Abstract
Objective
We sought to systematically review the literature on electronic nicotine delivery systems (ENDS, also called electronic cigarettes) awareness, use, reactions and beliefs.
Data sources
We searched five databases for articles published between 2006 and 1 July 2013 that contained variations of the phrases ‘electronic cigarette’, ‘e-cigarette’ and ‘electronic nicotine delivery’.
Study selection
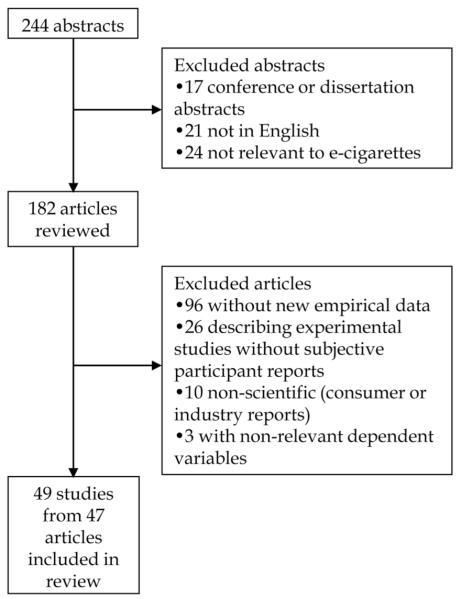
Of the 244 abstracts identified, we excluded articles not published in English, articles unrelated to ENDS, dissertation abstracts and articles without original data on prespecified outcomes.
Data extraction
Two reviewers coded each article for ENDS awareness, use, reactions and beliefs.
Data synthesis
49 studies met inclusion criteria. ENDS awareness increased from 16% to 58% from 2009 to 2011, and use increased from 1% to 6%. The majority of users were current or former smokers. Many users found ENDS satisfying, and some engaged in dual use of ENDS and other tobacco. No longitudinal studies examined whether ENDS serve as ‘gateways’ to future tobacco use. Common reasons for using ENDS were quitting smoking and using a product that is healthier than cigarettes. Self-reported survey data and prospective trials suggest that ENDS might help cigarette smokers quit, but no randomised controlled trials with probability samples compared ENDS with other cessation tools. Some individuals used ENDS to avoid smoking restrictions.
Conclusions
ENDS use is expanding rapidly despite experts’ concerns about safety, dual use and possible ‘gateway’ effects. More research is needed on effective public health messages, perceived health risks, validity of self-reports of smoking cessation and the use of different kinds of ENDS.
INTRODUCTION
Electronic nicotine delivery systems (ENDS), also called e-cigarettes or electronic cigarettes, are battery-operated devices that contain an inhalation-activated mechanism that heats a cartridge, producing vapour that the user, sometimes called a ‘vaper’, inhales. Liquid in the refillable cartridges typically has nicotine and humectants, although non-nicotine cartridges and disposable models are available. Notably, ENDS do not rely on combustion, meaning that users do not expose themselves or others to many of the harmful tobacco smoke constituents and particles produced by regular cigarettes.1 ENDS are controversial: safety information is sparse and inconsistent,2,3 regulation is in flux,4 and public interest is increasing rapidly5 despite the lack of research establishing ENDS’ long-term health effects or cessation properties for smokers. In addition, public health advocates are concerned that ENDS could act as a gateway to future smoking6 or prevent smokers from quitting by maintaining their nicotine addiction or deterring them from using existing, effective cessation tools.7 The ENDS literature is expanding rapidly, but to date no systematic review has summarised the findings across populations or identified gaps in the research. It is important to understand patterns of ENDS use across populations and time, and what beliefs and reactions drive either use or avoidance of ENDS. This review seeks to improve our understanding of who has used ENDS, how they feel about using them, and what both users and non-users think about this controversial product.
Safety of ENDS
With any nicotine or tobacco product, health and safety are primary public health concerns. However, evidence about the safety of ENDS, particularly related to the ‘e-liquid’ in the cartridges, is mixed. The US Food and Drug Administration (FDA) analysed the contents of ENDS cartridges2 and found four major tobacco-specific nitrosamines, a family of carcinogenic chemicals, but they reported only that these chemicals were detected, not whether the amounts detected reached harmful levels.8 A study of the effects of 40 different samples of refill liquids on pulmonary fibroblasts which model adult lung cells3 found tremendous variability in cytotoxicity even among individual samples from the same brand and flavour.
Experts disagree about the potential harms of propylene glycol, a chemical that serves as a humectant in e-liquid.9,10 While theatre fog is associated with impaired lung function,11 no studies have examined the long-term effects of inhaled propylene glycol in humans.7 Ingestion of or exposure to liquid nicotine from ENDS cartridges may also be unsafe. Many of the cartridges and the packets of e-liquid are not childproof,12 and children might be drawn to the candy- and fruit-flavoured e-liquids.13 If ingested by a child, high doses of nicotine can be fatal.13
Another concern is the lack of quality control standards. Multiple studies have detected nicotine in cartridges labelled nicotine-free.2,14,15 Some cartridges leak, are incorrectly or ambiguously labelled, or vary in content even though they are labelled as being the same brand and flavour.3,12
In sum, scientific consensus has yet to emerge about the degree of danger posed by ENDS. Making cartridges and e-liquids childproof and instituting quality control standards would help avoid some safety problems. Whether purposeful exposure, that is, ongoing use of ENDS, has harmful long-term consequences remains an open question.
Regulation of ENDS
Approaches to regulation vary widely. For example, Brazil bans the sale, import and advertising of ENDS, while Finland treats ENDS as medicinal products and bans only advertising.16 In the USA, the FDA is expected to propose deeming regulations in fall 2013.4 In the meantime, some local governments in the USA have taken action to prohibit sales to minors or otherwise restrict ENDS use.17 Given that ENDS vary widely, ranging from disposable models that can cost several dollars but have limited flavour and nicotine options to ‘personal vapourisers’ that can cost several hundred dollars but have hundreds of options for flavours and a wider variety of nicotine strengths, it will be important for policymakers to issue regulations that cover these different models.
Public interest in ENDS
The public has shown tremendous interest in ENDS, and the popular media has extensively covered ENDS.18,19 Celebrities have used them in movies and on television.20 Between January 2008 and February 2010, online searches for information on ENDS increased several hundred-fold.5 ENDS are extensively marketed online, promoted on YouTube videos and advertised on Twitter.21,22
One result of high levels of public interest is that ENDS have quickly become a big business.23–25 The current ENDS industry size is estimated to be $500 million in annual sales and increasing rapidly (expected to reach $1 billion by the end of 2013).23,24,26 ‘Big Tobacco’ companies entered the market when Lorillard purchased Blu eCigs, a major ENDS brand.27 R.J. Reynolds has also introduced their own ENDS line, and Altria (owner of Phillip Morris USA) plans to launch MarkTen ENDS in test markets in summer 2013.25
In sum, ENDS are increasingly popular, although their safety record is not yet established and regulation is still in flux. There is a need to understand what the public knows and believes about ENDS, as well as who uses ENDS and why. Health behaviour theories and the empirical literature show that beliefs and attitudes drive risky behaviour, including health-protective behaviours like vaccination28,29 and cancer screening30,31 and risk-taking behaviours like tanning32 and unprotected sex,33 so they may also be important motivators of ENDS use. This review does not address product safety or biological measurements of ENDS as we believe a separate, indepth review would better address these critical questions. Thus, the goal of this review is to synthesise research on the use of and beliefs about ENDS in order to identify gaps in the literature, inspire future research questions and understand the implications of these findings for public health efforts.
METHODS
Article searches
One investigator (JKP) searched PubMed, CINAHL, Web of Science, EMBASE and PsycInfo for articles published between 1 January 2006 (the year that ENDS became available in Europe and 1 year before they became available in the USA)34 and 1 July 2013. Search terms were: ‘electronic cigarette’ OR ‘electronic cigarettes’ OR ‘e-cigarette’ OR ‘e-cigarettes’ OR ‘electronic nicotine delivery.’ We selected this set of broad search terms as no relevant medical subject heading (MeSH term) existed at the time of this review. We also searched the reference sections of included articles.
Study selection
After removing duplicates, both authors reviewed the titles and abstracts (k=244), and conference or dissertation abstracts (k=17) or articles that were not in English (k=21) were discarded (see figure 1). Thirteen of the non-English articles had English-language abstracts; two of those appeared to be relevant to the review. The additional eight articles without English language abstracts appeared unlikely to contain relevant information based on translations of their titles or visual inspection of the article (eg, they contained no numbers). We also excluded articles that were not relevant to ENDS, typically because the search query identified articles with the phrase ‘i.e. cigarettes’ (k=24). In a second step, the first author reviewed the remaining abstracts and, when necessary, full articles, and conferred with the second author where eligibility was unclear. We excluded articles that did not contain original data about ENDS, such as commentaries, literature reviews and information about regulation (k=96); experiments or laboratory studies without descriptions of ‘natural’ patterns of use (ie, usage not instructed by the researcher) or subjective reports from participants on relevant dependent variables (k=26); not peer-reviewed, such as industry reports (k=10); or did not include appropriate dependent variables (ie, they reported data about internet search engines5,35 or pharmacies; k=3).36 We relied on the expanded Campbell approach to assess study quality, focusing on factors that bear on internal validity (study design) and external validity (sampling).37 We did not use a quality scoring system that yields a single score (eg, the Jadad scale)38 because of the exceptional breadth of methodologies and dependent variables across the studies and because single-score approaches combine distinct and important issues.
Figure 1.
Flow diagram of literature search and article identification.
Data abstraction
The first author coded the remaining articles (k=47) using a standardised data abstraction form. The second author or one of two additional coders reviewed each article, conferring with the first author in case of disagreements. Coders recorded ENDS awareness, natural patterns of use (ie, use outside of a lab setting, including dual use of ENDS with other tobacco products), subjective reactions to use (by users only), and beliefs or reasons for use (by users or non-users). We define ‘dual use’ as use of both ENDS and other tobacco products in the past 30 days. For the last two outcomes, coders also recorded whether the measure assessed: the perceived cost of ENDS, including the relative cost of ENDS and regular cigarettes (cost); the possibility that ENDS would serve as a gateway to other tobacco use (gateway); health, safety and side effects, including the relative safety of ENDS and regular cigarettes (health); quitting or reducing smoking or tobacco use because of ENDS (quit); the use of ENDS to avoid restrictions on smoking (restrict); the degree of satisfaction with ENDS’ taste, smell and quality (satisfaction); the extent to which ENDS have the same taste, smell or feeling of use as regular cigarettes (similar); and changes in withdrawal symptoms, desire to smoke and cravings (withdrawal). For example, the statement ‘e-cigarettes helped me quit smoking’ would be classified as quit in the category of reactions, while the statement ‘I started using e-cigarettes because I wanted to be healthier’ would be classified as health in the category of beliefs or reasons.
We selected these codes because they represented themes that were frequently reported by users or were specific public health concerns. For example, we coded for restrict because public health advocates are worried that people will use ENDS in order to bypass existing smoking regulations.7,39 Indeed, one ENDS brand highlights this benefit in its name: Smoking Everywhere. We also coded for similar because, as touted by advertisements for the product, ENDS can look, feel or taste like regular cigarettes as a way to appeal to smokers who might swap regular cigarettes for electronic ones. We review perceived cost of ENDS as the same objective (actual) cost might be prohibitively expensive for one user but negligible for another.
Because of the potential for industry affiliations and funding to influence conclusions in tobacco research,40,41 we coded that the study had a financial relationship with the ENDS industry if an ENDS manufacturer or distributor funded the study or supplied ENDS or cartridges to the researchers.
RESULTS
In all, 49 studies from 47 articles about ENDS met the inclusion criteria (see online supplementary table S1). The number of study participants ranged from 1 to 25 029. Of studies that reported location, most were conducted in the USA (k=23) or with participants from multiple countries (k=7). Other common locations included Italy (k=5) and the UK (k=4). A total of 25 studies used cross-sectional or repeated cross-sectional surveys, eight were laboratory experiments, five were case reports, four were observational, three were prospective trials, two used qualitative interviews and two used focus groups. Thirteen studies used probability sampling. Six studies relied on industry support; seven studies did not report this information. Detailed descriptions of study findings are provided in online supplementary appendix 1.
The studies had several important limitations. Nine studies recruited ENDS users in ways likely to over-represent satisfied users (eg, from online user forums, websites that sell ENDS or ENDS conventions42–50). It would be difficult to generalise to other populations based on some studies’ samples (eg, customers exiting shops in Prague;51 freshman and sophomore students recruited from one college lecture class;52 and YouTube videos of ENDS and cigarette users22). Fourteen studies did not report the date or location of data collection. Finally, although ENDS require some time to learn to use and models vary in quality, at least five laboratory studies tested only one model of ENDS or did not provide time for participants to learn to use the product.53–57
Awareness of ENDS
ENDS awareness is generally high and increasing. In three large national surveys with probability sampling, awareness of ENDS among US adults increased from 16% in 200958 to 32%–41% in 201058–60 and 58% in 2011.60 Men were more likely to be aware of ENDS than women in two of the three studies,58,59 and younger respondents were typically more likely to be aware of ENDS than older respondents.58–60 In all three studies, African-American participants were less likely to have heard of ENDS than white participants. Current smokers were always more likely to have heard of ENDS than never smokers and sometimes more likely than former smokers. For example, in a 2011 US study, 77% of current smokers, 65% of former smokers and 50% of never smokers had heard of ENDS.60
Studies in other countries found similar patterns of awareness. The International Tobacco Control (ITC) Four-Country Survey examined awareness of ENDS among probability samples of current and former smokers in 2010–2011 in the USA, UK, Canada and Australia. Across the four countries, younger, male, current smokers were more likely to have heard of ENDS than older, female, former smokers.61 Awareness was higher in the USA (73%) and UK (54%), where ENDS can be legally marketed and sold, than in Canada (40%) or Australia (20%), where they cannot be sold and may be more difficult to obtain.61 In a more recent survey of UK adults, awareness varied by smoking status; 79% of daily smokers compared with 38% of never smokers had heard of ENDS in 2012.62
Awareness among youth was variable. Only 10% of a probability sample of middle- and high-school students surveyed in 2008 in Korea were aware of ENDS.63 In contrast, 86% of Polish students (ages 15–24)64 and 70% of Midwestern US young adults (ages 20–28)65 had ever heard of ENDS in separate probability-based surveys. In the latter, men, current or former smokers, and participants with at least one close friend who smokes, were more likely to be aware of ENDS than their counterparts.65 Two-thirds (67%) of US adolescent boys ages 11–19 had heard of ENDS when surveyed in 2011.66 The discrepancy between the low rates of youth awareness in Korea and the high rates in Poland and the USA may be due to regional differences or the dates of data collection.
In addition to the ITC Four-Country survey, two other studies examined awareness only among current and former smokers. More than half (58%) of US smokers were aware of ENDS in a large 2010 survey.59 Most (86%) of a convenience sample of adults exiting stores in Prague, Czech Republic, after having purchased cigarettes had heard of ENDS.51
Only three studies reported sources of awareness. The most common sources were the internet, friends or personal contacts, and advertisements.42,50,63
Use of ENDS
Ever use
In large surveys, use of ENDS was low but increasing. Only 1% of US adults surveyed in 2009 had tried ENDS.58 Prevalence of use among the general US adult population was higher but still minimal (2%–3%) when assessed in 2010 in four national surveys with probability samples.58–60,67 In all of these studies, more current smokers had tried ENDS than former smokers or never smokers.59,60,67 Rates of use in the USA continued to rise in 2011 (6% overall) with the same gradient pattern by smoking status (1% of never smokers, 7% of former smokers and 21% of current smokers).60 Demographic correlates of use varied across studies. When these studies limited their samples to only smokers, ENDS use was unrelated to history of quit attempts in two studies,58,59 but smokers in one study who intended to quit in the next 6 months were more likely to have tried ENDS than smokers with no intention to quit.59
Multiple studies included only current and former smokers in their samples. Across the four countries surveyed by ITC, 8% of current and former smokers had ever tried ENDS.61 Daily heavy smokers had the highest use and long-term quitters had the lowest use. ENDS users were not more likely to have quit smoking since the previous wave of the ITC survey than non-users. Overall, 20% of adult US smokers had tried ENDS when surveyed as part of a 2011 probability panel.68 Unsuccessful quitters were more likely to have tried ENDS than those who had never tried to quit. In another probability panel of US smokers, 10% of cigarette-only smokers had tried ENDS, but 24% of dual cigar and cigarette users had done so.69 Current smokers in the Legacy Longitudinal Smoker Cohort were more likely to have tried ENDS than former smokers (6% vs 3%, respectively).59 Li and colleagues found that 7% of current smokers and recent quitters in New Zealand in 2011 had ever purchased ENDS (a proxy for use).70 Finally, in the most recent survey of ENDS use in 2012, 22% of current smokers in the UK had ever tried ENDS, compared with 4% of former smokers and <1% of never smokers.62 In many of these studies of current and former smokers, women61,68 and younger participants59,61,62,68,70 had higher rates of use than men and older participants. In some studies, ENDS use was not associated with quit attempts59,70 or quit intentions.59,61,68
In surveys with probability samples, use of ENDS by adolescents and young adults varied considerably by region and year, from less than 1% (US male adolescents in 2011 and Korean adolescents in 2008)63,66 to 21% (Polish high school and university students in 2010–2011).64 In other surveys with probability sampling, 5% of college students in North Carolina71 and 7% of young adults in the Midwestern US had tried ENDS.65 In general, the higher rate of ENDS use among Polish youth compared with US youth may relate, in part, to higher population-wide rates of tobacco use in Poland than in the USA.72,73 Across studies of youth, men,63–65,71 smokers63–65,71 and those with important others (friends, family or partners) who smoked64,65 were often more likely to have tried ENDS than their counterparts. In at least two studies, the relationship between ever use of ENDS and smoking status should be interpreted with caution due to the very low prevalence of smoking in the sample.63,66
Other reported rates of ever use of ENDS are difficult to interpret because of the nature of their samples. For example, 85% of a convenience sample of adults surveyed by Etter and Bullen had used ENDS, but the majority of their participants were recruited through online ENDS forums.44 Among callers to seven tobacco quitlines who responded to a follow-up survey (35% response rate), 31% had ever tried ENDS, and users were less likely to have quit smoking since their initial call to the quit-line than non-users.74
Current use
Adults consistently reported low current use (ie, in the past 30 days). Around 1% of respondents were current ENDS users in three 2010 US national, probability-based surveys.58,59,67 As with ever use of ENDS, current smokers were more likely to be current ENDS users than either former or never smokers.58,59,62 In surveys limited to current and former smokers, rates varied from 3% (across four countries in the 2010–2011 ITC survey)61 to 8% (USA in 2011).68 In a convenience sample of callers to tobacco quitlines, 9% of current and former smokers reported that they currently used ENDS some days or every day.74
Youth also reported low current use. Only 1% of young adults in the Midwestern USA65 and 2% of North Carolina college students71 were current ENDS users in 2010–2011 and 2009, respectively. Possibly reflecting the overall higher rates of smoking in Poland compared with the USA, a higher percentage of high school and university students (7%) were current ENDS users.64
Dual use
In population-based surveys, most current (or past 30 days) smokers were not current (or past 30 days) ENDS users. In two 2010 probability samples of US adults, 4%59 and 6%58 of current smokers had used ENDS in the past 30 days. More than 11% of those who were current users of more than one tobacco product (eg, cigarettes, cigars, snuff, hookah) had used ENDS in the past month.58 In another probability sample, 11% of Polish youth and young adults who were current smokers had used ENDS in the past 30 days.64 In a large survey with UK adults based on an opt-in survey panel, 3% of daily smokers in 2010 and 7% of daily smokers in 2012 used ENDS.62
Dual use of ENDS with regular cigarettes was fairly common in convenience samples. In surveys with convenience samples of dedicated ENDS users, 12%–34% of ENDS users were current smokers.42,44,46,47 Of 179 ENDS users, 6% used hookah, snuff or some other non-cigarette tobacco product.47 However, among a sample of people who had bought ENDS 6 months earlier, 35% of current ENDS users did not smoke cigarettes, suggesting up to 65% dual use.48 Finally, in three prospective trials with convenience samples, dual use of ENDS and regular cigarettes appeared to be common, with some of the smokers who reduced their consumption of cigarettes continuing to use ENDS at the end of the study.75–77
Amount and duration of use
Daily use among ENDS users was common43–47,55 in all but two studies.48,74 It is difficult to further quantify the amount of use because, unlike regular smoking which can be measured by the number of cigarettes smoked, ENDS use has no clear metric. An individual does not usually ‘vape’ an entire cartridge of one ENDS in a single sitting. Some studies quantified use by estimating puffs per day (range 120–236),42–45 while others reported the number of bouts of use per day (median of 20 per day46 or 67% use more than 15 times per day).47 Measurements of the number of e-liquid cartridges (range 0–4)75–77 or millilitre of e-liquid used per day (range 3–5 mL)42,55 are difficult to interpret because cartridges leak, vary in strength both within and across brands, and require different levels of vacuum to inhale.3,12,78 Another metric for quantifying use is puff duration. Two studies, one in a laboratory55 and one that examined 73 YouTube videos,22 found that ENDS users take longer puffs on ENDS than conventional smokers do on regular tobacco cigarettes.
Ten studies reported how long participants had been using ENDS.42–47,50,55,74,79 Estimates varied from less than 1 month of prior use for 62% of callers to state quitlines74 to a mean of 13 months of use in a convenience-based survey of experienced ENDS users (n=104) conducted in 2011.46 We are not aware of any data about the extent or amount of use of disposable ENDS, although five studies described the use of modified ENDS (sometimes called ‘mods’) or personal vapourisers that do not mimic the appearance of regular cigarettes.42,46,49,50,79
Subjective reactions to using ENDS
Cost
ENDS users’ experiences with the cost savings from using ENDS in lieu of regular tobacco cigarettes are inconsistent.43,50 In open-ended survey questions, some participants in a convenience sample survey of ENDS users (n=81) said that they found ENDS to be less expensive than cigarettes (10 comments), while others said they were too expensive (14 comments).43 Most dedicated ENDS users interviewed at a convention (n=15) found them less expensive than cigarettes.50 Finally, among Czech smokers who had tried but stopped using ENDS, 13% did so because they found ENDS to be too expensive.51
Gateway use
Among the 179 Polish ENDS users surveyed online in 2009, 25 reported that they were non-smokers when they previously began using ENDS. Of those, 20% (n=5) currently smoked cigarettes at the time of the survey.47
Health and safety
Many users report positive changes in their health after they begin using the product. In surveys, interviews and case reports, users often describe improved breathing,42,43,50 less coughing,42,43,50 fewer sore throats,43 and improvements in overall health and fitness.43,50,80 In one case report, switching from cigarettes to ENDS alleviated a patient’s previously elevated white blood cell count (neutrophilia).81
Some users also report experiencing side effects from using ENDS. As of the first quarter of 2012, the FDA had received 47 reports about adverse events related to ENDS use.82 Of these, they classified eight as serious adverse events, including pneumonia and chest pain, and 39 as minor, including headache and cough. Of the 405 health effects reported by users in an online ENDS forum, 326 were negative; the most frequently reported problems were in the mouth, throat, respiratory system and neurological system.83 One case report described a patient developing lipid pneumonia,84 and another described a patient experiencing heart arrhythmia from using ENDS.85
In surveys with non-probability samples, laboratory research and other case reports, the majority of reported side effects were minor, including mouth or throat irritation/dryness,42–44,53 cough,47,80,86 vertigo,43,53 headache43,47,53 and nausea.43,53 In three prospective trials in which smokers tried ENDS for 6 or 12 months, there were no serious adverse events.75–77 Many of the minor side effects experienced at baseline, including cough,75–77 mouth and throat irritation,75,77 and headache,76,77 lessened considerably or resolved completely by the end of the study period.
Quitting or reducing tobacco use
Although successful quitting was generally not associated with ENDS use in large surveys,58,61,68,74 in convenience sample surveys, focus groups, case studies and interviews with dedicated ENDS users, they often reported that using the product helped them quit smoking42–44,46–50,80,81,86 or significantly reduce tobacco use,42,44,48 often despite being heavy smokers or having failed quit attempts in the past.43,44,46,48–50,80,81,86 Unlike quitting smoking, reducing smoking may not represent a positive public health outcome, given that it may indicate dual use of ENDS and regular cigarettes.
Only three longitudinal studies have examined smokers’ use of ENDS to quit smoking. Two of the three prospective trials were uncontrolled. In the first, a 12-month trial with 14 patients being treated for schizophrenia, seven of 14 participants reduced their smoking by at least 50% and two others quit smoking entirely.76 In the second, a 6-month prospective trial of 40 smokers, 13 were lost to follow-up, 13 reduced their cigarette consumption by at least 50% and an additional nine participants quit smoking entirely.75 Another prospective trial randomly assigned 300 smokers to use either ENDS with nicotine or ENDS without nicotine.77 At the end of the 12-month period, 11% of smokers using ENDS with nicotine had quit and 10% reduced their cigarette consumption by at least 50%, while 4% of the non-nicotine group had quit and 12% had reduced their consumption. More than a third were lost to follow-up. The differences in cessation between groups were not statistically significant. The results of these trials should be interpreted with caution given that only one randomised participants to conditions (and it did not include a comparison condition with an alternative quit aid), and all three relied on convenience samples in a limited geographical setting.
Restrictions on smoking
The extent to which smokers use ENDS to avoid smoking restrictions was not clear. About a third (36%) of ENDS users in one survey said that they frequently used ENDS in places where smoking was banned.42 In contrast, in another survey of ENDS enthusiasts (n=104) recruited from a convention, 90% said they were able to use where smoking was banned, although they did not clarify how often they did so.46 In a third survey, a substantial number of daily ENDS users from a variety of countries reported using their ENDS at work (71%) or in cafes, restaurants or bars (43%), but it is difficult to interpret these results with respect to avoiding smoking restrictions given that they vary by country.44
Satisfaction
Satisfaction with ENDS that contained nicotine was moderate in most laboratory studies53,56,57,79 and very high in surveys of committed ENDS users.42–44,49,50 Users often mentioned taste and flavour. For example, more than 90% of users surveyed by Etter and Bullen liked the taste of ENDS.44 Smokers who used ENDS in prospective trials had mixed reactions75,77 as did many smokers interviewed in Prauge.51 Finally, in five studies, ENDS users expressed concerns about the quality of ENDS they used, including leaking cartridges or broken components.43,44,50,51,75 Some stopped using ENDS because of problems with the devices.44,51
Similarity to regular cigarettes
Studies showed little agreement about how much users of ENDS thought they look, feel or taste like cigarettes, as well as whether similarity to cigarettes was a benefit or a drawback. In small focus groups with former smokers (n=11), users mentioned that they liked how using ENDS mimicked the feel of smoking cigarettes, and that they swapped regular cigarettes for ENDS as part of the same daily routines (eg, used after a meal).49 The similarity between the products also made it easier to switch from one to the other. Other ENDS users recruited at a convention (n=15) noted that their desire for ENDS to mimic regular cigarettes had changed over time; although they began using ENDS that looked and felt like cigarettes, most transitioned to using personal vapourisers that did not look like cigarettes.50 In a large convenience sample survey, over a half of the participants said they used ENDS ‘in a similar manner’ as cigarettes.42 A small number of users stopped using ENDS because they did not have the same flavour,43 but others preferred the flavour of ENDS to regular cigarettes.46 During bouts of use of ENDS as part of lab studies (n=20 and n=32), participants’ ratings of ‘mild as own brand’56,57 and ‘taste like own brand’57 increased as they continued to use the product. In direct comparisons, about two-thirds of users in separate convenience sample surveys rated ENDS as equally or more satisfying than cigarettes.42,47
Withdrawal symptoms, desire to smoke and cravings
ENDS typically provided some relief of smokers’ nicotine cravings and withdrawal symptoms. In six lab studies, participants who had been tobacco abstinent for 2–12 h reported that using nicotine-containing ENDS reduced their desire to smoke or cravings during their inlaboratory use session,53,56,57,79,87,88 although this was not true in one study.54 In some cases, this reduction was shown to be greater for nicotine-containing ENDS than for non-nicotine ENDS53,87,88 or for just holding unlit regular cigarettes or ENDS.56,88 Interestingly, this reduction in desire to smoke or lessening of cravings occurred even in some cases where objectively measured nicotine uptake was low or modest.53,56 Evidence for the alleviation of specific withdrawal symptoms in these lab studies was inconsistent.53,56,57,79,87,88
Dedicated ENDS users frequently reported that using ENDS successfully reduced their cravings to smoke and some withdrawal symptoms.42–44,46,49,50 For example, experienced vapers interviewed at the Midwest Vapefest ‘routinely described relief of nicotine cravings within 5 minutes of vaping’ and said they could comfortably go long periods of time between bouts of use without experiencing withdrawal symptoms.50 However, in a large online survey with current and former users, 33% of users said they stopped using because ENDS did not reduce their cravings.44 Finally, in the three prospective trials in Italy that followed smokers who began using ENDS for 6–12 months, participants experienced few or no withdrawal symptoms.75–77
Beliefs about and reasons for using ENDS
Cost
ENDS users’ beliefs about the cost savings from using ENDS in lieu of regular tobacco cigarettes are inconsistent. In multiple convenience sample surveys from the USA and other countries, a small per cent of users said they first tried or used ENDS for cost savings,42,43,47,74 although a much higher per cent reported this reason in another convenience sample survey.44 More than half (53%) of UK smokers, including a mix of users and non-users, believed that ENDS might be too expensive.62
Gateway use
One study documented beliefs about ENDS as gateways to use of tobacco products. Young adult smokers and non-smokers in focus groups in Minnesota, USA, believed that ENDS and other novel tobacco products, including snus and dissolvable tobacco, might appeal to non-smokers who have ‘always wanted to know the feeling of a cigarette’.89 They felt that these products could lead non-smokers to become smokers.
Health and safety
Many users believe that ENDS are healthier than regular cigarettes for themselves42–44,46,47,49,50 or for others,46 and they use ENDS for this reason. For example, of 179 Polish adult ENDS users in a convenience sample, 82% believed that ENDS were less dangerous than cigarettes, and an additional 15% believed that ENDS were ‘absolutely safe’.47 Typically, only a handful of users are concerned about the potential negative health effects or toxicity of ENDS.43,44
Respondents in surveys that include non-users generally indicate slightly less confidence in the healthfulness of ENDS. In three probability sample surveys of adult current and former smokers, 70%–85% of those who were aware of ENDS believed they were less harmful than regular cigarettes.59,61 In a non-probability survey of adult smokers in the UK, 71% held this belief, although 21% still felt that ENDS might not be safe enough.62 Only a third of adult New Zealand smokers believed that ENDS were less harmful than regular cigarettes.70
In multiple studies of students and young adults, 23%–55% believed that ENDS were safer than regular cigarettes.64,65,71 In focus groups, young adults who had never tried ENDS expressed mixed beliefs about whether ENDS were equivalent to or less harmful than regular cigarettes.89
Examining Twitter accounts related to smoking cessation, Prochaska and colleagues also found a range of beliefs about the healthfulness of ENDS, including tweets with health warnings as well as tweets touting health benefits.90
Quitting or reducing tobacco use
The majority of ENDS users believe that ENDS can help people quit or reduce smoking, and they often use ENDS themselves for this reason.42–44,46,47,49,61,62,74,80 Two surveys with probability sampling described beliefs about the cessation properties of ENDS. Among Midwestern young adults (ages 20–28), almost half (45%) of those who had heard of ENDS agreed that they can help people quit smoking.65 A third of New Zealand smokers believed that ENDS could help smokers quit, and 58% said they would be willing to try ENDS for that reason.70
In two studies in which researchers provided smokers with ENDS (both n=40), these smokers later said that they would recommend ENDS to friends or family who wished to quit smoking.53,75 Some commercial and personal Twitter accounts also promoted ENDS as a quit tool.90 Finally, in focus groups of young adult non-users (n=66), participants expressed differing opinions about whether ENDS could be used as a cessation device, and some spontaneously recounted anecdotes about people they knew who quit smoking using ENDS.89
Restrictions on smoking
ENDS users have conflicting beliefs about using ENDS to avoid smoking restrictions. In some surveys, only a small per cent of users describe this as a motivation.42,43,47,74 However, in other surveys, more than 40% of respondents said they used ENDS for this reason.46,61,62
In small focus groups, non-users noted that an advantage of ENDS and other novel tobacco products (snus, dissolvable tobacco) is the ability to get around smoking bans and use them in places where one cannot smoke.89
Satisfaction
Some vapers use ENDS for the pleasure of the experience.43 Smokers who have not tried ENDS had mixed opinions about their potential satisfaction. About one third believed that ENDS might taste unpleasant.62
Similarity to regular cigarettes
Some smokers and users liked that ENDS resembled or felt like regular cigarettes, while others considered this a drawback.42,43,62,74 Certain elements of the vaping and smoking experiences were clearly different. Because ENDS are more complex than cigarettes, interviewed users (n=15) noted that, unlike cigarettes, they required a learning curve to use properly.50 In focus groups, some young adult smokers mentioned that the social experience of smoking might not be replicated with ENDS.89
Withdrawal symptoms, desire to smoke and cravings
Beliefs about the addictive properties of ENDS vary. About a quarter of Midwestern young adults, most of whom were non-users, believed that ENDS are less addictive than regular cigarettes.65 In contrast, in an internet-based survey of a convenience sample of Polish ENDS users, 60% believed that ENDS were less addictive than regular cigarettes, and an additional 7% believed that ENDS were not addictive at all.47 However, more than a half believed they were addicted to ENDS.
Very few (8%) ever users in another online survey said they were afraid of becoming addicted to ENDS, although 4% of the sample stated that they used ENDS because they were unable to stop using them.44 More than half (60%) of UK smokers, only some of whom had used ENDS, believed that ENDS might satisfy the desire to smoke.62 Indeed, some vapers used ENDS to relieve their cravings or alleviate their withdrawal symptoms.43,44,46,50,74
Other beliefs
Three other important themes emerged in the literature. First, concerns about personal appearance (eg, preventing yellow teeth) or odour (eg, clothes not smelling like smoke) sometimes motivated interest in or use of ENDS.43,44,47,49,50,62,75,89 Second, ENDS users felt a sense of camaraderie with one another; they gathered at vaping conventions46,50 and in online forums where they shared information, recommendations and personal experiences.43,49,50 However, a small minority of ENDS users were concerned about the social acceptability of ENDS use and felt embarrassed about using the product.42,51 University students, few of whom had tried ENDS, viewed ENDS use as more socially acceptable than smoking.52 Third, some ENDS enthusiasts were concerned that the product will be banned.43,44,50 We did not find any arguments by users that ENDS should be specifically exempted from smoke-free indoor air laws.
CONCLUSIONS
As the public health community settles an internal debate over the safety and cessation properties of ENDS, interest and use by the public are increasing rapidly. Consistently across the literature, current and former smokers were more likely to be aware of and use ENDS than non-smokers, although quit intentions and attempts were often not associated with ENDS use. In surveys and interviews with dedicated ENDS users, users were generally satisfied with the product and believed it was healthier than regular cigarettes. Current and former smokers believed that ENDS could help them quit smoking, and a significant proportion reported that ENDS already helped them reduce the amount they smoke or quit entirely. Dedicated ENDS users who are or were smokers often commented that ENDS alleviate their cravings to smoke, and they felt healthier because of using ENDS. Some evidence suggested that smokers use ENDS in order to avoid smoking restrictions, but no longitudinal studies have evaluated whether ENDS serve as a gateway to future tobacco use. In lab-based studies, smokers often reported that ENDS reduced their desire to smoke and alleviated some withdrawal symptoms, although they experienced minor side effects. In prospective trials testing ENDS as a potential quit aid, a moderate number of smokers were able to quit, but many appeared to engage in dual use of ENDS and regular cigarettes.
Some of the reviewed studies received either funding from ENDS companies or used ENDS that these companies donated. Prior systematic reviews have found that financial relationships may influence study findings. For example, nearly all studies funded by the tobacco industry found a relationship between smoke-free restaurant and bar laws and reduced sales or employment in the hospitality industry; none of the non-industry supported studies found this association.40 Although we detected no apparent pattern of results associated with ENDS industry funding, it remains important to be aware of possible conflicts of interest when interpreting these findings.
The literature on ENDS described in this review suggests several important questions that future research should address. First, future research should identify effective messages for discouraging ENDS use among vulnerable populations, given the beliefs and attitudes identified in our review. Two specific vulnerable populations are non-smokers who could begin smoking as a result of developing nicotine addiction from ENDS use and smokers who use ENDS only as a bridge to their next cigarette. If we better understand why ENDS may be attractive to some vulnerable populations (eg, teenagers think ENDS are fashionable),89 we can craft and deliver effective messages that deter use.
Another question for future research is how well perceived health risks of using ENDS correspond to objective risks, and whether beliefs about these risks change as additional safety data become available. Future safety studies will hopefully clarify some of the conflicting findings of past studies.2,3,9 Current ENDS users who are former smokers frequently state that they use ENDS because they are healthier than regular cigarettes. If future safety studies find evidence of long-term harms, will these ENDS users stop using? If not, can we improve how we communicate safety information? Designing appropriate warning labels on ENDS packages could be an important first step.
A third important research question is whether ENDS users’ self-reports of successful smoking cessation match evidence from the currently ongoing randomised controlled trials (RCTs) evaluating the efficacy of ENDS as a smoking cessation tool. Caponnetto and colleagues conducted an RCT, but it did not include an arm testing an alternative to ENDS (ie, nicotine replacement therapies (NRTs) or other proven smoking cessation methods).77 Bullen and colleagues recently described the protocol for their study in which adult smokers who want to quit are randomly assigned to use ENDS with 16 mg nicotine cartridges, 21 mg nicotine patches or placebo (ENDS with 0 mg nicotine).91 The primary outcome is the proportion of participants who maintain smoking abstinence 6 months after the start of the study, but investigators will also assess reductions in smoking, safety of the ENDS and patches, and perceptions of the products. This RCT and other similar studies are critical to understanding whether, as users have reported, ENDS can serve as harm reduction tools.
Finally, it will be important to understand how users of the various kinds of ENDS differ. ENDS are available in many different varieties. For example, there are disposable ENDS, often available at gas stations for only a few dollars, and more expensive refillable models that require an initial investment for a starter kit. Some ENDS mimic the appearance of regular cigarettes, while some modified ENDS do not resemble cigarettes. Understanding whether and why different types of ENDS appeal to different populations could assist efforts to regulate these products. For example, if young people who have not already initiated tobacco use prefer inexpensive, readily available disposable models, restricting the sale of these models would be a key public health priority.
Several additional research questions, beyond the scope of the material covered in this review, will also be important to answer. One key question is whether ENDS are safe to use. Observing adverse events in RCTs, such as the one described by Bullen and colleagues,91 will help to determine this. What is the best way to measure ENDS use (eg, number of puffs vs number of e-liquid cartridges used) so that findings can be compared across studies? Do ENDS act as gateway devices, causing individuals who would not otherwise use tobacco to initiate use? More than 10% of college students who reported using ENDS had never smoked a conventional cigarette,71 and we do not yet know whether these young people will start smoking as a result of their experience with ENDS. Prospective cohort studies, particularly with adolescents and young adults, are necessary to track patterns of use of ENDS and other tobacco products. Other important questions posed by a recent Cancer Research UK report include: how will future regulation impact quit attempts using ENDS; do ENDS ‘re-normalise’ smoking; and are ENDS undermining the use of other NRTs in smoking cessation attempts?16
Limitations to this systematic review include that, because the quality of the studies included in this review varies tremendously, readers should interpret the findings with care. In addition, as we reviewed only articles written in English and indexed electronically or cited in papers we reviewed, the review may have missed some relevant articles. While we provide an overview of findings across the literature, we did not conduct a meta-analysis to provide a quantitative synthesis due to the small number of studies and variability in their designs and measures. Also, the codes we developed may not have included all relevant areas and some findings may have overlapped across codes. Finally, because this literature is young and evolving quickly, the conclusions of this review may not capture all of these changes.
In sum, concerns about ENDS include their safety, lack of regulation, possibility of gateway use, and potential for dual use or avoidance of existing smoking restrictions. However, harm reduction advocates note that ENDS may be less harmful—and are highly unlikely to be more harmful—to smokers than regular cigarettes which are a proven cause of morbidity and mortality.8,92 Thus, the concerns about ENDS must be balanced with the possibility that ENDS could prove to be a valuable harm reduction tool for addicted adult smokers, provided they do not encourage dual use or prevent other cessation efforts. Furthermore, as we learn more about the safety of ENDS and their efficacy as a quit tool, we will hopefully be able to design better tobacco control and cessation programmes in the future.
Supplementary Material
What this paper adds.
Electronic nicotine delivery systems (ENDS) could be a harm-reducing alternative to regular cigarettes among adult tobacco smokers, but they could also be used to avoid smoking restrictions or act as a gateway to other tobacco use. This systematic review found that:
ENDS awareness and use are increasing.
Users frequently report improved health and a high degree of satisfaction with the product.
Many users say that ENDS helped them quit smoking, but this claim has not yet been evaluated by randomised controlled trials comparing ENDS with other cessation tools.
Future research should examine whether perceptions of ENDS’ safety change as more objective safety data become available, what public health messages are best for discouraging use among vulnerable populations and whether different types of ENDS (eg, disposable vs refillable) attract different user populations.
Acknowledgements
We thank Emily A Elstad and Laura E Bach for their assistance in coding the articles. We thank Martin Mozes for his help with translation.
Funding Support for this study was provided by the Cancer Control Education Program at UNC Lineberger Comprehensive Cancer Center (R25 CA57726).
Footnotes
Contributors JKP and NTB designed and conducted the systematic review. JKP drafted the manuscript. NTB assisted with revisions.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Benowitz NL. Smokeless tobacco as a nicotine delivery device: harm or harm reduction? Clin Pharmacol Ther. 2011;90:491–3. doi: 10.1038/clpt.2011.191. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration . Evaluation of e-cigarettes. DPATR-FY-09-23; 2009. [Google Scholar]
- 3.Bahl V, Lin S, Xu N, et al. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–37. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration Public Health Focus—Electronic Cigarettes (e-Cigarettes) http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm172906.htm.
- 5.Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med. 2011;40:448–53. doi: 10.1016/j.amepre.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Grana RA. Electronic Cigarettes: A New Nicotine Gateway? J Adolesc Health. 2013;52:135–6. doi: 10.1016/j.jadohealth.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Cobb NK, Abrams DB. E-cigarette or drug-delivery device? Regulating novel nicotine products. N Engl J Med. 2011;365:193–5. doi: 10.1056/NEJMp1105249. [DOI] [PubMed] [Google Scholar]
- 8.Rodu B. The scientific foundation for tobacco harm reduction, 2006–2011. Harm Reduct J. 2011;8:19. doi: 10.1186/1477-7517-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laugesen M. Safety report on the Ruyan® e-cigarette cartridge and inhaled aerosol. Health New Zealand Ltd; 2008. [Google Scholar]
- 10.Riker CA, Lee K, Darville A, et al. E-cigarettes: promise or peril? Nurs Clin North Am. 2012;47:159–71. doi: 10.1016/j.cnur.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Varughese S, Teschke K, Brauer M, et al. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am J Ind Med. 2005;47:411–18. doi: 10.1002/ajim.20151. [DOI] [PubMed] [Google Scholar]
- 12.Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control. 2011;20:47–52. doi: 10.1136/tc.2010.037259. [DOI] [PubMed] [Google Scholar]
- 13.Cobb NK, Byron MJ, Abrams DB, et al. Novel nicotine delivery systems and public health: the rise of the “e-cigarette”. Am J Public Health. 2010;100:2340–2. doi: 10.2105/AJPH.2010.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadwiger ME, Trehy ML, Ye W, et al. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr. 2010;1217:7547–55. doi: 10.1016/j.chroma.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr R T. 2011;34:1442–58. [Google Scholar]
- 16.de Andrade M, Hastings G. Tobacco harm reduction and nicotine containing products: research priorities and policy directions. Cancer Research UK; 2013. [Google Scholar]
- 17.Gilroy J. Electronic Cigarette Regulations in King County, Washington. Paper presented at: National Conference on Tobacco or Health; Kansas City, Missouri. August 15–17, 2102. [Google Scholar]
- 18.Tierney J. E-cigarettes help smokers quit, but they have some unlikely critics. The New York Times. 2011 [Google Scholar]
- 19.Mishori R. E-cigarettes: can they help you quit? Parade Magazine. Parade Publications; New York, NY: 2009. [Google Scholar]
- 20.Grana RA, Glantz SA, Ling PM. Electronic nicotine delivery systems in the hands of Hollywood. Tob Control. 2011;20:425–6. doi: 10.1136/tc.2011.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Ann Intern Med. 2010;153:607–9. doi: 10.7326/0003-4819-153-9-201011020-00011. [DOI] [PubMed] [Google Scholar]
- 22.Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22:103–6. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- 23.Modi N, Schmid B, Miller R. UBS investment research: clearing the smoke on E-cigarettes. UBS; 2012. [Google Scholar]
- 24.Herzog B, Metrano B, Gerberi J. Equity research tobacco talk survey—E-cigarettes a promising opportunity. Wells Fargo Securities. 2012 [Google Scholar]
- 25.Kamerow D. Big Tobacco lights up e-cigarettes. BMJ. 2013;346:f3418. doi: 10.1136/bmj.f3418. [DOI] [PubMed] [Google Scholar]
- 26.Esterl M, Kell J. Big tobacco takes up e-cigs. Wall Street J April. 2013;26:B1. [Google Scholar]
- 27.Lorillard Inc . Reports Third Quarter 2012 Results. Greensboro, NC: 2012. [Google Scholar]
- 28.Brewer NT, Chapman GB, Gibbons FX, et al. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26:136–45. doi: 10.1037/0278-6133.26.2.136. [DOI] [PubMed] [Google Scholar]
- 29.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev Med. 2007;45:107–14. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 30.McCaul KD, Branstetter AD, Schroeder DM, et al. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. 1996;15:423–9. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 31.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 32.Holman DM, Watson M. Correlates of intentional tanning among adolescents in the United States: a systematic review of the literature. J Adolesc Health. 2013;52(5 suppl):S52–9. doi: 10.1016/j.jadohealth.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheeran P, Abraham C, Orbell S. Psychosocial correlates of heterosexual condom use: a meta-analysis. Psychol Bull. 1999;125:90–132. doi: 10.1037/0033-2909.125.1.90. [DOI] [PubMed] [Google Scholar]
- 34.Timeline—A quick look into electronic cigarette history 2012 [accessed 21 Mar 2013]; http://blog.blucigs.com/timeline-a-quick-look-into-electronic-cigarette-history/
- 35.Huang J, Zheng R, Emery S. Assessing the impact of the national smoking ban in indoor public places in China: evidence from quit smoking related online searches. PloS one. 2013;8:e65577. doi: 10.1371/journal.pone.0065577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidenberg AB, Hong W, Liu J, et al. Availability and range of tobacco products for sale in Massachusetts pharmacies. Tob Control. 2012;22:372–5. doi: 10.1136/tobaccocontrol-2012-050591. [DOI] [PubMed] [Google Scholar]
- 37.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Houghton Mifflin; Boston, MA: 2002. [Google Scholar]
- 38.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 39.Henningfield JE, Zaatari GS. Electronic nicotine delivery systems: emerging science foundation for policy. Tob Control. 2010;19:89–90. doi: 10.1136/tc.2009.035279. [DOI] [PubMed] [Google Scholar]
- 40.Scollo M, Lal A, Hyland A, et al. Review of the quality of studies on the economic effects of smoke-free policies on the hospitality industry. Tob Control. 2003;12:13–20. doi: 10.1136/tc.12.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19:465–80. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawkins L, Turner J, Roberts A, et al. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013;108:1115–25. doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- 43.Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–28. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 45.Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38:1219–20. doi: 10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- 46.Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65:1037–42. doi: 10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 47.Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an Internet survey. Drug Alcohol Rev. 2013;32:133–40. doi: 10.1111/j.1465-3362.2012.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: results from an online survey. Am J Prev Med. 2011;40:472–5. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: a qualitative approach. Addict Sci Clin Pract. 2013;8:5. doi: 10.1186/1940-0640-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McQueen A, Tower S, Sumner W. Interviews with “vapers”: Implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13:860–7. doi: 10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- 51.Kralikova E, Kubatova S, Truneckova K, et al. The electronic cigarette: what proportion of smokers have tried it and how many use it regularly? Addiction. 2012;107:1528–9. doi: 10.1111/j.1360-0443.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 52.Trumbo CW, Harper R. Use and perception of electronic cigarettes among college students. J Am Coll Health. 2013;61:149–55. doi: 10.1080/07448481.2013.776052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bullen C, McRobbie H, Thornley S, et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 54.Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19:87–8. doi: 10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farsalinos KE, Romagna G, Tsiapras D, et al. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–14. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vansickel AR, Cobb CO, Weaver MF, et al. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–53. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012;107:1493–500. doi: 10.1111/j.1360-0443.2012.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Regan AK, Promoff G, Dube SR, et al. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22:19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- 59.Pearson JL, Richardson A, Niaura RS, et al. E-cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–66. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King BA, Alam S, Promoff G, et al. Awareness and ever use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob Res. 2013;15:1623–7. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44:207–15. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dockrell M, Morison R, Bauld L, et al. E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res. 2013;15:1737–44. doi: 10.1093/ntr/ntt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho JH, Shin E, Moon SS. Electronic-cigarette smoking experience among adolescents. J Adolesc Health. 2011;49:542–6. doi: 10.1016/j.jadohealth.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879–85. doi: 10.1542/peds.2011-3448. [DOI] [PubMed] [Google Scholar]
- 65.Choi K, Forster J. Characteristics associated with awareness, perceptions, and use of electronic nicotine delivery systems among young US Midwestern adults. Am J Public Health. 2013;103:556–61. doi: 10.2105/AJPH.2012.300947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pepper JK, Reiter PL, McRee AL, et al. Adolescent males’ awareness of and willingness to try electronic cigarettes. J Adolesc Health. 2013;52:144–50. doi: 10.1016/j.jadohealth.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMillen R, Maduka J, Winickoff J. Use of emerging tobacco products in the United States. J Environ Public Health. 2012:989474. doi: 10.1155/2012/989474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103:923–30. doi: 10.2105/AJPH.2012.301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richardson A, Xiao H, Vallone DM. Primary and dual users of cigars and cigarettes: Profiles, tobacco use patterns and relevance to policy. Nicotine Tob Res. 2012;14:927–32. doi: 10.1093/ntr/ntr306. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Bullen C, Newcombe R, et al. The use and acceptability of electronic cigarettes among New Zealand smokers. N Z Med J. 2013;126:48–57. [PubMed] [Google Scholar]
- 71.Sutfin EL, McCoy TP, Morrell HE, et al. Electronic cigarette use by college students. Drug Alcohol Depend. 2013;131:214–21. doi: 10.1016/j.drugalcdep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention Vital signs: current cigarette smoking among adults aged ≥18 years — United States, 2005–2010. MMWR. 2011;60:1207–12. [PubMed] [Google Scholar]
- 73.Bogdanovica I, Godfrey F, McNeill A, et al. Smoking prevalence in the European Union: a comparison of national and transnational prevalence survey methods and results. Tob Control. 2011;20:e4. doi: 10.1136/tc.2010.036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787–91. doi: 10.1093/ntr/ntt061. [DOI] [PubMed] [Google Scholar]
- 75.Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10:446–61. doi: 10.3390/ijerph10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PloS one. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res. 2010;12:905–12. doi: 10.1093/ntr/ntq114. [DOI] [PubMed] [Google Scholar]
- 79.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–70. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caponnetto P, Polosa R, Russo C, et al. Successful smoking cessation with electronic cigarettes in smokers with a documented history of recurring relapses: a case series. J Med Case Reports. 2011;5:585. doi: 10.1186/1752-1947-5-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farsalinos KE, Romagna G. Chronic idiopathic neutrophilia in a smoker, relieved after smoking cessation with the use of electronic cigarette: a case report. Clin Med Insights Case Rep. 2013;6:15–21. doi: 10.4137/CCRep.S11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen I. FDA summary of adverse events on electronic cigarettes. Nicotine Tob Res. 2013;15:615–16. doi: 10.1093/ntr/nts145. [DOI] [PubMed] [Google Scholar]
- 83.Hua M, Alfi M, Talbot P. Health-related effects reported by electronic cigarette users in online forums. J Med Internet Res. 2013;15:e59. doi: 10.2196/jmir.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012;141:1110–13. doi: 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 85.Monroy AE, Hommel E, Smith ST, et al. Paroxysmal atrial fibrillation following electronic cigarette use in an elderly woman. Clinical Geriatrics. 2012;20:28–32. [Google Scholar]
- 86.Caponnetto P, Polosa R, Auditore R, et al. Smoking cessation with e-cigarettes in smokers with a documented history of depression and recurring relapses. Int J Clin Med. 2011;2:281–4. doi: 10.1186/1752-1947-5-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dawkins L, Turner J, Crowe E. Nicotine derived from the electronic cigarette improves time-based prospective memory in abstinent smokers. Psychopharmacology. 2013;227:377–84. doi: 10.1007/s00213-013-2983-2. [DOI] [PubMed] [Google Scholar]
- 88.Dawkins L, Turner J, Hasna S, et al. The electronic-cigarette: Effects on desire to smoke, withdrawal symptoms and cognition. Addict Behav. 2012;37:970–3. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Choi K, Fabian L, Mottey N, et al. Young adults’ favorable perceptions of snus, dissolvable tobacco products, and electronic cigarettes: findings from a focus group study. Am J Public Health. 2012;102:2088–93. doi: 10.2105/AJPH.2011.300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prochaska JJ, Pechmann C, Kim R, et al. Twitter=quitter? An analysis of Twitter quit smoking social networks. Tob Control. 2012;21:447–9. doi: 10.1136/tc.2010.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bullen C, Williman J, Howe C, et al. Study protocol for a randomised controlled trial of electronic cigarettes versus nicotine patch for smoking cessation. BMC Public Health. 2013;13:210. doi: 10.1186/1471-2458-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.