Abstract
Nuclear receptors (NRs) act as ligand-inducible transcription factors which regulate the expression of target genes upon binding to cognate response elements. The ligand-dependent activity of the NR activation function AF-2 is believed to be mediated to the transcription machinery through transcriptional mediators/intermediary factors (TIFs). We report here the cloning of the 160 kDa human nuclear protein TIF2, which exhibits all properties expected for a mediator of AF-2: (i) it interacts in vivo with NRs in an agonist-dependent manner; (ii) it binds directly to the ligand-binding domains (LBDs) of NRs in an agonist- and AF-2-integrity-dependent manner in vitro; (iii) it harbours an autonomous transcriptional activation function; (iv) it relieves nuclear receptor autosquelching; and (v) it enhances the activity of some nuclear receptor AF-2s when overexpressed in mammalian cells. TIF2 exhibits partial sequence homology with the recently isolated steroid receptor coactivator SRC-1, indicating the existence of a novel gene family of nuclear receptor transcriptional mediators.
Full text
PDF
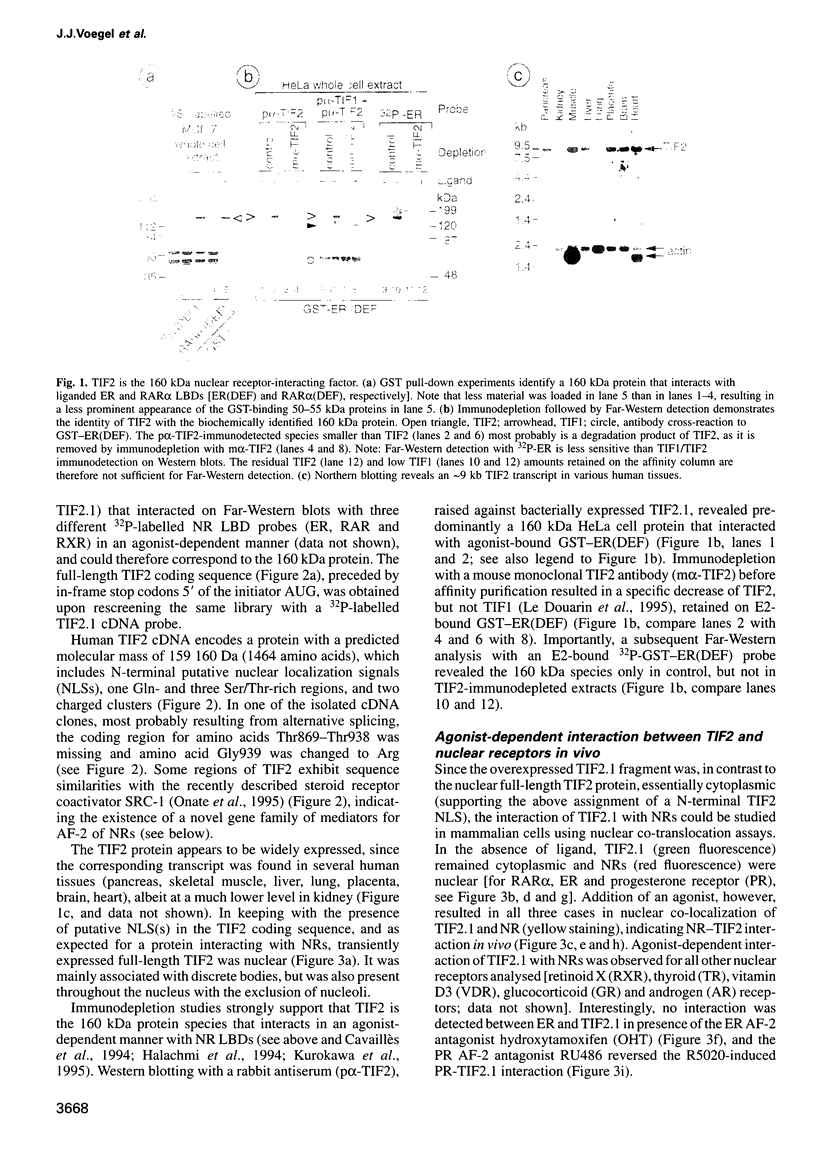

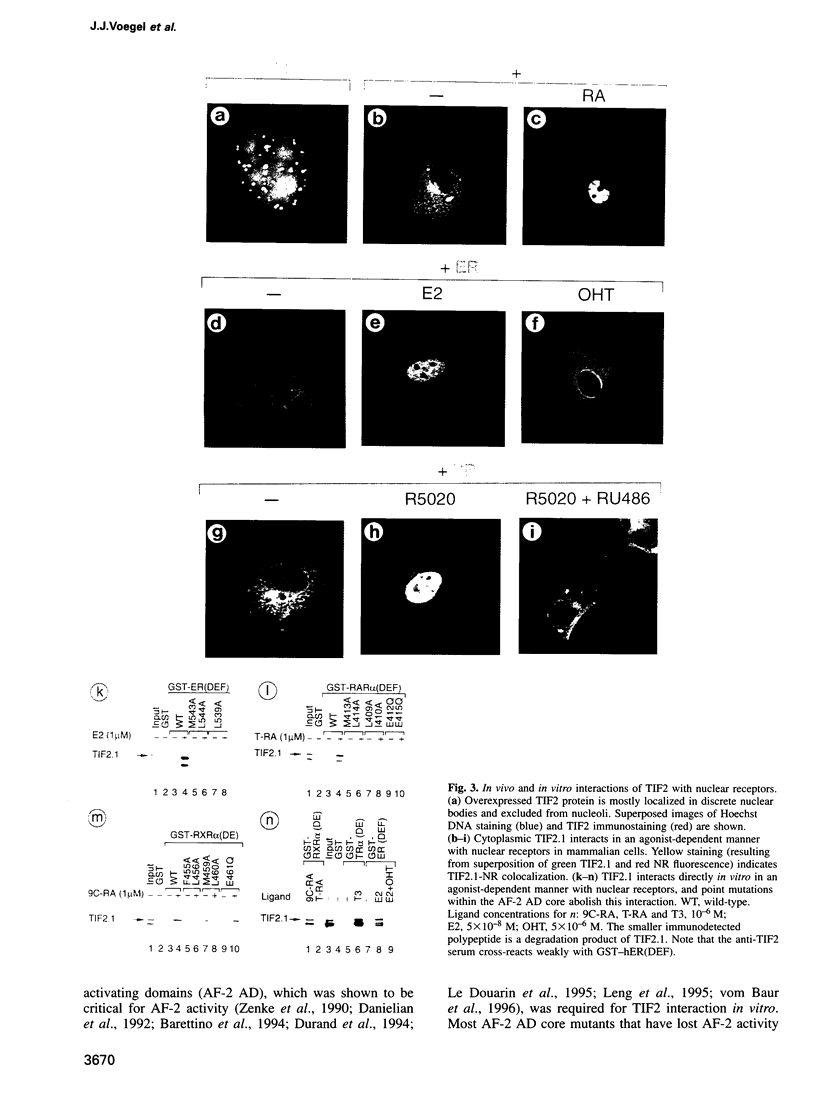





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barettino D., Vivanco Ruiz M. M., Stunnenberg H. G. Characterization of the ligand-dependent transactivation domain of thyroid hormone receptor. EMBO J. 1994 Jul 1;13(13):3039–3049. doi: 10.1002/j.1460-2075.1994.tb06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M., Herrlich P., Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995 Dec 15;83(6):851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990 Sep;9(9):2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquel M. T., Kumar V., Stricker C., Chambon P., Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989 Apr 11;17(7):2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguet W., Ruff M., Chambon P., Gronemeyer H., Moras D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature. 1995 Jun 1;375(6530):377–382. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- Cavaillès V., Dauvois S., Danielian P. S., Parker M. G. Interaction of proteins with transcriptionally active estrogen receptors. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10009–10013. doi: 10.1073/pnas.91.21.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillès V., Dauvois S., L'Horset F., Lopez G., Hoare S., Kushner P. J., Parker M. G. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995 Aug 1;14(15):3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Y., Penco S., Ostrowski J., Balaguer P., Pons M., Starrett J. E., Reczek P., Chambon P., Gronemeyer H. RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation. EMBO J. 1995 Mar 15;14(6):1187–1197. doi: 10.1002/j.1460-2075.1995.tb07102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. P., Shemshedini L., Durand B., Noy N., Chambon P., Gronemeyer H. Pure and functionally homogeneous recombinant retinoid X receptor. J Biol Chem. 1994 Oct 14;269(41):25770–25776. [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B., Saunders M., Gaudon C., Roy B., Losson R., Chambon P. Activation function 2 (AF-2) of retinoic acid receptor and 9-cis retinoic acid receptor: presence of a conserved autonomous constitutive activating domain and influence of the nature of the response element on AF-2 activity. EMBO J. 1994 Nov 15;13(22):5370–5382. doi: 10.1002/j.1460-2075.1994.tb06872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer H., Laudet V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2(11):1173–1308. [PubMed] [Google Scholar]
- Halachmi S., Marden E., Martin G., MacKay H., Abbondanza C., Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994 Jun 3;264(5164):1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- Jacq X., Brou C., Lutz Y., Davidson I., Chambon P., Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994 Oct 7;79(1):107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- Kastner P., Mark M., Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995 Dec 15;83(6):859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Kastner P., Perez A., Lutz Y., Rochette-Egly C., Gaub M. P., Durand B., Lanotte M., Berger R., Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 1992 Feb;11(2):629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., Söderström M., Hörlein A., Halachmi S., Brown M., Rosenfeld M. G., Glass C. K. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995 Oct 5;377(6548):451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- Le Douarin B., Zechel C., Garnier J. M., Lutz Y., Tora L., Pierrat P., Heery D., Gronemeyer H., Chambon P., Losson R. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 1995 May 1;14(9):2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Choi H. S., Gyuris J., Brent R., Moore D. D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995 Feb;9(2):243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Ryan F., Swaffield J. C., Johnston S. A., Moore D. D. Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature. 1995 Mar 2;374(6517):91–94. doi: 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- Leng X., Blanco J., Tsai S. Y., Ozato K., O'Malley B. W., Tsai M. J. Mouse retinoid X receptor contains a separable ligand-binding and transactivation domain in its E region. Mol Cell Biol. 1995 Jan;15(1):255–263. doi: 10.1128/mcb.15.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Evans R. M. The RXR heterodimers and orphan receptors. Cell. 1995 Dec 15;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995 Dec 15;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengus G., May M., Jacq X., Staub A., Tora L., Chambon P., Davidson I. Cloning and characterization of hTAFII18, hTAFII20 and hTAFII28: three subunits of the human transcription factor TFIID. EMBO J. 1995 Apr 3;14(7):1520–1531. doi: 10.1002/j.1460-2075.1995.tb07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Friant S., Nakshatri H., Chambon P. RARs and RXRs: evidence for two autonomous transactivation functions (AF-1 and AF-2) and heterodimerization in vivo. EMBO J. 1993 Jun;12(6):2349–2360. doi: 10.1002/j.1460-2075.1993.tb05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell. 1992 Sep 18;70(6):1007–1019. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- Oñate S. A., Tsai S. Y., Tsai M. J., O'Malley B. W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995 Nov 24;270(5240):1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Renaud J. P., Rochel N., Ruff M., Vivat V., Chambon P., Gronemeyer H., Moras D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995 Dec 14;378(6558):681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- Seipel K., Georgiev O., Schaffner W. Different activation domains stimulate transcription from remote ('enhancer') and proximal ('promoter') positions. EMBO J. 1992 Dec;11(13):4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasset D., Tora L., Fromental C., Scheer E., Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990 Sep 21;62(6):1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- Thummel C. S. From embryogenesis to metamorphosis: the regulation and function of Drosophila nuclear receptor superfamily members. Cell. 1995 Dec 15;83(6):871–877. doi: 10.1016/0092-8674(95)90203-1. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Wagner R. L., Apriletti J. W., McGrath M. E., West B. L., Baxter J. D., Fletterick R. J. A structural role for hormone in the thyroid hormone receptor. Nature. 1995 Dec 14;378(6558):690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Wurtz J. M., Bourguet W., Renaud J. P., Vivat V., Chambon P., Moras D., Gronemeyer H. A canonical structure for the ligand-binding domain of nuclear receptors. Nat Struct Biol. 1996 Jan;3(1):87–94. doi: 10.1038/nsb0196-87. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- vom Baur E., Zechel C., Heery D., Heine M. J., Garnier J. M., Vivat V., Le Douarin B., Gronemeyer H., Chambon P., Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 1996 Jan 2;15(1):110–124. [PMC free article] [PubMed] [Google Scholar]








