Abstract
Short-chain fatty acids (SCFAs) are fermentation end products produced by the intestinal microbiota and have anti-inflammatory and histone deacetylase–inhibiting properties. Recently, a dual relationship between the intestine and kidneys has been unraveled. Therefore, we evaluated the role of SCFA in an AKI model in which the inflammatory process has a detrimental role. We observed that therapy with the three main SCFAs (acetate, propionate, and butyrate) improved renal dysfunction caused by injury. This protection was associated with low levels of local and systemic inflammation, oxidative cellular stress, cell infiltration/activation, and apoptosis. However, it was also associated with an increase in autophagy. Moreover, SCFAs inhibited histone deacetylase activity and modulated the expression levels of enzymes involved in chromatin modification. In vitro analyses showed that SCFAs modulated the inflammatory process, decreasing the maturation of dendritic cells and inhibiting the capacity of these cells to induce CD4+ and CD8+ T cell proliferation. Furthermore, SCFAs ameliorated the effects of hypoxia in kidney epithelial cells by improving mitochondrial biogenesis. Notably, mice treated with acetate-producing bacteria also had better outcomes after AKI. Thus, we demonstrate that SCFAs improve organ function and viability after an injury through modulation of the inflammatory process, most likely via epigenetic modification.
Keywords: ischemia-reperfusion, SCFA, kidney disease, epigenetic modifications, microbiota
AKI is an inflammatory process frequently observed in hospitalized patients. AKI is associated with the development of CKD and causes distress to the patient.1,2 AKI induced by ischemia and reperfusion injury (IRI) is closely linked to the activation of tubular epithelial and endothelial cells by endogenous danger signals released after cell stress and death due to enhanced production of reactive oxygen species (ROS), among other inducers.3 Additionally, IRI involves the migration and activation of innate and adaptive immune cells into the kidneys.
Increased ROS production disrupts the ratio of oxidant/antioxidant enzymes, leading to mitochondrial-mediated apoptotic cell death. Activation of endothelial cells enhances adhesion molecule expression, which recruits immune cells, contributing to ROS production.3 Similarly, kidney tubular epithelial cells (TECs) and resident antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs), can produce cytokines and chemokines upon activation at the site of inflammation. Furthermore, APCs increase the expression of costimulatory molecules and migrate to the draining lymph node to activate CD4+ and CD8+ T lymphocytes, thereby contributing to tissue damage.4 Our group and others have demonstrated the role of different immune cell populations in kidney IRI.5–9
Recently, an intimate connection between the intestine and the kidneys has been proposed.10,11 Established data have already shown that modification of microbiota composition could affect the outcome of glomerulopathies, and recent data indicate that renal inflammation is associated with the production of uremic toxins by the intestine and with augments intestinal permeability, leading to the onset of systemic inflammation.12,13 In this sense, molecules produced by local intestinal flora could have a direct effect on kidney injuries.
Short-chain fatty acids (SCFAs) are end products produced from the fermentation of complex carbohydrates by the intestinal microbiota, especially by anaerobic bacteria.14 The most abundant SCFAs are acetate, propionate, and butyrate. Locally, SCFAs are energy sources for colonocytes. However, they can reach the bloodstream.15 SCFA treatment has ameliorated colitis, airway disease, and metabolic syndrome in diet-induced obese mice.16–18
So far, researchers suggested that SCFAs may operate in two manners: by binding G-protein membrane receptors (GPR41 and GPR43),19,20 or by entering cells directly through transporter channels in the cellular membrane and working as histone deacetylase (HDAC) inhibitors. Thus, they could act by modulating epigenetic processes.21
Because AKI has an important inflammatory component yet SCFAs have anti-inflammatory properties, we investigate whether SCFAs treatment could protect mice from IRI. Furthermore, we investigated whether this protection could involve direct modulation of the inflammatory process and/or amelioration of the oxidative stress presented in this model.
Results
SCFA Treatment Ameliorates Kidney Function after IRI
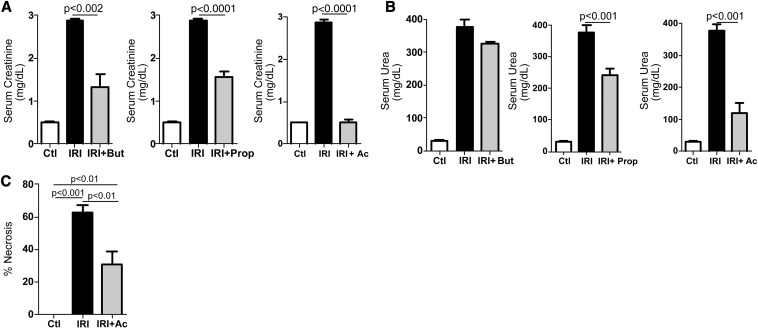
To investigate the role of SCFAs in IRI, mice were subjected to IRI and treated with SCFAs. SCFA treatment diminished levels of serum creatinine and urea after IRI, with acetate treatment resulting in the best protection (Figure 1, A and B). SCFA treatment did not interfere in kidney function or inflammatory parameters (Supplemental Figure 1, A–C). Hereafter, we consider in vivo analyses only for acetate treatment. Histopathologic analysis showed that acetate treatment preserved kidney structure as reflected in the reduced necrosis score in kidney tubular epithelial cells (Figure 1C).
Figure 1.
SCFA ameliorates renal function. Mice (n=5) were subjected to kidney IRI and treated with acetate (Ac), propionate (Prop), and butyrate (But) at 0.5 hour before ischemia and at the moment of reperfusion (200 mg/kg each). Serum creatinine (A) and urea (B) were measured after 24 hours. (C) Quantification of necrosis in tubular epithelial cells. Ctl, control group.
Acetate Treatment Reduces Cellular Stress and Local and Systemic Inflammation
Acetate protection was associated with decreased ROS production (Figure 2A). Cytokines and chemokines were also diminished locally (Figure 2, B and C) and systemically (Figure 2D). Additionally, low levels of mRNA of toll-like receptor 4 and its endogenous ligand, biglycan (Figure 2E), and lesser activation of the NF-κB pathway were observed (Figure 2F). As a consequence, low levels of activated neutrophils and macrophages (myeloperoxidase in kidney tissue), a low frequency of infiltrating macrophages (CD11b+F4/80+), and a low frequency of activated DCs (CD11c+CD40+) (Figure 2, G and H) were observed in acetate-treated mice. Thus, acetate treatment diminished cellular stress and inflammation in kidney IRI.
Figure 2.
Acetate treatment decreases cellular stress, cytokine and chemokine production, and cellular infiltrates. Mice (n=5) were subjected to kidney IRI and treated with acetate. (A) Glutathione reduced (GSS) and glutathione oxidized (GSSH) ratio. mRNA levels measured by real-time PCR (B) and protein levels measured by BioPlex (C) of proinflammatory cytokines and chemokines in kidney tissue and serum protein levels of the proinflammatory cytokines and chemokines (D). (E) mRNA levels of TLR4 and biglycan measured by real-time PCR in kidney tissue. (F) Right, Western blot analysis in kidney tissue for IκBα and β-actin; each band quantified (optical densitometry) for each protein in the group and normalized by quantification of β-actin for the respective group. (G) Myeloperoxidase (MPO) levels in kidney tissue. (H) Percentage of activated DCs (CD11c+/CD40+) and macrophages (CD11b+/F4/80+) in kidney tissue analyzed by flow cytometry. Ac, acetate; Ctl, control; KC, Cxcl1; MCP-1, monocyte chemotactic protein-1.
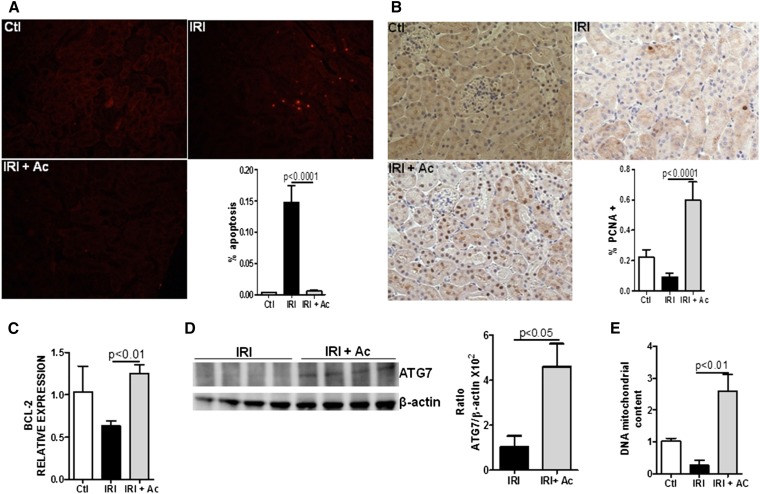
Acetate Treatment Reduces Apoptosis and Increases Autophagy and Tubular Proliferating Cells
Apoptosis is a frequent event in kidney IRI. Acetate treatment diminished the number of apoptotic cells in kidney tissue (Figure 3A) but increased proliferation in kidney epithelial cells (Figure 3B) and BCL-2 gene expression (Figure 3C). Moreover, acetate treatment increased the levels of ATG-7 protein, which is involved in the autophagy pathway (Figure 3D). Supporting these findings was an increase in mitochondrial DNA in kidney tissue after acetate treatment compared with the IRI group (Figure 3E). Taken together, these results show that acetate treatment inhibits apoptosis and increases cell proliferation, potentially through autophagy activation.
Figure 3.
Acetate treatment decreases apoptosis levels and increases tubular proliferating cells and activation of the autophagy pathway. (A) Immunofluorescence of apoptosis levels measured by TUNEL in kidney tissue. The percentage of apoptosis was measured relative to the photographic area (original magnification, ×20). (B) Immunohistochemistry for proliferating cell nuclear antigen in kidney tissue. The percentage of positive staining for the molecule was measured relative to the photographic area (original magnification, ×20). (C) Real-time PCR in kidney tissue for BCL-2. (D) Western blot analysis in kidney tissue for ATG-7 and β-actin. The ratio of ATG-7/β-actin was calculated through quantification of each band (optical densitometry) in the group and normalized to the quantification of β-actin. (E) Ratio of mitochondrial DNA (mtDNA) and genomic DNA (gDNA) in kidney tissue. n=5 per group. Ac, acetate; Ctl, control; PCNA, proliferating cell nuclear antigen; TUNEL, terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling.
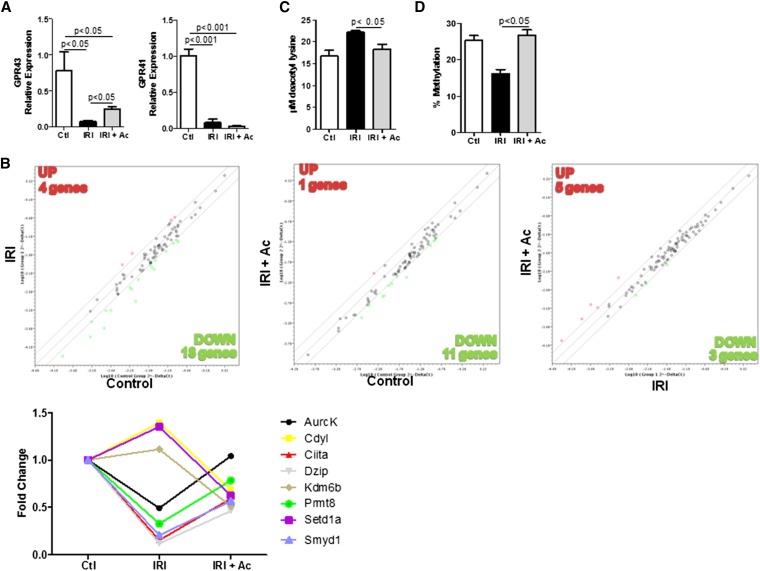
SCFA Receptors and Epigenetic Modifications in Kidney Tissue
Gene expression analysis for SCFA receptors revealed no difference in GPR41 expression, whereas the levels of GPR43 increased after acetate treatment (Figure 4A). Additionally, the PCR-based array showed that acetate treatment modulated the expression of genes encoding enzymes involved in epigenetic modifications (Figure 4B). Another important function for these SCFAs is their ability to act as HDAC inhibitors. Acetate treatment inhibited the activity of HDACs (Figure 4C) in kidney IRI. Another important epigenetic process is DNA methylation. Global methylation status decreased in kidney tissue undergoing IRI, and acetate treatment reversed this process (Figure 4D).
Figure 4.
Acetate treatment modulates epigenetic modification and upregulates GPR43 expression. (A) Real-time PCR of GPR41 and GPR43 in kidney tissue. (B) PCR-based array for the expression of genes encoding chromatin modification enzymes in kidney tissue. Comparison of gene expression levels for these genes between IR versus control (left), IR plus acetate versus IR (middle) and IR plus acetate versus IR (right). Up- and downregulated gene expression is shown in relation to the y axis group. (C) Measurement of histone deacetylase activity in kidney tissue. (D) Global DNA methylation in kidney tissue. n=5 per group Ac, acetate; Ctl, control.
Evaluation of SCFAs in DCs In Vitro
Having observed in vivo a lower frequency of activated DCs in kidney tissue after IRI, we sought to determine whether SCFAs may modulate these cells in vitro. SCFA treatment reduced the expression of the costimulatory molecules CD80 and CD40 in bone marrow DCs (BM-DCs) (Figure 5, A and B). These data corroborated the in vivo finding (Figure 2H). Furthermore, we evaluated whether this reduction is functional. We pretreated APCs from RAGKO mice with LPS, with or without SCFAs, for 24 hours. Treatment of APCs with LPS plus SCFAs was sufficient to reduce the proliferation of CD8+ and CD4+ cells (Figure 5, C and D). Therefore, SCFA treatment modulates the activation and function of APCs.
Figure 5.
SCFA treatment decreases activation of BM-DCs and inhibits APC function. (A) BM-DCs were generated with GM-CSCFs (20 ng/ml) and on the seventh day were stimulated with LPS (20 ng/ml in the presence of acetate (Ac), propionate (Pr), and butyrate (But) for 24 hours. Histogram of the percentage of positive cells (A) and the mean fluorescence intensity (B) for the costimulatory molecules CD80, CD86, and CD40 (C) Spleen cells from RAGKO mice (APCs) were pretreated with LPS (20 ng/ml) in the presence of acetate, propionate, and butyrate for 24 hours. After, APCs were washed and cocultivated with carboxyfluorescein succinimidyl ester (CSFE)–labeled spleen cells from BALB/c mice for 4 days. (D) Percentage of proliferating CFSE-labeled CD4 and CD8 cells. NP, nonproliferating cells; PROL, proliferating cells. **P<0.01; ***P<0.001.
Evaluation of SCFA Treatment in a Kidney Epithelial Cell Line
It is well known that TEC activation enhances inflammation in kidney tissue after IRI. To date, SCFA action in TECs has not been reported. Thus, we investigated whether SCFA treatment is effective in these cells. TECs stimulated with an inflammatory cocktail and treated with SCFAs had reduced NFκB activation (percentage of p65+ cells) and nitric oxide production (Figure 6, A and B). ROS production in TECs induced by ischemia in the presence of SCFAs was diminished, whereas mitochondrial function was enhanced (Figure 7). Moreover, translocation of the transcription factor hypoxia-inducible factor (HIF)-1α to the nucleus, a hallmark of hypoxia, was diminished in the nuclei of cells treated with SCFAs after 24 hours of hypoxia (Figure 8A), and there were low levels of lactate in the culture supernatant (Figure 8B). Expression of the HIF-1α target gene (VEGF) was increased after hypoxia and decreased after SCFAs treatment (Figure 8B). Taken together, these findings indicate that SCFA treatment is effective in TECs and diminishes hypoxia and inflammation in these cells.
Figure 6.
SCFA treatment inhibits NFκb activation and nitric oxide production in epithelial kidney cell line. MM55.k kidney epithelial cells were stimulated with inflammatory cocktail (LPS, 10 µg/ml; zymosan, 10 µg/ml; IL-6, 50 ng/ml; IL-1β, 50 ng/ml, and TNF-α, 100 ng/ml) in the presence of acetate (Ac; 25 mM), propionate (Pr; 12 mM), and butyrate (But; 3.2 mM) for 24 hours and were evaluated by flow cytometry for (A) left: overlay histogram of NFκb activation measured through phosphorylation of the p65 subunit (percentage of p65+ cells) and right: percentage of the p65+ cells and (B) left: overlay histogram nitric oxide production (DAF-FM Diacetate) [4-Amino-5-Methylamino-2′,7′-Difluorofluorescein Diacetate] and right: mean fluorescence intensity of the DAF-positive cells. *P<0.05.
Figure 7.
SCFA treatment inhibits ROS production in an epithelial kidney cell line after hypoxia. HK-2 human kidney epithelial cells were seeded on a cover slip and subjected to hypoxia for 24 hours in the presence of acetate (Ac; 25 mM), propionate (Pr; 12 mM), and butyrate (But, 3.2 mM). Thirty minutes before completion of the 24 hours, cells were incubated with Hypoxyprobe and MitoSOX markers and analyzed with confocal microscopy. Scale bar, 10 µm.
Figure 8.
SFCA treatment inhibits HIF-1α translocation to the nucleus, lactate production, and VEGF expression under hypoxia. HK-2 human kidney epithelial cells were seeded on a cover slip and subjected to hypoxia for 24 hours in the presence of acetate (Ac; 25 mM), propionate (Pr; 12 mM), and butyrate (But, 3.2 mM). (A) Cells were labeled with anti-human HIF-1α followed by FITC-labeled secondary antibodies and analyzed with confocal microscopy. Scale bar, 10µm. (B) Left lactate levels after hypoxia were measured in culture supernatant. Right relative expression of VEGF in normoxia and hypoxia. *P<0.05; ***P<0.001.
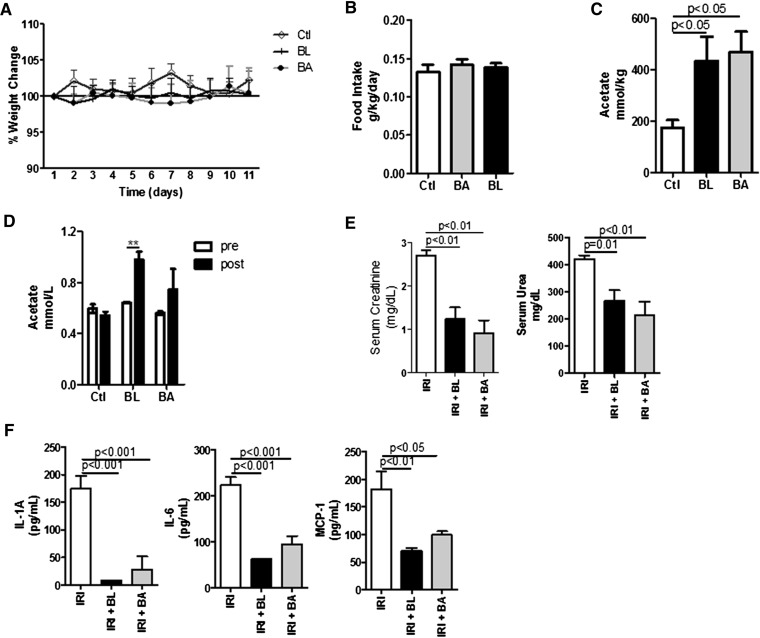
Acetate-Producing Bacteria Treatment Ameliorates Kidney Function after IRI
Because commensal bacteria produce SCFAs, we treated mice with Bifidobacterium adolescentis or B. longum, which are acetate producers. The bacteria (probiotics) treatment did not alter weight change, food intake, or kidney function during the treatment period (Figure 9, A and B, Supplemental Figure 2B). The treatment seemed to be effective because we detected the bacteria in their niche, although in very low frequency (Supplemental Figure 2A). Additionally, the probiotics treatment increased acetate levels in feces from the large intestine and in plasma (Figure 9, C and D). The animals treated with acetate-producing bacteria and subjected to kidney IRI had low serum levels of creatinine and urea (Figure 9E). This was accompanied by low levels of cytokines and chemokines in the serum of bacteria-treated mice (Figure 9F). Therefore, treatment with acetate-producing bacteria increases acetate production and protects mice from kidney IRI.
Figure 9.
Acetate-producing bacteria ameliorate AKI induced by IR. Mice (n=5 per group) were administered B. longum or B. adolescentis (108 bacteria) by gavage for 10days before euthanasia and were subjected to kidney IRI. (A) Weight change and (B) food intake during 10 days before surgery induction. (C) Acetate levels measured in the intestinal (colon) content. (D) Acetate levels measured from plasma samples harvested pre and post probiotic treatment. (E) Serum levels of creatinine and urea in mice subjected to kidney IRI. (F) Serum levels of proinflammatory cytokines and chemokines in mice subjected to kidney IRI, treated or untreated with B. longum (BL) or B. adolescentis (BA). Ctl, control. **P<0.01.
Discussion
Kidney IRI physiopathology has a complex network of events that involves a well known and important inflammatory process that includes tubular epithelial, endothelial, and resident immune cells. In addition to the high mortality rate in hospitalized patients, the extent of this event has been believed to be a risk factor associated with worse outcomes.1 Thus, its prevention is extremely important. In this study, we investigated whether SCFAs, which have anti-inflammatory properties, could ameliorate kidney IRI.
Treatment with SCFAs, especially acetate, reduced kidney damage after kidney IRI. To our knowledge, this is the first study demonstrating the protective role of SCFAs in kidney IRI. Other studies have observed a reduction in kidney injury in other models after SCFA treatment.22,23 The higher protection observed after acetate treatment might be due to the composition of acetate, favoring the rapid entry of the molecule into the metabolic pathway, whereas other types of SCFAs need to be converted into precursor molecules. The concentrations used in this study agree with the physiologic concentrations observed for these molecules in the intestine.24 SCFA treatment was also protective in colitis and lung injury models.16,17 SCFAs can reach the bloodstream14 even when produced and taken up by local cells in the intestine, but their presence in kidney cells has not been reported.
Inflammation and apoptosis are hallmarks of this model, and acetate treatment inhibited both processes. This is an expected result because SCFAs have previously been found to possess anti-inflammatory properties. The reduction of apoptosis levels could be due to inflammation reduction or enhancement of the autophagy activation pathway. Restricted deletion of ATG7 in TECs showed increased susceptibility to kidney injury in cisplatin-induced AKI.25 Additionally, in cancer cells, butyrate treatment induced autophagy as a defense strategy to retard mitochondrial apoptosis-induced cell death.26 We observed lower apoptosis levels and higher levels of the ATG-7 protein after acetate treatment, suggesting that acetate could induce autophagy and thereby retard apoptosis.
Two mechanisms have been proposed for the operation of SCFAs: as a ligand for GPR41 and GPR43 or as an HDAC inhibitor. The latter is believed to occur in a GPR-independent manner.21 In our study, GPR41 had similar mRNA levels with kidney IRI and acetate treatment, whereas GPR43 levels were increased. Considering the other proposed function of SCFAs, HDAC activity was decreased in kidney tissues and the expression of chromatin modification enzymes was modulated.
Among the differentially expressed genes, none of them encoded for an HDAC, which suggests that HDAC inhibition exerted by SCFAs may be only at the protein level. Other studies have observed modulation of HDAC activity by acetate.27 It is not fully understood whether HDAC inhibition by SCFAs is GPR41/43 dependent. Recently, HDAC inhibition was partially GPR43 dependent in colon tissue,28 but other researchers have observed HDAC inhibition in a GPR43-independent manner.29 Although we observed high expression of GPR43 in our model, its expression has been reported as minimal or null in kidney tissue.30,31 Thus, we believe that acetate might be regulating the inflammatory process by regulating epigenetic modification in a GPR-independent manner.
Another interesting finding in our work is the increase of global methylation of DNA after acetate treatment. One limitation of this finding is that this assay is unspecific, barring speculation as to which regions and consequently target genes could be altered by modifications in methylation status. Because we do not know whether this effect is direct or indirect, future investigations are necessary to unravel the possible relationship between GPR activation, HDAC inhibition, and DNA methylation by SCFAs in the kidney.
To further investigate possible SCFA protection, we examined the role of SCFAs in immune cells and a kidney epithelial cell line. As observed in vivo, SCFA treatment reduced the expression of the costimulatory molecule CD40 and (to a lesser extent) CD80 in BM-DC. Other studies have also demonstrated a reduction in DC development and activation.32–34 Furthermore, we observed that SCFA-treated APC induced less proliferation of CD4+ and CD8+ T lymphocytes, demonstrating that the modulation of DC treated with SCFAs was functional. DC links innate immunity with adaptive immunity, so lower activation of DC is important to reduce lymphocyte activation. Our group reported that Th1-lymphocyte factors, such as IFN-γ and IL-12, are important for AKI development in kidney IRI.7 Thus, it is plausible to speculate that low frequencies of activated DC and reduced lymphocyte activation occur in vivo.
Kidney ischemia is an important event that contributes to kidney damage because of ROS production and inflammation.4 SCFA treatment reduced NFκb activation and nitric oxide production in TECs. Moreover, lower production of ROS, lower HIF-1α translocation, and lower lactate production after hypoxia in the presence of SCFAs were observed. Lactate production and HIF-1α activation are directly associated.35 The expression of GPRs in kidney tissue is minimal,30,31 which suggests that SCFAs could operate via an epigenetic mechanism in these cells, as observed in vivo. Another possibility is that SCFAs ameliorate mitochondrial function after kidney ischemia, through SCFAs metabolism in these cells, providing energy. This idea is supported by the finding of increased mitochondrial DNA in kidney tissue undergoing IRI after acetate treatment (Figure 3E). Butyrate seems to have exerted a greater effect in vitro than in vivo. Not all SCFAs could bind to the same receptor, as shown recently for the receptor Olr78, which can be activated by acetate and propionate and is involved in kidney BP regulation after binding of SCFAs.36 This new finding raises the question of whether acetate could protect from kidney IRI through Olr78 activation. If so, this could explain, at least for butyrate treatment, the better results observed in vitro because Olr78 is not expressed in epithelial cells. Either way, SCFA treatment can modulate the pathways involved in ischemia injury and inflammation in TEC in vitro.
Finally, we treated animals with acetate-producing bacteria and observed an improvement of kidney function. B. longum, but not B. adolescentis, was recently reported to reduce death in a model of enterohemorrhagic Escherichia coli infection, which was associated with acetate production.37 Here, both types of bacteria treatment protected from kidney IRI. One explanation is that although B. adolescentis produces less acetate, the amount was enough to protect from kidney IRI. However, further investigation will be necessary to clarify this issue. It is important to mention that because these treatments were performed without previous antibiotic administration, and because we have found very low frequencies of these probiotics in intestine (none of them close to the mucus region of treated mice), these bacteria probably did not colonize the intestine. However, future studies are necessary to elucidate this issue. Germ-free mice are more susceptible to kidney IRI.38 In this study, we demonstrated that metabolites from microbiota are also important to control inflammatory processes in distant organs where SCFAs are not produced. Acetate administration can also protect from colitis in the absence of microbiota,15 whereas the administration of SCFAs in wild-type mice also protects from other diseases.17,22,23 Despite the importance of the presence of microbiota in maintaining intestinal homeostasis39 and preventing inflammation, it appears that an increase in the concentration of SCFAs could enhance this protection. It is already known that gut microbiota can change composition rapidly through diet alteration.40 Augmented SCFA levels could be reached through changes of diet, and probiotics have been investigated as alternative therapeutic strategies in some contexts, strengthening the idea that acetate-producing bacteria could become a tool for management or prevention of inflammatory processes.
In conclusion, acetate treatment diminishes inflammation in kidney epithelial and immune cells and ameliorates kidney IRI, most likely through modulation of epigenetic processes.
Concise Methods
Mice
Male C57BL/6 (H-2Ab) mice were purchased from Federal University of São Paulo, UNIFESP (n=5 per group). All animal procedures were performed in accordance with the Brazilian Committee for Experimental Animals, and the institutional ethics committee on animal use of the University of São Paulo approved the study (number 121/2011).
AKI Model SCFA/Probiotics Treatment
Bilateral kidney IRI was performed as previously reported.41 SCFAs (pH 7.4, diluted in PBS) were administered individually in two intraperitoneal dosages (200 mg/kg), 30 minutes before ischemia and at the moment of reperfusion.
Renal Function
Serum creatinine was measured by the Jaffe modified method, and serum urea was measured using a Labtest Kit (Minas Gerais, Brazil) according to the manufacturer’s instructions.
Assessment of Apoptosis
Apoptotic cells (terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling) were detected through the Cell Death Detection Kit TMR Red (Roche Diagnostics GmbH, Mannheim, Germany).
Immunohistochemistry
All steps to perform immunohistochemistry were performed as reported.42 Details are provided in the full methods section (see Supplemental Material).
Flow Cytometry
Kidney tissue was processed as recently published.43 See the full methods section (Supplemental Material).
Detection of Myeloperoxidase in Renal Tissue
Myeloperoxidase in renal tissue was estimated as previously described by Hillegass et al.44
BM-DC Generation/Maturation and Coculture
BM-DCs were generated as recently described.45 See the full methods section (Supplemental Material).
Global Methylation and Evaluation of Mitochondrial DNA
Global methylation was performed according to the method of Sharma et al.46 See the full methods section (Supplemental Material).
For detailed information about the other assays performed in this manuscript, see full methods section (Supplemental Material).
Statistical Analyses
The data are presented as mean and SD. A t test and ANOVA (with Bonferroni post-test) were used for comparisons between two and three or more groups, respectively. P<0.05 was considered to indicate a statistically significant difference. All graphs and statistical analyses were performed using Graph-Pad PRISM (GraphPad Software Inc., La Jolla, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Mario Cruz for technical assistance and Centro de Facilidades à Pesquisa (CEFAP/USP).
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant numbers 2011/01016-2 and 12/02270-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Renal Immunopathogy Laboratory CNPq/Inserm and Complex Fluids INCT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Gut Feeling in AKI: The Long Arm of Short–Chain Fatty Acids,” on pages 1755–1757.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030288/-/DCSupplemental.
References
- 1.Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Singbartl K, Kellum JA: AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 81: 819–825, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Jang HR, Ko GJ, Wasowska BA, Rabb H: The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 87: 859–864, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD: Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H: B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 7.de Paiva VN, Monteiro RM, Marques VP, Cenedeze MA, Teixeira VP, dos Reis MA, Pacheco-Silva A, Câmara NO: Critical involvement of Th1-related cytokines in renal injuries induced by ischemia and reperfusion. Int Immunopharmacol 9: 668–672, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Monteiro RM, Camara NO, Rodrigues MM, Tzelepis F, Damião MJ, Cenedeze MA, Teixeira VP, dos Reis MA, Pacheco-Silva A: A role for regulatory T cells in renal acute kidney injury. Transpl Immunol 21: 50–55, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Anders HJ, Andersen K, Stecher B: The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 83: 1010–1016, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Vitetta L, Gobe G: Uremia and chronic kidney disease: The role of the gut microflora and therapies with pro- and prebiotics. Mol Nutr Food Res 57: 824–832, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Vitetta L, Linnane AW, Gobe GC: From the gastrointestinal tract (GIT) to the kidneys: Live bacterial cultures (probiotics) mediating reductions of uremic toxin levels via free radical signaling. Toxins (Basel) 5: 2042–2057, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramezani A, Raj DS: The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ: Colonic health: Fermentation and short chain fatty acids. J Clin Gastroenterol 40: 235–243, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Pomare EW, Branch WJ, Cummings JH: Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest 75: 1448–1454, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR: Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ: Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Vinolo MA, Rodrigues HG, Festuccia WT, Crisma AR, Alves VS, Martins AR, Amaral CL, Fiamoncini J, Hirabara SM, Sato FT, Fock RA, Malheiros G, dos Santos MF, Curi R: Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab 303: E272–E282, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ: The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M: Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R: Regulation of inflammation by short chain fatty acids. Nutrients 3: 858–876, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machado RA, Constantino LS, Tomasi CD, Rojas HA, Vuolo FS, Vitto MF, Cesconetto PA, de Souza CT, Ritter C, Dal-Pizzol F: Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transplant 27: 3136–3140, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Zhang B, Hong X, Zhang X, Kong X: Histone deacetylase inhibitor, sodium butyrate, attenuates gentamicin-induced nephrotoxicity by increasing prohibitin protein expression in rats. Eur J Pharmacol 707: 147–154, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT: Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z: Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y, Chen Y, Jiang H, Nie D: Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ 18: 602–618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L: The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91–119, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS: The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoyama M, Kotani J, Usami M: Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26: 653–661, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G: The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 4: 1829, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson NE, Kotarsky K, Owman C, Olde B: Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun 303: 1047–1052, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V: Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 285: 27601–27608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nascimento CR, Freire-de-Lima CG, da Silva de Oliveira A, Rumjanek FD, Rumjanek VM: The short chain fatty acid sodium butyrate regulates the induction of CD1a in developing dendritic cells. Immunobiology 216: 275–284, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Morinobu A, Horiuchi M, Liu J, Kumagai S: Butyrate inhibits functional differentiation of human monocyte-derived dendritic cells. Cell Immunol 253: 54–58, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Sonveaux P, Copetti T, De Saedeleer CJ, Végran F, Verrax J, Kennedy KM, Moon EJ, Dhup S, Danhier P, Frérart F, Gallez B, Ribeiro A, Michiels C, Dewhirst MW, Feron O: Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 7: e33418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ: Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 110: 4410–4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H: Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H: Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol 297: F1457–F1465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maloy KJ, Powrie F: Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298–306, 2011 [DOI] [PubMed] [Google Scholar]
- 40.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ: Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correa-Costa M, Azevedo H, Amano MT, Gonçalves GM, Hyane MI, Cenedeze MA, Renesto PG, Pacheco-Silva A, Moreira-Filho CA, Câmara NO: Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS ONE 7: e49569, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braga TT, Correa-Costa M, Guise YF, Castoldi A, de Oliveira CD, Hyane MI, Cenedeze MA, Teixeira SA, Muscara MN, Perez KR, Cuccovia IM, Pacheco-Silva A, Gonçalves GM, Camara NO: MyD88 signaling pathway is involved in renal fibrosis by favoring a TH2 immune response and activating alternative M2 macrophages. Mol Med 18: 1231–1239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castoldi A, Braga TT, Correa-Costa M, Aguiar CF, Bassi ÊJ, Correa-Silva R, Elias RM, Salvador F, Moraes-Vieira PM, Cenedeze MA, Reis MA, Hiyane MI, Pacheco-Silva Á, Gonçalves GM, Saraiva Câmara NO: TLR2, TLR4 and the MYD88 signaling pathway are crucial for neutrophil migration in acute kidney injury induced by sepsis. PLoS ONE 7: e37584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C: Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods 24: 285–295, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Moraes-Vieira PM, Larocca RA, Bassi EJ, Peron JP, Andrade-Oliveira V, Wasinski F, Araujo R, Thornley T, Quintana FJ, Basso AS, Strom TB, Câmara NO: Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur J Immunol 44: 794–806, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S, De Carvalho DD, Jeong S, Jones PA, Liang G: Nucleosomes containing methylated DNA stabilize DNA methyltransferases 3A/3B and ensure faithful epigenetic inheritance. PLoS Genet 7: e1001286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.