasant smelling, easily and forever available, biofriendly and non-toxic alternative fixative in oral cytology.
Acknowledgments
The authors appreciate and thank the subjects for their cooperation. The study was presented in the First National Midterm Conference - Indian Association of Oral and Maxillofacial Pathologist, 2013, at the Saveetha Dental College, Chennai.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dhingra V, Mehrotra R. Historical development of oral cytology. In: Mehrotra R, editor. Oral Cytology: A Concise Guide. New York: Springer; 2013. pp. 5–9. [Google Scholar]
- 2.Kumarasinghe MP, Constantine SR, Hemamali RL. Methanol as an alternative fixative for cytological smears. Malays J Pathol. 1997;19:137–40. [PubMed] [Google Scholar]
- 3.Ozkan N, Salva E, Cakalağaoğlu F, Tüzüner B. Honey as a substitute for formalin? Biotech Histochem. 2012;87:148–53. doi: 10.3109/10520295.2011.590155. [DOI] [PubMed] [Google Scholar]
- 4.Olaitan PB, Adeleke OE, Ola IO. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr Health Sci. 2007;7:159–65. doi: 10.5555/afhs.2007.7.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roach M. Stiff: The Curious Lives of Human Cadavers. Paw Print. 2010:52–3. [Google Scholar]
- 6.McCarthy J. The antibacterial effects of honey. Am Bee J. 1995:171–2. [Google Scholar]
- 7.Avwioro G, Bankole J, Iyiola S, Avwioro T, Akinola G. One of the properties of honey in wound healing is prevention of autolysis. Der Pharmacia Lettre. 2010;2:321–5. [Google Scholar]
- 8.Molan PC. Antibacterial activity of honey: 1. The nature of antibacterial activity. Bee World. 1992;73:5–28. [Google Scholar]
- 9.Molan PC. Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World. 1999;80:80–92. [Google Scholar]
- 10.Al-Maaini R, Bryant P. The effectiveness of honey as a substitute for formalin in the histological fixation of tissue. J Histotechnol. 2006;29:173–6. [Google Scholar]


