Abstract
The effects of endothelium-derived hyperpolarizing factors have been attributed to cytochrome P450–derived epoxyeicosatrienoic acids (EETs), but the regulation and role of EETs in endothelial dysfunction remain largely unexplored. Hypertension is a primary risk factor for renal dysfunction, which is frequently accompanied by various systemic diseases induced by endothelial dysfunction in the microcirculation. We previously reported that the endothelial growth factor midkine (MK) enhances hypertension in a model of CKD. Here, we investigated the hypothesis that MK regulates EET activity and thereby BP. MK gene-deleted mice were resistant to hypertension and developed less glomerulosclerosis and proteinuria after administration of a nitric oxide synthase (NOS) inhibitor in the setting of uninephrectomy. The hypertension observed in uninephrectomized wild-type mice after NOS inhibition was ameliorated by anti-MK antibody. MK-deficient mice produced higher amounts of EETs, and EETs dominantly regulated BP in these mice. Furthermore, MK administration to MK-deficient mice recapitulated the BP control observed in wild-type mice. EETs also dominantly regulated renal blood flow, which may influence renal function, in MK-deficient mice. Taken together, these results suggest that the MK/EET pathway is physiologically engaged in BP control and could be a target for the treatment of hypertension complicated by endothelial dysfunction.
Keywords: hypertension, endothelium-derived hyperpolarizing factor, endothelial cell, CKD
Hypertension is a major risk factor for nephrosclerosis as well as cardiovascular and cerebral vascular diseases. Regardless of the primary disease process, renal dysfunction and CKD are frequently accompanied by various systemic disease induced by endothelial dysfunction in the microcirculation, eventually resulting in high mortality and morbidity rates. The endothelium produces both vasoconstrictors and vasodilators. The endothelial vasodilators include nitric oxide (NO), PGI2, and endothelium-derived hyperpolarizing factor (EDHF),1–4 but NO has been the most extensively investigated. Notably, cardiovascular diseases and diabetes show evidence of reduced endothelial NO production. The NO synthase (NOS) inhibitor N(ω)-nitro-L-arginine methylester (L-NAME) induces endothelial dysfunction in animals, which in turn leads to cardiovascular disorders accompanied by hypertension.5 Thus, L-NAME–treated animals are a useful model to investigate the mechanisms underlying such disorders. In contrast with NO, the biologic/clinical significance of EDHF remains largely unexplored. Because the vasorelaxation activity of EDHF is especially important in the small arteries,6–10 EDHF is considered to play a critical role in organ blood flow and BP, particularly when NO production is compromised. Although there is still ongoing debate on the characteristics of EDHF, it is now accepted that cytochrome P450 (CYP)–derived eicosanoids, epoxyeicosatrienoic acids (EETs), function as EDHFs.11–13 However, the physiologic regulation of EETs and the pathogenesis of disorders associated with defects in EETs remain unknown.
The growth factor midkine (MK) has been implicated in various biologic and pathologic events. It promotes tumorigenesis and inflammation, and protects the heart and the brain from infarction.14–17 We recently reported that MK enhances hypertension in the 5/6 renal ablation model of CKD by upregulation of pulmonary angiotensin-converting enzyme (ACE).18 Because MK is expressed by endothelial cells,15,18 we hypothesized that MK might play a fundamental role in BP control aside from its role via the renin-angiotensin system (RAS). Here, we found that a model of endothelial dysfunction induced by NOS inhibition showed no difference in the RAS between genotypes, and uncovered that endothelial MK is a physiologic regulator of the release of EETs, and is thus a regulator of EDHF release.
Results
Hypertension Induced by NOS Inhibition Is Attenuated in Mdk−/− Mice
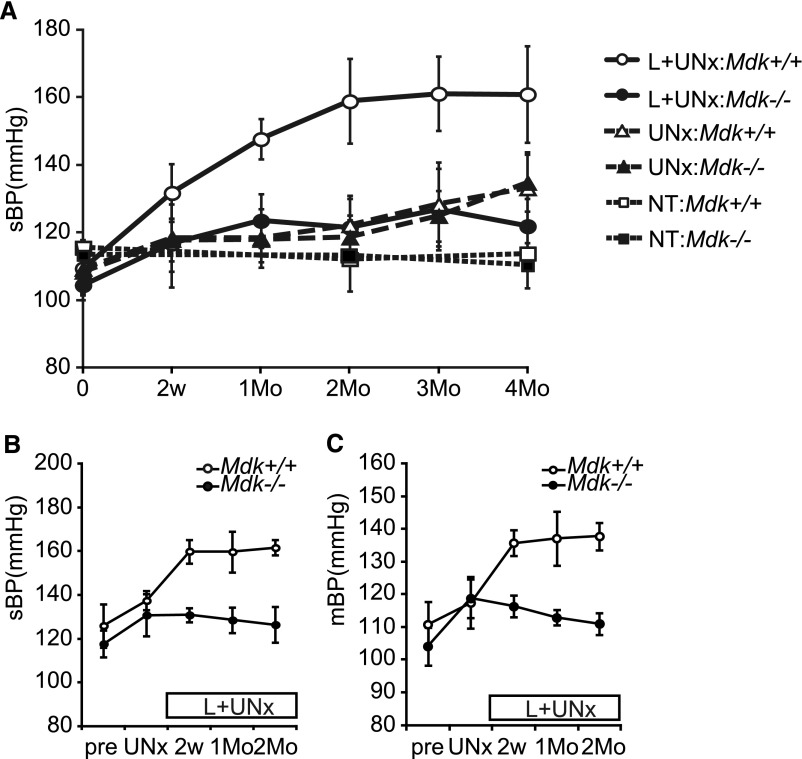
There was no difference in basal BP between nontreated wild-type (Mdk+/+) and MK-deficient (Mdk−/−) mice (113.7±9.1 mmHg in Mdk+/+ versus 110.1±6.7 mmHg in Mdk−/−) (Figure 1A; open and closed squares). BP remained unchanged after 2 months of uninephrectomy (UNx), and rose modestly after 4 months of UNx in both Mdk+/+ and Mdk−/− mice (ΔBP 23.8±7.6 mmHg in Mdk+/+ versus 26.2±6.9 mmHg in Mdk−/−) (Figure 1A; open and closed triangles). At no time point was there a significant difference between the genotypes.
Figure 1.
Hypertension induced by a NOS inhibitor is attenuated in Mdk−/− mice. (A) Systolic BP (sBP) is measured by the tail-cuff method in conscious mice at 0 and 2 weeks, and at 1, 2, 3, and 4 months after L+UNx (Mdk+/+, n=7; Mdk−/−, n=8), UNx alone (Mdk+/+, n=10; Mdk−/−, n=10), or NT (Mdk+/+, n=8; Mdk−/−, n=6). (B and C) Systolic BP (B) and mean BP (C) are measured in conscious mice by the radiotelemetry system before UNx (Mdk+/+, n=4; Mdk−/−, n=4), at 2 weeks after UNx (Mdk+/+, n=4; Mdk−/−, n=4), and at 2 weeks (Mdk+/+, n=4; Mdk−/−, n=3), 1 month (Mdk+/+, n=4; Mdk−/−, n=3), and 2 months (Mdk+/+, n=3; Mdk−/−, n=3) after L-NAME administration. Data are presented as the mean±SEM. P=0.002 (Mdk+/+ versus Mdk−/−) in A; P=0.02 (Mdk+/+ versus Mdk−/−) in B; and P=0.01 (Mdk+/+ versus Mdk−/−) in C. sBP, systolic BP; NT, no treatment; mBP, mean BP; pre, before UNx.
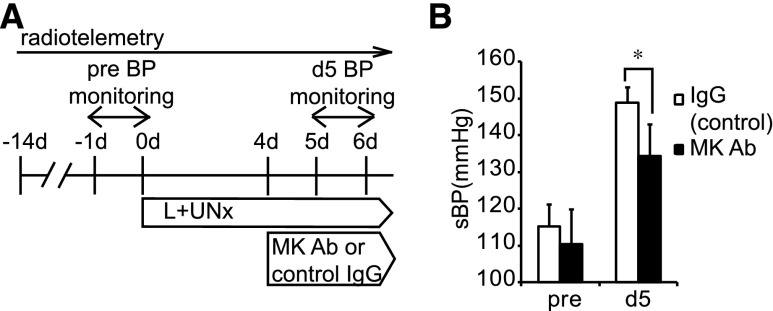
By contrast, the combination of UNx and the NOS inhibitor L-NAME (L+UNx) induced a striking increase in BP in Mdk+/+ mice but not in Mdk−/− mice, whose BP was increased only very modestly (ΔBP 51.5±14.2 mmHg in Mdk+/+ versus 17.4±9.3 mmHg in Mdk−/−) (Figure 1A; open and closed circles). The BP of Mdk+/+ was significantly higher than that of Mdk−/− after L+UNx. These conclusions from tail-cuff measurements of BP were confirmed by radiotelemetry (Figure 1, B and C). However, there was no difference in heart rate between Mdk+/+ and Mdk−/− mice (Supplemental Figure 1A). Systemic administration of anti-MK antibody significantly suppressed the BP elevation induced by L+UNx, supporting the idea that MK was vital in the BP control after L+UNx (Figure 2).
Figure 2.
Hypertension induced by NOS inhibition is treated by anti-MK antibody. (A) Protocol for treatment of hypertension induced by L+UNx with anti-MK antibody. (B) Hypertension induced by L+UNx is significantly suppressed by anti-MK antibody compared with control IgG. Data are presented as the mean±SEM (n=6, each). *P<0.05 versus control IgG. Ab, antibody; sBP, systolic BP, pre, before UNx.
Renal Injury under NOS Inhibition Is Dependent on BP
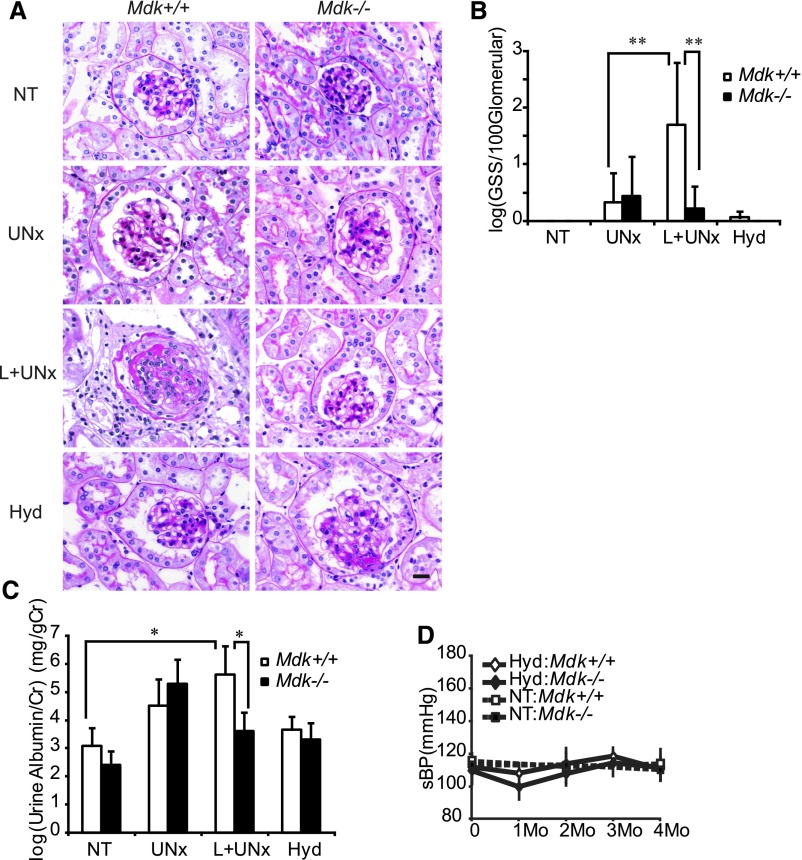
L+UNx induced glomerulosclerosis (Figure 3, A and B) and proteinuria (Figure 3C) in Mdk+/+ but not Mdk−/− mice, whereas BUN was not changed in either group (Table 1). The weights of the kidney and heart were increased significantly in Mdk+/+ and Mdk−/− after L+UNx (Table 1). To assess the role of hypertension in glomerulosclerosis and proteinuria, mice were treated with hydralazine (0.4 mg/dl in Mdk+/+, 0.2 mg/dl in Mdk−/−) and L+UNx. Hydralazine reduced BP in Mdk+/+ mice after L+UNx (Figure 3D), and ameliorated glomerulosclerosis and proteinuria (Figure 3, A–C). We concluded that the kidney injury was dependent on hypertension in this model.
Figure 3.
Renal injury under NOS inhibition is dependent on BP. (A) A representative result of the glomerular histology by PAS staining is shown. L+UNx induces glomerular sclerosis in Mdk+/+ but not Mdk−/− mice. Hydralazine ameliorates the glomerulosclerosis. (B) Semiquantitative analysis of the glomerular sclerosis score (NT: Mdk+/+, n=5; Mdk−/−, n=5; UNx: Mdk+/+, n=9; Mdk−/−, n=10; L+UNx: Mdk+/+, n=7; Mdk−/−, n=8; and Hyd: Mdk+/+, n=10; Mdk−/−, n=8). Data are transformed by log(x+1). (C) Proteinuria corrected by urine creatinine with log-transformation is also increased in Mdk+/+ but not Mdk−/− mice after L+UNx. Hydralazine ameliorates proteinuria (NT: Mdk+/+, n=7; Mdk−/−, n=5; UNx: Mdk+/+, n=9; Mdk−/−, n=9; L+UNx: Mdk+/+, n=8; Mdk−/−, n=8; Hyd: Mdk+/+, n=10; Mdk−/−, n=8). (D) BP is controlled by hydralazine (0.4 mg/dl in Mdk+/+ mice, 0.2 mg/dl in Mdk−/−) after L+UNx (Hyd). Data in B–D are presented as the mean±SEM. *P<0.05; **P<0.01. PAS, periodic acid–Schiff; Cr, creatinine; sBP, systolic BP. Bar, 20 μm in A.
Table 1.
Body weight, left kidney weight, heart weight, and BUN
| Group | Body Wt (g) | Kidney Wt/Body Wt | Heart Wt/Body Wt | BUN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 d | 2 wk | 1 mo | 2 mo | 3 mo | 4 mo | (mg/g)a | (mg/g)b | (mg/dl)c | ||
| NT | Mdk+/+ | ND | ND | ND | ND | ND | 35.6±2.2 | 6.6±0.6 | 4.2±0.2 | 28.3±1.3 |
| Mdk−/− | ND | ND | ND | ND | ND | 32.8±1.7 | 7.0±0.9 | 4.1±0.1 | 24.9±4.8 | |
| UNx | Mdk+/+ | 30.1±1.9 | 30.1±1.6 | 30.6±1.7 | 31.7±1.6 | 32.1±1.5 | 32.4±1.7 | 11.3±0.8d | 5.8±0.5d | 37.1±10.4 |
| Mdk−/− | 26.4±2.1 | 26.8±1.5 | 27.0±1.8 | 28.9±2.3 | 28.4±1.7 | 29.3±1.7d | 11.2±0.7d | 5.6±0.3d | 33.7±3.0 | |
| L+UNx | Mdk+/+ | 28.1±1.4 | 28.2±2.1 | 29.2±1.3 | 29.1±2.0 | 30.2±1.7 | 29.5±2.1e | 11.2±0.9d | 5.9±0.9d | 30.1±2.2 |
| Mdk−/− | 26.7±2.1 | 25.7±1.4 | 26.2±0.9 | 26.4±1.6 | 28.2±1.0 | 28.9±0.6d | 10.3±0.9d | 5.5±0.5d | 33.7±3.9 | |
| Hyd | Mdk+/+ | 29.3±2.1 | 27.4±2.2 | 29.0±1.8 | 28.9±1.5 | 31.5±1.0 | 32.2±1.8 | 9.4±0.5e | 5.4±0.3e | 35.4±1.8 |
| Mdk−/− | 26.8±1.3 | 24.5±0.8 | 26.4±1.2 | 26.5±1.6 | 28.4±2.5 | 28.4±1.5d | 9.3±0.7 | 5.0±0.4 | 32.6±4.2 | |
Values are the mean±SEM. ND, not determined.
NT: Mdk+/+, n=15; Mdk−/−, n=13; UNx: Mdk+/+, n=10; Mdk−/−, n=10; L+UNx: Mdk+/+, n=7; Mdk−/−, n=8; and Hyd: Mdk+/+, n=10; Mdk−/−, n=8.
NT: Mdk+/+, n=7; Mdk−/−, n=7; UNx: Mdk+/+, n=10; Mdk−/−, n=10; L+UNx: Mdk+/+, n=6; Mdk−/−, n=7; and Hyd: Mdk+/+, n=10; Mdk−/−, n=8,
NT: n=3; UNx: n=5; L+UNx: n=4; and Hyd: n=4.
P<0.01 versus NT.
P<0.05 versus NT.
The NO Axis Is Not Involved in the Hypertensinogenic Action of MK
The data indicated that MK was required for the development of hypertension in UNx mice after NOS inhibition. This occurred despite a similar decrease in urinary nitrate/nitrite of L+UNx in Mdk+/+ and Mdk−/− mice (Supplemental Figure 2A). In addition, there were no changes in cyclic guanosine monophosphate, a second messenger of NO (Supplemental Figure 2B). NO activity can be reduced by reaction with reactive oxygen species; however, there were no genotypic differences associated with changes in SOD activity (Supplemental Figure 2D) or changes in urine 8-OhdG (Supplemental Figure 2C), which is a product of reactive oxygen species reaction with deoxyguanosine, in L+UNx mice, and no genotypic differences associated with changes in the expression of Nox and p22phox (Supplemental Figure 2, E–H). We concluded that although NOS inhibition was required to uncover the role of MK in permitting the development of hypertension in UNx mice, the NO axis itself did not mediate this hypertensinogenic action of MK.
EETs Dominate the BP Control of Mdk−/− Mice
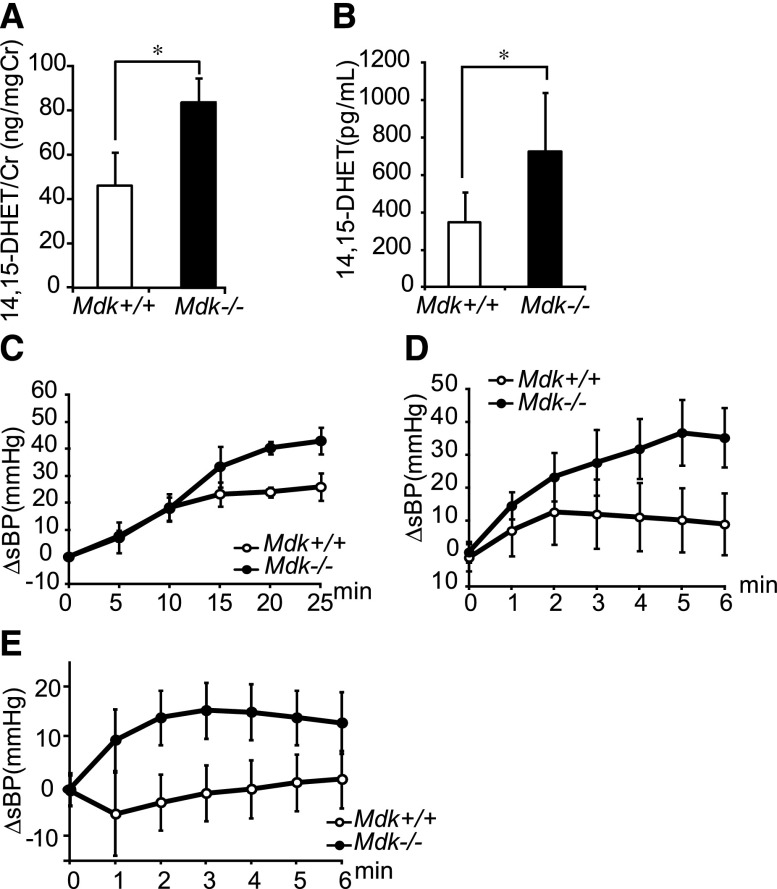
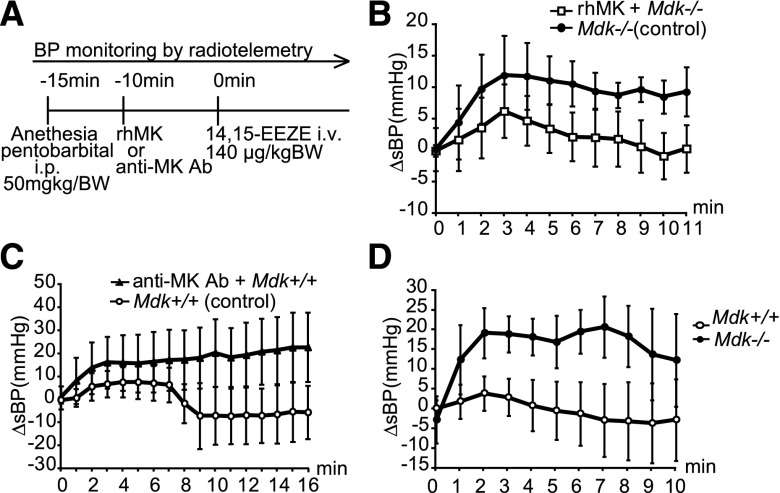
The failure of NO to explain the effects of MK on BP prompted a study of PGI2 and EETs.3 Because the expressions of PGI2 synthase were similar in Mdk−/− and Mdk+/+ mice (Supplemental Figure 3), we investigated the role of EETs. Remarkably, the urine excretion of 14,15-dihydroxyeicosatrienoic acid (DHET), a metabolite of EETs, was almost twice as high in Mdk−/− mice compared with Mdk+/+ mice (Figure 4A). Consistent with this, endothelial cells cultured from Mdk−/− showed enhanced release of 14,15-DHET (Figure 4B). To confirm the involvement of EETs in the maintenance of BP in Mdk−/− mice, we examined the effects of the KCa channel blocker charybdotoxin. EETs exert their vasodilator actions through KCa channels. Intraperitoneal or intravenous administration of charybdotoxin elevated the BP significantly more in Mdk−/− than in Mdk+/+ mice (Figure 4, C and D). This was likely due to ongoing activation of KCa by EETs in Mdk−/− mice. The 14,15-EET competitive analog 14,15-EE-5(Z)-E (EEZE) increased BP selectively in Mdk−/− mice, but no difference in heart rate between Mdk+/+ and Mdk−/− mice was observed (Figure 4E, Supplemental Figure 1B).
Figure 4.
EETs are upregulated in Mdk−/− mice. (A) Urine 14,15-DHET corrected by urine creatinine is higher in Mdk−/− mice than Mdk+/+ mice (n=5, each). (B) 14,15-DHET secretion from endothelial cells is increased in Mdk−/− mice (n=8, each). (C and D) BP change (ΔsBP) after intraperitoneal (n=3) (C) or intravenous (n=6) (D) injection of charybdotoxin (KCa blocker) is significantly increased in Mdk−/− compared with Mdk+/+. P=0.04 in C (Mdk+/+ versus Mdk−/−) and P<0.001 in D (Mdk+/+ versus Mdk−/−). (E) ΔsBP after intravenous injection of 14,15-EEZE (a 14,15-EET antagonist) is significantly increased in Mdk−/− mice but not Mdk+/+ mice (Mdk+/+, n=7; Mdk−/−, n=9). P<0.001 (Mdk+/+ versus Mdk−/−). Data are presented as the mean±SEM. *P<0.05 versus Mdk+/+. Cr, creatinine; sBP, systolic BP.
We concluded that endogenous MK downregulated the production of EETs, and that overproduced EETs rendered Mdk−/− mice resistant to hypertension, especially after NOS inhibition. To test this hypothesis, Mdk−/− mice were pretreated with exogenous human MK, which was found to suppress the BP elevation induced by 14,15-EEZE (Figure 5, A and B). Moreover, although a significant difference was not detected, we could observe that MK antibody sensitized Mdk+/+ mice to the EET antagonist, and induced higher BP compared with mice untreated with the antibody (Figure 5, A and C). Because there was no difference in MK expression in the kidney between control and L+UNx (Supplemental Figure 4), steadily expressed MK may be enough for EET production.
Figure 5.
MK is involved in BP control through EETs. (A) Protocol for pretreatment with MK or anti-MK antibody. (B) Pretreatment with exogenous human MK suppresses the BP elevation induced by 14,15-EEZE injection (n=6, each). P=0.03 (Mdk+/+ versus Mdk−/−). (C) Pretreatment with anti-MK antibody in Mdk+/+ mice increases BP after 14,15-EEZE (MK Ab, n=6; control, n=3). (D) ΔsBP after intravenous injection of an adenosine A2AR antagonist, ZM241385, is significantly elevated in Mdk−/− but not Mdk+/+ (n=7, each). P=0.03 (Mdk+/+ versus Mdk−/−). Data in B–D are presented as the mean±SEM. rhMK, recombinant human MK; Ab, antibody; BW, body weight; sBP, systolic BP.
The findings that the excretion of the EET metabolite 14,15-DHET was increased in Mdk−/− mice (Figure 4, A and B), and that both the KCa channel blocker charybdotoxin and the 14,15-EET competitive analog 14,15-EEZE increased BP in Mdk−/− mice (Figure 4, C–E) suggested that MK suppresses the generation of EETs, rather than enhancing their metabolism. Indeed, the kidney mRNA expression of soluble epoxide hydrolase (sEH), which is responsible for conversion of biologically active EETs to DHETs, was not changed (Supplemental Figure 5A).
In humans, CYP2J2 and CYP2C8 are responsible for the production of EETs.19,20 However, the responsible epoxygenase remains elusive in mice. We investigated mouse members of CYP2J and 2C families, which might be involved in EET biosynthesis according to the literature.21–24 mRNA expression of CYP2C44, CYP2C29, CYP2C38, CYP2J5, CYP2J6, CYP2J9, and CYP2J13 in the kidney were not different between genotypes (Supplemental Figure 5, B–H). CYP2C39 was not detected by RT-PCR. MK might not control CYP expression directly. So we hypothesized that MK controlled another pathway that affected EET synthesis.
There was a report that the vasodilation of adenosine is induced by the adenosine A2A receptor (A2AR),25 whose effects are mediated via stimulation of EET synthesis.26 Activation of the A2AR stimulates EET synthesis, and contributes to vasodilation.25,26 Because the A2AR antagonist ZM241385 induced a striking elevation of BP in Mdk−/− but not Mdk+/+ mice (Figure 5D), we concluded that MK is required to mediate the effect of A2AR, likely through the generation of EETs.
EETs Promote the Renal Blood Flow of Mdk−/− Mice
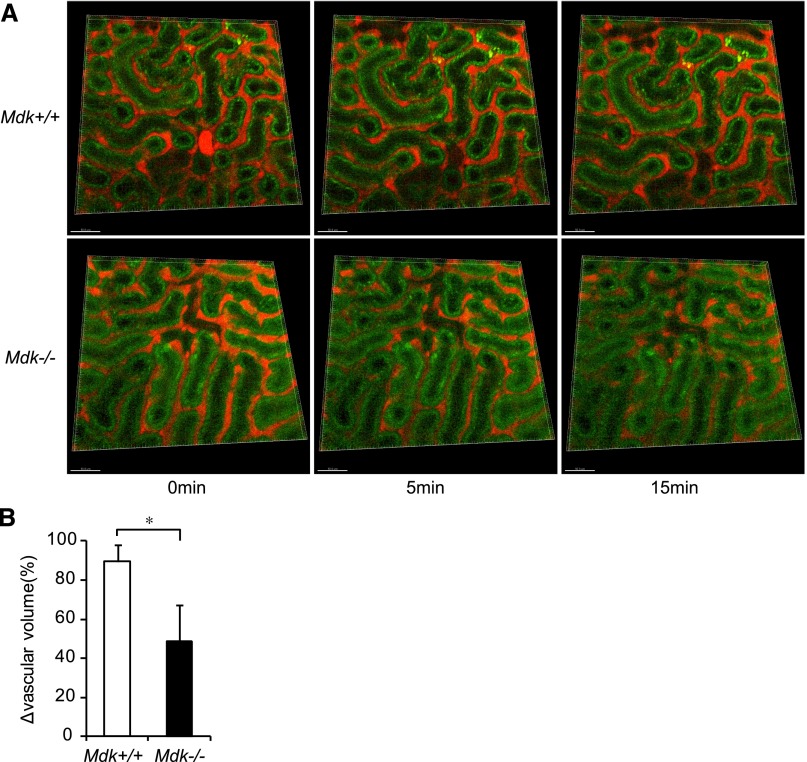
To evaluate the influence of EETs on the kidney, we assessed renal blood flow via intravital imaging by multiphoton excitation microscopy under administration of an EET antagonist. We intravenously injected rhodamine-dextran to see blood flow, since it is known that 70 kD dextran does not penetrate the wall of blood vessels. Peritubular capillaries were observed as red signals after injection of rhodamine-dextran. Green signals indicated intrinsic fluorescence of tubular cells (Figure 6A). Quantitative analysis of capillary volume was calculated using Imaris software (Figure 6B). Capillary volume was decreased in both Mdk+/+ and Mdk−/− mice soon after 14,15-EEZE injection (5 minutes), and was recovered in Mdk+/+ mice at 15 minutes. By contrast, the decline in capillary volume remained irreversible in Mdk−/− mice at the same time point (Figure 6A). Thus, there was a significant difference in the decrease of blood flow between Mdk+/+ and Mdk−/− mice at 15 minutes (Figure 6B).
Figure 6.
Peritubular capillary blood flow of the kidney surface is estimated with multiphoton-intravital microscopy under administration of an EET antagonist. (A) Red signals indicate peritubular capillaries, whereas green signals indicate intrinsic fluorescence of tubular cells. (B) The vascular volume percentage at 15 minutes compared from 0 minutes is significantly smaller in Mdk−/− than in Mdk+/+ mice. Quantitative analysis of capillary volume is carried out using Imaris software (Mdk+/+, n=4; Mdk−/−, n=4). *P<0.05 Mdk+/+ versus Mdk−/−. Data are presented as the mean±SEM.
The Sympathetic Nervous System, But Not the RAS, Is Complementary Upregulated in Mdk−/− Mice
The maintenance of a normal BP in Mdk−/− mice despite overproduction of the vasodilator EETs suggested the activation of a compensatory mechanism. To address this question, we investigated vasoconstrictor pathways. Although MK upregulated pulmonary ACE after 5/6 renal ablation,18 ACE expression and activity was not different between Mdk+/+ and Mdk−/− mice after L+UNx (Supplemental Figure 6, A and B). Moreover, the renal renin expression and the plasma aldosterone concentration were also similar in Mdk−/− and Mdk+/+ mice (Supplemental Figure 6, C and D). The renal expression of the aldosterone receptor and sgk-1, its downstream signaling pathway, were also similar in Mdk+/+ and Mdk−/− mice (Supplemental Figure 6, E and F).
To examine whether the MK/EET pathway is independent of RAS, we utilized an ACE inhibitor model. We administered ACE inhibitor enarapril with the dosage (120 mg/L in drinking water, 16 mg/kg per day) that is reported to fully suppress ACE but does not decrease BP.27,28 Even after two weeks of enarapril treatment, Mdk−/− mice injected with 14,15-EEZE showed a tendency of BP elevation. (Supplemental Figure 7).
The other vasoconstrictor produced by endothelial cells, the renal expression of endothelin-1 also was not different between Mdk+/+ and Mdk−/− mice after L+UNx (Supplemental Figure 8A). Renal sodium excretion was also similar (Supplemental Figure 8B).
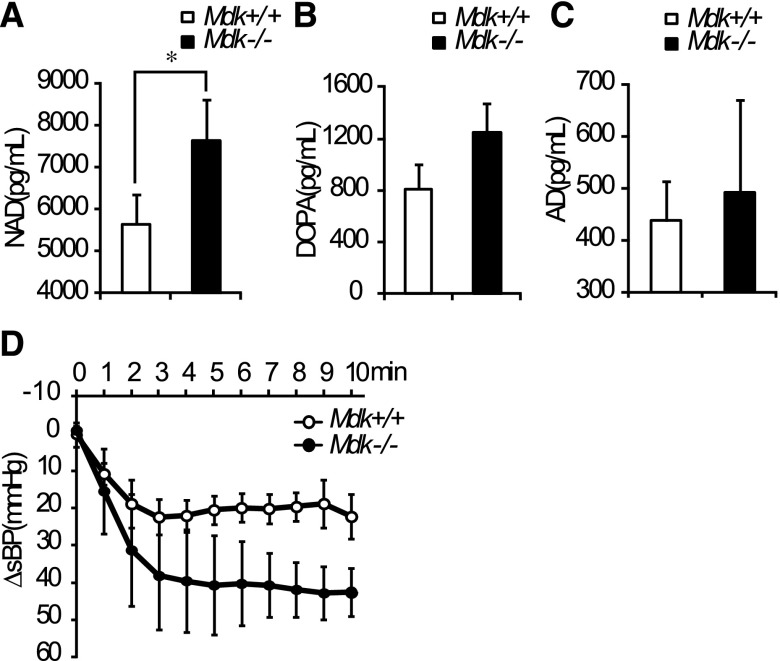
By contrast, plasma noradrenaline was significantly higher in Mdk−/− than in Mdk+/+ mice (Figure 7A), although dopamine and adrenaline were not significantly changed (Figure 7, B and C). We observed that the intravenous injection of hexamethonium, a nicotinic acetylcholine receptor antagonist, tended to induce a sharper decrease in BP in Mdk−/− mice. On the other hand, no difference in heart rate between Mdk+/+ and Mdk−/− mice (Figure 7D and Supplemental Figure 1C). These data suggest that enhanced sympathetic nerve activity maintains BP in Mdk−/− mice despite elevated EETs.
Figure 7.
The sympathetic nervous system is activated in Mdk−/− mice. (A–C) Serum catecholamine (noradrenaline [A], dopamine [B], and adrenaline [C]) concentrations (n=5, each). (D) sBP change (ΔsBP) after intravenous administration of hexamethonium, a nicotinic acetylcholine receptor antagonist, is larger in Mdk−/− mice than in Mdk+/+ mice (n=4, each). P=0.08 (Mdk+/+ versus Mdk−/−). Data are presented as the mean±SEM. NAD, noradrenaline; DOPA, dopamine; AD, adrenaline; sBP, systolic BP.
We previously reported that MK was involved in controlling inflammatory cells, especially monocytes and regulatory T cells.16,29 Furthermore, activation of immune mechanisms has been implicated in the pathogenesis of many forms of hypertension.30
Macrophage/monocyte infiltration and tumor necrosis factor-α expression in the kidney were slightly increased after L-NAME administration (not significant), but were not different among genotypes (Supplemental Figure 9, A and D). To explore regulatory T cell expansion in the kidney, we investigated Foxp3 and Ctla4 mRNA expression in the whole kidney. Foxp3 and Ctla4 were not different between Mdk+/+ and Mdk−/− in the nontreated and L+UNx groups (Supplemental Figure 9, B and C). Taken together, our data suggest that the immune system may not be involved in the difference between Mdk+/+ and Mdk−/− in the L+UNx model.
Discussion
Here, we show that MK suppresses the generation of EETs from endothelial cells. This pathway is physiologically engaged in normal mice, because the BP of Mdk−/− mice increases abruptly after blockade of EET synthesis or signaling. We identified two pathways that together can conceal this potent effect of MK. First, loss of endothelial NO unmasks the upregulation of the endothelial EET/EDHF pathway in Mdk−/− mice. Second, ongoing endogenous vasodilation produced by EETs stimulated by MK ablation elicits a compensatory activation of the sympathetic nervous system.
In contrast with the 5/6 kidney ablation utilized in the previous study,18 NOS inhibition did not show any difference in the RAS between wild-type and Mdk−/− mice. Therefore, the function of MK in the endothelial EET/EDHF pathway was clearly manifested after NOS inhibition in this study. The inter-relationship between NO and EDHF has not been fully defined,3 but the synthesis of EDHF was enhanced after NOS inhibition and was inhibited by supplementation with NO.31,32 Endothelium-dependent dilation by NO plays a major role in the large arteries, whereas EDHF exerts a critical effect on the small arteries and arterioles.10,33 Mice transgenic for the human CYP2J2 and CYP2C8 genes, which encode EET synthase, are resistant to the effects of NOS inhibition after L-NAME despite normal basal BPs.34 These data complement our findings that MK regulates the BP response to NOS inhibition in UNx mice by downregulation of EET synthesis.
The vasodilation and BP-lowering effect of adenosine are induced by A2AR,25 whose effects are mediated via stimulation of EET synthesis.26 We asked whether MK directly regulated the expression of the adenosine A2AR in renal endothelial cells. Quantitative RT-PCR revealed that the mRNA expression of the adenosine A2AR was not affected by MK (data not shown). Therefore, MK may not influence this receptor expression, but probably regulates its function through regulating molecules interacting with or acting downstream of the adenosine A2AR. Our results highlight that MK regulates this A2AR-EETs axis, although the underlying mechanisms including the receptor and the intracellular signaling pathways for EETs remain to be explored.35 MK-deficient mice would be a useful model to study the regulation of EETs.
Endothelial dysfunction is characterized by reduced vasodilation,36 which in turn leads to the reduction of blood flow. Because EETs are involved in dilation of small arteries,7,11,37,38 it is supposed that EETs may play a role in the axis of endothelial dysfunction and reduced vasodilation. As shown Figure 6, an EET antagonist significantly suppressed peritubular capillary blood flow in Mdk−/− mice, but not in wild-type mice. This suggests that EETs produced in renal endothelial cells in Mdk−/− mice actively promote dilation of small arteries, and consequently not only suppress BP but also promote blood flow of peritubular capillaries that are peripheral to the small arteries. Although EETs can inhibit inflammation,39–41 inflammation did not appear to contribute to the phenotype difference in Mdk+/+ and Mdk−/− mice in the model utilized (Supplemental Figure 9). Because it is widely accepted that a reduced renal blood flow leads to glomerulosclerosis and proteinuria, it is conceivable that MK deletion triggers the production of EETs, which in turn prevent renal injury through increasing blood flow and suppressing BP.
Endothelial dysfunction is a critical insult underlying a variety of diseases, including hypertension and CKD, but lacks a targeted therapy. The control of hypertension does not fully restore endothelial function. Although ACE inhibitors and angiotensin receptor blockers can prevent vascular remodeling,33 they do not fully restore endothelial function, perhaps because of the “aldosterone-escape phenomenon.”42,43 Therefore, a novel drug for endothelial dysfunction has been awaited, and endothelium-derived vasodilators such as EETs are intriguing targets.44 EETs can reduce BP by a combination of a reduction in renal tubular sodium reabsorption45–47 and dilation of blood vessels.7,11,37,38 EETs also inhibit inflammation and platelet aggregation.39–41 Thus, MK-targeted therapy may treat not only hypertension, but also other diseases complicated by endothelial dysfunction, such as cardiovascular diseases, cerebrovascular diseases, CKD, and diabetes.
The three principal eicosanoid pathways in the arachidonate cascade are the cyclooxygenase, the lipoxygenase, and the CYP pathway.35 The CYP pathway is composed of two enzymatic pathways: hydroxylases and epoxygenases. The CYP hydroxylases generate hydroxyeicosatetraenoic acids, which are proinflammatory and cause vasoconstriction. On the other hand, CYP epoxygenases generate EETs, which are metabolized to the inactive DHETs by sEH. Therefore, the inhibition of sEH should increase EETs, and exert beneficial effects. Indeed, sEH inhibitors have been developed and shown to reduce BP.35,48 However, one study demonstrated that a sEH inhibitor increased not only EETs but also 20-hydroxyeicosatetraenoic acid, and did not achieve sufficient beneficial effects,49 which raises concern about the therapeutic potential for sEH inhibitors. Our study has demonstrated that anti-MK antibody ameliorates hypertension induced by NOS inhibition, and has identified MK as another potential target to regulate EETs that could be a target for the treatment of hypertension associated with endothelial dysfunction.
Concise Methods
Animals
Animal care and experimental procedures were approved by the Animal Experimentation Committee of the Nagoya University Graduate School of Medicine and were conducted according to the Nagoya University Regulations for Experiments. Mice deficient in Mdk were generated as previously described.50 Mdk+/− mice of 129sv background were mated, and littermates obtained were divided into two groups (Mdk+/+ and Mdk−/−) for experiments. Mdk+/− mice were not used for experiments. Experiments were performed on 10- to 14-week-old male mice weighing 25–35 g that were housed under controlled environmental conditions and maintained with standard food and water.
MK Protein and Anti-MK Antibody Preparation
Recombinant human MK and goat polyclonal antibody against MK were produced from yeast as previously described.51–53
L-NAME Model
Mice were randomly assigned to one of the following four groups: nontreatment, UNx, L- L+UNx, or hydralazine administration with L-NAME after UNx (Hyd).
UNx was performed as previously described.18 In the L+UNx group, L-NAME (Sigma-Aldrich) administration was started at a dose of 1 mg/ml in drinking water on the day of UNx. The Hyd group received L-NAME plus hydralazine (Sigma-Aldrich) in drinking water after UNx. We adjusted the dose of hydralazine while maintaining a normal BP (0.4 mg/dl in Mdk+/+ mice, 0.2 mg/dl in Mdk−/− mice). We quantified drug uptake by weighing the drinking bottles and calculating daily water intake. During the treatment, water intake averaged 160.3±19.2 ml/kg body wt per day in the L+UNx group of Mdk+/+ mice, 166.3±16.8 ml/kg body wt per day in the L+UNx group of Mdk−/− mice, 151.0±24.5 ml/kg body wt per day in the Hyd group of Mdk+/+ mice, and 147.5±19.7 ml/kg body wt per day in the Hyd group of Mdk−/− mice, and there was no statistically significant difference among genotypes.
BP was assessed as the mean value of eight consecutive measurements using a tail-cuff sphygmomanometer (BP-98A; Softron) at 2 weeks and 1, 2, 3, and 4 months as previously described.18
Eighteen-hour urine samples were collected every 4 weeks in metabolic cages.
Mice were euthanized at 4 months and the kidney, lung, heart, and aorta were removed rapidly, and processed for histology, protein extraction, and RNA extraction. For protein extraction, each sample was snap-frozen in liquid nitrogen, and stored at −80°C. Serum were separated by centrifugation (500g for 10 minutes) and stored at −80°C.
Urinary albumin was quantified by an ELISA (Albwell M; Exocell Inc.), and urinary creatinine was quantified by the Jaffé method (Creatinine companion; Exocell Inc.).
BP Monitoring by a Radiotelemetry System
BP was measured in conscious, unrestrained mice by radiotelemetry using an indwelling transducer-tipped catheter (TA11PA-C10; Data Sciences International) according to the manufacturer’s instructions. The mice were allowed to recover from surgery for 2 weeks, and then the BP and heart rate measurements for pretreatment were obtained continuously for 2 days, and UNx was performed. At 2 weeks after UNx, BP measurements for UNx were obtained continuously for 2 days, and then L-NAME administration in drinking water (1 mg/ml) was started. Continuous recordings for 2 days were performed at 2 weeks and 1 and 2 months after L-NAME was started. The values for 2 days were averaged for statistical comparison across genotypes.
Hypertension Treatment with Anti-MK Antibody
At 2 weeks after surgical insertion of a transducer-tipped catheter, BP measurements for pretreatment were obtained. L-NAME administration (1 mg/ml) was started on the day of UNx. At 4 days after L+UNx, anti-MK antibody (5.0 μg/h) or goat IgG (Abcam, Inc.) (5.0 μg/h) as a control was infused into mice using osmotic pumps (1007D; Alzet). One day after administration of MK antibody or IgG, BP was measured.
Histology
The removed kidneys were fixed in 10% formalin and embedded in paraffin. The sections were stained with periodic acid–Schiff and Masson’s trichrome. High-power fields were used to examine the sections for evidence of focal sclerosis. The semiquantitative glomerular sclerosis score was calculated by counting 100 glomeruli under ×400 magnification (grade 0 to +4) and summing the grades for each kidney as follows: grade 0, no sclerosis in the glomerulus; grade 1, sclerosis of up to 25% of the glomerulus; grade 2, sclerosis of 25%–50% of the glomerulus; grade 3, sclerosis of 50%–75% of the glomerulus; and grade 4, sclerosis of 75%–100% of the glomerulus.18
14,15-DHET Measurements
The concentrations of urine and medium 14,15-DHET were determined by an ELISA kit (Detroit R&D, Inc.).
In Vivo EET Antagonist Injection Experiment
Charybdotoxin (Peptide Institute, Osaka, Japan) was dissolved with saline. 14,15-EEZE (Cayman Chemical) in ethanol was evaporated under a gentle stream of nitrogen to change the solvent and supplemented with DMSO, and then diluted with saline to a final concentration of 0.02 μg/μl in 2% DMSO. The adenosine A2AR blocker, ZM 241385 (Sigma-Aldrich), was dissolved in DMSO and diluted further with saline to a final concentration of 100 mg/ml in 5% DMSO.
A transducer-tipped catheter was surgically inserted into 10- to 12-week-old male mice as described above, in order to record BP simultaneously with drug injection. We started the experiments 24 hours after the surgery. After stabilization of BP by 15 minutes of anesthesia with pentobarbital (50 mg/kg body wt, intraperitoneally), each of the following drugs was injected into a tail vein at the indicated dose: charybdotoxin, 0.4 mg/kg body wt intraperitoneally; charybdotoxin, 75 μg/kg body wt intraperitoneally; 14,15-EEZE, 140 μg/kg body wt intraperitoneally; and ZM 241385, 5 mg/kg body wt intraperitoneally.
For the MK supplementation experiment, Mdk−/− mice were pretreated by intraperitoneal administration of recombinant human MK in PBS or PBS alone (control) at 10 minutes before 14,15-EEZE injection. For the MK inhibition experiment, Mdk+/+ mice were pretreated by intraperitoneal administration of anti-MK antibody in saline or saline alone (control) at 10 minutes before 14,15-EEZE injection. In both experiments, the doses of pentobarbital and 14,15-EEZE were the same as described above.
Primary Endothelial Cell Isolation
Kidneys from young adult Mdk+/+ and Mdk−/− mice were harvested for isolation of primary endothelial cells, as previously described.34,54 After reaching confluence, cells were washed with PBS and cultured for 12 hours in FBS-free medium. These cells were exposed to 10 μM Ca2+ ionophore (A23187; Wako, Osaka, Japan) for 15 minutes to stimulate AA release, and then the cells and medium were harvested.
Multiphoton-Intravital Renal Peritubular Capillary Imaging
Intravital microscopy of mouse kidney was performed using a protocol modified from a previous report.55 Mice were anesthetized with pentobarbital (40 mg/kg body wt, subcutaneously), and the animals were placed on a heating pad to maintain a constant body temperature (37°C). The hair in the back was removed with hair removal lotion. Left flank incisions were made, and the left kidney was moved out. A 24G indwelling cannula (Thermo Fisher Scientific) was placed into the tail vein. The kidney was immobilized in a custom-made holder. The imaging system was composed of an A1R MP multiphoton microscope (Nikon), driven by Mai Tai Deep See (Newsport) tuned to 830 nm, and an upright microscope equipped with a ×25 water immersion objective (Apo LWD 25×/1.10W; Nikon).Vessels were visualized by intravenous injection of 70 kD Rhodamine conjugated-dextran (Invitrogen) detected using a 595/50 nm filter. Tubular cells were detected by intrinsic fluorescence through a 525/50 nm filter (for FITC). Image stacks were acquired at 3 µm z-spacing to cover a volume of 509.12×509.12×12.0 µm.
Before 14,15-EEZE (166 µg/kg body wt) was injected into the tail vein, the image at 0 minutes was taken. Time-lapse images were acquired at 5 minutes, and 15 minutes after 14,15-EEZE injection. Quantitative analysis of capillary volume was calculated using Imaris software (Bitplane).
Sympathetic Nervous System Evaluation
Blood was obtained from 10- to 14-week-old male nontreated mice, and serum catecholamines were measured by SRL, Inc. (Tokyo, Japan).
Under BP monitoring with a radiotelemetry system as described above, anesthesia with pentobarbital (30 mg/kg body wt, intraperitoneally) was administered for 7 minutes, and then a ganglionic blocker, hexamethonium, was injected into the tail vein (15 mg/kg body wt).
Statistical Analyses
Results are expressed as the mean±SEM. Statistical difference was assessed by a one-way ANOVA followed by a post hoc Tukey–Kramer test for multiple comparisons, and by an unpaired t test for comparisons between two groups. Longitudinal data such as BP were analyzed with a linear mixed-effects model including treatment group, measurement time, an interaction term between them, and baseline value. P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank N. Suzuki, N. Asano, and Y. Sawa for their excellent technical assistance.
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research on Innovative Areas 23110002 to K.K.; Grants-in-Aid 18390099 and 20390092 to K.K., 22590886 to W.S., and 19590947 to Y.Y.; and funds from the Global COE Program to Nagoya University), the Ministry of Health, Labour and Welfare of Japan (Grant-in-Aid for Progressive Renal Diseases Research, Research on Intractable Disease), the Robert A. Welch Foundation (grant GL 625910 to J.R.F.), the US National Institutes of Health (grants GM31278 to J.R.F., and DK49870, DK36079, and HL68686 to C.S.W.), and the George E. Schreiner Chair of Nephrology (funds to C.S.W.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121259/-/DCSupplemental.
References
- 1.Furchgott RF, Zawadzki JV: The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Ferrige AG, Moncada S: Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Campbell WB, Falck JR: Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Zou H, Harrington JJ, Shire AM, Rego RL, Wang L, Campbell ME, Oberg AL, Ahlquist DA: Highly methylated genes in colorectal neoplasia: Implications for screening. Cancer Epidemiol Biomarkers Prev 16: 2686–2696, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Zatz R, Baylis C: Chronic nitric oxide inhibition model six years on. Hypertension 32: 958–964, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagao T, Illiano S, Vanhoutte PM: Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-L-arginine in rats. Am J Physiol 263: H1090–H1094, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R: Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR: Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol 7: 2364–2370, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Arima S, Endo Y, Yaoita H, Omata K, Ogawa S, Tsunoda K, Abe M, Takeuchi K, Abe K, Ito S: Possible role of P-450 metabolite of arachidonic acid in vasodilator mechanism of angiotensin II type 2 receptor in the isolated microperfused rabbit afferent arteriole. J Clin Invest 100: 2816–2823, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikawa Y, Stepp DW, Chilian WM: In vivo location and mechanism of EDHF-mediated vasodilation in canine coronary microcirculation. Am J Physiol 277: H1252–H1259, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Campbell WB, Gebremedhin D, Pratt PF, Harder DR: Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Zeldin DC: Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276: 36059–36062, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Fleming I, Busse R: Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension 47: 629–633, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kadomatsu K, Tomomura M, Muramatsu T: cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun 151: 1312–1318, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Horiba M, Kadomatsu K, Nakamura E, Muramatsu H, Ikematsu S, Sakuma S, Hayashi K, Yuzawa Y, Matsuo S, Kuzuya M, Kaname T, Hirai M, Saito H, Muramatsu T: Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J Clin Invest 105: 489–495, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S, Muramatsu T: Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol 167: 3463–3469, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Kadomatsu K, Muramatsu T: Midkine and pleiotrophin in neural development and cancer. Cancer Lett 204: 127–143, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hobo A, Yuzawa Y, Kosugi T, Kato N, Asai N, Sato W, Maruyama S, Ito Y, Kobori H, Ikematsu S, Nishiyama A, Matsuo S, Kadomatsu K: The growth factor midkine regulates the renin-angiotensin system in mice. J Clin Invest 119: 1616–1625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC: Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem 271: 3460–3468, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Zeldin DC, DuBois RN, Falck JR, Capdevila JH: Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys 322: 76–86, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Roman RJ: P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Baragatti B, Schwartzman ML, Angeloni D, Scebba F, Ciofini E, Sodini D, Ottaviano V, Nencioni S, Paolicchi A, Graves JP, Zeldin DC, Gotlinger K, Luin S, Coceani F: EDHF function in the ductus arteriosus: Evidence against involvement of epoxyeicosatrienoic acids and 12S-hydroxyeicosatetraenoic acid. Am J Physiol Heart Circ Physiol 297: H2161–H2168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun P, Antoun J, Lin DH, Yue P, Gotlinger KH, Capdevila J, Wang WH: Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake. Hypertension 59: 339–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pidkovka N, Rao R, Mei S, Gong Y, Harris RC, Wang WH, Capdevila JH: Epoxyeicosatrienoic acids (EETs) regulate epithelial sodium channel activity by extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated phosphorylation. J Biol Chem 288: 5223–5231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M: Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Liclican EL, Doumad AB, Wang J, Li J, Falck JR, Stier CT, Jr, Carroll MA: Inhibition of the adenosine2A receptor-epoxyeicosatrienoic acid pathway renders Dahl salt-resistant rats hypertensive. Hypertension 54: 1284–1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bro S, Binder CJ, Witztum JL, Olgaard K, Nielsen LB: Inhibition of the renin-angiotensin system abolishes the proatherogenic effect of uremia in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 27: 1080–1086, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Hirsch AT, Talsness CE, Smith AD, Schunkert H, Ingelfinger JR, Dzau VJ: Differential effects of captopril and enalapril on tissue renin-angiotensin systems in experimental heart failure. Circulation 86: 1566–1574, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Takeuchi H, Sonobe Y, Jin S, Mizuno T, Miyakawa S, Fujiwara M, Nakamura Y, Kato T, Muramatsu H, Muramatsu T, Suzumura A: Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory T cell population. Proc Natl Acad Sci U S A 105: 3915–3920, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzielak DJ: The immune system and hypertension. Hypertension 19[Suppl]: I36–I44, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Campbell WB, Falck JR, Gauthier K: Role of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor in bovine coronary arteries. Med Sci Monit 7: 578–584, 2001 [PubMed] [Google Scholar]
- 32.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R: Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94: 3341–3347, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Schiffrin EL, Park JB, Intengan HD, Touyz RM: Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation 101: 1653–1659, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC: Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J 24: 3770–3781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imig JD, Hammock BD: Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8: 794–805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endemann DH, Schiffrin EL: Endothelial dysfunction. J Am Soc Nephrol 15: 1983–1992, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Imig JD, Falck JR, Wei S, Capdevila JH: Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res 38: 247–255, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Falck JR, Kodela R, Manne R, Atcha KR, Puli N, Dubasi N, Manthati VL, Capdevila JH, Yi XY, Goldman DH, Morisseau C, Hammock BD, Campbell WB: 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: Influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem 52: 5069–5075, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK: Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis BB, Thompson DA, Howard LL, Morisseau C, Hammock BD, Weiss RH: Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A 99: 2222–2227, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krötz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, Campbell WB, Pohl U: Membrane-potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol 24: 595–600, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Bomback AS, Klemmer PJ: The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol 3: 486–492, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Struthers AD: Aldosterone escape during angiotensin-converting enzyme inhibitor therapy in chronic heart failure. J Card Fail 2: 47–54, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Imig JD: Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol 289: F496–F503, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH: Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2414–2420, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartzman ML, Martasek P, Rios AR, Levere RD, Solangi K, Goodman AI, Abraham NG: Cytochrome P450-dependent arachidonic acid metabolism in human kidney. Kidney Int 37: 94–99, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC: Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem 273: 29254–29261, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Imig JD: Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev 24: 169–188, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Shen HC, Ding FX, Wang S, Deng Q, Zhang X, Chen Y, Zhou G, Xu S, Chen HS, Tong X, Tong V, Mitra K, Kumar S, Tsai C, Stevenson AS, Pai LY, Alonso-Galicia M, Chen X, Soisson SM, Roy S, Zhang B, Tata JR, Berger JP, Colletti SL: Discovery of a highly potent, selective, and bioavailable soluble epoxide hydrolase inhibitor with excellent ex vivo target engagement. J Med Chem 52: 5009–5012, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Nakamura E, Kadomatsu K, Yuasa S, Muramatsu H, Mamiya T, Nabeshima T, Fan QW, Ishiguro K, Igakura T, Matsubara S, Kaname T, Horiba M, Saito H, Muramatsu T: Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells 3: 811–822, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, Okamoto K, Oda M, Sakuma S, Aikou T, Muramatsu H, Kadomatsu K, Muramatsu T: Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer 83: 701–706, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muramatsu H, Muramatsu T: Purification of recombinant midkine and examination of its biological activities: Functional comparison of new heparin binding factors. Biochem Biophys Res Commun 177: 652–658, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Maehara H, Kaname T, Yanagi K, Hanzawa H, Owan I, Kinjou T, Kadomatsu K, Ikematsu S, Iwamasa T, Kanaya F, Naritomi K: Midkine as a novel target for antibody therapy in osteosarcoma. Biochem Biophys Res Commun 358: 757–762, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW: Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol 162: 1591–1601, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN: Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458: 524–528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.