Abstract
Background:
Both chronic kidney disease (CKD) and its treatment can affect a wide range of tissues and systems. It directly or indirectly affects flow, concentrations and composition of saliva. Hemodialysis can effectively minimize most of these complications to some extent.
Aims:
The main aim of this study was to know the salivary content of sodium, potassium, calcium, urea, bicarbonate and oral manifestations in patients with CKD.
Materials and Methods:
For this study, 50 patients diagnosed with CKD and 50 systemically and periodontally healthy individuals were subjected to a detailed general and intraoral examination. Whole un-stimulated saliva samples of all the selected subjects were collected and subjected to calcium (Ca), phosphorous (P), sodium (Na), potassium (K), bicarbonate and urea analysis.
Statistical Analysis Used:
Paired t-test, Mann–Whitney test.
Results:
Among 50 study subjects, 26 subjects had reduced salivary flow in the range of 0.1–0.4 ml/min. Intraoral examination of the study subjects revealed pallor, increased deposition of calculus, bleeding gums, metallic taste, hypoplasia of teeth and fissured tongue. There was a significant difference between healthy and prehemodialysis patients in the salivary sodium, potassium, calcium, phosphorus, urea levels and the difference was insignificant in relation to bicarbonate levels.
Conclusions:
Alterations in salivary calcium, phosphorous, urea, sodium, potassium levels were significantly higher in the study groups when compared to control groups and the difference was insignificant in relation to bicarbonate level. The increased levels in dialysis patients correlated with renal disease severity.
Keywords: Chronic renal failure, hemodialysis, saliva
INTRODUCTION
Once discarded focal infection theory, proposed by William Hunter, has been gaining significant support nowadays from various evidence based studies, conducted to know the two-way relationship between oral infection and systemic health. This new paradigm shift could be explained by the term periodontal medicine, coined by Williams and Offenbacher.[1] In periodontal medicine, till now limited literature is available regarding salivary composition and oral manifestation of patients with end stage renal disease.
About 60,000 persons annually lose their lives due to kidney-related diseases. Glomerulonephritis constitutes 54.7% of kidney diseases, pyelonephritis accounts for 12.3% of renal failures and others about 33%. In general, patients with end stage kidney disease, especially those on hemodialysis show a wide range of clinical symptoms and signs including biochemical changes such as hyperkalemia, hyperphosphatemia and hypocalcaemia and hormonal disturbances like secondary hyperparathyroidism, low activity of 1,25(OH)2 Vitamin D.[2]
Both chronic kidney disease (CKD) and its treatment can affect a wide range of tissues and systems, resulting in nervous, cardiovascular, respiratory, endocrine, hemopoietic, gastrointestinal and urological complications. It directly or indirectly affects flow, concentrations and composition of saliva. Hemodialysis can effectively minimize most of these complications to some extent.[2,3,4] So, the present study was directed toward the study of salivary content of sodium, potassium, calcium, urea, phosphorous, bicarbonate and oral manifestations in patients with CKD.
MATERIALS AND METHODS
The present study was carried out on 100 subjects who visited the Department of Periodontics, MNR Dental College and Hospital and Department of Nephrology, MNR Medical College at Sangareddy, in accordance with the Helsinki declaration of 1975, as revised in 2002. Subjects were included in the study after obtaining an informed consent. The ethical approval was obtained from the Institutional Review Board of MNR Dental College and Hospital, Sangareddy, Telangana, India.
A total number of 50 patients diagnosed with CKD (study group), 50 systemically and periodontally healthy subjects (control group) were selected and subjected to a detailed general and intraoral examination, and the relevant data was recorded in a specially designed proforma. Saliva samples from the study group and the control group were collected from the Department of Nephrology, MNR Medical College and Department of Periodontics, MNR Dental College and Hospital, Sangareddy respectively.
Whole un-stimulated saliva samples of all the selected subjects were collected before dialysis (i.e., before 8 am), and subjects were refrained from taking food before sample collection. The saliva was collected in a transparent glass test tube and allowed to settle for 5 min. Color of saliva was estimated by visual examination, and it ranged from transparent or clear to opaque white/opaque red. Flow rate was calculated as the volume collected divided by the time required for the collection, which ranged from 0.5 ml/min to 3.5 ml/min. The pH of saliva was tested with paper strips and recorded according to the corresponding color developed on the paper strip.
Salivary sodium and potassium levels were estimated by using the principle of flame photometry with flame photometer. Salivary estimation of bicarbonate was done by titration method. Left out amount of hydrochloric acid after reacting with saliva, was back titrated with sodium hydroxide using phenol red indicator and was indicative of salivary bicarbonate levels.
Salivary calcium and the salivary phosphorus were estimated by end point colorimetry. At alkaline pH, calcium binds with ortho cresolphthalein complexone to form a bluish purple complex equivalent at 578 nm. Phosphorous reacts with molybdate to form phosphomolybdate. The increase in absorbance, due to the formation of this complex is measured at 340 nm. Estimation of urea was done photometrically by Berthelot method. This method offers a high degree of specificity with precision due to urease activity and high sensitivity due to high molar absorption of the final color (yellow).
RESULTS
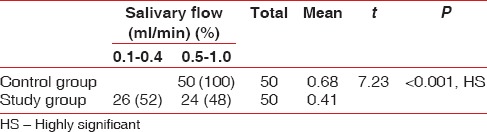
Salivary flow was notably decreased in 24 hemodialysis patients of study group compared to no changes in salivary flow of the control group (50 subjects). The mean salivary flow in control and study subjects was 0.68 and 0.41 respectively [Table 1].
Table 1.
Salivary flow
Oral manifestation
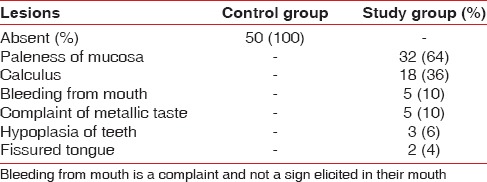
In the present study, oral symptoms were seen as follows: Paleness of mucosa was observed in 32 subjects, hypoplasia of teeth in 3 (6%), metallic taste 5 (10%), fissured tongue 2 (4%). Poor oral hygiene status and gingival bleeding were observed in 22 subjects [Table 2].
Table 2.
Oral manifestations
Comparison of healthy and prehemodialysis patients
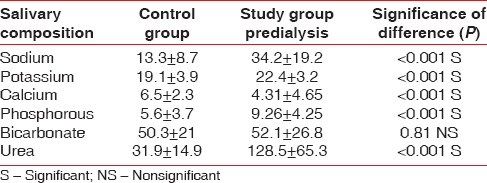
There was a significant difference between healthy and prehemodialysis patients in salivary sodium (13.3 ± 8.7; 34.2 ± 19.2), potassium (19.1 ± 3.9; 22.4 ± 3.2), calcium (6.5 ± 2.3; 4.31 ± 4.65), phosphorus (5.6 ± 3.7; 26 ± 4.25), urea levels (31.9 ± 14.9; 128.5 ± 65.3) and the difference were insignificant in relation to bicarbonate level (50.3 ± 21; 52.1 ± 26.8) [Table 3].
Table 3.
Comparison of healthy controls and hemodialyzed subjects (pre) Mann-Whitney test
DISCUSSION
Chronic kidney disease is a life threatening disorder that may result from a number of causes, many of which ultimately compromise renal function to the extent that patient will require either a kidney transplant or hemodialysis.[5] CKD patients exhibit oral manifestations, recognition of which is important since they may be indicators of the presence or extent of the disease. This may be useful to the clinician in diagnosing the disorder, determining treatment requirements or assessing the prognosis of the disease.[5]
Saliva is a unique fluid and its significance as a diagnostic medium has advanced exponentially in the past decade. The use of saliva as a diagnostic fluid has been tremendously influenced by current technological advances.[6] Whole saliva can be collected noninvasively and by individuals with limited training. No special equipment is needed for collection of the fluid. Diagnosis of the disease via analysis of saliva is a potentially valuable tool for children and older adults since the collection of the fluid is associated with fewer compliance problems compared with the collection of blood. Further, analysis of saliva may provide a cost effective approach for the screening of large populations.[6]
Therefore, this study was designed to gain further insight into quantitative changes and composition of saliva in CKD patients. The saliva samples were analyzed for sodium, potassium, calcium, phosphorous, bicarbonate and urea. In the present study, the age and sex of the patients were not considered as criteria. 50 documented cases of CKD as study subjects were chosen for the study.
Salivary flow
The mean salivary flow rate among control subjects in the present study was 0.68 ± 0.08 ml/min which correlated with the value of 0.45 ± 0.25 ml/min seen in control group of a study conducted by Kho et al.[7] However, these values were slightly higher than the control group of a study conducted by Epstein SR et al. where the range was from 0.09 ± 0.07 ml/min to 0.78 ± 0.39 ml/min.[7] This could be due to whole saliva was collected in the present study as compared to Epstein SR et al. where saliva was collected from the major salivary glands individually, both with stimulation and without stimulation.
Among the study subjects, mean salivary flow was 0.41 ± 0.12 ml/min, which was comparable with the results obtained by Kho et al.[7] (0.30 ± 0.18 ml/min) and also with the results obtained by Epstein et al.[8] where the range was 0.04 ± 0.03–0.36 ± 0.34 ml/min. Reduced salivary flow among the study subjects could be related to decrease in fluid intake, or a combination of direct involvement of the salivary glands, chemical inflammation, dehydration, drugs and Kussmaul breathing.
Oral manifestations
In the present study, subjects presented with different manifestations like paleness of mucosa, bleeding gums, increased deposition of calculus, uremic odor, hypoplasia of teeth, fissured tongue and petechiae that correlates with the oral findings among study subjects of the Kho et al.[7] and that of Epstein et al.[8]
Antoniades et al.,[9] Huffer et al.,[10] Greenberg and Cohen,[11] Kelly et al.,[12] Clark,[13] De Rossi and Glick[14] and McCreary et al.[15] reported mucosal pallor in renal failure patients. Mucosal pallor is secondary to the anemia precipitated by a lack of erythropoietin production, bone marrow depression and reduced red cell survival times.
The oral lesions may be a reaction of toxins in the tissues, or to the action of ammonia, or to irritating ammonium compounds formed by the action of bacteria on urea.[14] Gingival bleeding, as seen in 5 study subjects, may be a result of the use of anticoagulants and quantitative and qualitative changes of platelets.[7]
In the present study, 18 study subjects showed heavy calculus deposition on their teeth, which correlates with the study group of Epstein et al. Urea may have been a factor in calculus formation. The elevated levels of phosphate and protein may also have contributed.[8]
In the present study, 12 study subjects showed a uremic fetor, an ammonia odor. This finding correlates with the study group of Kho et al. This uremic fetor, which is typical of uremic patients, is caused by the high concentration of urea in the saliva.[7]
Dental caries
In the present study, only 11 study subjects had dental caries, as compared to 32 subjects in the control group. Mueller et al.[16] and Peterson et al.[17] were also of opinion that massive amounts of urea in saliva led to increased neutralizing capacity, thus release of free amino acids would be inhibited once the plaque become saturated with urea. Since the salivary urea concentration remains quite high even after dialysis, it could conceivably sustain this cariostatic effect. Salivary urea had been shown to inhibit caries because of its antibacterial properties and its inhibitory effect on plaque formation.
In the present study, three subjects showed enamel hypoplasia. Enamel hypoplasia in patients with renal insufficiency was typical of that seen in patients with calcium deficiency, may be because of calcium depletion, which occurs partly due to reduction in glomerular filtration of phosphate. Renal insufficiency can also impair the kidneys capacity to convert 25-hydroxycholecalciferol, a metabolic product of Vitamin D, to its most active form 1, 25-dihydroxycholecalciferol. Since the latter increased calcium absorption from the intestine, decreased levels of it will reflect in further calcium depletion.
Salivary sodium
Nine control subjects of Epstein et al.[8] showed sodium level ranging from 2.9 ± 1.2 mM/l to 26 ± 18 mM/l which could not be compared to present study mean value of 13.3 ± 8.7 mM/l. However, in study subjects sodium values ranged from 5.1 ± 2.0 mM/l to 15 ± 11 mM/l, which was far less, from the present study. In this study, saliva samples were collected between dialysis visits and separately from the major salivary glands.
Salivary sodium concentration is directly related to the salivary flow rate.[8] In the present study, only 26 study subjects had reduced salivary flow rate, and the remaining 24 had normal salivary flow. It was also stated that Na/K ratio in saliva is further modified by the enhanced reabsorption in the ducts and also regulated by aldosterone, cortisone and parathyroid hormone.[18]
The study subject's salivary sodium were linearly correlating with the Earlbaum and Quinton but his values were far less when compared to the present study. This could be because of the method of collection of saliva and laboratory analysis was different from the present study.[19]
Results of the study by Shasha SM et al. were far less than the present study for the control, prehemodialysis.[2] This could be due to the less sample size, that is, 10 in his study and method of analysis was different, that is, flame photometry.
Salivary potassium
In the present study, the mean value of the control group was 19.1 ± 3.9 mM/l and comparable with the values of Epstein et al.[8] study ranging from 17 ± 3 to 26 ± 4 mM/l. In the present study, mean value of salivary potassium before dialysis was 22.4 ± 3.2 mM/l and comparable with that of parotid stimulated and sub-mandibular stimulated saliva samples of Epstein et al.[8] study. Parotid un-stimulated saliva in his study showed higher value of 35 ± 7 mM/l, though the saliva was collected from each gland, potassium level showed statistical significance with P < 0.05.
In a study by Earlbaum and Quinton,[19] the potassium values for the control group were 19.9 ± 4.6 mM/l and correlated exactly with the values of present study control group. For study group, the potassium values were 27.1 ± 6.0 mM/l, showed slightly higher values than the present study. This could be because the saliva samples were collected from the parotid gland, but in our study, whole saliva was used. Control grouP values of the present study were comparable with Shasha et al.,[2] but 36.5 ± 12.0 mM/l of prehemodialysis, were higher than our study. Since in this study, sample size was 10, done among Israelis when compared to the present study done among 50 South Indians.
Salivary calcium
The salivary calcium level of the control group in the present study was 6.5 ± 2.3 mg% which is comparable with the control group of Shasha et al.[2] which was 5.2 ± 1.8 mg%, but not with that of Epstein et al.[8] where the values were high. The salivary calcium concentration of prehemodialysis patients of Earlbaum and Quinton[19] (2.8 ± 0.76 mg%), Shasha et al.[2] (2.3 ± 2.0 mg%) and that of Obry et al.[20] (1.64 ± 0.32 mg%) were far less when compared to the present study values of 4.31 ± 4.65, which was 2–3 times higher.
The mechanism by which calcium appears in the saliva at concentration that are substantially below that of plasma, whether these ions are secreted as a result of active/passive forces cannot be answered without electrochemical data in both acinar and ductal lumens. According to Obry et al.[20] considerable lowering in calcium concentration explains that, apart from a cellular response enhancing a leak in electrolyte which would appear to return to normalcy by dialysis. In contradiction to this, Shasha SM et al.[2] stated that treatment with hemodialysis has no effect on calcium concentration among patients. The salivary calcium values obtained by Epstein et al.[8] are higher when compared to the present study.
Significantly lower calcium concentration observed in our study patients could be due to decreased formation of 1, 25-dihydroxycholecalciferol, the active metabolite of Vitamin D which leads to diminished production of calcium binding protein in the intestinal mucosa there by depressing calcium absorption and potentiating hypocalcemic state with an increase in serum parathyroid hormone level.[2,3,4]
Salivary phosphorous
The salivary phosphorous level of the control group in the present study was 5.6 ± 3.7, which was comparable with the control group of Epstein et al.[8] The salivary concentration of phosphorous of prehemodialysis patients in the present study was 9.26 ± 4.25. Phosphate values are significantly increased in the dialysis patients. This elevation is a reflection of the reduced flow rate since salivary phosphate concentration is inversely related to flow rate.[8]
Salivary bicarbonate
In the present study, the mean values of the control group were 50.3 ± 21 mM/l and comparable with the values obtained by Epstein et al.[8] study ranging from 45 ± 3 to 49 ± 4 mM/l. In this study, prehemodialysis values of bicarbonate were 52.1 ± 26.8 mM/l and comparable with the results of parotid stimulated and sub-mandibular stimulated saliva samples of Epstein et al.,[8] parotid un-stimulated saliva also showed higher value of 61 ± 7 mM/l. This could be because the saliva samples were collected from the parotid gland, but in our study, whole saliva was used.
Salivary urea
Dahlberg et al.[21] demonstrated that parotid and serum ratio for urea were consistent and saliva could be used to monitor the dialysis procedure per se Peterson et al.[17] and Obry et al.[20] stated that salivary urea and uric acid are passively diffused from plasma and reflects the blood levels.
Epstein et al.[8] stated that urea level was significantly high among dialyzed subjects, and the values in his study ranged from 7 ± 2–17 ± 7 mg% to 60 ± 36–93 ± 45 mg% for the control and study group respectively. The salivary urea level of the control group in the present study was 31.9 ± 14.9 mg%.
The range of salivary urea in prehemodialysis patients in the present study was 25.3–292 mg% with mean of 128.5 ± 65.3 mg% where as in Obry et al.[20] it ranged from 189 mg% to 814 mg% with mean of 513 ± 210 mg%. The range of the salivary urea in posthemodialysis patients in Obry et al.[20] study was from 107 mg% to 362 mg% with a mean of 110 ± 48 mg%. This disparity could be due to a different method of analysis of saliva adopted by Obry et al.
A very substantial part of the drop in the urea concentration in saliva, between pre and posthemodialysis may find its explanation in the extra renal purification system.[20] The values for salivary urea in the present study were striking, because the mean salivary urea for pre and posthemodialysis was at least 4 times and 2 times higher respectively, when compared to the control subjects. This may signify that natural excretion occurs from salivary glands, partially replacing normal renal function, thus accounting for high concentration of urea found in saliva. Most of the urea in whole saliva was found to be present as ammonia and its buffering action is an important factor in determining the caries resistance in children. Epstein et al. inferred that this could be a factor in the formation of heavy calculus among hemodialysis patients.[8]
SUMMARY AND CONCLUSION
Alterations in salivary calcium (Ca), phosphorous (P), urea, sodium (Na), potassium (K) levels were significantly higher in the study groups when compared to control groups and the difference was insignificant in relation to bicarbonate level. The increased levels in dialysis patients correlated with renal disease severity and therefore salivary flow rate, dental caries prevalence and calculus deposition may be reflection of presence of urea in saliva as found in these patients. Since this study was under taken on a small sample, size, definite conclusions cannot be drawn. Hence, further study from a large group is necessary.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Williams RC, Offenbacher S. Periodontal medicine: The emergence of a new branch of periodontology. Periodontol 2000. 2000;23:9–12. doi: 10.1034/j.1600-0757.2000.2230101.x. [DOI] [PubMed] [Google Scholar]
- 2.Shasha SM, Ben Aryeh H, Angel A, Gutman D. Salivary content in hemodialysed patients. J Oral Med. 1983;38:67–70. [PubMed] [Google Scholar]
- 3.Naylor GD, Fredericks MR. Pharmacologic considerations in the dental management of the patient with disorders of the renal system. Dent Clin North Am. 1996;40:665–83. [PubMed] [Google Scholar]
- 4.Gavaldá C, Bagán J, Scully C, Silvestre F, Milián M, Jiménez Y. Renal hemodialysis patients: Oral, salivary, dental and periodontal findings in 105 adult cases. Oral Dis. 1999;5:299–302. doi: 10.1111/j.1601-0825.1999.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 5.Wysocki GP, Daley TD. A case of primary oxaluria. J Oral Surg. 1982;4:65–7. [Google Scholar]
- 6.Kaufman E, Lamster IB. The diagnostic applications of saliva – A review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 7.Kho HS, Lee SW, Chung SC, Kim YK. Oral manifestations and salivary flow rate, pH, and buffer capacity in patients with end-stage renal disease undergoing hemodialysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:316–9. doi: 10.1016/s1079-2104(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 8.Epstein SR, Mandel I, Scopp IW. Salivary composition and calculus formation in patients undergoing hemodialysis. J Periodontol. 1980;51:336–8. doi: 10.1902/jop.1980.51.6.336. [DOI] [PubMed] [Google Scholar]
- 9.Antoniades DZ, Markopoulos AK, Andreadis D, Balaskas I, Patrikalou E, Grekas D. Ulcerative uremic stomatitis associated with untreated chronic renal failure: Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:608–13. doi: 10.1016/j.tripleo.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Huffer WE, Kuzela D, Popovtzer MM. Metabolic bone disease in chronic renal failure. I Dialyzed uremics. Am J Pathol. 1975;78:365–84. [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg MS, Cohen G. Oral infection in immunosuppressed renal transplant patients. Oral Surg Oral Med Oral Pathol. 1977;43:879–85. doi: 10.1016/0030-4220(77)90080-9. [DOI] [PubMed] [Google Scholar]
- 12.Kelly WH, Mirahmadi MK, Simon JH, Gorman JT. Radiographic changes of the jawbones in end stage renal disease. Oral Surg Oral Med Oral Pathol. 1980;50:372–81. doi: 10.1016/0030-4220(80)90423-5. [DOI] [PubMed] [Google Scholar]
- 13.Clark DB. Dental findings in patients with chronic renal failure. An overview. J Can Dent Assoc. 1987;53:781–5. [PubMed] [Google Scholar]
- 14.De Rossi SS, Glick M. Dental considerations for the patient with renal disease receiving hemodialysis. J Am Dent Assoc. 1996;127:211–9. doi: 10.14219/jada.archive.1996.0171. [DOI] [PubMed] [Google Scholar]
- 15.McCreary CE, Flint SR, McCartan BE, Shields JA, Mabruk M, Toner ME. Uremic stomatitis mimicking oral hairy leukoplakia: Report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:350–3. doi: 10.1016/s1079-2104(97)90242-0. [DOI] [PubMed] [Google Scholar]
- 16.Mueller WA, Courts FJ, Tapley PM. Relationship of salivary urea to caries incidence in CRF patients. J Dent Res. 1984;63:10–4. [Google Scholar]
- 17.Peterson S, Woodhead J, Crall J. Caries resistance in children with chronic renal failure: Plaque pH, salivary pH, and salivary composition. Pediatr Res. 1985;19:796–9. doi: 10.1203/00006450-198508000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Mangos JA, McSherry NR. Micropuncture study of sodium and potassium excretion in rat parotid saliva: Role of aldosterone. Proc Soc Exp Biol Med. 1969;132:797–801. doi: 10.3181/00379727-132-34311. [DOI] [PubMed] [Google Scholar]
- 19.Earlbaum AM, Quinton PM. Elevated divalent ion concentrations in parotid saliva from chronic renal failure patients. Nephron. 1981;28:58–61. doi: 10.1159/000182107. [DOI] [PubMed] [Google Scholar]
- 20.Obry F, Belcourt AB, Frank RM, Geisert J, Fischbach M. Biochemical study of whole saliva from children with chronic renal failure. ASDC J Dent Child. 1987;54:429–32. [PubMed] [Google Scholar]
- 21.Dahlberg WH, Sreebny LM, King B. Studies of parotid saliva and blood in hemodialysis patients. J Appl Physiol. 1967;23:100–8. doi: 10.1152/jappl.1967.23.1.100. [DOI] [PubMed] [Google Scholar]


