Abstract
Human peer relations provide tangible benefits including food and protection, as well as emotional benefits. While social exclusion poses a threat to all of these benefits, the psychological threat is particularly susceptible to modulation by the relation of the excluders to the excluded person. The current study used functional magnetic resonance imaging to explore the effects of manipulating the gender relation of participants to their excluders during an interactive ball toss game. Ventral anterior cingulate cortex activation was higher during exclusion by same-gender peers, while right ventrolateral prefrontal cortex activation negatively correlated with self-reported distress in other-gender exclusion. Results imply that exclusion by one’s own gender is fundamentally different from exclusion by the opposite gender, and suggest a regulatory role for ventrolateral prefrontal cortex in response to out-group exclusion. Individual differences in implicit gender attitudes modulated neural responses to exclusion. The importance of these findings to investigations of social cognition is discussed.
Keywords: Social Exclusion, Group Membership, Ventral ACC, Right ventrolateral PFC
Introduction
All social animals derive primary, physical benefits from social affiliations including protection, access to shared resources and access to health promoting social actions (e.g. grooming). Because ostracism threatens access to these primary benefits in humans as well as in other animals (Goodall, 1986; Lancaster, 1986; Williams, 2007), the ability to detect and respond to social exclusion is an evolutionarily important trait. However, humans also derive secondary, psychological benefits from inclusion. Being associated with a specific, valued peer group promotes positive self-regard, thus humans are motivated to build and maintain social ties for the physical and psychological benefits they bestow, and will suffer when these ties are threatened (Baumeister & Leary, 1995). Negative feedback from other individuals is upsetting because of the threat it poses to affiliative bonds and to the physical and psychological benefits they entail.
Behavioral evidence has demonstrated the negative psychological effects of social exclusion (Boyes & French, 2009; Sebastian, Viding, Williams, & Blakemore, 2010; van Beest & Williams, 2006; Williams, Cheung, & Choi, 2000; Wirth & Williams, 2009; Zadro, Williams, & Richardson, 2004, 2005). In addition, neuroimaging studies have begun to identify neural correlates of this negative response to ostracism, including activations in anterior insula, anterior cingulate cortex, and right ventrolateral prefrontal cortex (Bolling et al., 2010, in press; Eisenberger, Lieberman, & Williams, 2003; Masten et al., 2009, 2011; Onoda et al., 2009, Sebastian et al., 2010, in press). Specifically, previous studies have shown that activation to social exclusion in anterior cingulate cortex (ACC) is correlated with self-reported distress (Eisenberger et al., 2003; Masten et al., 2009). However, the nature of the excluding group is also meaningful, especially in the context of secondary, psychological benefits of social inclusion. Being excluded by one’s “in-group” (a group with which one shares some meaningful characteristic) may be perceived as more threatening than being excluded by an “out-group” because of the psychological benefits that one’s in-group provides (Baumeister & Leary, 1995). Thus, the current study aimed to investigate the neural correlates of differential responses to social exclusion by one’s own versus opposite gender in typical adults using functional magnetic resonance imaging (fMRI).
Investigations of the effects of group membership on negative feedback offer evidence of the protective nature of attributing negative behaviors from out-group members to discrimination. Behavioral studies on gender group membership have found that self-esteem and projected depression were decreased when participants could attribute negative behavior to gender discrimination (Major et al., 2003; Crocker et al., 1991). The buffering nature of gender group membership was even seen when the sex of the feedback provider was unknown, and only his or her identification as sexist (versus not sexist) was provided (Crandall et al., 2000). Moreover, social exclusion by an out-group of opposite gender excluders (compared to mixed gender excluders) was shown to decrease the negative psychological effects of exclusion (Wittenbaum, Schulman, & Braz, 2010). Neuroimaging work on racial group membership corroborates behavioral findings. One study looking specifically at social exclusion by members of a different race (blacks being excluded by whites) identified regions of dorsal and rostral ACC active to exclusion which were modulated by the extent to which an individual attributed the exclusion to racial biases (Masten, Telzer, & Eisenberger, 2011). Though this work only looked at one type of out-group exclusion, Krill & Platek (2009) compared brain activation to same-and other-race social exclusion and demonstrated that brain responses to exclusion in ACC were increased when participants were excluded by members of their own (versus other) race. Thus, our prediction for the current study is that social exclusion by one’s own gender will be more distressing, eliciting more ACC activation, than exclusion by the opposite gender, as the potential to attribute other-gender exclusion to group discrimination may serve as a buffer from the negative effects of ostracism.
Further behavioral work elucidating the nature of psychological protective mechanisms in gender discrimination by McCoy and Major (2003) showed that individual differences in gender self-identification modulated the effect of discrimination attribution on depression and self-esteem, such that individuals with high gender identification showed lower buffering effects. In social ostracism more specifically, manipulation of the permanence of the excluding out-group membership (permanent versus temporary) affected the persistence of negative mood following exclusion in a similar manner. Permanent (more salient) group membership caused longer persistence of negative affect than temporary (less salient) group membership (Wirth & Williams, 2009). In relation, neuroimaging work on social exclusion and racial group membership found that individuals with higher implicit positive biases towards their own race showed increased amygdala activation during exclusion by another race and decreased activation during exclusion by their own race (Krill & Platek, 2009). Thus, individual differences in attitudes about interpersonal affiliations may modulate the psychological benefits of group membership, also affecting the negative consequences of social exclusion. More specifically, stronger associations between one’s gender and positive valence or self-concept may modulate the neural responses to exclusion by one’s own or the opposite gender in medial prefrontal regions often shown to be active during social exclusion (compared to fair play). Further, if brain responses to exclusion vary on the basis of the gender of the excluders, we expect the effects of implicit gender concepts in these regions to also vary based on the gender of the excluders in relation to the excluded member.
The current study explored the differential neural correlates of social exclusion by one’s own versus opposite gender. Further, measures of gender self-identity and biases were used to explore individual differences in gender-manipulated responses to ostracism. To date, this is the first neuroimaging study to examine the differential effects of gender group membership on processing social exclusion. The negative psychological effects of social exclusion, including increases in self-deflating behavior, social avoidance, and depression (Laird et al., 2001; Gazelle and Ladd, 2003; Gazelle and Rudolph, 2004) and decreases in prosocial behavior and general mental health (Twenge et al., 2007; Rigby, 2000) highlight the importance of identifying individual and situational factors that modulate this experience. In addition, detecting neural correlates of protective psychological mechanisms in responses to exclusion will inform future attempts to take advantage of factors which ameliorate negative effects of social exclusion.
In order to elicit an experience of social exclusion, the present study used a version of Cyberball, a computer-based interactive game in which participants throw a ball with two other players, and are periodically included or excluded from the interaction (Williams et al., 2000). Participants were informed of the gender of their fellow players by pictures displayed on the screen throughout the game. Each participant played two rounds of Cyberball, one with players of his or her own gender, and one with players of the opposite gender. A within-participant design could then be used to investigate differences in the experience of social exclusion as a function of gender group membership. To investigate if individual differences in implicit gender attitudes affect gender group manipulations of social exclusion, the current study utilized two versions of the IAT that assessed individual differences in gender self-identification and positive gender biases. Thus, the hypothesis in the current study was that social exclusion by one’s own gender would elicit a stronger emotional and neural response than exclusion by the opposite gender. Further, prior work would predict that the extent to which one identifies with their own (versus opposite) gender would modulate responses to social exclusion dependant on the gender of the excluders in relation to the excluded participant.
Methods
Participants
Twenty-four healthy adults participated in the current study (13 males, 24.93 ± 3.98 years). From this original sample, four participants were excluded from analyses, one for excessive motion (> 2mm drift from starting position), one for having prior knowledge of the Cyberball game, one for failing to throw the ball on 12 (out of 20) trials during one of the games, and one for periodically falling asleep during the games. After these exclusions, twenty participants remained (11 males, 24.99 ± 3.91 years). Of these twenty adults, 11 played Cyberball with players of their same gender first, while 9 played with the opposite gender first. One of these adults admitted to believing that the players in the Cyberball games were real people, while the remaining participants voiced suspicions that the players were not real people. While it would be ideal to have all participants believe they were playing with real people, previous research has shown that self-reported distress levels in a Cyberball game where participants knew the fictitious nature of the players were comparable to distress levels in a Cyberball game where participants believed they were playing with real people (Zadro, Williams, & Richardson, 2004).
Experimental Design
Cyberball
During a functional magnetic resonance imaging (fMRI) scan, participants played two rounds of an interactive ball-toss game called Cyberball, each lasting 5 minutes and consisting of 10 continuous, alternating 30 second blocks of fair play and exclusion (for full methods see Bolling et al., 2011). In each game, players were depicted as images of adult faces with neutral expressions, taken from NimStim set of face stimuli (Tottenham et al., 2009), and matched on self-reported ethnicity to the participant. In the “same-gender” manipulation, participants played with members of their own gender, while in the “other-gender” manipulation, participants played with members of the opposite gender. Pictures were normed on attractiveness in a separate subset of adults (n = 20, 10 males and 10 females) so that within each ethnicity group, average values of attractiveness of the two same-gender players did not significantly differ from the average attractiveness value of the two other-gender players. The order of games (same-gender and other-gender) was counterbalanced between participants.
Implicit Association Task
Prior to each scan, participants completed two IATs (Greenwald, McGhee, & Schwartz, 1998). One IAT assessed implicit associations between gender associated nouns (e.g. truck, skirt) and self-concept (e.g. me, them), while the other IAT assessed implicit associations between gender (e.g. boy, lady) and valence (e.g. excellent, nasty). Words were normed in a separate subset of adult participants (n = 18) such that all words used significantly varied from a median rating of 4 on a 1 to 7 Likert scale representing the two poles of the construct they were used to represent. The IAT task consisted of a blank screen with a category presented in each of the top corners (i.e. “good” and “bad”). A series of single words appeared in the center of the screen that participants were instructed to sort based on what category they fit into, using the “p” and “q” keys on a keyboard. Each IAT consisted of 2 practice sections (20 trials each), where words were sorted based on only one dimension (i.e. “good” versus “bad”), followed by an association section where words were sorted based on two dimensions simultaneously (i.e. “good” or “female” versus “bad” or “male”; 60 trials). After the association section, another training section (40 trials) occurred where the location on the screen (right versus left corner) of one of the dimensions was switched, followed by another association section where the combination of paired words from each dimension were switched from the first association section (60 trials). Supplemental Figure 1 depicts a sample progression of IAT sections in one task. The extended length of the third practice section was chosen to represent an appropriate number of trials to eliminate order effects of switching the category location (Nosek, Greenwald, & Banaji, 2005). To further address order effects, two versions of each IAT existed such that the order of the association pairings was counterbalanced between participants.
Self-report questionnaires
Immediately following the completion of both rounds of Cyberball, while still in the scanner, participants were prompted to answer 12 questions about the nature of their experience during the games. Questions assessing the amount of exclusion-related distress were a previously-used subset of items from the Need Threat Questionnaire (SED-Q; Bolling et al., 2011), with two items modified to assess differences in exclusion responses to males and females separately. Questions were delivered visually and auditorily, with responses ranging from “not at all” to “extremely” on a 1 to 5 Likert scale.
Imaging Protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. Whole-brain T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1900 ms; TE = 2.96 ms; flip angle = 9°; FOV = 256 mm; image matrix 256 mm2; voxel size =1 × 1 × 1 mm; 160 slices; NEX = 1). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 64 mm2; voxel size = 3.4 × 3.4 × 4 mm; 34 slices) sensitive to blood oxygen level dependent (BOLD) contrast.
Data Analysis
IAT analysis
For each of the two IATs administered, reaction time and accuracy information was recorded using E-prime 2.0 software (Psychological Software Tools, Inc., Pittsburgh, PA). These data points were used to calculate IAT d-scores, generated using the modified algorithm proposed by Greenwald et al. (2003).
fMRI analysis
Data were preprocessed and analyzed using BrainVoyager QX 2.0.08 (Brain Innovation, Maastricht, the Netherlands). The first five volumes acquired in the functional scan were discarded from analysis to allow for T1 equilibrium. Following this, preprocessing of functional data included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4 mm Gaussian kernel, linear trend removal, and temporal high-pass filtering (GLM with Fourier basis set, using 2 cycles per time course). The processed functional data sets were coregistered to within-session anatomical images, which were subsequently normalized to Talairach space. Estimated motion plots depicting head drift from the position at first volume acquisition were generated for each participant to identify movement and eliminate runs with head drift greater than 2 mm in any direction or 2 degrees of rotation (for which one participant was eliminated).
Confounding brain activity associated with each participant’s ball throwing events was modeled and regressed out prior to all subsequent analyses (see Bolling et al., 2011 for methods). To identify brain regions responsive to the Cyberball task manipulation, voxel-wise first-level statistics were computed for each participant’s functional runs using a general linear model (GLM). Predictors for social exclusion and fair play were entered into the GLM. The model time course for each predictor was computed by convolving a boxcar function (defined as 1 during each condition, and 0 otherwise) with a double-gamma hemodynamic response function (HRF). In addition, time series capturing motion in each of the 6 directions and rotations were also included as predictors of no interest to further account for motion. Predictor beta values derived from the first-level analyses were subsequently entered into multi-participant random-effects analyses described below.
Whole Brain Analyses
Whole-brain analyses were restricted to voxels within the extent of the template MNI brain which had previously been normalized to Talairach space, thus constituting a whole-brain mask. Statistical maps from the second level random-effects analysis were set at a threshold of p < 0.05. Correction for multiple comparisons was done at the cluster-level. A cluster-size threshold was computed using an iterative Monte Carlo simulation to estimate an acceptable cluster-level false-positive rate (Forman et al., 1995; Goebel, Esposito, & Formisano, 2006). After 1000 iterations, a minimum cluster-size was chosen, corresponding to a false positive rate of α < .05.
Region of Interest Analyses
Individual regions of interest (ROIs) were created for ventral ACC, dorsal ACC, right and left anterior insula, and right ventrolateral prefrontal cortex (vlPFC). The right and left anterior insula ROIs were each created by combining the right (or left) ventral and dorsal anterior insula regions functionally defined by Deen, Pitskel, & Pelphrey (2010). The ventral ACC ROI was modified from the ACC region defined by Talairach database (Lancaster et al., 1997, 2000) by excluding all voxels above the plane z = 9. The dorsal ACC region was the remainder of this Talairach-defined ACC region (all voxels above the plane z = 9). The vlPFC ROI was modified from the right middle/inferior frontal gyrus regions defined by the Talairach database by restricting the region to the extent of the MNI brain. For each region, the average beta value by condition was computed for each participant. The participant-wise averages were used in subsequent analyses.
Results
IAT results
IAT d-scores were calculated for the association between one’s own gender and self-concept (mean d-score of 0.40 ± 0.54; higher d-score implies a stronger association) and the association between one’s own gender and positive valence (mean score of 0.35 ± 0.60). D-scores in each IAT significantly correlated (r = .89, p < .0001). In females, the mean d-score for the female-self association was 0.90 (±0.30) and the mean d-score for the female-good association was 0.89 (±0.31). In males, the mean d-score for the male-self association was −0.02 (±0.40) and the mean d-score for the male-good association was −0.09 (± 0.38). None of these scores significantly correlated with age (p > .05).
Distress Questionnaire
Total scores on the distress questionnaire (12 items, score range 12–60) were calculated for each participant. In addition, four items were extracted, two corresponding specifically to same-gender exclusion, and two corresponding specifically to other-gender exclusion (“I felt excluded in the first (second) round”, I felt the male (female) players interacted with me a lot”). These items were used to create two sub-scores for each participant, relating to same- and other-gender exclusion distress (2 items, score range 2–10). The average total distress score (sum of all 12 items) was 36.78 (± 7.38). The average scores on the same-gender and opposite-gender subscales were 6.33 (± 1.53) and 6.57 (± 1.28), respectively. Total scores and sub-scores on the questionnaire did not differ by gender, and other-gender and same-gender distress scores sub-scores were not significantly different.
fMRI results
Social Exclusion versus Fair Play
To compare brain activation during social exclusion to fair play regardless of computer players’ gender, functional runs for same- and other-gender were combined for each participant, and social exclusion was contrasted with fair play in a multi-participant GLM. Results of this analysis were assessed at a statistical threshold of p < 0.05, with a cluster threshold of 50 contiguous functional voxels corresponding to α < 0.05 (Figure 1, Table 1). Regions that were more active to social exclusion included bilateral hippocampus, bilateral central sulcus, bilateral posterior insula, ventral ACC extending to medial prefrontal cortex and dorsal anterior cingulate cortex, left ventrolateral prefrontal cortex, left superior and middle temporal gyrus, cuneus extending to lateral occipital cortex, restrosplenial cortex, and right postcentral gyrus. In contrast, regions more active to fair play included right lateral parietal cortex, left intraparietal sulcus, bilateral middle frontal gyrus extending to dorsomedial prefrontal cortex, bilateral anterior insula extending into striatum, bilateral precuneus, and left cerebellum.
Figure 1.
Activation in the contrast of social exclusion > fair play collapsed across gender conditions (n = 20). Regions in orange showed greater activation in social exclusion compared to fair play. Regions in blue showed greater activation in fair play compared to social exclusion (p < .05, k = 1,350).
Table 1.
Activation in Cyberball. Regions identified in a full brain contrast of social exclusion to fair play collapsed across gender conditions. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Cluster sizes are reported in functional voxels. Abbreviations: superior temporal gyrus (STG), ventral anterior cingulate cortex (vACC), ventrolateral prefrontal cortex (vlPFC), medial temporal gyrus (MTG), middle frontal gyrus (MFG), inferior frontal gyrus (IFG), medial prefrontal cortex (mPFC), inferior parietal sulcus (IPS), dorsal anterior cingulate cortex (dACC), lateral occipital cortex (LOC), dorsomedial prefrontal cortex (dmPFC).
| X | Y | Z | size | t | p | |
|---|---|---|---|---|---|---|
| social exclusion > fair play | ||||||
| Right hippocampus / Retrosplenial Cortex | 30 | −49 | 4 | 919 | 7.9 | < 0.001 |
| Left hippocampus / Posterior Insula | −33 | −19 | −20 | 532 | 4.94 | 0.00009 |
| Left STG / MTG | −39 | −16 | −17 | 475 | 5.29 | 0.000042 |
| Left vlPFC | −42 | 26 | −8 | 64 | 4.51 | 0.000241 |
| Left central sulcus | −18 | −28 | 49 | 319 | 3.91 | 0.000935 |
| Right central sulcus | 45 | −13 | 37 | 58 | 3.61 | 0.00185 |
| Cuneus / Retrosplenial Cortex / LOC | −45 | −76 | 19 | 1784 | 6.86 | 0.000002 |
| Right posterior insula | 51 | −10 | 7 | 465 | 4.79 | 0.000134 |
| Left posterior insula | −48 | −37 | 16 | 232 | 4.71 | 0.000152 |
| Right postcentral gyrus | 21 | −43 | 55 | 124 | 4.1 | 0.000616 |
| Left vACC / mPFC /dACC | −3 | 29 | −8 | 760 | 5.64 | 0.000019 |
| Left IFG | −51 | 17 | 25 | 102 | 3.59 | 0.00197 |
| fair play > social exclusion | ||||||
| Right lateral parietal cortex | 42 | −52 | 37 | 1161 | −7.14 | 0.000001 |
| Right MFG / dmPFC | 39 | 38 | 34 | 2351 | −6.31 | 0.000005 |
| Left MFG | −36 | 41 | 16 | 289 | −5.5 | 0.000026 |
| Right anterior insula / Striatum | 21 | 2 | 10 | 338 | −6.1 | 0.000007 |
| Left anterior insula / Striatum | −30 | 17 | 7 | 162 | −4.55 | 0.00022 |
| Right precuneus | 9 | −64 | 43 | 193 | −4.79 | 0.000129 |
| Left precuneus | −9 | −67 | 46 | 56 | −4.05 | 0.000681 |
| Left cerebellum | −24 | −58 | −26 | 536 | −7.08 | 0.000001 |
| Left IPS | −57 | −37 | 34 | 375 | −4.63 | 0.000183 |
Brain regions showing modulation in their response to exclusion as a function of the online players’ gender were identified by contrasting the magnitude of the exclusion effect between same- and other-gender games, i.e. (same-gender exclusion – same-gender fair play) – (other-gender exclusion – other-gender fair play). This subtraction was done for each participant, taking advantage of the within-subject design of the experiment. Results of this analysis were assessed at a statistical threshold of p < 0.05, corrected for multiple comparisons with a cluster threshold of 34 contiguous functional voxels (Figure 2, Table 2). Regions more active to exclusion by same-gender players included ventral ACC, bilateral precuneus, cerebellum, paracentral lobule, left postcentral gyrus, and cuneus. In turn, the one region that was more active to exclusion by other-gender players was the right striatum. Investigating the nature of these differences, beta values were extracted from each of these regions, and the contrast of social exclusion – fair play was compared to zero in each game (same- and other-gender) separately. Regions where the significant difference between same- and other-gender exclusion was driven by significant activation to exclusion (versus fair play) in same-gender Cyberball included ventral ACC, left precuneus, cerebellum, paracentral lobule, left postcentral gyrus, and cuneus. Regions with significant activation in same-gender exclusion and significant deactivation in other-gender exclusion (both compared to fair play) included right precuneus and left cerebellum. Finally, right striatum was significantly more active to fair play (compared to social exclusion) only in same-gender Cyberball. The bar graph in Figure 3 is not independent of the between-game analysis, and serves only to illuminate the nature of the same- versus other-gender difference in ventral ACC driving the aforementioned between-game difference.
Figure 2.

Activation in the contrast of same-gender exclusion (versus fair play) > other-gender exclusion (versus fair play) (n = 20). Regions in orange showed greater activation in social exclusion by one’s own gender compared to fair play. Regions in blue showed greater activation in social exclusion by one’s opposite gender compared to fair play (p < .05, k = 918). The bar graph on the right represents a non-independent visual depiction of the activation to exclusion > fair play in each Cyberball game with error bars depicting standard error.
Table 2.
Activation to in-group and out-group exclusion in Cyberball. Regions identified in a full brain contrast of social exclusion to fair play in each gender condition (same and other). Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Cluster sizes are reported in functional voxels. Abbreviations: anterior cingulate cortex (ACC).
| X | Y | Z | size | t | p | |
|---|---|---|---|---|---|---|
| social exclusion > fair play | ||||||
| same-gender > other-gender | ||||||
| Right Precuneus | 18 | −46 | 22 | 52 | 3.74 | 0.000591 |
| Left Precuneus | −3 | −67 | 19 | 79 | 4.73 | 0.000029 |
| Ventral ACC | −9 | 44 | −1 | 117 | 4.35 | 0.000095 |
| Cerebellum | 3 | −61 | −30 | 41 | 2.57 | 0.014259 |
| Left Cerebellum | −38 | −79 | −20 | 54 | 1.78 | 0.083308 |
| Paracentral Lobule | −6 | −37 | 43 | 46 | 2.39 | 0.021626 |
| Left Postcentral gyrus | −24 | −34 | 67 | 42 | 3.28 | 0.002205 |
| Left Postcentral Gyrus | −42 | −20 | 52 | 34 | 3 | 0.004639 |
| Cuneus | 3 | −82 | 22 | 116 | 4.25 | 0.000127 |
| other-gender > same-gender | ||||||
| Right Striatum | 21 | 5 | 10 | 53 | −4.3 | 0.000111 |
Figure 3.
Activation in the contrast of social exclusion > fair play correlating with gender-self IAT scores (n = 20). Regions in orange showed greater activation to social exclusion correlated with greater implicit association between one’s gender and self-concept. Regions in blue showed less activation to social exclusion correlated with greater implicit association between one’s gender and self-concept (p < .05, k = 918).
Correlations with Implicit Associations
Subsequent analyses aimed to investigate the relationship between brain activation to social exclusion by either one’s own or the opposite gender and implicit associations between one’s own gender and self-concept or positive valence. D-scores from each IAT (gender-self and gender-good) were used as covariates in whole brain correlation analyses within each Cyberball game (same-gender and other-gender). For consistency, results of these correlation analyses were reported at the same statistical threshold as the same- versus other-gender exclusion contrast (p < .05, k = 918).
Whole-brain covariate analyses identified regions that significantly correlated with scores on each of the two IATs. In same-gender Cyberball, greater associations between one’s own gender and self- concept correlated positively with activation during exclusion (versus inclusion) in bilateral superior frontal gyrus. Regions showing a negative correlation included bilateral precentral and postcentral sulcus, right dorsal ACC, posterior cingulate cortex, left paracentral lobule, left ventral ACC and ventromedial PFC, and left temporal pole (Figure 3). In the same game, associations between one’s own gender and positive valence correlated positively with activation during exclusion (versus inclusion) in right middle occipital gyrus and left middle temporal gyrus. Regions showing a negative correlation included bilateral precentral sulcus, right postcentral sulcus, dorsomedial PFC, left dorsolateral and ventrolateral PFC, left posterior cingulate cortex, and left anterior insula (Figure 4). In other-gender Cyberball, associations between one’s own gender and self concept correlated positively with activation during exclusion in right superior temporal gyrus, posterior cingulate sulcus, ventral striatum, medial prefrontal cortex, and left posterior cingulate cortex (Figure 3). Comparably, in this game, associations between one’s own gender and positive valence correlated positively with activation during exclusion in bilateral superior temporal sulcus, left superior temporal gyrus, right middle occipital cortex, right postcentral gyrus, ventral striatum, left inferior frontal gyrus, left posterior cingulate cortex, and medial prefrontal cortex (Figure 4). It is worth noting that regions showing correlations with IAT scores and activation in the contrast of exclusion – fair play do not necessarily show significant differences in activation between exclusion and fair play.
Figure 4.
Activation in the contrast of social exclusion > fair play correlating with gender-valence IAT scores (n = 20). Regions in orange showed greater activation to social exclusion correlated with greater implicit association between their gender and positive valence. Regions in blue showed less activation to social exclusion correlated with greater implicit association between one’s gender and positive valence (p < .05, k = 918).
Regions of Interest
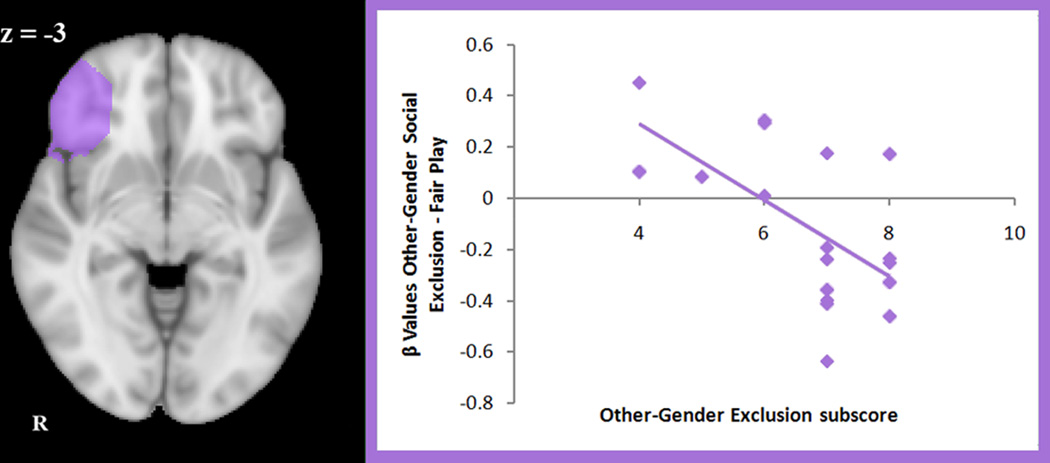
Specific ROI analyses in ventral ACC, dorsal ACC, right and left anterior insula and right vlPFC were used to investigate correlations between self-report distress scores and brain activation. Independently defined ROIs were used in order to visualize these correlations in an independent way, since the regions of interest were chosen a priori. Using the other-gender exclusion sub-score, distress caused by other-gender exclusion correlated negatively with activation in right vlPFC (r = −0.62, p = 0.007). Using the same-gender exclusion sub-score, distress caused by same-gender exclusion correlated negatively with activation in dorsal ACC (r = −0.47, p = 0.05). In dorsal ACC, a correlation between activation to same-gender exclusion and total distress scores was also significant (r = −0.54, p = 0.01). No correlations between activation and distress scores (both total and subscores) were significant in ventral ACC, right or left anterior insula (p > .05). No correlations existed with activation in right vlPFC in same-gender Cyberball (p > .05). During other-gender Cyberball, a correlation between activation in dorsal ACC and other-gender distress sub-scores trended towards significance (r = −0.47, p > .055).
Discussion
The current study replicated and extended previous work identifying the neural correlates of processing social exclusion by investigating the effects of group membership on these neural responses. Building on past neuroimaging work that showed differences in brain responses to social exclusion based on the race of the excluders in relation to the participant (Krill & Platek, 2009), the current study found similar differences based on the gender relation of the excluders in ventral ACC. In addition, the present study identified individual differences in implicit gender attitudes that modulated brain responses to exclusion based on the gender relation of the excluders. The identification of individual and situational factors which modulate responses to social exclusion is especially informative to the understanding of factors which may intensify or ameliorate the negative psychological and neural effects of ostracism.
Results from the contrast of social exclusion versus fair play collapsed across gender conditions replicated past studies identifying the neural correlates of processing social exclusion (Bolling et al., 2010, in press; Eisenberger et al., 2003; Masten et al., 2009, 2011; Onoda et al., 2009, Sebastian et al., 2010, in press). The activation of this network of brain regions was complemented by self-report measures confirming that participants felt distressed following exclusion. The average total rating of exclusion in the present study was 36.78 (sum of all 12 questionnaire items), corresponding to an average item rating of 3.06 (“1” = no distress, “5” = extreme distress). Thus, the present study was successful in eliciting an experience of social exclusion, independent of the gender of the excluders. Though we did not identify a difference in self-reported distress to same-gender versus other-gender exclusion, previous work has similarly reported the failure of self-report measures of distress to out-group exclusion to correlate with brain responses to exclusion (Masten, Telzer, & Eisenberger, 2011). Instead, this study found that observational measures of distress yielded more meaningful results in correlations with brain responses to exclusion. Thus, there may be components of the experience of exclusion specifically by members of an out-group that may affect the self-reporting of distress.
The primary question in the present study was whether the neural response to social exclusion would be modulated by the gender of the excluders in relation to the participant. To address this question, we utilized a within-participant design to contrast neural activation to social exclusion by one’s own versus opposite gender. This analysis identified several regions that were more active when participants were excluded by players of their own gender. In particular, a region of ventral ACC encompassing subgenual to pregenual sub-regions showed significant activation to same-gender exclusion and not other-gender exclusion (Figure 1). Ventral ACC (most specifically the subgenual ACC) has been implicated in the processing of negative emotion, especially sadness (Liotti et al., 2000; Phan, Wager, Taylor, & Liberzon, 2002; Shafritz, Collins, & Blumberg, 2006), and has been shown to correlate with self-reported distress during social exclusion (Masten et al., 2009; Onoda et al., 2009). While we cannot be certain that ventral ACC activation in the current study corresponds to greater distress during exclusion by members of one’s own gender, we are inclined to interpret the lack of activation in this region during exclusion by the opposite gender as indication that the gender of the excluding players significantly alters the experience of social exclusion. In addition, this finding is consistent with work by Krill and Platek (2009) identifying a region of ACC that was more active to social exclusion by same-race (compared to other-race) players.
Differential neural responses to social exclusion by one’s own gender suggest that this experience is fundamentally different from exclusion by the opposite gender. This conclusion is in line with the conjecture that social exclusion is distressing because of the threat it poses to interpersonal relations, especially the threat it poses on the human need to belong (Baumeister and Leary, 1995). Interpersonal bonds with other conspecifics may be evolutionarily important to survival, but the psychological importance of bonds between an individual and members of their in-group (same gender in this case) predicts that the psychological threat ostracism poses should be greater when one is excluded by members of their own gender. However, negative psychological effects of gender discrimination are not uncommon, especially in women (Landrine, Klonoff, Gibbs, Manning, & Lund, 1995; Schmitt, Branscombe, & Postmes, 2003). Specifically, behavioral studies have demonstrated that individuals who strongly identified with their own gender showed more pronounced effects of negative feedback from opposite gender peers (McCoy & Major, 2003). Thus, we predicted that implicit attitudes about gender valence and self-concept would modulate neural activation to social exclusion in medial prefrontal brain regions shown to be active during social exclusion compared to fair play, and sensitive to the gender manipulation in the current study.
In the current study we identified several regions where activation to exclusion covaried with individual implicit gender attitudes. While being excluded by members of the opposite gender, activation in medial prefrontal cortex (mPFC) positively correlated with one’s implicit gender-self association. A similar region also correlated with one’s association with their own gender and positive valence, as this association correlated highly with gender-self implicit attitudes. The region of mPFC that was more active to out-group exclusion in people with greater gender-self associations overlapped substantially with the ventral ACC/mPFC region shown to be active during general social exclusion. As we hypothesized that activation in this region during social exclusion may be related to the affective response to ostracism, these correlation results may reflect that the buffering effect of being excluded by the opposite gender is less successful in people who highly identify with their own gender. Exclusion as a discriminatory offense to one’s gender may be perceived more personally in participants with high gender-self associations.
The relationship between implicit attitudes about gender and activation during social exclusion was markedly different when participants were excluded by players of their own gender. Namely, stronger self-gender associations correlated with lower activation to same-gender exclusion in ventral ACC and ventromedial prefrontal cortex, showing the opposite pattern to what was found in correlations with activation during other-gender exclusion. Further, the same correlation pattern was shown in dorsal ACC, another region previously associated with the affective processing of social exclusion (Eisenberger et al., 2003). Similar results were seen in covariate analyses with gender-valence implicit attitudes. These results are comparable to decreased amygdala activation found in people with high own-race positive biases during exclusion by members of one’s own race (Krill & Platek, 2009). While speculative, one might take these results as an example of the buffering effects (lower activation in affective brain regions) of holding a strong positive association with one’s own gender in the case when members of that gender are providing negative feedback in the form of social exclusion.
During social ostracism, modulation of activation by the gender of the excluders in brain regions associated with affective processing supports the notion that exclusion by the opposite gender provides a buffering effect against negative consequences of social exclusion. In a study of the effects of being excluded by members of another race, Masten, Telzer, & Eisenberger (2011) identified regions active during social exclusion that were modulated by the extent to which participants attributed the exclusion to race including dorsal and rostral ACC. This modulation was hypothesized to relate to buffering effects of attributing exclusion to race. Further, this study along with other past research on social exclusion (Eisenberger et al., 2003, Masten et al. 2009) identified inverse correlations between right vlPFC and self-reported or observed distress, leading to the hypothesis that right vlPFC has a role in emotion regulation during ostracism. Thus, the psychologically buffering effects of attributing out-group exclusion to discrimination may involve this region. Indeed, activation in a structurally defined region of right vlPFC during other-gender exclusion correlated negatively with the sub-score of the SE-Q relating to exclusion by the opposite gender specifically. No such correlation existed in this region during same-gender exclusion. While there was no difference in activation of right VLPFC between same- and other-gender exclusion, the existence of a significant correlation with self-reported distress only during exclusion by the opposite gender corroborates our hypothesis that amelioration of the negative psychological consequences of exclusion by the opposite gender involves the successful recruitment of regulatory brain regions, specifically right vlPFC. Activation to same-gender exclusion in a structurally-defined region of dorsal ACC showed a negative correlation with same-gender distress sub-scores as well as total distress scores. A similar relationship existed in other-gender exclusion that trended towards significance. These results suggest that increased activation in dorsal ACC during social exclusion by either gender is associated with lower self-reported distress. While dorsal regions of ACC have been implicated in processing Cyberball exclusion (Eisenberger et al., 2003) and have been more generally associated with cognitive (versus emotional) processing (Bush, Luu, & Posner, 2000), cognitive processes during social exclusion may decrease distress levels by allowing participants to reappraise the negative situation.
While the results of the current study provide strong evidence for the brain mechanisms involved in processing social exclusion which are modulated by the relational gender identity of the excluders, the study did leave several questions for further investigation. First, while self-report measures confirmed that participants were distressed following the experience of social exclusion, there was not a significant correlation between self reported distress and activation in ventral ACC during exclusion. This is most likely due to the fact that distress measures were taken following exclusion but not inclusion, and so no baseline measure of distress existed for each participant. The present study utilized an alternating block design necessary in neuroimaging research to minimize order effects and scanner drift, but the alternating design did not allow for a baseline measure of distress to be obtained following social inclusion. In addition, the use of one distress measure at the end of the extended experience of transient exclusion might capture reported distress at its lowest point (after 10 minutes and 20 experiences of exclusion). Thus, our finding that significant distress existed at the end of two Cyberball games suggests that initial distress to exclusion was probably even higher. The present study also failed to find a difference between distress levels following same- versus other-gender exclusion. Ideally, a full distress questionnaire after each experience of exclusion (same-gender and other-gender) would provide the most power in identifying affective differences in these ostracism experiences. The results of this paper do suggest that the experience of exclusion by one’s own gender is processed differently in the brain than exclusion by the opposite gender, and thus, all hypotheses discussed about the relative distressing nature of these experiences are inferentially based on our interpretation of the data.
As it follows, while self-report measures revealed that participants felt distressed by the experience of exclusion, only one adult admitted to believing that the online players were real people. While previous literature has shown that participants are significantly affected by social exclusion despite knowing the fictitious nature of the excluding players (Zadro et al., 2004), having participants believe that they are being excluded by real people increases the ecological validity of the ostracism experience. The current study demonstrated a neural response to exclusion that is similar to previous investigations of social rejection where participants did believe they were being excluded by real people (Eisenberger et al., 2003; Masten et al., 2009). This suggests that a more convincing manipulation of social exclusion would not have yielded substantially different regions of neural activation, but may have resulted in stronger emotional responses to the rejection, potentially yielding more robust results than those revealed in the current study.
While the current study used gender as a means to manipulate group membership, we did not extensively investigate gender differences in these effects. Post-hoc exploratory analyses revealed that exclusion by same gender players elicited greater ventral ACC activation in the whole group as well as separately in both males (Talairach coordinate of peak voxel: 6, 41, 1; p = 0.004, 119 functional voxels) and females (Talairach coordinate of peak voxel: −6, 23, −8; p = 0.01,4 functional voxels). However, two regions showed significantly greater differential activation to same- versus other- gender exclusion in males compared to females (dorsal ACC, Talairach coordinates: 2, 31, 30; cerebellum, Talairach coordinates: −10, −38, −30). These gender differences were significant at p < .05 corrected to α < .05 with a cluster threshold of 37 voxels. We could not determine if the identified gender difference in average IAT scores was a result of the small within-gender sample sizes, or if this difference would replicate in a larger sample of adults. Thus, this difference was not further probed in the current study. Voxel-wise t-tests revealed that none of the regions identified in either game as correlating with IAT scores showed significant gender-differences during same- or other-gender exclusion at an uncorrected threshold of p < .05. To this end, we are confident that the results of the IAT correlations were not driven solely by bimodal gender differences. Future investigations of gender differences in implicit gender associations as well as brain responses to social exclusion would be important in extending our understanding of how gender differences and group membership interact in social exclusion. While we did not anticipate the high correlation between scores on the two IATs, this result is not surprising, as past work has shown that implicit attitudes about one’s self correlate with implicit attitudes about one’s own gender (Rudman, Greenwald, & McGhee, 2001), suggesting that stronger self-gender associations might enhance this effect. Work reviewing studies using the IAT have noted a self-positivity bias (Greenwald et al., 2002), which may translate to an own-gender-positivity bias that is more strongly expressed in people with a strong gender self concept as we see in the current results. While unsurprising, the correlation between IAT measures renders our whole-brain correlations with each IAT measure separately slightly redundant, and thus the current study focused mainly on an interpretation of the gender-self IAT.
While studies of social cognition have extensively investigated interpersonal processes such as the understanding of others’ emotions, intentions and actions (see Blakemore, Winston, & Frith, 2004 for review), much of this investigation has ignored the effects of group membership on such processes. Understanding the neural mechanisms by which people grasp and respond to social interactions is vital in comprehending how humans function in day-to-day life, as humans are an innately social species. The characteristic (i.e. gender) relation between individuals engaging in social interaction is an under-investigated facet of social cognition. The current study demonstrated that one ubiquitous interpersonal relational factor, gender, modulates neural responses to social exclusion. This finding highlights the importance of investigating this modulation in other social cognitive processes.
Conclusions
The present study both replicated and extended previous research on the neural correlates of social exclusion. We replicated findings implicating ventral ACC, PCC, and insula in processing social exclusion (Bolling et al., 2010, in press; Eisenberger et al., 2003; Masten et al., 2009, 2011; Onoda et al., 2009, Sebastian et al., 2010, in press). Most importantly, we identified regions modulated by the gender relation of the excluders to the excluded participants. Our neuroimaging results substantiate past behavioral work showing the buffering effects of attributing negative feedback to discrimination with potential neural correlates for this phenomenon. First, ventral ACC, a region previously associated with the affective experience of social exclusion, was more active to exclusion by one’s own (versus opposite) gender. Second, individual differences in implicit associations with one’s own gender may have muted this buffering effect, demonstrated by increased activation in mPFC to exclusion by the opposite gender. Last, we propose activation of right vlPFC as a brain mechanism underlying the amelioration of psychological effects of exclusion because of its inverse correlation with distress scores specifically during exclusion by the opposite gender. Because previous research has shown the involvement of ventral ACC and right VLPFC in differentially processing exclusion by one’s own and other race peers (Krill & Platek, 2009; Masten, Telzer, & Eisenberger, 2011), we hypothesize that the modulation of activation in these regions by gender in the current study may relate to differences in the experiences of exclusion by in-group versus out-group members more generally.
Supplementary Material
Supplemental Figure 1: A sample progression of one IAT task. Each screen depicts a section of the task comprised of a series of sorting trials. Dimension categories are examples from the gender-valence IAT task.
Figure 5.
Region of interest analysis of activation in the contrast of other-gender social exclusion > fair play (n = 20). The image on the left depicts the structural region of interest. The scatterplot on the right illustrates the negative correlation between activation to social exclusion (versus fair play) in right ventrolateral prefrontal cortex and self-reported distress from exclusion by the opposite gender (r = −0.62, p = .007)
Table 3.
Activation to social exclusion correlating with IAT scores. Regions identified as correlating with IAT scores in a full brain contrast of social exclusion to fair play in each gender condition (same and other). Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Cluster sizes are reported in functional voxels. Abbreviations: ventral anterior cingulate cortex (vACC), superior frontal gyrus (SFG), dorsal anterior cingulate cortex (dACC), posterior cingulate cortex (PCC), superior temporal gyrus (STG), prefrontal cortex (PFC), middle occipital gyrus (MOG), middle temporal gyrus (MTG), ventrolateral prefrontal cortex (vlPFC), dorsolateral prefrontal cortex (dlPFC), superior temporal sulcus (STS), inferior frontal gyrus (IFG).
| X | Y | Z | size | r | p | |
|---|---|---|---|---|---|---|
| Gender-Self IAT score | ||||||
| Same | ||||||
| Right SFG | 18 | 29 | 55 | 56 | 0.67 | 0.001106 |
| Left SFG | −18 | 45 | 49 | 51 | 0.71 | 0.000467 |
| Left vACC / vmPFC | −12 | 20 | −8 | 249 | −0.76 | 0.000087 |
| Right precentral / postcentral sulcus | 24 | −16 | 52 | 458 | −0.76 | 0.000097 |
| Left precentral / postcentral sulcus | −12 | 26 | 34 | 292 | −0.74 | 0.000191 |
| Right dACC | 15 | 41 | 28 | 42 | −0.66 | 0.001699 |
| PCC | −9 | −34 | 28 | 53 | −0.65 | 0.001779 |
| Left paracentral lobule | −15 | −22 | 52 | 45 | −0.76 | 0.000087 |
| Left temporal pole | −48 | 5 | −32 | 59 | −0.72 | 0.000419 |
| Other | ||||||
| Right STG | 45 | 23 | −14 | 69 | 0.66 | 0.001541 |
| Posterior cingulate sulcus | 12 | −22 | 46 | 45 | 0.67 | 0.001167 |
| Ventral striatum | 21 | 20 | −5 | 79 | 0.74 | 0.000205 |
| Medial PFC | −15 | 44 | 16 | 49 | 0.59 | 0.006217 |
| Left PCC | −18 | −43 | 22 | 48 | 0.82 | 0.00001 |
| Gender-Good IAT score | ||||||
| Same | ||||||
| Right MOG | 22 | −100 | 4 | 114 | 0.65 | 0.002135 |
| Left MTG | −48 | −28 | −8 | 43 | 0.77 | 0.00008 |
| Dorsomedial PFC | −12 | 29 | 34 | 110 | −0.79 | 0.000041 |
| Left PCC | −9 | −34 | 28 | 40 | −0.59 | 0.005882 |
| Left anterior insula | −12 | 20 | −11 | 37 | −0.65 | 0.001986 |
| Left vlPFC | −18 | 65 | 13 | 79 | −0.66 | 0.00171 |
| Left dlPFC | −33 | −1 | 37 | 51 | −0.67 | 0.001285 |
| Right precentral / postcentral sulcus | 24 | −16 | 52 | 323 | −0.81 | 0.000016 |
| Left precentral sulcus | −57 | −13 | 34 | 54 | −0.65 | 0.001776 |
| Other | ||||||
| Right STS | 63 | −22 | 13 | 121 | 0.7 | 0.000594 |
| Left STS | −67 | −37 | 10 | 85 | 0.71 | 0.000432 |
| Left STG | 45 | 23 | −14 | 73 | 0.66 | 0.001626 |
| Right middle occipital cortex | 30 | −67 | 7 | 50 | 0.67 | 0.001268 |
| Right postcentral gyrus | 12 | −40 | 61 | 118 | 0.7 | 0.000522 |
| Ventral Striatum | 21 | 20 | −5 | 41 | 0.7 | 0.000566 |
| Medial PFC | −15 | 44 | 16 | 53 | 0.71 | 0.000484 |
| Left PCC | −18 | −43 | 22 | 84 | 0.78 | 0.000054 |
| Left IFG | −39 | 8 | 10 | 56 | 0.66 | 0.001477 |
Table 4.
Words used in the implicit association tasks, and the format of the task. Asterisk denotes blocks of the task where reaction times were used to calculate the final d-score for each participant. “Left” and “Right” categories refer to the corner of the screen in which each category was located (See supplemental Figure 1).
| Section | Left | Right | trials |
|---|---|---|---|
| construct practice | female | male | 20 |
| construct practice | self | other | 20 |
| association practice* | female + self | male + other | 20 |
| association test* | female + self | male + other | 40 |
| construct practice | other | self | 40 |
| association practice* | female + other | male + self | 20 |
| association test* | female + other | male + self | 40 |
| Words | |||
| gender-self IAT | |||
| female | male | self | other |
| bake | hammer | I | other |
| ballet | sports | me | their |
| barbie | tie | mine | them |
| dolls | tools | my | themself |
| dress | trains | myself | themselves |
| makeup | truck | self | they |
| pink | video games | ||
| skirt | wrestling | ||
| gender-valence IAT | |||
| female | male | good | bad |
| daughter | boy | amazing | appalling |
| female | brother | awesome | awful |
| girl | father | beautiful | dreadful |
| lady | gentleman | excellent | gross |
| maiden | guy | fantastic | horrible |
| miss | lad | magnificent | nasty |
| mother | male | super | poor |
| sister | man | wonderful | terrible |
| woman | son | ||
References
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Blakemore S-J, Winston J, Frith U. Social cognitive neuroscience: where are we heading? Trends in Cognitive Sciences. 2004;8:216–222. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, Pelphrey KA. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Kaiser MD, Wyk BCV, Wu J, Mayes LC, Pelphrey KA. Enhanced neural responses to rule violation in children with autism: A comparison to social exclusion. Developmental Cognitive Neuroscience. doi: 10.1016/j.dcn.2011.02.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes ME, French DJ. Having a Cyberball: using a ball-throwing game as an experimental social stressor to examine the relationship between neuroticism and coping. Personality and Individual Differences. 2009;47:396–401. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Crocker J, Voelkl K, Testa M, Major B. Social stigma: The affective consequences of attributional ambiguity. Journal of Personality and Social Psychology. 1991;60:218–228. doi: 10.1037//0022-3514.64.1.60. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with Brain Voyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality and Social Psychology. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR, Rudman LA, Farnham SD, Nosek BA, Mellott DS. A unified theory of implicit attitudes, stereotypes, self-esteem, and self-concept. Psychological Review. 2002;109:3–25. doi: 10.1037/0033-295x.109.1.3. [DOI] [PubMed] [Google Scholar]
- Krill A, Platek SM. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Frontiers in Evolutionary Neuroscience. 2009;1:1–7. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kotchunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrine H, Klonoff EA, Gibbs J, Manning V, Lund M. Physical and psychiatric correlates of gender discrimination. Psychology of Women Quarterly. 1995;19:473–492. [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biological Psychiatry. 2000;30:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Major B, Kaiser CR, McCoy SK. It not my fault: When and why attributions to prejudice protect self-esteem. Personality and Social Psychology Bulletin. 2003;29:772–781. doi: 10.1177/0146167203029006009. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Development and Psychopathology. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI. An fMRI investigation of attributing negative social treatment to racial discrimination. Journal of Cognitive Neuroscience. 2011;23:1042–1051. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- Nosek BA, Greenwald AG, Banaji MR. Understanding and using the implicit association test: II. Method variables and construct validity. Personality and Social Psychology Bulletin. 2005;31:166–180. doi: 10.1177/0146167204271418. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rudman LA, Greenwald AG, McGhee DE. Implicit self-concept and evaluative implicit gender stereotypes: Self and ingroup share desirable traits. Personality and Social Psychology Bulletin. 2001;27:1164–1178. [Google Scholar]
- Schmitt MT, Branscombe NR, Postmes T. Women’s emotional responses to the pervasiveness of gender discrimination. European Journal of Social Psychology. 2003;33:297–312. [Google Scholar]
- Sebastian CL, Roiser JP, Tan GCY, Viding E, Wood NW, Blakemore S-J. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain and Behavior. 2010;9:62–637. doi: 10.1111/j.1601-183X.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore S-J. Developmental influences on the neural bases of responses to social rejection: Implications of social neuroscience for education. NeuroImage. doi: 10.1016/j.neuroimage.2010.09.063. (in press) [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore S-J. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Wirth JH, Williams KD. 'They don't like our kind': Consequences of being ostracized while possessing a group membership. Group Processes and Intergroup Relations. 2009;12:111–127. [Google Scholar]
- Wittenbaum GM, Schulman HC, Braz ME. Social ostracism in task groups: the effects of group composition. Small Groups Research. 2010;41:330–353. [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental and Social Psychology. 2004;40:560–567. [Google Scholar]
- Zadro L, Williams KD, Richardson R. Riding the ‘O’ train: comparing the effects of ostracism and verbal dispute on targets and sources. Group Processes and Intergroup Relations. 2005;8:125–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: A sample progression of one IAT task. Each screen depicts a section of the task comprised of a series of sorting trials. Dimension categories are examples from the gender-valence IAT task.