Abstract
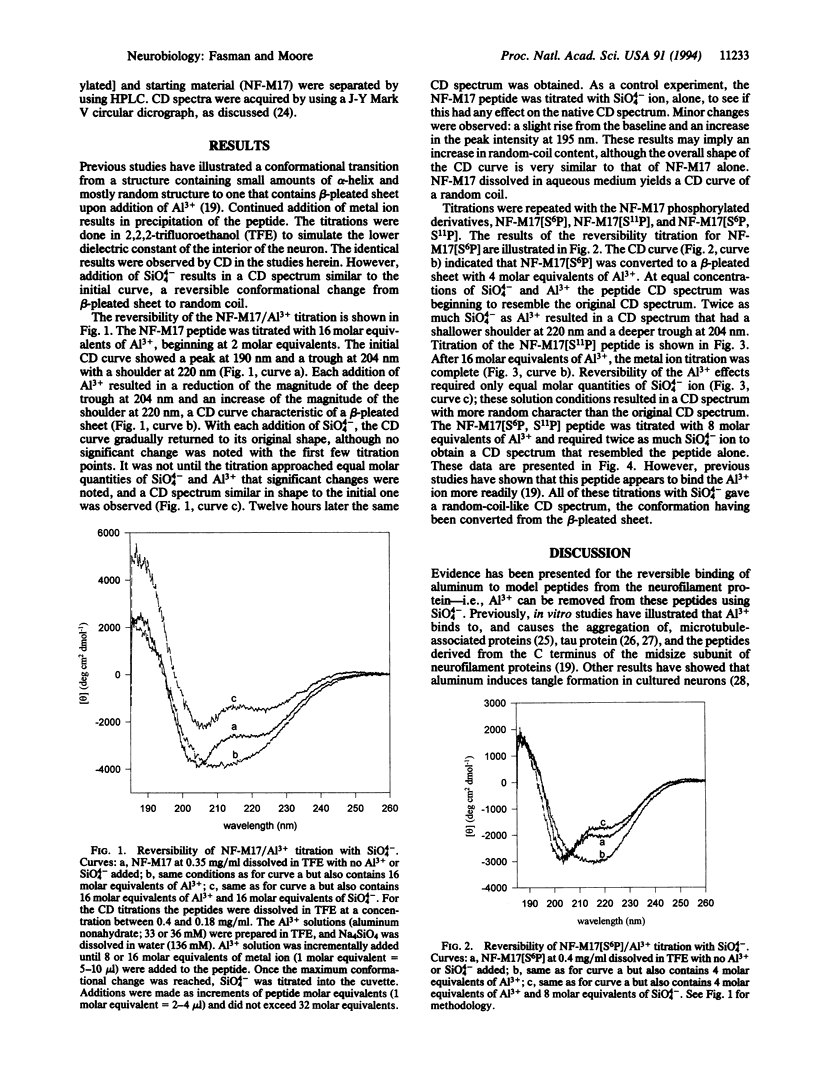
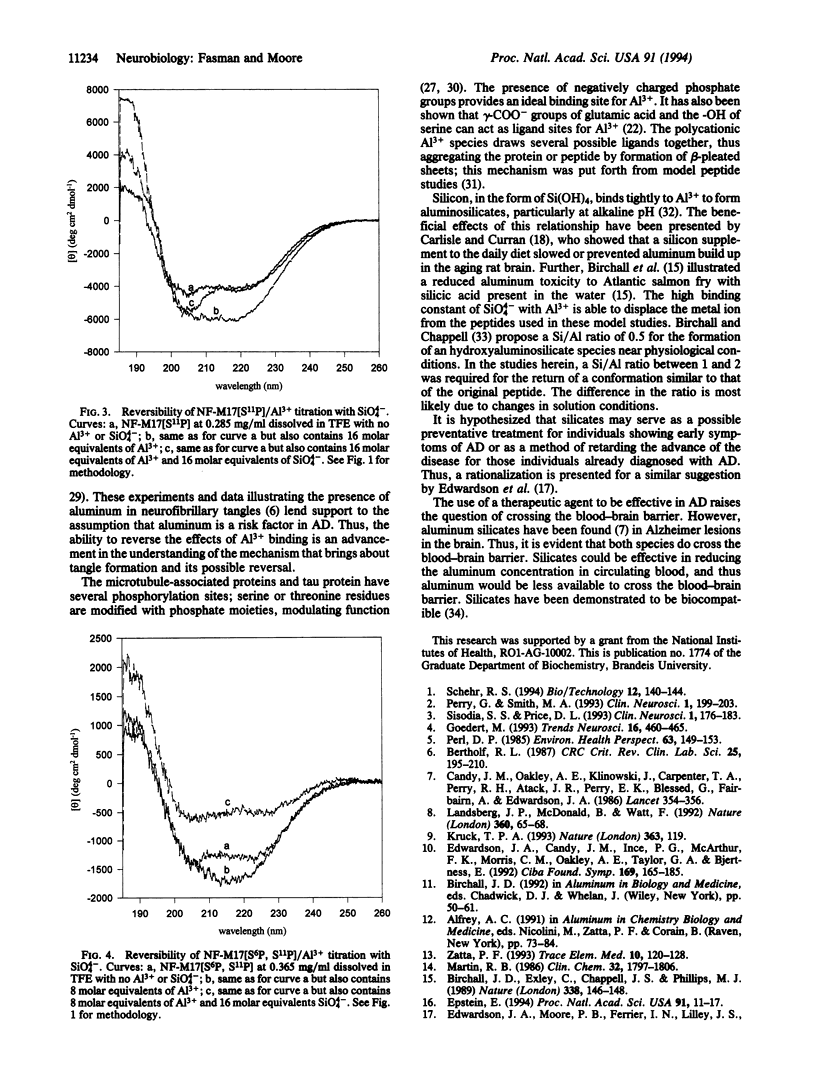
Neurofibrillary tangles are one of two lesions found in the brain of Alzheimer disease victims. With synthetic peptide fragments of human neurofilament NF-M17 (Glu-Glu-Lys-Gly-Lys-Ser-Pro- Val-Pro-Lys-Ser-Pro-Val-Glu-Glu-Lys-Gly, phosphorylated and unphosphorylated), CD studies were done to examine the effect of sodium orthosilicate on the conformational state produced by Al3+ on fragments of neuronal proteins. Previous studies had shown a conformational transition from alpha-helix and random to beta-pleated sheet upon addition of Al3+ to both phosphorylated and unphosphorylated peptides. If sufficient quantities of Al3+ are added, the peptide precipitates from solution. The ability to reverse or slow the progression of aggregation was examined. Al3+ binding was reversed with 1-2 molar equivalents of sodium orthosilicate (with respect to Al3+), altering the conformation from beta-sheet to random coil and resulting in a CD spectrum similar to that of the initial peptide. The tight binding of the SiO4(4-) with the Al3+ provides the mechanism for this transition. These results provide additional information toward understanding the role of aluminum in the Alzheimer diseased brain and suggest the investigation of the possible use of silicates as a therapeutic agent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., el-Sebae A. K., Shalloway D. Aluminum-induced nonenzymatic phospho-incorporation into human tau and other proteins. J Biol Chem. 1993 Jun 5;268(16):11976–11981. [PubMed] [Google Scholar]
- Bertholf R. L. Aluminum and Alzheimer's disease: perspectives for a cytoskeletal mechanism. Crit Rev Clin Lab Sci. 1987;25(3):195–210. doi: 10.3109/10408368709105882. [DOI] [PubMed] [Google Scholar]
- Birchall J. D., Chappell J. S. The chemistry of aluminum and silicon in relation to Alzheimer's disease. Clin Chem. 1988 Feb;34(2):265–267. [PubMed] [Google Scholar]
- Candy J. M., Oakley A. E., Klinowski J., Carpenter T. A., Perry R. H., Atack J. R., Perry E. K., Blessed G., Fairbairn A., Edwardson J. A. Aluminosilicates and senile plaque formation in Alzheimer's disease. Lancet. 1986 Feb 15;1(8477):354–357. doi: 10.1016/s0140-6736(86)92319-6. [DOI] [PubMed] [Google Scholar]
- Carlisle E. M., Curran M. J. Effect of dietary silicon and aluminum on silicon and aluminum levels in rat brain. Alzheimer Dis Assoc Disord. 1987;1(2):83–89. doi: 10.1097/00002093-198701020-00003. [DOI] [PubMed] [Google Scholar]
- Díaz-Nido J., Avila J. Aluminum induces the in vitro aggregation of bovine brain cytoskeletal proteins. Neurosci Lett. 1990 Mar 2;110(1-2):221–226. doi: 10.1016/0304-3940(90)90815-q. [DOI] [PubMed] [Google Scholar]
- Edwardson J. A., Candy J. M., Ince P. G., McArthur F. K., Morris C. M., Oakley A. E., Taylor G. A., Bjertness E. Aluminium accumulation, beta-amyloid deposition and neurofibrillary changes in the central nervous system. Ciba Found Symp. 1992;169:165–185. doi: 10.1002/9780470514306.ch10. [DOI] [PubMed] [Google Scholar]
- Edwardson J. A., Moore P. B., Ferrier I. N., Lilley J. S., Newton G. W., Barker J., Templar J., Day J. P. Effect of silicon on gastrointestinal absorption of aluminium. Lancet. 1993 Jul 24;342(8865):211–212. doi: 10.1016/0140-6736(93)92301-9. [DOI] [PubMed] [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 1993 Nov;16(11):460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- Hench L. L., Wilson J. Biocompatibility of silicates for medical use. Ciba Found Symp. 1986;121:231–246. doi: 10.1002/9780470513323.ch14. [DOI] [PubMed] [Google Scholar]
- Hollósi M., Otvös L., Jr, Urge L., Kajtár J., Perczel A., Laczkó I., Vadász Z., Fasman G. D. Ca(2+)-induced conformational transitions of phosphorylated peptides. Biopolymers. 1993 Mar;33(3):497–510. doi: 10.1002/bip.360330316. [DOI] [PubMed] [Google Scholar]
- Hollósi M., Urge L., Perczel A., Kajtár J., Teplán I., Otvös L., Jr, Fasman G. D. Metal ion-induced conformational changes of phosphorylated fragments of human neurofilament (NF-M) protein. J Mol Biol. 1992 Feb 5;223(3):673–682. doi: 10.1016/0022-2836(92)90983-q. [DOI] [PubMed] [Google Scholar]
- Inouye H., Fraser P. E., Kirschner D. A. Structure of beta-crystallite assemblies formed by Alzheimer beta-amyloid protein analogues: analysis by x-ray diffraction. Biophys J. 1993 Feb;64(2):502–519. doi: 10.1016/S0006-3495(93)81393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruck T. P. Aluminium--Alzheimer's link? Nature. 1993 May 13;363(6425):119–119. doi: 10.1038/363119a0. [DOI] [PubMed] [Google Scholar]
- Landsberg J. P., McDonald B., Watt F. Absence of aluminium in neuritic plaque cores in Alzheimer's disease. Nature. 1992 Nov 5;360(6399):65–68. doi: 10.1038/360065a0. [DOI] [PubMed] [Google Scholar]
- Langui D., Anderton B. H., Brion J. P., Ulrich J. Effects of aluminium chloride on cultured cells from rat brain hemispheres. Brain Res. 1988 Jan 12;438(1-2):67–76. doi: 10.1016/0006-8993(88)91324-8. [DOI] [PubMed] [Google Scholar]
- Langui D., Probst A., Anderton B., Brion J. P., Ulrich J. Aluminium-induced tangles in cultured rat neurones. Enhanced effect of aluminium by addition of maltol. Acta Neuropathol. 1990;80(6):649–655. doi: 10.1007/BF00307634. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Martin R. B. The chemistry of aluminum as related to biology and medicine. Clin Chem. 1986 Oct;32(10):1797–1806. [PubMed] [Google Scholar]
- Otvos L., Jr, Tangoren I. A., Wroblewski K., Hollosi M., Lee V. M. Reversed-phase high-performance liquid chromatographic separation of synthetic phosphopeptide isomers. J Chromatogr. 1990 Jul 20;512:265–272. doi: 10.1016/s0021-9673(01)89493-0. [DOI] [PubMed] [Google Scholar]
- Perl D. P. Relationship of aluminum to Alzheimer's disease. Environ Health Perspect. 1985 Nov;63:149–153. doi: 10.1289/ehp.8563149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevelige P. E., Jr, Fasman G. D. Structural studies of acetylated and control inner core histones. Biochemistry. 1987 May 19;26(10):2944–2955. doi: 10.1021/bi00384a041. [DOI] [PubMed] [Google Scholar]
- Schehr R. S. Therapeutic approaches to Alzheimer's disease. An informal survey of promising drug discovery strategies. Biotechnology (N Y) 1994 Feb;12(2):140–144. doi: 10.1038/nbt0294-140. [DOI] [PubMed] [Google Scholar]
- Scott C. W., Fieles A., Sygowski L. A., Caputo C. B. Aggregation of tau protein by aluminum. Brain Res. 1993 Nov 19;628(1-2):77–84. doi: 10.1016/0006-8993(93)90940-o. [DOI] [PubMed] [Google Scholar]
- Shen Z. M., Perczel A., Hollósi M., Nagypál I., Fasman G. D. Study of Al3+ binding and conformational properties of the alanine-substituted C-terminal domain of the NF-M protein and its relevance to Alzheimer's disease. Biochemistry. 1994 Aug 16;33(32):9627–9636. doi: 10.1021/bi00198a031. [DOI] [PubMed] [Google Scholar]



