Abstract
The role of Foxp3+ regulatory T cells (Tregs) in operational tolerance remains elusive, as initial results revealed an increased frequency of this subset in tolerant patients but no functional differences compared with immunosuppressed recipients. In addition, recent studies of regulatory B cells strongly suggest that Tregs may not have a central role in kidney transplantation tolerance. However, recent investigations of the crucial role of Foxp3 demethylation in Treg function and the possibility of identifying distinct Foxp3 T cell subsets prompted us to more thoroughly characterize Tregs in operationally tolerant patients. Thus, we studied the level of demethylation of the Foxp3 Treg-specific demethylated region (TSDR) in circulating CD4+ T cells and analyzed Treg subset frequency in tolerant patients, healthy volunteers, patients with stable graft function under immunosuppression, and chronically rejecting recipients. We observed a higher proportion of CD4+ T cells with demethylated Foxp3 and a specific expansion of CD4+ CD45RA− Foxp3hi memory Tregs exclusively in tolerant patients. The memory Tregs of tolerant recipients exhibited increased Foxp3 TSDR demethylation, expressed higher levels of CD39 and glucocorticoid-induced TNF-related receptor, and harbored greater suppressive properties than memory Tregs from patients with stable graft function. Taken together, our data demonstrate that operationally tolerant patients mobilize an array of potentially suppressive cells, including not only regulatory B cells but also Tregs. Our results also indicate that tolerant patients have potent CD4+CD45RA− Foxp3hi memory Tregs with a specific Foxp3 TSDR demethylation pattern, which may contribute to the maintenance of graft tolerance.
Keywords: kidney transplantation, immunology, pathology, tolerance
Transplantation tolerance is defined as the maintenance of stable allograft function in the absence of immunosuppressive therapy. An increasing number of “operationally tolerant patients” have been described in the literature, demonstrating that this state exists in humans.1–3 Analyses of these patients suggest the potential involvement of multiple cell subsets, including B and T cell subsets, in the development and/or maintenance of tolerance. We and others previously reported an increased number of B cells4 and the overexpression of numerous genes related to the proliferation, maturation, and differentiation of these cells.5–8 In parallel, some studies, including ours, also suggest a potential role for regulatory T cells (Tregs) in these patients, with overexpression of several genes associated with regulatory functions.6,8–10 In particular, stable patients and recipients with ongoing chronic rejection display lower levels and proportions of CD4+ CD25+ Foxp3+ T cells compared with operationally tolerant patients and healthy volunteers.9,11 However, when polyclonally stimulated, Tregs from tolerant patients failed to exhibit an increase in suppressive properties compared with Tregs from other patients.9 Thus, although the potency of Tregs for the prevention of graft rejection and the induction of transplant tolerance has been clearly demonstrated in experimental models,12–14 the role of these cells in the clinical setting is yet to be precisely defined.
Despite the central role of Foxp3 in Treg homeostasis, recent data demonstrated that the expression of this marker is not sufficient to generate stable and functional Tregs.15–17 In fact, the epigenetic status of the Foxp3 gene locus is closely linked to the differentiation and stability of Tregs.18 Epigenetic modifications, including histone modifications, chromatin interactions, and DNA methylations, play important roles in Treg cell differentiation.18,19 Mounting evidence demonstrates that specific DNA demethylation of the Treg-specific demethylated region (TSDR) and chromatin modifications of the Foxp3 gene occur in Tregs.20,21 Demethylation of the Foxp3 region is not only important for the induction and stabilization of Foxp3 expression but also ensures the differentiation and suppressive function of Tregs.22,23 In this article, we characterize the profile of Tregs in circulating blood from operationally tolerant patients and investigate the Foxp3 demethylation level of these cells in conjunction with their suppressive properties. To our knowledge, this study is the first report of an enhanced frequency of CD45RA− Foxp3hi memory Tregs (mTregs) expressing CD39 and GITR and exhibiting increased Foxp3 TSDR demethylation and stronger suppressive properties in operationally tolerant patients.
Results
Clinical Characteristics of Transplant Patients
Three groups of transplant recipients were included in the study: 13 operationally tolerant (TOL) patients, 33 patients with stable graft function under immunosuppression (STA), and 19 patients with symptoms of chronic rejection (RC). In addition, 15 healthy volunteers (HVs) were included as a control (See “patients” in the Material and Methods section). TOL is defined as patients with stable kidney graft function (i.e., creatinemia <150 mmol/L and proteinuria <1 g/24 h) in the absence of immunosuppression for at least 1 year (Supplemental Table 1). All patients were matched for sex and age to obtain homogenous groups (Table 1). A well functioning graft is defined by stable levels of creatinine <150 µM/L and proteinuria <1 g/24 h for at least 3 years. Immunosuppressive treatment was stopped in TOL patients due to noncompliance (n=8), post-transplant lymphoproliferative disorder (n=3), calcineurin inhibitor toxicity (n=1), and meningoencephalitis (n=1). RC recipients had creatinemia >150 µM/L and/or proteinuria >1 g/24 h and were histopathologically and serologically classified according to the updated Banff classification criteria.24,25 In addition, 17 of 19 patients exhibited graft glomerulopathy, with 11 RC patients exhibiting positive C4d staining and 4 patients exhibiting anti-donor–specific antibodies (Table 1). Apart from two TOL patients, all renal grafts had been obtained from cadaveric donors. No significant differences in cancer, infection, HLA mismatches, and the number of acute rejection episodes were found among the three groups of patients. All clinical parameters are provided in Table 1.
Table 1.
Demographic and clinical characteristics of the individuals and patients enrolled in this study
| Demographic and Transplant Characteristics | Group | P Value | |||
|---|---|---|---|---|---|
| HV | TOL | STA | RC | ||
| Participants, n | 15 | 13 | 33 | 19 | |
| Donor type, n | |||||
| Cadaveric | — | 13 | 33 | 19 | |
| Living | — | 2 | — | — | |
| Recipient age, yr | 41 (37–45) | 55 (40–84) | 51 (18–73) | 51 (25–80) | 0.88 |
| Sex | 0.33 | ||||
| Women | 4 | 6 | 16 | 11 | |
| Men | 11 | 7 | 17 | 8 | |
| Time after transplantation, mo | — | 226 (114–347) | 129 (67–285) | 89 (26–241) | <0.001 |
| Creatinine level, μmol/L | — | 105 (63–216) | 107 (80–214) | 187 (109–957) | <0.001 |
| Proteinuria level, g/24 h | — | 0.2 (0–1.69) | 0.13 (0.03–0.39) | 1.6 (0.2–4.61) | <0.001 |
| HLA mismatch | — | 3 (0–5) | 3 (0–6) | 4 (0–6) | 0.23 |
| Cytomegalovirus infection | — | 6 (46.1) | 9 (27.3) | 4 (21.05) | 0.38 |
| Cancer | — | 5 (36.4) | 9 (31.0) | 3 (15.8) | 0.32 |
| Time of immunosuppression cessation, yr | — | 8 (2–15) | — | — | |
| Episode of acute rejection | — | 2 (15.4) | 5 (15.2) | 2 (10.5) | 0.88 |
| Reason for cessation | |||||
| Medical condition | — | 5 (38.5) | — | — | |
| Noncompliance | — | 8 (61.5) | — | — | |
| Chronic rejection | |||||
| C4d staining | — | — | — | 11 (57.9) | |
| Graft glomerulopathy | — | — | — | 17 (89.5) | |
| Donor-specific antibodies, n | — | 2 (15.4) | — | 4 (21.1) | |
| Immunosuppressive therapy | |||||
| Calcineurin inhibitor | — | 33 | 16 | ||
| Mycophenolate mofetil/mycophenolate sodium | — | 9 | 9 | ||
| Corticotherapy | — | 11 | 10 | ||
| Azathioprine | — | 8 | — | ||
Data are given as the median (range) or n (%), unless otherwise indicated.
CD4+ T Cells from Tolerant Patients Have Higher Levels of DNA Demethylation in the Treg-Specific Foxp3 Region
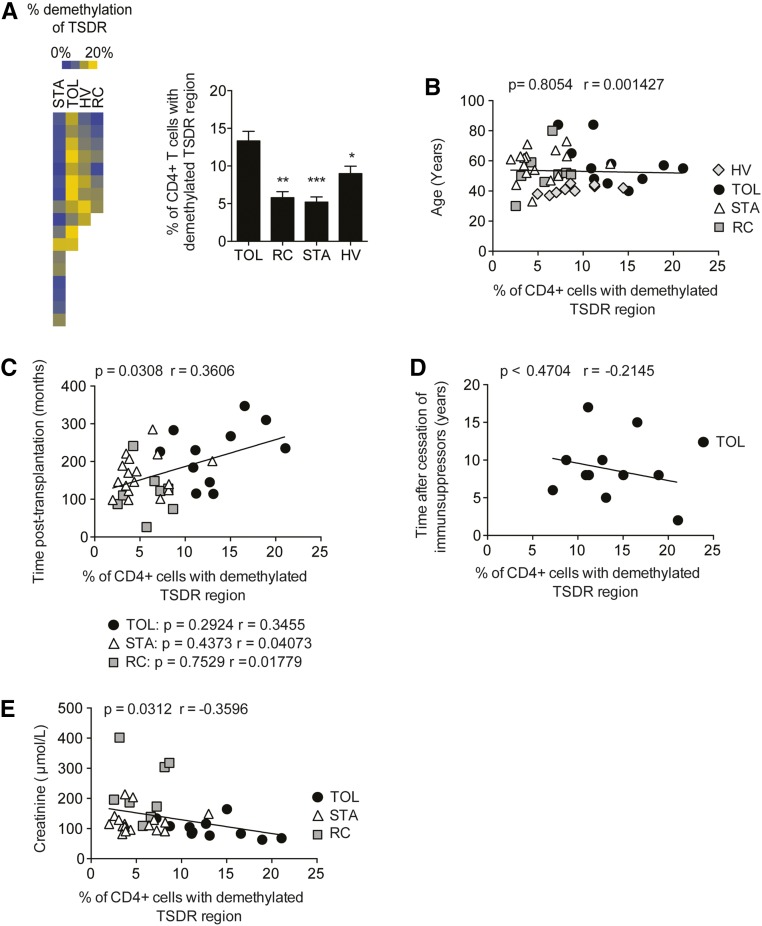
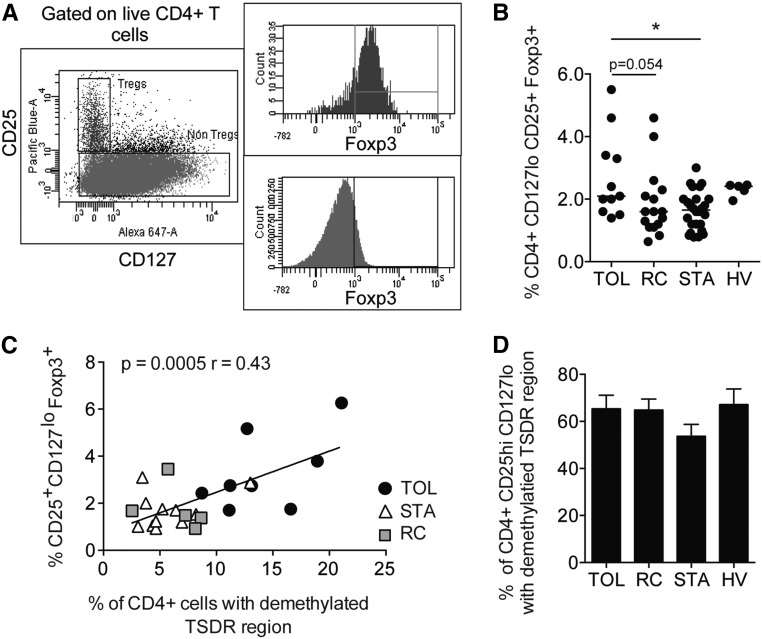
Because Foxp3 demethylation is correlated with the function of Tregs, we analyzed Foxp3 TSDR demethylation in purified CD4+ T cells from transplanted patients and HVs. The percentage of CD4+ T cells with Foxp3 demethylation was significantly higher in the TOL group (n=11; 13.33%±1.276%) compared with the STA (n=17; 5.21%±0.677%; P<0.001), RC (n=8; 5.788%±0.806%; P<0.01), and HV (n=9; 8.996%±0.978%; P<0.05) groups (Figure 1A). To investigate whether Foxp3 demethylation levels were age dependent, we analyzed the percentage of CD4+ cells with a demethylated TSDR according to the age of the four groups of patients. No correlation was found between age, time after transplantation, and demethylation of the Foxp3 region in our groups (Figure 1B). Interestingly, no correlation was found between the percentage of CD4+ T cells with a demethylated TSDR region and thelength of time since the cessation of immunosuppressors, showing that the percentage of CD4+ with a demethylated TSDR does not result from the reduced immunosuppression that occurs in patients who are further post-transplant (Figure 1C). The level of Foxp3 demethylation inversely correlated with the level of creatinemia (P=0.03, r=−0.36), suggesting a close link between graft integrity and Treg function (Figure 1D). Because Tregs have been defined primarily by the expression of CD25, CD127, and Foxp3, we then quantified the frequency of circulating Tregs based on the expression of these markers.26 A higher proportion of CD4+ CD25+ CD127lo Foxp3+ Tregs was found in the blood of TOL patients (3.11%±0.47%) compared with STA patients (1.88%±0.14%; P<0.05) (Figure 2A). A similar trend was observed upon comparison with RC samples, but this difference was not statistically significant (P=0.05). Finally, the total CD4+ CD25+ CD127lo Treg frequency in all transplant recipients correlated with the Foxp3 demethylation observed in circulating CD4+ cells (Figure 2B). Because of this correlation, we investigated the TSDR demethylation status of Foxp3 in FACS-sorted CD4+ CD25+ CD127lo T cells. No difference in Foxp3 demethylation was found between the three groups of patients and the HV group (Figure 2C).
Figure 1.
Operational tolerance is associated with a higher percentage of CD4+ T cells with demethylated Foxp3. (A) Purified circulating CD4+ T cells from the HV (n=9), TOL (n=11), STA (n=17), and RC (n=8) groups are subjected to quantitative PCR to determine the percentage of TSDR demethylation. (B) Correlation between the percentage of TSDR demethylation and age. (C) Correlation between the percentage of TSDR demethylation and time after transplantation. (D) Correlation between the percentage of TSDR demethylation and time after the cessation of immunosuppressors. (E) Correlation between the percentage of TSDR demethylation and levels of creatinine (E). *P<0.05; **P<0.01; ***P<0.001.
Figure 2.
Study of CD25hi CD127low Tregs. (A) Gating strategy for the identification of total Tregs. (B) Percentage of Tregs in the blood of individuals from the HV (n=5), TOL (n=10), STA (n=26), and RC (n=15) groups. (C) Correlation between the percentage of TSDR demethylation in CD4+ T cells and the percentage of total Tregs in the TOL (n=8), STA (n=11), and RC (n=5) groups. (D) Percentage of purified circulating CD25hi CD127low Tregs with TSDR demethylation, as measured using quantitative PCR in the TOL (n=8), STA (n=11), and RC (n=5) groups (D). *P<0.05.
Operationally Tolerant Patients Have a Significantly Higher Level of Circulating CD4+ CD45RA− Foxp3hi mTregs
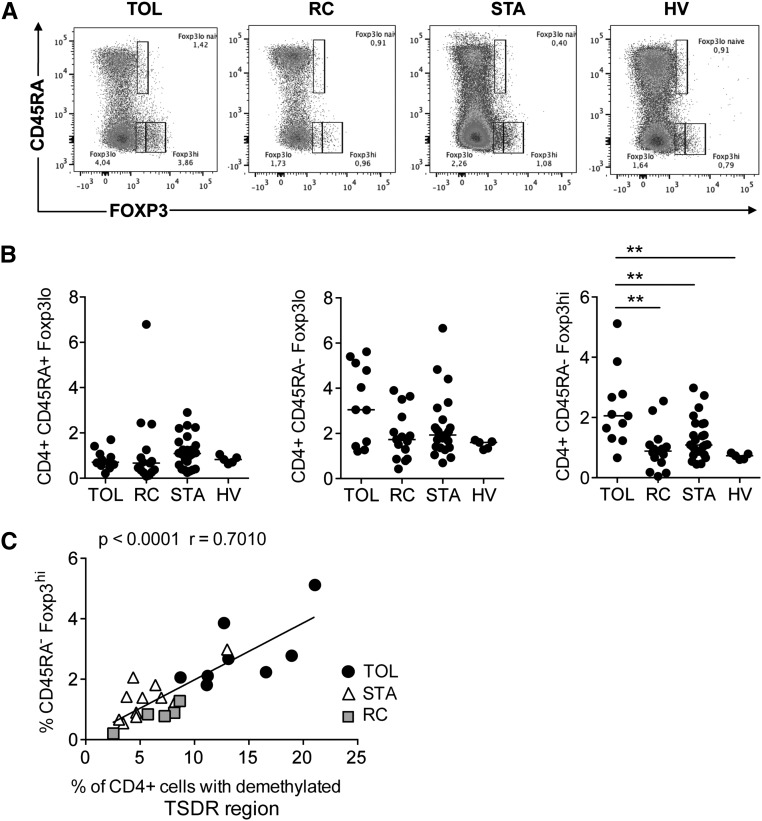
Many studies have attempted to identify distinct Treg subsets based on their phenotypic and functional properties.16,27,28 On the basis of the expression of CD45RA, CD25, CD127, and Foxp3, one can distinguish CD45RA+ Foxp3lo CD25+ CD127lo naive Tregs, CD45RA− Foxp3hi CD25hi CD127lo mTregs, and CD45RA− Foxp3lo CD25+ CD127+ non-Tregs16 (Supplemental Figure 1, A and B). Each population has been reported to exhibit specific Foxp3 epigenetic modifications and functional properties.16 To investigate whether differences in Treg subsets existed in our cohort of patients, we quantified these populations using flow cytometry. This approach revealed a significantly higher frequency of CD45RA− Foxp3hi mTregs in the TOL group (Figure 3, A and B). Interestingly, the percentage of mTregs strongly correlates with the percentage of CD4+ T cells with demethylated Foxp3 in our patients (P<0.001, r=0.70) (Figure 3C), suggesting a potential role of this subset in operational tolerance.
Figure 3.
CD45RA− Foxp3hi mTregs are significantly expanded in the blood of TOL patients. (A and B) Quantification of Treg subsets in the blood of individuals from the HV (n=5), TOL (n=10), STA (n=26), and RC (n=15) groups. (C) Correlation between the percentage of TSDR demethylation in CD4+ T cells and the percentage of CD45RA+ Foxp3+ mTregs in the TOL (n=8), STA (n=11), and RC (n=5) groups. **P<0.01.
Circulating mTregs from Tolerant Recipients Express Higher Levels of CD39 GITR and Exhibit Higher Foxp3 Demethylation Levels and Greater Suppressive Function
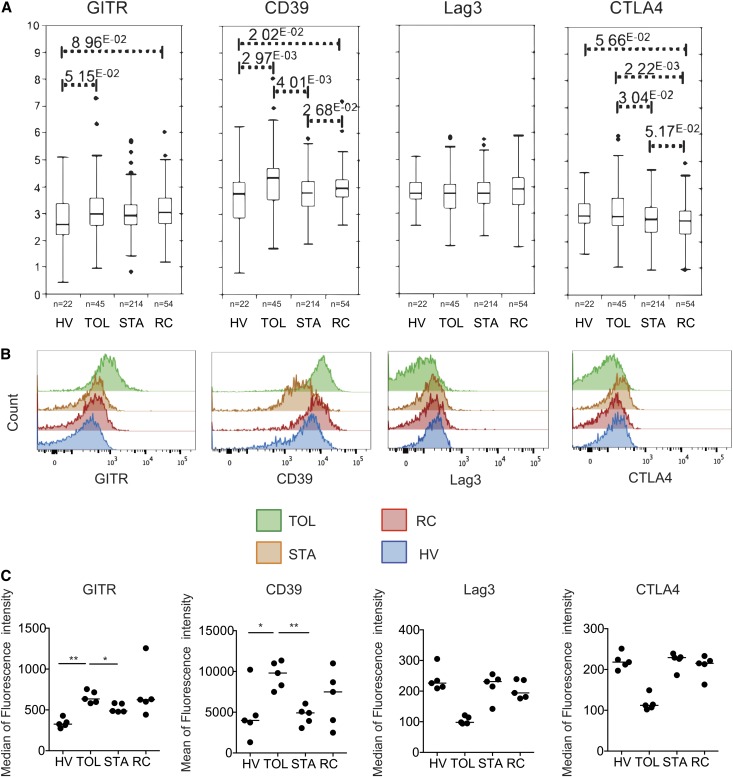
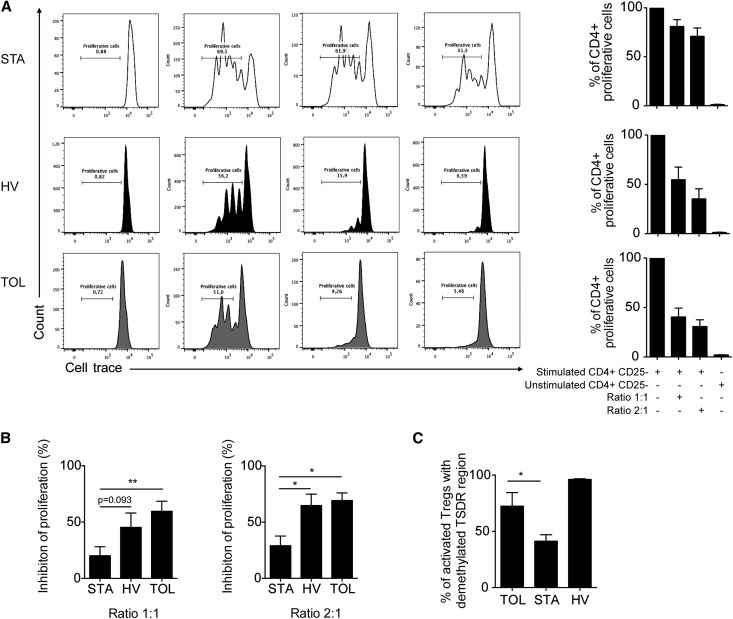
Given the greater proportion of mTregs in TOL patients and the correlation of this subset with Foxp3 demethylation in the total CD4+ T cell population, we characterized this Treg subset in more detail in transplant recipients. As a first step, we studied the expression of four Treg molecules that were previously reported to be overexpressed on activated mTregs: GITR, Lag3, CD39, and CTLA4.29 A meta-analysis of our previous transcriptomic studies6,30 reveals higher expression of CTLA4, CD39, and GITR in the blood of the TOL group compared with the other groups (Figure 4A). Consistent with this finding, we used flow cytometry to confirm the overexpression of CD39 and GITR on the surface of mTregs from TOL patients compared with the STA and HV groups (Figure 4, B and C). In a second step, we assessed the in vitro suppressive potency of these cells. MTregs were purified and sorted based on their CD4+ CD45RA− CD25hi phenotypes16 (Supplemental Figure 1B). We then measured the extent of cell trace dilution among FACS-sorted labeled CD4+ CD25− effector T cells that were cocultured with CD4+ CD45RA− CD25hi mTregs (at ratios of 1:1 and 2:1) and stimulated for 4 days with coated anti-CD3 in the presence of feeder cells. We found that at both ratios, CD4+ CD45RA− CD25hi mTregs from the TOL and HV groups were highly suppressive; by contrast, these cells were not suppressive and were unable to control CD4+ CD25− effector T cell proliferation in the STA group (Figure 5, A and B). In addition, increased demethylation of the Foxp3 locus was observed in mTregs from TOL patients (72.4%±12.1%) compared with STA patients (36.3%±7.1%) (P<0.05) (Figure 5C). Collectively, these data demonstrate that mTregs from TOL patients are fully functional and overexpress CD39 and GITR. These characteristics are linked to a higher level of demethylation at the Foxp3 locus. By contrast, these cells are nonfunctional in STA patients.
Figure 4.
Expression of Treg-related molecules in TOL recipients. (A) Expression of CD39, GITR, CTLA4, and Lag3 in PBMCs from the TOL, HV, STA, and RC groups. The data are obtained from a meta-analysis of previous microarray studies. (B and C) Expression of CD39, GITR, CTLA4, and Lag3 on the surface of circulating mTregs from the TOL, HV, STA, and RC groups. *P<0.05; **P<0.01.
Figure 5.
mTregs from TOL are fully functional. (A) Coculture of mTregs with activated trace-labeled CD4+ CD25– T responder cells in the presence of feeders at the indicated suppressor/responder cell ratios. (B) Percentage of inhibition of T cell proliferation in the TOL (n=6), HV (n=6), and STA (n=6) groups. (C) Percentage of TSDR demethylation in CD25hi CD45RA− mTregs from the HV (n=3), TOL (n=4), and STA (n=14) groups (D). *P<0.05; **P<0.01.
Discussion
The identification of mechanisms of tolerance in stable graft recipients would significantly improve long-term clinical outcomes by reducing immunosuppressive drug comorbidity.31,32 Although the phenomenon of operational tolerance offers a unique model for the regulation of the immune response in humans, the mechanisms responsible for the maintenance of tolerance in these patients remain unclear. We and others previously demonstrated the possible involvement of regulatory cells, including regulatory B cells (Bregs)33 and Tregs,4,8,9,34 in this process. We initially reported a greater number of circulating CD4+ CD25hi Foxp3+ cells in blood from patients with operational tolerance,9 and previous transcriptomic studies highlighted higher mRNA levels of Foxp3 in the blood, grafts, and urine of tolerant patients compared with other transplant recipients and with HVs.6,7,35 In accordance with these findings, lower frequencies of Tregs have been correlated with poor graft outcomes.4,11,34,36 Despite accumulating data from various experimental models, the present clinical results are insufficient to prove that Tregs play a role in the maintenance of tolerance in operationally tolerant recipients. In this article, we report on increased Foxp3 demethylation and a higher frequency of circulating mTregs in the blood of tolerant recipients compared with nontolerant equivalents and healthy controls. Moreover, we provide the first evidence, to our knowledge, that operationally tolerant patients harbor mTregs with higher suppressive function compared with other transplant patients. Consequently, our data provide a better understanding of operational tolerance by shedding light on the potent role of mTregs in long-term graft survival.
Although Foxp3 controls differentiation and function of Tregs,37–39 recent evidence suggests that the expression of Foxp3 alone may not be sufficient to maintain the suppressive function of Tregs over time.15,16,40 Other factors, such as DNA demethylation at the Foxp3 gene locus, have been reported to be crucial for the stability and function of Tregs.18 Interestingly, we report here that operationally tolerant patients have significantly higher levels of CD4+ T cells with demethylated Foxp3 compared with nontolerant transplant recipients and HVs, confirming that this feature is not treatment dependent but is specifically associated with operational tolerance. This finding is confirmed by the observed correlation between graft function (assessed based on levels of creatinemia) and the percentage of demethylated TSDR in CD4+ T cells from operationally tolerant recipients. Accordingly, high levels of TSDR in CD4+ T cells from the kidney graft were associated with a favorable long-term graft outcome.41 We also found a higher frequency of total circulating CD4+ CD25+ Foxp3+ CD127lo cells in the blood of tolerant recipients. However, Foxp3 demethylation was similar in the overall Treg population in all groups of patients and the HVs. This finding may explain why previous studies of Tregs from operationally tolerant recipients failed to indicate a stronger suppressive function for these cells in vitro.9 These studies, including ours, were essentially based on an overall characterization of Tregs using a consensual phenotype that was based primarily on higher expression of CD25 and lower expression of CD127.8,9,11 This phenotype is likely overly simplistic considering the known heterogeneity and plasticity of Tregs.16,28,42
A recent study identified three distinct Foxp3-expressing T cell subsets in humans.16 We can distinguish distinct memory CD4+ CD45RA− FOXP3hi and naïve CD4+ CD45RA+ FOXP3lo Treg cell subsets with strong suppressive functions, as well as nonsuppressive CD4+ CD45RA− FOXP3lo T cells that can secrete inflammatory cytokines after polyclonal activation. The suppressive activities of mTregs are positively correlated with the DNA methylation status of the Foxp3 locus.16 Consistently, our data demonstrate that the percentage of CD4+ T cells with demethylated Foxp3 is strongly correlated with the level of mTregs in transplant patients. Interestingly, this Treg subset is increased in the blood of tolerant recipients but not other groups. In addition, our investigations report on higher levels of Foxp3 demethylation and stronger suppressive functions of mTregs from tolerant recipients and HVs compared with stable patients. This difference with stable patients could result from the effect of immunosuppressive therapy on Tregs. However, studies describing the effects of immunosuppression on these cells are contradictory.41,43 Whereas high doses of calcineurin inhibitors appear to alter TSDR demethylation and Treg function in pediatric liver recipients,43 the presence of demethylated Foxp3+ Tregs in kidney recipients with subclinical rejection (even with interstitial fibrosis and tubular atrophy) is associated with a favorable long-term allograft outcome, independent of immunosuppressive therapy.41 This second result is consistent with our findings. Moreover, the fact that mTregs are specifically increased in TOL patients and not in HVs suggests that the generation and expansion of alloantigen-specific mTregs occur in TOL patients. This finding may explain why the mTreg frequencies of these patients are higher than those observed in HVs, whereas the suppressive functions of these cells are similar on a per-cell level. This finding is of special interest for graft tolerance, given the therapeutic potential of Tregs in transplantation.44,45
Our work also demonstrates that phenotypic, functional, and epigenetic investigations of Tregs are complementary to the evaluation of the role of these cells in immunopathology. A recent comprehensive genome-wide study in humans revealed that mTregs are also characterized by a specific signature that consists of upregulated genes involved in immune regulation, such as GITR, CTLA-4, CD39, TGF-β1, LAG3, and IL-10.29 Some of these genes were previously reported to be upregulated in blood from operationally tolerant patients, reinforcing our observations.6 We found increased expression of GITR and CD39 on the surface of mTregs from TOL patients compared with STA patients and HVs. This result is consistent with the growing body of evidence that demonstrates the role of CD39 in immunologic tolerance46 and in control of inflammation and graft rejection.47,48 Indeed, CD39+ Tregs have been demonstrated to catalyze the cleavage of ATP to AMP, which is then further cleaved to adenosine, leading to the control of T cell activation.49 In combination with our findings, these data suggest a potential role for CD39+ Tregs in transplantation tolerance.
Although an overall understanding of operational tolerance remains to be elaborated, we know that operationally tolerant patients display a specific transcriptomic and cellular signature that is biased toward Tregs and Bregs with inhibitory transcriptional and phenotypic profiles.6–8,10 This finding suggests that tolerance may arise from the action of multiple mechanisms that work in concert and lead to the emergence of well functioning suppressive cells. In this article, we demonstrated that mTregs may play a role in this complex system of regulation. Foxp3 expression, Foxp3 demethylation, and Treg function are critically affected by many mechanisms.18 For example, the duration of T-cell receptor stimulation is instrumental for Treg DNA demethylation.23 In addition, other signals favor the stabilization and the suppressive phenotype of Tregs,18 such as TGF-β, which is known to induce suppressive T cells in vitro.50 However, TGF-β–induced Tregs are not stable15,23 and fail to induce complete demethylation of the Foxp3 locus.23 However, continuous exposure to TGF-β in conjunction with T-cell receptor stimulation can progressively induce complete Foxp3 demethylation in Tregs in vivo.23 Consequently, one could hypothesize that continuous exposure to alloantigens in a pro-TGF-β environment could contribute to the generation of in vivo antigen-specific Tregs in operationally tolerant patients. Interestingly, we previously reported that >40% of the differential expression of genes that is observed in blood from tolerant patients is dependent on the TGF-β pathway.6 Moreover, recent data demonstrate that immune cells from tolerant patients exhibit the strongest suppressive responses in a delayed-type hypersensitivity model and that the regulatory mechanism is TGF-β dependent.51 Finally, B cells are also potential candidates for involvement in transplantation tolerance.33 The recent description of Bregs that can induce, maintain, and expand Tregs strengthens this idea.52 A link between Bregs and Tregs in transplantation tolerance was suggested in recent work.51 All of these mechanisms could be responsible for an amplification loop that leads to the stabilization of Tregs through the continuous maintenance of Foxp3 demethylation.
In summary, these data reveal new findings concerning Foxp3 epigenetic modifications in Tregs that exhibit a specific phenotype and immune regulatory properties in patients with operational tolerance. How these factors and extracellular stimuli interact with each other to maintain functional Tregs during transplantation tolerance remains to be established. Undoubtedly, understanding the mechanisms that regulate the stability of Tregs and their epigenetic modifications will allow the identification of new techniques for promoting Treg function and tolerance in organ transplant recipients.
Concise Methods
Patients and Study Cohorts
The University Hospital Ethical Committee of Nantes and the Committee for the Protection of Patients from Biologic Risks approved this study. All patients who participated in this study gave informed consent. A total of 65 age-matched kidney transplant patients were included in the study, and a summary of the clinical data is presented in Table 1. The criteria for operational tolerance were described in detail elsewhere.1 TOL is defined as patients with stable kidney graft function (i.e., creatinemia <150 mmol/L and proteinuria <1 g/24 h) in the absence of immunosuppression for at least 1 year.
Antibodies, Flow Cytometry, and Cell Sorting
The following antibodies and reagents were used for flow cytometry and cell sorting: CD45-PO (Caltag; Invitrogen, Darmstadt, Germany); CD3-Alexa Fluor 700, anti-CD45-PE, and CD4-PB (both from BD Biosciences, Heidelberg, Germany); CD3 Brillant Violet 605, CD4 APC-Cy7, CD45RA-PeCy7, CCR7-PE, CD25 Brillant Violet 421, and CD127-Alexa Fluor 647 (Biolegend, Inc., San Diego, CA); Foxp3-PercpCy5.5, GITR-APC, CD39-APC, Lag3-Pe, and CTLA4-Pe (eBioscience, San Diego, CA); anti-CD4-FITC (Miltenyi Biotec, Bergisch Gladbach, Germany); and anti-CD25-Alexa Fluor 647 (anti-CD25 from Immunotech, Marseille, France).
Foxp3 TSDR Quantification
Genomic DNA was isolated from PBMC samples, purified CD4+ T cells and FACS-sorted Tregs using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Real-time PCR was performed to quantify the percentage of the Foxp3 TSDR, as described elsewhere.53
Suppression Assay
For this assay, 96-well round-bottom plates were coated with 0.5 µg/ml of anti-CD3 (OKT3 mAb), and 1×105 CD4− irradiated feeder cells were subsequently added to the plates. CD4+CD25− responder T cells were labeled with Cell Trace Violet (Invitrogen, Cergy Pontoise, France) according to the manufacturer’s instructions, and 1×104 cells were seeded. Suppressive activity was assessed by the addition of 1×104 or 2×104 CD4+ CD25hi CD45RA− unlabeled mTregs per well. After 96 hours of culture, the cells were stained with the anti-CD4-FITC antibody, and the proliferation of CD4+ Cell Trace Violet–labeled cells was assessed via flow cytometry using an LSRII cytometer and FACSDiva software (BD Biosciences, Mountain View, CA).
Statistical Analyses
We compared patient group characteristics using chi-squared testing as appropriate. The nonparametric Kruskal–Wallis test was used for comparisons of >2 groups. Differences were defined as statistically significant when P<0.05, P<0.01, and P<0.001. Correlations were analyzed using linear regression. All statistical analysis was performed using GraphPad Prism software (version 6).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the participating patients and their families, whose trust, support, and cooperation were essential for the collection of the data used in this study. We also thank Drs. G. Blancho, L. Braun, D. Cantarovitch, R. Crochette, J. Dantal, M. Hourmant, B. Hurault de Ligny, H. Janbon, G. Lefrancois, C. Legendre, B. Le Mauff, H. Le Monies De Sagazan, A. Meurette, M.-C. Moal, C. Noel, M. Rabant, J.F. Subra, J. Sayegh, A. Testa, F. Villemain, and J. Zuber for their help in this study. Finally, we thank the Cytocell Cytometry Facility in Nantes for expert technical assistance.
This work was supported by grants from the French Research Ministry (to F.B.), the Centaure Foundation, the French Agency of Biomedicine, and the European Society of Transplantation. This research was also supported by a University Hospital Institute (IHU)-European Center for Transplantation and Immunotherapy Services (CESTI) project, which received French Government financial support managed by the National Research Agency via the “Investment in the Future” program (ANR-10-IBHU-005). The IHU-CESTI project is also supported by Nantes Metropole and the Pays de la Loire Region.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050480/-/DCSupplemental.
References
- 1.Brouard S, Pallier A, Renaudin K, Foucher Y, Danger R, Devys A, Cesbron A, Guillot-Guegen C, Ashton-Chess J, Le Roux S, Harb J, Roussey G, Subra JF, Villemain F, Legendre C, Bemelman FJ, Orlando G, Garnier A, Jambon H, Le Monies De Sagazan H, Braun L, Noël C, Pillebout E, Moal MC, Cantarell C, Hoitsma A, Ranbant M, Testa A, Soulillou JP, Giral M: The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant 12: 3296–3307, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Orlando G, Hematti P, Stratta RJ, Burke GW, 3rd, Di Cocco P, Pisani F, Soker S, Wood K: Clinical operational tolerance after renal transplantation: Current status and future challenges. Ann Surg 252: 915–928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roussey-Kesler G, Giral M, Moreau A, Subra JF, Legendre C, Noël C, Pillebout E, Brouard S, Soulillou JP: Clinical operational tolerance after kidney transplantation. Am J Transplant 6: 736–746, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Louis S, Braudeau C, Giral M, Dupont A, Moizant F, Robillard N, Moreau A, Soulillou JP, Brouard S: Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation 81: 398–407, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Pallier A, Hillion S, Danger R, Giral M, Racapé M, Degauque N, Dugast E, Ashton-Chess J, Pettré S, Lozano JJ, Bataille R, Devys A, Cesbron-Gautier A, Braudeau C, Larrose C, Soulillou JP, Brouard S: Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 78: 503–513, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Brouard S, Mansfield E, Braud C, Li L, Giral M, Hsieh SC, Baeten D, Zhang M, Ashton-Chess J, Braudeau C, Hsieh F, Dupont A, Pallier A, Moreau A, Louis S, Ruiz C, Salvatierra O, Soulillou JP, Sarwal M: Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A 104: 15448–15453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, Hernandez-Fuentes MP, Turka LA, Seyfert-Margolis VL, Immune Tolerance Network ST507 Study Group : Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120: 1836–1847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk HD, Soulillou JP, Hernandez-Fuentes MP, Lechler RI: Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120: 1848–1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braudeau C, Racape M, Giral M, Louis S, Moreau A, Berthelot L, Heslan M, Ashton-Chess J, Soulillou JP, Brouard S: Variation in numbers of CD4+CD25highFOXP3+ T cells with normal immuno-regulatory properties in long-term graft outcome. Transpl Int 20: 845–855, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Moraes-Vieira PM, Takenaka MC, Silva HM, Monteiro SM, Agena F, Lemos F, Saitovitch D, Kalil J, Coelho V: GATA3 and a dominant regulatory gene expression profile discriminate operational tolerance in human transplantation. Clin Immunol 142: 117–126, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Moraes-Vieira PM, Silva HM, Takenaka MC, Monteiro SM, Lemos F, Saitovitch D, Kalil J, Coelho V: Differential monocyte STAT6 activation and CD4(+)CD25(+)Foxp3(+) T cells in kidney operational tolerance transplanted individuals. Hum Immunol 71: 442–450, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Lin CY, Graca L, Cobbold SP, Waldmann H: Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol 3: 1208–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y: Regulatory T cells and organ transplantation. Semin Immunol 16: 119–126, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Graca L, Cobbold SP, Waldmann H: Identification of regulatory T cells in tolerated allografts. J Exp Med 195: 1641–1646, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C: Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity 27: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S: Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30: 899–911, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY: Foxp3-dependent programme of regulatory T-cell differentiation. Nature 445: 771–775, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ohkura N, Kitagawa Y, Sakaguchi S: Development and maintenance of regulatory T cells. Immunity 38: 414–423, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Morikawa H, Ohkura N, Vandenbon A, Itoh M, Nagao-Sato S, Kawaji H, Lassmann T, Carninci P, Hayashizaki Y, Forrest AR, Standley DM, Date H, Sakaguchi S, FANTOM Consortium : Differential roles of epigenetic changes and Foxp3 expression in regulatory T cell-specific transcriptional regulation. Proc Natl Acad Sci U S A 111: 5289–5294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J: DNA methylation controls Foxp3 gene expression. Eur J Immunol 38: 1654–1663, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY: Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toker A, Engelbert D, Garg G, Polansky JK, Floess S, Miyao T, Baron U, Düber S, Geffers R, Giehr P, Schallenberg S, Kretschmer K, Olek S, Walter J, Weiss S, Hori S, Hamann A, Huehn J: Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol 190: 3180–3188, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S: T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37: 785–799, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA: CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203: 1701–1711, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN: Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest 116: 2423–2433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M: A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest 115: 1953–1962, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidl C, Hansmann L, Lassmann T, Balwierz PJ, Kawaji H, Itoh M, Kawai J, Nagao-Sato S, Suzuki H, Andreesen R, Hayashizaki Y, Forrest AR, Carninci P, Hoffmann P, Edinger M, Rehli M, FANTOM consortium : The enhancer and promoter landscape of human regulatory and conventional T-cell subpopulations. Blood 123: e68–e78, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Braud C, Baeten D, Giral M, Pallier A, Ashton-Chess J, Braudeau C, Chevalier C, Lebars A, Léger J, Moreau A, Pechkova E, Nicolini C, Soulillou JP, Brouard S: Immunosuppressive drug-free operational immune tolerance in human kidney transplant recipients: Part I. Blood gene expression statistical analysis. J Cell Biochem 103: 1681–1692, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Braza F, Soulillou JP, Brouard S: Gene expression signature in transplantation tolerance. Clin Chim Acta 413: 1414–1418, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Dantal J, Soulillou JP: Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med 352: 1371–1373, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Chesneau M, Michel L, Degauque N, Brouard S: Regulatory B cells and tolerance in transplantation: from animal models to human. Front Immunol 4: 497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaier M, Seissler N, Schmitt E, Meuer S, Hug F, Zeier M, Steinborn A: DR(high+)CD45RA(-)-Tregs potentially affect the suppressive activity of the total Treg pool in renal transplant patients. PLoS ONE 7: e34208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker LE, de Oliveira Biazotto F, Conrad H, Schaier M, Kihm LP, Gross-Weissmann ML, Waldherr R, Bierhaus A, Nawroth PP, Zeier M, Morath C: Cellular infiltrates and NFκB subunit c-Rel signaling in kidney allografts of patients with clinical operational tolerance. Transplantation 94: 729–737, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Zuber J, Brodin-Sartorius A, Lapidus N, Patey N, Tosolini M, Candon S, Rabant M, Snanoudj R, Panterne C, Thervet E, Legendre C, Chatenoud L: FOXP3-enriched infiltrates associated with better outcome in renal allografts with inflamed fibrosis. Nephrol Dial Transplant 24: 3847–3854, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD: The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–21, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Hori S, Nomura T, Sakaguchi S: Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK: Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19: 345–354, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Bestard O, Cuñetti L, Cruzado JM, Lucia M, Valdez R, Olek S, Melilli E, Torras J, Mast R, Gomà M, Franquesa M, Grinyó JM: Intragraft regulatory T cells in protocol biopsies retain foxp3 demethylation and are protective biomarkers for kidney graft outcome. Am J Transplant 11: 2162–2172, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, Fazekas de Saint Groth B: Persistence of naive CD45RA+ regulatory T cells in adult life. Blood 107: 2830–2838, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Akimova T, Kamath BM, Goebel JW, Meyers KE, Rand EB, Hawkins A, Levine MH, Bucuvalas JC, Hancock WW: Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients. Am J Transplant 12: 3449–3461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood KJ, Bushell A, Hester J: Regulatory immune cells in transplantation. Nat Rev Immunol 12: 417–430, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, Aversa F, Martelli MF: Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117: 3921–3928, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Antonioli L, Pacher P, Vizi ES, Haskó G: CD39 and CD73 in immunity and inflammation. Trends Mol Med 19: 355–367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crikis S, Lu B, Murray-Segal LM, Selan C, Robson SC, D’Apice AJ, Nandurkar HH, Cowan PJ, Dwyer KM: Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant 10: 2586–2595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC: Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204: 1257–1265, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk K: Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 110: 1225–1232, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM: Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198: 1875–1886, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes LD, Jankowska-Gan E, Sheka A, Keller MR, Hernandez-Fuentes MP, Lechler RI, Seyfert-Margolis V, Turka LA, Newell KA, Burlingham WJ: Donor-specific indirect pathway analysis reveals a B-cell-independent signature which reflects outcomes in kidney transplant recipients. Am J Transplant 12: 640–648, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosser EC, Blair PA, Mauri C: Cellular targets of regulatory B cell-mediated suppression. Mol Immunol 62: 296–304, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, Baron U, Stauch D, Kotsch K, Pratschke J, Hamann A, Loddenkemper C, Stein H, Volk HD, Hoffmüller U, Grützkau A, Mustea A, Huehn J, Scheibenbogen C, Olek S: Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res 69: 599–608, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.