Abstract
Both shared and unique genetic risk factors underlie the two symptom domains of attention deficit hyperactivity disorder (ADHD): inattention and hyperactivity-impulsivity. The developmental course and relationship to co-occurring disorders differs across the two symptom domains, highlighting the importance of their partially distinct etiologies. Familial cognitive impairment factors have been identified in ADHD, but whether they show specificity in relation to the two ADHD symptom domains remains poorly understood. We aimed to investigate whether different cognitive impairments are genetically linked to the ADHD symptom domains of inattention versus hyperactivity-impulsivity. We conducted multivariate genetic model fitting analyses on ADHD symptom scores and cognitive data, from go/no-go and fast tasks, collected on a population twin sample of 1312 children aged 7-10. Reaction time variability (RTV) showed substantial genetic overlap with inattention, as observed in an additive genetic correlation of 0.64, compared to an additive genetic correlation of 0.31 with hyperactivity-impulsivity. Commission errors (CE) showed low additive genetic correlations with both hyperactivity-impulsivity and inattention (genetic correlations of 0.17 and 0.11, respectively). The additive genetic correlation between RTV and CE was also low and non-significant at −0.10, consistent with the etiological separation between the two indices of cognitive impairments. Overall, two key cognitive impairments phenotypically associated with ADHD symptoms, captured by RTV and CE, showed different genetic relationships to the two ADHD symptom domains. The findings extend a previous model of two familial cognitive impairment factors in combined subtype ADHD by separating pathways underlying inattention and hyperactivity-impulsivity symptoms.
Keywords: ADHD, Genetics, Reaction time variability, Commission errors, Inattention, Hyperactivity/impulsivity
Introduction
Two behavioural symptom domains underlie the current conceptualisation of attention-deficit/hyperactivity disorder (ADHD): inattention and hyperactivity-impulsivity (APA 2000). Previous twin analyses on ADHD symptom scores indicate that 55-80% of the genetic influences on inattention also influence hyperactive-impulsivity, with the remaining genetic influences reflecting those that are unique to each symptom domain (Greven et al. 2011; McLoughlin et al. 2007, 2011; Wood et al. 2009). Despite the substantial shared genetic component, converging evidence highlights the importance of the partially distinct etiologies, as the two ADHD domains show differential phenotypic and etiological relations with co-occurring neurodevelopmental and behavioural problems. For example, reading difficulties are linked predominantly to inattention (Paloyelis et al. 2010; Willcutt et al. 2007), and oppositional behaviours to hyperactivity-impulsivity (Newcorn et al. 2001; Wood et al. 2009). Furthermore, hyperactivity-impulsivity decreases relative to inattention throughout development in both clinical (Biederman et al. 2000; Todd et al. 2008) and population (Larsson et al. 2006) samples.
The emerging knowledge of the shared and unique etiological influences on the two ADHD symptom domains raises questions about how this maps onto cognitive impairments, particularly those that index the familial risk for ADHD. In a recent large-scale investigation of ADHD and control sibling pairs, we obtained evidence for two familial cognitive impairment factors in ADHD (Kuntsi et al. 2010). The larger familial factor, accounting for 85% of the familial variance of ADHD, captured 98-100% of the familial influences on mean reaction time (RT) and reaction time variability (RTV) (Kuntsi et al. 2010). This factor separated from a second familial factor that captured 62-82% of the familial influences on omission and commission errors (on a go/no-go task) and accounted for 13% of the familial variance of ADHD. Drawing on the arousal-attention (Johnson et al. 2007; O’Connell et al. 2008) and developmental (Halperin and Schulz 2006; Halperin et al. 2008) models of ADHD, we proposed that the first factor (RT) may represent bottom-up arousal dysregulation and the second factor (errors) top-down control of sustained attention and inhibition (Kuntsi et al. 2010). However, this study, based on a clinical sample of probands with combined subtype ADHD, was unable to examine the specificity that the cognitive impairment factors may have in relation to inattention and hyperactivity-impulsivity symptoms considered separately.
Previous comparisons of cognitive performance between the inattentive and combined subtypes of ADHD have failed to identify clearly distinguishable cognitive profiles (Carr et al. 2010). Empirical approaches to ADHD subtypes indicate that many inattentive subtype cases reflect sub-threshold combined type ADHD and should not be treated as a separate category (Todd et al. 2001). Furthermore, ADHD subtypes are unstable, with many combined subtype cases being re-classified as inattentive subtype as they grow older (Biederman et al. 2000). A more strictly defined pure inattentive subtype was, however, linked to early attentional problems and inconsistent performance, whereas inhibition difficulties were observed across ADHD subgroups (Adams et al. 2008; Carr et al. 2010).
The present study applies a multivariate twin model fitting approach on a population twin sample to investigate inattentive and hyperactive-impulsive symptoms separately. Using the twin sample, we previously found that associations between ADHD symptoms and the cognitive impairments of slow and variable RTs and commission errors (CE) (Kuntsi et al. 2009; Wood et al. 2010a) were similar to those observed in a large clinical sample of ADHD combined subtype cases (Andreou et al. 2007; Kuntsi et al. 2010; Uebel et al. 2010; Wood et al. 2010b). In both samples we have recently also shown that RTV difference scores, which capture the ADHD-sensitive improvement in RTV (for example under rewarded conditions (Andreou et al. 2007; Kuntsi et al. 2009; Uebel et al. 2010)), measure largely the same etiological process as RTV under baseline condition (Kuntsi et al. 2013), supporting theories emphasizing the malleability of the observed high RTV.
We now address two new questions. First, using multivariate twin model fitting, we investigate if there are differential etiological associations between the previously identified cognitive impairments and the two symptom domains of ADHD considered separately. Secondly, we examine whether the etiological separation between impaired RT performance and CE (Kuntsi et al. 2010) is confirmed in a population twin sample.
Methods
Sample and Procedure
Participants are members of the Study of Activity and Impulsivity Levels in children (SAIL), a general population sample of twins aged 7-10 years. They were recruited from the Twins’ Early Development Study (TEDS (Trouton et al. 2002) a birth cohort study which invited parents of all twins born in England and Wales during 1994-1996 to enroll. The TEDS families are representative of the UK population with respect to parental occupation, education and ethnicity (Oliver and Plomin 2007).
TEDS families were invited to take part if they fulfilled the following SAIL project inclusion criteria: twins’ birthdates between September 1, 1995, and December 31, 1996; lived within a feasible travelling distance from the research centre; White European ethnic origin (to reduce population heterogeneity for molecular genetic studies); recent participation in TEDS, as indicated by return of questionnaires at either 4- or 7-year data collection point; no extreme pregnancy, perinatal difficulties, specific medical syndromes, chromosomal anomalies or epilepsy; not participating in other current TEDS substudies; and not on stimulant or other neuropsychiatric medications.
Of the 1,230 suitable families contacted, 672 families (55%) agreed to participate. Thirty-two individual children were subsequently excluded due to: IQ < 70, epilepsy, obsessive-compulsive disorder, autism or other neurodevelopmental disorder, illness during testing or placement on stimulant medication for ADHD. The final sample consisted of 1,312 individuals: 257 monozygotic (MZ) twin pairs, 181 same-sex dizygotic (DZ) and 206 opposite-sex DZ twin pairs, as well as 24 singletons coming from pairs with one of the twins excluded. Data for the 24 singleton twins were also used in the structural equation modeling (Neale et al. 2006a). Participants were invited to our research centre for a cognitive assessment, where ratings on the Conners’ scale were collected from parents. Teachers’ ratings on the Conners’ scale were obtained through the post. The mean age of the sample was 8.83 years (SD=0.67), and half of the sample were girls (51%). Children’s IQs ranged from 70 to 158 (mean=109.34, SD=14.72). Parents of all participants gave informed consent following procedures approved by the Institute of Psychiatry Ethical Committee.
The families visited the research centre for the assessments. Two testers assessed the twins simultaneously in separate testing rooms. The tasks were administered in a fixed order as part of a more extensive test session, which in total (including breaks) lasted approximately 2.5 h.
Measures
Rating Scales
Parents and teachers were asked to complete the Long Versions of Conners’ Parent and Teacher Rating Scales (Conners et al. 1998a, 1998b). From both scales, we used the 9-item inattention and 9-item hyperactivity-impulsivity DSM-IV ADHD symptom subscales, obtaining summed parent and teacher ratings on the corresponding subscales. Teacher ratings were missing for 151 individuals and parent ratings for two individuals.
Wechsler Intelligence Scales for Children, Third Edition (WISC-III) (Weschler 1991)
The vocabulary, similarities, picture completion and block design subtests from the WISC-III were used to obtain an estimate of the child’s IQ (prorated following procedures described by Sattler (Sattler 1992).
The go/no-go task (Borger and van der Meere 2000; Kuntsi et al. 2005; van der Meere et al. 1995)
On each trial, one of two possible stimuli appeared for 300 ms in the middle of the computer screen. The child was instructed to respond only to the ‘go’ stimuli and to react as quickly as possible, but to maintain a high level of accuracy. The proportion of ‘go’ stimuli to ‘no-go’ stimuli was 4:1. The participants performed the task under three conditions (slow, fast and incentive), matched for length of time on task. Herein we present data from the slow condition, which had an inter-stimulus interval of 8 s and consisting of 72 trials, and the fast condition, with an inter-stimulus interval of 1 second and consisting of 462 trials. The order of presentation of the slow and fast conditions varied randomly across participants. The variables obtained from the task are mean RT (MRT), standard deviation of RTs (RTV), commission errors (CE), and omission errors.
The fast task (Andreou et al. 2007; Kuntsi et al. 2005, 2006)
The baseline condition, with a fore period of 8 seconds and consisting of 72 trials, followed a standard warned four-choice RT task (Leth-Steensen et al. 2000). A warning signal (four empty circles, arranged side by side) first appeared on the screen. At the end of the fore period of 8 s (presentation interval for the warning signal), the circle designated as the target signal for that trial was filled (coloured) in. The child was asked to make a compatible choice by pressing the response key that directly corresponded in position to the location of the target stimulus. After a response, the stimuli disappeared from the screen and a fixed inter-trial interval of 2.5 s followed. Speed and accuracy were emphasized equally. If the child did not respond within 10 s, the trial was terminated. A comparison condition with a fast event rate (1 s) and incentives followed the baseline condition (Andreou et al. 2007). The variables obtained from the task are MRT and standard deviation of RTs, herein reported for the baseline condition.
Selection of Cognitive Variables for Analyses
To limit the total number of variables, to create psychometrically robust variables (Kuntsi et al. 2006) and to enable a comparison to our previous findings using the same tasks in a clinically diagnosed sample (Kuntsi et al. 2010), summed scores were obtained across two tasks or conditions as follows: unstandardized MRT and RTV across fast task baseline condition and go/no-go task slow condition, and percentage of CE across go/no-go task slow and fast conditions. Omission errors on the go/no-go task were rare in this population sample and therefore were not included, in line with previous analyses on this sample (Kuntsi et al. 2006, 2009). Summed variables were regressed to correct for the effects of age and sex (a standard twin modeling procedure) and the residuals used in analysis. Cognitive variables were further regressed for IQ. Although our previous analyses indicated that the majority of genetic influences shared between ADHD and cognitive variables were independent of those shared with IQ (Kuntsi et al. 2010; Wood et al. 2010b), regressing for IQ ensured we controlled for any small mediating effects of IQ that were not the focus of present analyses, consistent with our previously adopted approach (Kuntsi et al. 2010).
Statistical Analyses
Overview of the Twin Method
In univariate analyses, correlations between members of a twin pair for each trait are used to apportion phenotypic variance to additive genetic (A), dominant genetic (D) or shared environment (C), and child-specific environment (E) components (which also subsumes measurement error) (Neale and Cardon 1992; Plomin et al. 2001). Based on the assumptions that (a) MZ twins are genetically identical and therefore share 100% of genetic variation, whereas DZ twins share, on average, 50% of their segregating alleles contributing to A and 25% contributing to D, and (b) both MZ and DZ pairs share 100% of their C but are discordant for E, the phenotypic variance for a trait is partitioned into constituent A, C/D and E influences (when only twin pairs reared together are used, the available information allows the estimation of only a C or D component at a time). Greater phenotypic similarity between MZ twins compared to DZ twins suggests genetic influences on trait variance. If the phenotypic similarity of MZ twins is more than twice that of DZ twins, this suggests the presence of D, otherwise only A is suggested. DZ twin correlations greater than half the MZ twin correlations suggest the presence of C. The extent to which MZ twins are not 100% concordant for a trait reflects E (Rijsdijk and Sham 2002).
Structural equation modeling provides a tool for the formal estimation of variance components (A, C/D and E parameters) and for testing alternative models describing possible component contributions to trait variance or covariance. In multivariate genetic analyses, as well as partitioning the phenotypic variance of single traits, it is also the covariance between traits that is decomposed into A, C/D and E influences following exactly the same logic as above and using the ratio of MZ:DZ differences in cross-twin cross-trait correlations, (e.g. inattention ratings in twin 1 with RTV scores in twin 2) (Rijsdijk and Sham 2002).
Genetic Structural Equation Models
The structural equation modeling program Mx (Neale et al. 2006a) was used. With the exception of CE (skew: −0.12), all residual summed scores were positively skewed (1.06 to 1.92) and were transformed to approximate a normal distribution (using the optimised minimal skew command; Stata version 10 (Stata 2007)).
Saturated Phenotypic Model
The saturated model fully describes the data using the maximum number of free parameters, modelling the observed means and variances without dissecting variance or covariance into etiological components, and provides a baseline comparison for subsequent genetic models. We constrained this model in accordance with assumptions of the genetic method (that is, means and variances within traits and phenotypic correlations were equated across twins in a pair and zygosity groups) to obtain phenotypic correlations representative of the whole sample while taking into account the non-independence of the data (i.e. data of related subjects).
Parameter Selection for Multivariate Genetic Analyses
Univariate modelling was used to inform the choice of parameters for the multivariate models (e.g. the choice of C or D parameters) and to test for sex effects. As multivariate models have increased power over univariate models (Schmitz et al. 1998), we do not present parameter estimates from univariate models. In the univariate analyses, an ACE model provided the best fit for cognitive measures, while ADE models (with scalar sex differences) fitted the ADHD subscale ratings best. Due to the lack of qualitative or quantitative sex differences in the univariate analyses beyond scalar differences, the computational intensity of modeling sex effects and additional power issues (Neale et al., 2006b), only scalar differences between males and females were allowed in the multivariate models, by pre- and post-multiplying male phenotypic variances by a scaling factor.
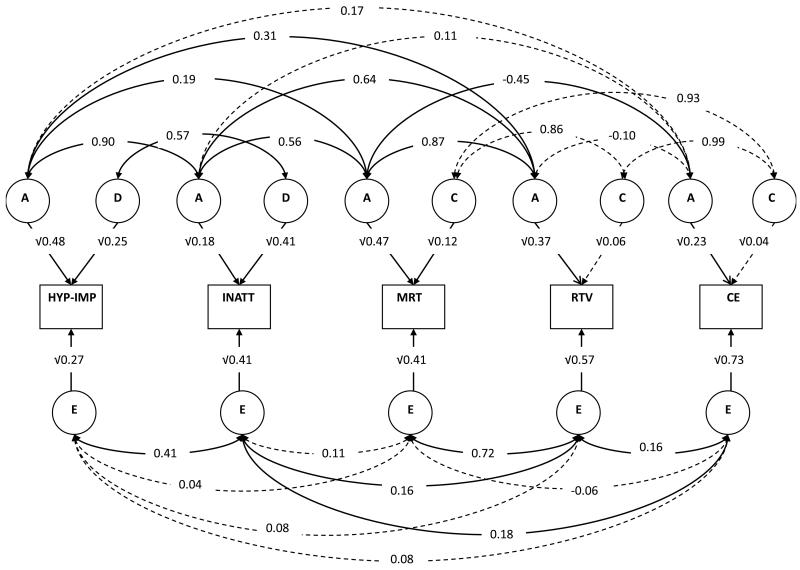
Correlated Factor Solution of the Full Cholesky Decomposition Model (Fig. 1)
Figure 1. Standardised solution of the full correlated factor model.
Note: Significant parameters are indicated with solid lines and non-significant parameters with dotted lines; Abbreviations: HYP-IMP: Hyperactivity-impulsivity; INATT: inattention; MRT: mean reaction time; RTV: reaction time variability; CE: commission errors. Model presented for one twin only for ease of presentation.
A triangular decomposition was run and converted to the mathematical equivalent correlated factor solution (Loehlin 1996), in which the order of traits is arbitrary. The mathematical solution estimates the degree of overlapping etiological factors between two traits, with etiological correlations that vary from 0 (indicative of no overlap) to 1 (reflecting complete overlap), irrespective of the extent to which they are shared with other traits in the model.
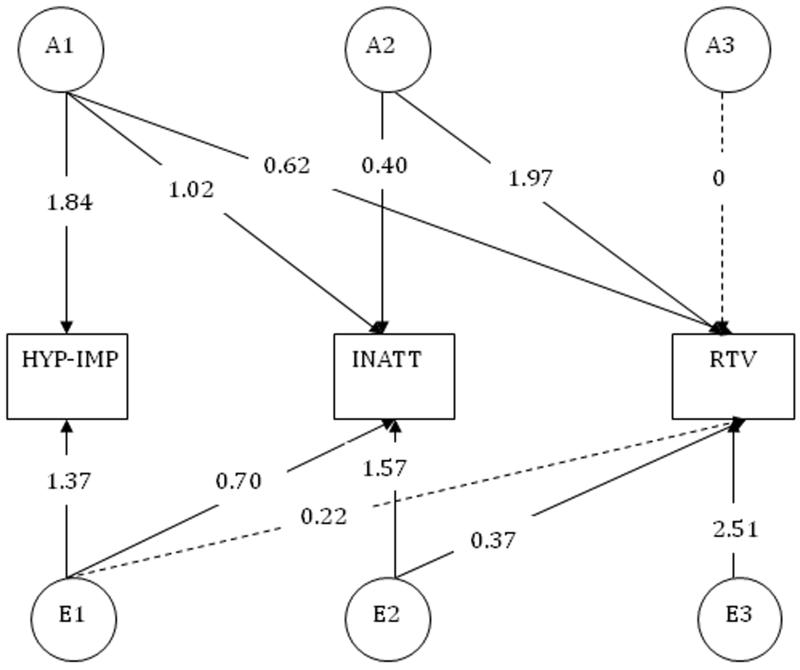
Cholesky Decomposition Model (Fig. 2)
Figure 2. Additive genetic and individual-specific environmental parameter estimates from the three-variable Cholesky model.
Note: Unstandardised parameter estimates; significant parameters are indicated with solid lines and non-significant parameters with dotted lines; Abbreviations: HYP-IMP: Hyperactivity-impulsivity; INATT: inattention; RTV: reaction time variability; Model presented for one twin only for ease of presentation and for only shared components additive genetic (A) and individual-specific environmental (E) influences only
In the Cholesky, a triangular decomposition is used, to decompose the variance in each phenotype and covariance between the phenotypes into A, D/C and E influences. The ordering of the traits in the Cholesky model was decided a priori: to ascertain how much of the overlap between inattentiveness and cognitive data is due to etiological influences that are independent of influences underlying hyperactivity-impulsivity, hyperactivity-impulsivity is assigned as the first measured variable. As such, for these analyses we present the triangular (Cholesky) decomposition.
Results
Means and standard deviations of ADHD ratings and cognitive data are given in Table 1. Due to variance differences between the genders, means and standard deviations are presented separately for males and females. Maximum likelihood twin-pair correlations between ADHD ratings and cognitive data are given in Table 2, and are presented together for males and females due to the lack of quantitative or qualitative sex differences.
Table 1. Means and standard deviations.
| Hyperactivity- impulsivitya |
Inattention a | MRT b | RTV b | CE c | |
|---|---|---|---|---|---|
| Means and (standard deviations) d | |||||
| MZM | 11.06 (8.61) | 12.70 (8.95) | 1481.79 (322.16) | 619.06 (350.81) | 116.52 (34.29) |
| MZF | 6.74 (5.89) | 7.79 (6.51) | 1587.52 (310.24) | 629.94 (364.15) | 96.42 (31.47) |
| DZM | 11.53 (9.64) | 14.25 (11.14) | 1497.49 (322.12) | 631.01 (376.52) | 115.59 (32.90) |
| DZF | 7.32 (6.49) | 9.06 (7.88) | 1551.48 (314.56) | 628.04 (359.12) | 95.61 (33.14) |
Note: MRT: mean reaction time; RTV; reaction time variability; CE: commission errors;
Sum of parent and teacher ratings;
Sum of unstandardised data scores across fast task baseline and go/no-go slow conditions;
Sum of percentages of CE across go/no-go slow and fast conditions;
raw data; MZ data in bold, DZ data in italic typeface
Table 2. Twin pair correlations (and 95% confidence intervals).
| Hyperactivity- impulsivity a |
Inattention a | MRT b | RTV b | CE c | |
|---|---|---|---|---|---|
| MZ / DZ | Twin 1 | ||||
| Twin 2 | |||||
| Hyperactivity- Impulsivity a |
0.73 (0.66 - 0.76)
0.30 (0.20 - 0.32) |
0.16 (0.08 - 0.24) | 0.01 (−0.06 - 0.09) | 0.04 (−0.03 - 0.08) | 0.05 (−0.03 - 0.09) |
| Inattention a | 0.45 (0.38 - 0.54) |
0.62 (0.53 - 0.68)
0.08 (0.02 - 0.15) |
0.01 (−0.06 - 0.03) | 0.03 (−0.04 - 0.09) | 0.01 (−0.06 - 0.08) |
| MRT b | 0.09 (0.02 - 0.19) | 0.19 (0.12 - 0.29) |
0.60 (0.51 - 0.73)
0.33 (0.23 - 0.33) |
0.23 (0.14 - 0.26) | 0.01 (−0.06 - 0.08) |
| RTV b | 0.13 (0.06 - 0.14) | 0.18 (0.11 - 0.21) | 0.44 (0.36 - 0.46) |
0.44 (0.34 - 0.48)
0.22 (0.12 - 0.27) |
0.06 (−0.02 - 0.06) |
| CE c | 0.05 (−0.03 - 0.12) | 0.04 (−0.05 – 0.11) | −0.09 (−0.16 - −0.08) | 0.02 ( −0.06 - 0.04) |
0.28 (0.17 - 0.39)
0.13 (0.03 - 0.23) |
Note: MRT: mean reaction time; RTV; reaction time variability; CE: commission errors;
Sum of parent and teacher ratings;
Sum of unstandardised data scores across fast task baseline and go/no-go slow conditions;
Sum of percentages of CE across go/no-go slow and fast conditions; Estimated using maximum likelihood estimation; MZ data in bold, DZ data in italic typeface
Parameter estimates are presented in Table 3 and Fig. 1 from the full correlated factors solution of the Cholesky decomposition (to avoid artificially inflating parameters, estimates from the full model are provided) and non-significance is indicated by confidence intervals that include zero. The genetic variance within cognitive variables and the genetic correlations between symptom domains and cognitive variables refer to additive genetic effects, as dominant genetic effects do not contribute to the variation of cognitive variables of their covariation with ADHD symptom domains. Additive genetic correlations in particular indicated a different pattern of association with the two ADHD symptoms for RT variables versus CE, with the strongest genetic association observed between RTV and inattention (ra=0.64). A moderate additive genetic association was also observed between RTV and hyperactivity-impulsivity symptoms (ra=0.31). In contrast, we found lower additive genetic correlations for CE, although there was less differentiation with symptom domains, with correlations of 0.11 and 0.17 for inattention and hyperactivity-impulsivity, respectively.
Table 3. Phenotypic correlations, parameter estimates, and derived covariance variance components (and 95% confidence intervals) from the correlated factors solution of the Cholesky model.
| Hyperactivity- impulsivity |
Inattention | MRT | RTV | CE | |
|---|---|---|---|---|---|
| Phenotypic correlations | |||||
|
| |||||
| Inattention | 0.58 (0.54 - 0.62) | ||||
| MRT | 0.10 (0.04 - 0.17) | 0.21 (0.15 – 0.27) | |||
| RTV | 0.16 (0.10 - 0.22) | 0.24 (0.18 – 0.30) | 0.79 (0.76 - 0.81) | ||
| CE | 0.09 (0.03 - 0.15) | 0.12 (0.06 – 0.18) | −0.11 (−0.17 - −0.05) | 0.12 (0.07 - 0.18) | |
|
| |||||
| Additive genetic influences | |||||
|
| |||||
| Hyperactivity- impulsivity |
0.48 (0.10 - 0.76) | 0.27 (46%) | 0. 09 (87%) | 0.13 (81%) | 0.06 (61%) |
| Inattention | 0.90 (0.39 - 0.99) | 0.18 (0.05 – 0.40) | 0.16 (78%) | 0.17 (68%) | 0.02 (19%) |
| MRT | 0.19 (0.03 - 0.47) | 0.56 (0.29 – 0.94) | 0.47 (0.28 - 0.62) | 0.36 (46%) | * |
| RTV | 0.31 (0.13 - 0.72) | 0.64 (0.33 – 1.00) | 0.87 (0.72 – 1.00) | 0.37 (0.15 – 0.51) | * |
| CE | 0.17 (−0.06 - 0.57) | 0.11 (−0.38 - 0.49) | −0.45 (−0.96 - −0.03) | −0.10 (−0.91 - 0.36) | 0.23 (0.03 - 0.36) |
|
| |||||
| Dominant genetic influences (Hyperactivity-impulsivity, inattention) / Common environmental influences (MRT, RTV, CE) | |||||
|
| |||||
| Hyperactivity- impulsivity |
0.25 (0.00 - 0.63) | 0.18 (31%) | - | - | - |
| Inattention | 0.57 (0.57 - 1.00) | 0.41 (0.17 – 0.57) | - | - | - |
| MRT | - | - | 0.12 (0.01 - 0.27) | 0.07 (9%) | * |
| RTV | - | - | 0.86 (−1.00 – 1.00) | 0.06 (0.00 – 0.23) | * |
| CE | - | - | 0.93 (−1.00 – 1.00) | 0.99 (−1.00 – 1.00) | 0.04 (0.00 - 0.20) |
|
| |||||
| Child-specific environmental influences | |||||
|
| |||||
| Hyperactivity- impulsivity |
0.27 (0.22 - 0.34) | 0.14 (24%) | 0.01 (13%) | 0.03 (19%) | 0.04 (39%) |
| Inattention | 0.41 (0.28 - 0.52) | 0.41 (0.33 – 0.51) | 0.05 (22%) | 0.08 (32%) | 0.10 (82%) |
| MRT | 0.04 (−0.09 - 0.17) | 0.11 (−0.01 - 0.24) | 0.41 (0.34 - 0.49) | 0.35 (45%) | * |
| RTV | 0.08 (−0.04 - 0.20) | 0.16 (0.05 – 0.27) | 0.72 (0.66 - 0.77) | 0.57 (0.48 – 0.67) | * |
| CE | 0.08 (−0.04 - 0.20) | 0.18 (0.07 – 0.29) | −0.06 (−0.17 - 0.05) | 0.16 (0.05 – 0.27) | 0.73 (0.63 - 0.83) |
Note: In the upper part of the table, the phenotypic correlations are given. In the next quarter, additive genetic estimates (with 95% CIs) of each variable are given in bold on the diagonal. The additive genetic correlations between pairs of variables (with 95% CIs) are given below the diagonal. The contribution of additive genetic factors to the phenotypic correlation between variables is given above the diagonal, with the percentage of the phenotypic correlation that is due to additive genetic effects in brackets. The same information is presented for dominant genetic/shared environmental and child-specific environmental influences in the third and lower quarters of the table, respectively.
It was not possible to formally estimate these proportions, due to the presence of both positive and negative etiological correlations between MRT and CE, and CE and RTV. However, the phenotypic correlation between CE and RTV is 0.12, of which −0.03 is due to A, 0.05 is due to C, and 0.10 is due to E. The phenotypic correlation between MRT and CE is −0.12, of which −0.15 is due to A, 0.06 is due to C, and −0.03 is due to E.
The vast majority (68% to 87%) of the phenotypic covariance between RT-related factors and either ADHD behavioural dimension was due to shared genetic (additive) effects. A greater degree of differentiation was observed when partitioning the contribution of shared genetic factors to the phenotypic covariation of CE for ADHD symptom domains (inattention (19%) and hyperactivity-impulsivity (61%)).
Given that the strongest genetic correlation between symptom scores and a cognitive variable emerged between inattention and RTV, this was investigated further in the Cholesky decomposition. Specifically, we wanted to test with the Cholesky decomposition how much of the etiological association between RTV and inattention was independent of hyperactivity-impulsivity. This can be estimated by summing the product of Cholesky additive genetic/individual-specific environmental paths that are not shared with hyperactivity-impulsivity, and taking them as a percentage of the total additive genetic/individual-specific environmental covariance between inattention and RTV data (C and D do not underlie both inattention and RTV and so do not contribute to the covariation between these two traits).
Using the parameter estimates from the Cholesky decomposition (Figure 2), we estimated that 55% of the genetic covariance between inattention and RTV occurred independently of genetic effects underlying hyperactivity-impulsivity: ((0.40*1.97)/(0.40*1.97)+(1.02*0.62) = 0.79/(0.79+0.63) = 0.79/1.42 = 0.55). In a similar vein, 79% of the individual-specific environmental covariance between RTV and inattention was independent of E underlying hyperactivity-impulsivity.
Discussion
We investigated the genetic associations of the two ADHD symptom domains of inattention and hyperactivity-impulsivity with key cognitive impairments known to be associated with the familial risk for ADHD. Multivariate twin model fitting identified two cognitive processes phenotypically associated with ADHD symptoms, captured by reaction time variability (RTV) and commission errors (CE), which showed different genetic relationships to the two ADHD symptom domains.
The findings are consistent with our previous report on two familial cognitive impairment factors in ADHD (Kuntsi et al. 2010), but further extend the previous observations by investigating the two ADHD symptom dimensions separately and by using a twin design that can distinguish between genetic and shared environmental effects that underlie familial influences.
Our previous analyses on a large ADHD and control sibling-pair sample indicated that RT measures index a large familial cognitive impairment factor in ADHD that accounts for 85% of the familial influences on ADHD (Kuntsi et al. 2010). Here we show, with a large population-based twin sample, that the RTV-ADHD association reflects largely additive genetic influences that RTV shares with inattention (ra=0.64) (a similar pattern was observed for MRT). A moderate additive genetic association was also observed between RTV and hyperactive-impulsive symptoms (ra=0.31). However, our further analyses showed that just over half (55%) of the additive genetic covariance between RTV and inattention was independent of genetic influences on hyperactivity-impulsivity. This degree of separation is notable, given the strong genetic correlation between inattention and hyperactivity symptoms, as reported previously (Greven et al. 2011; McLoughlin et al. 2007, 2011; Paloyelis et al. 2010; Wood et al. 2009) and further confirmed here (ra=0.90). Our findings also confirm the previous observation (Wood et al. 2010a) that MRT indexes largely the same genetic liability as RTV, observed in the high additive genetic correlation of 0.87.
The second, smaller familial cognitive impairment factor in ADHD in our previous analyses captured commission errors (CE), as well as omission errors, and accounted for 13% of the familial influences on ADHD (Kuntsi et al. 2010). However, the current results provide no evidence to suggest that the CE-ADHD association reflects a stronger association of CE with either hyperactivity-impulsivity or inattention; both additive genetic correlations were overall low (rg= 0.17 and 0.11, respectively) and non-significant. Further twin studies are required to clarify whether the low genetic correlations between CE and the ADHD symptom domains would emerge as significant in larger samples, although we note the consistency between the current and previous findings in the degree of genetic/familial association between CE and ADHD symptoms (Kuntsi et al. 2010).
Finally, the current findings demonstrate the etiological separation between the two indices of cognitive impairments, since there were no significant shared additive genetic influences across RTV and CE (ra=−0.10, ns). This is consistent with the etiological separation that was identified in the previous study using combined type children and adolescents with ADHD, their siblings and control sibling pairs.
Our findings converge with previous studies using clinical phenotypes in highlighting the importance of both shared and unique etiological pathways on the two symptom domains of ADHD. A recent analysis comparing factor models of ADHD symptoms in adolescents found that a general combined factor with separable inattention and hyperactivity-impulsivity dimensions best explained the symptom data (Toplak et al. 2009); a pattern of findings that is reflected in the shared and unique genetic effects that influence inattention and hyperactivity-impulsivity. Here we demonstrate the degree of specificity that the cognitive impairment factors have in their genetic association with inattention and hyperactivity-impulsivity symptoms. The two cognitive impairments in ADHD may also interplay throughout development, leading to different outcomes for ADHD as individuals pass from childhood into adulthood (Halperin and Schulz 2006; Halperin et al. 2008). Within such a developmental model, the finding that RTV, reflecting an early-onset enduring deficit (Halperin and Schulz 2006; Halperin et al. 2008), is associated specifically with inattention, may explain the developmental persistence of the inattentive symptom domain (Biederman et al. 2000; Larsson et al. 2006; Todd et al. 2008). The possible role of the cognitive processes described here in mediating the association of the two ADHD symptom domains with different patterns of comorbidity is an important direction for future research that arises from these findings.
A limitation of the study is that teacher ratings were missing for 151 individuals. Strengths of this study include the use of a population sampling strategy that is free from potential referral effects, which might bias estimates of the etiological associations between co-occurring behavioural and cognitive phenotypes. We adopted a quantitative approach to the analysis of ADHD symptoms, which reflects the continuous nature of ADHD symptoms in the population. The similarity between the findings presented here and the previous study using clinical cases of ADHD provides further evidence that ADHD reflects the extreme and impairing tail of quantitative traits for inattention and hyperactivity-impulsivity (Chen et al. 2008). This has implications for our understanding of the nature of ADHD by demonstrating the quantitative nature of ADHD at both the behavioural, cognitive and etiological level. This further emphasizes the importance of linking symptoms to impairments when defining the clinical condition (NICE 2008), and supports the further use of population sampling strategies for investigating the separate neurobiological processes that underlie the clinical condition.
ACKNOWLEDGMENTS
We thank the TEDS-SAIL families, who give their time and support so unstintingly. We also thank research team members Keeley Brookes, Rebecca Gibbs, Hannah Rogers, Eda Salih, Greer Swinard, Kate Lievesley, Kayley O’Flynn, Suzi Marquis, Rebecca Whittemore, Xiaohui Xu, and everyone on the TEDS team.
FUNDING: The Study of Activity and Impulsivity Levels in children (SAIL) was funded by a Wellcome Trust grant GR070345MF.
REFERENCES
- Adams ZW, Derefinko KJ, Milich R, Fillmore MT. Inhibitory functioning across ADHD subtypes: recent findings, clinical implications, and future directions. Developmental Disabilities Research Review. 2008;14(4):268–275. doi: 10.1002/ddrr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37(12):1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders, 4 edition, text revision. 4th ed. APA; Washington: 2000. [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157(5):816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Borger N, van der Meere JJ. Visual behaviour of ADHD children during an attention test: an almost forgotten variable. Journal of Child Psychology and Psychiatry. 2000;41(4):525–532. doi: 10.1017/s0021963000005655. [DOI] [PubMed] [Google Scholar]
- Carr L, Henderson J, Nigg JT. Cognitive control and attentional selection in adolescents with ADHD versus ADD. Journal of Child Psychology and Psychiatry. 2010;39(6):726–740. doi: 10.1080/15374416.2010.517168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, et al. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(8):1450–1460. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998a;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998b;26(4):279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Greven CU, Rijsdijk FV, Plomin R. A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. Journal of Abnormal Child Psychology. 2011;39(2):265–275. doi: 10.1007/s10802-010-9451-9. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49(9):958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45(4):630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Andreou P, Ma J, Borger NA, van der Meere JJ. Testing assumptions for endophenotype studies in ADHD: reliability and validity of tasks in a general population sample. BMC Psychiatry. 2005;5:40. doi: 10.1186/1471-244X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Borger N, van der Meere JJ, Rijsdijk F, et al. Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychological Medicine. 2006;36(11):1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, van der Meere JJ, Asherson P. Why cognitive performance in ADHD may not reveal true potential: findings from a large population-based sample. Journal of the International Neuropsychological Society. 2009;15(4):570–579. doi: 10.1017/S135561770909081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, et al. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Archives of General Psychiatry. 2010;67(11):1159–1167. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Frazier-Wood AC, Banaschewski T, Gill M, Miranda A, Oades RD, et al. Genetic analysis of reaction time variability: room for improvement? Psychological Medicine. 2013 doi: 10.1017/S0033291712002061. Epub ahead of print. PMID: 22975296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Lichtenstein P, Larsson JO. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(8):973–981. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104(2):167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky Approach: a cautionary note. Behavior Genetics. 1996;26(1):65–69. [Google Scholar]
- McLoughlin G, Ronald A, Kuntsi J, Asherson P, Plomin R. Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. Journal of Abnormal Child Psychology. 2007;35(6):999–1008. doi: 10.1007/s10802-007-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin G, Rijsdijk F, Asherson P, Kuntsi J. Parents and teachers make different contributions to a shared perspective on hyperactive-impulsive and inattentive symptoms: a multivariate analysis of parent and teacher ratings on the symptom domains of ADHD. Behavior Genetics. 2011;41(5):668–679. doi: 10.1007/s10519-011-9473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Cardon L. Methodology for genetic studies of twins and families. Kluwer; Dordrecht: 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: statistical modeling. 7th ed. Department of Psychiatry; Richmond, VA: 2006a. [Google Scholar]
- Neale MC, Roysamb E, Jacobsom K. Multivariate genetic analysis of sex limitation and GxE interactions. Twin Research and Human Genetics. 2006b;9(4):481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcorn JH, Halperin JM, Jensen PS, Abikoff HB, Arnold LE, Cantwell DP, et al. Symptom profiles in children with ADHD: effects of comorbidity and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(2):137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- NICE . Attention Deficit Hyperactivity Disorder: The NICE guideline on diagnosis and managment of ADHD in children, young people and adults. The British Psychological Society and The Royal College of Psychiatrists; London: 2008. [Google Scholar]
- O’Connell RG, Bellgrove MA, Dockree PM, Lau A, Fitzgerald M, Robertson IH. Self-Alert Training: volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia. 2008;46(5):1379–1390. doi: 10.1016/j.neuropsychologia.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Oliver BR, Plomin R. Twins’ Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Research and Human Genetics. 2007;10(1):96–105. doi: 10.1375/twin.10.1.96. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Rijsdijk F, Wood AC, Asherson P, Kuntsi J. The genetic association between ADHD symptoms and reading difficulties: the role of inattentiveness and IQ. Journal of Abnormal Child Psychology. 2010;38(8):1083–1095. doi: 10.1007/s10802-010-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 4th ed. Worth; New York: 2001. [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Briefings in Bioinformatics. 2002;3(2):119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: WAIC-III and WPPSI-R Supplement. Jerome M Sattler; San Diego: 1992. [Google Scholar]
- Schmitz S, Cherny SS, Fulker DW. Increase in power through multivariate analyses. Behavior Genetics. 1998;28(5):357–363. doi: 10.1023/a:1021669602220. [DOI] [PubMed] [Google Scholar]
- Stata . Stata Statistical Software Release 9.0: Survey Data Manual. Stata Corporation; College Station, TX: 2007. [Google Scholar]
- Todd RD, Rasmussen ER, Neuman RJ, Reich W, Hudziak JJ, Bucholz KK, et al. Familiality and heritability of subtypes of attention deficit hyperactivity disorder in a population sample of adolescent female twins. American Journal of Psychiatry. 2001;158(11):1891–1898. doi: 10.1176/appi.ajp.158.11.1891. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Todorov AA, Neuman RJ, Reiersen AM, Henderson CA, et al. Predictors of stability of attention-deficit/hyperactivity disorder subtypes from childhood to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):76–85. doi: 10.1097/chi.0b013e31815a6aca. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Pitch A, Flora DB, Iwenofu L, Ghelani K, Jain U, et al. The unity and diversity of inattention and hyperactivity/impulsivity in ADHD: evidence for a general factor with separable dimensions. Journal of Abnormal Child Psychology. 2009;37(8):1137–1150. doi: 10.1007/s10802-009-9336-y. [DOI] [PubMed] [Google Scholar]
- Trouton A, Spinath FM, Plomin R. Twins early development study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Research and Human Genetics. 2002;5(5):444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 2010;51(2):210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meere JJ, Stemerdink N, Gunning B. Effects of presentation rate of stimuli on response inhibition in ADHD children with and without tics. Perceptual and Motor Skills. 1995;81(1):259–262. doi: 10.2466/pms.1995.81.1.259. [DOI] [PubMed] [Google Scholar]
- Weschler D. Wechsler Intelligence Scale for Children 3rd edn. Psychological Corporation; London: 1991. [Google Scholar]
- Willcutt, Pennington B, Olson RK, DeFries JC. Understanding comorbidity: a twin study of reading disability and attention-deficit/hyperactivity disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B(6):709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- Wood AC, Rijsdijk F, Asherson P, Kuntsi J. Hyperactive-impulsive symptom scores and oppositional behaviours reflect alternate manifestations of a single liability. Behavior Genetics. 2009;39(5):447–460. doi: 10.1007/s10519-009-9290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Asherson P, van der Meere JJ, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychological Medicine. 2010a;40(6):1027–1037. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, et al. The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychological Medicine. 2010b;41(4):861–871. doi: 10.1017/S003329171000108X. [DOI] [PMC free article] [PubMed] [Google Scholar]