Abstract
Background
The presence of hydrogen peroxide (H2O2)-producing lactobacilli in the vagina is associated with decreased rates of preterm birth and HIV acquisition. We hypothesize that this is due to immunomodulatory effects of these species.
Methods
Concentrations of IL1β, IL6, IL8, secretory leukocyte protease inhibitor (SLPI) and human beta defensin 2 (HBD2) were quantified from vaginal swabs from 4 groups of women: women with and without bacterial vaginosis (BV) by Nugent score, further stratified by detection of H2O2-producing lactobacilli by semi-quantitative culture. Ten quantitative PCR assays characterized presence and quantity of select Lactobacillus and BV-associated species in each group. Levels of immune markers and bacteria were compared between the four groups using ANOVA, Kruskal-Wallis, Mann Whitney U or chi-square tests.
Results
Swabs from 110 women from four groups were included: 26 had a normal Nugent score (BV−), and no H2O2-producing lactobacilli detected(H2O2−), 47 were BV−, H2O2+, 27 BV+, H2O2− and 10 BV+, H2O2+. The groups were similar in age, marital status and reproductive history, but not ethnicity: the BV−, H2O2− group had more Caucasian participants(p = 0.02). In women with and without BV, IL1β was lower in the H2O2+ groups. HBD2 was lowest in BV+ H2O2− women and highest in BV−, H2O2−. SLPI was lower in women with BV, and did not differ by the presence of H2O2–producing lactobacilli. In regression analysis higher quantities of L. crispatus were associated with lower quantities of IL1β. Detection and quantity of BV-associated species by qPCR was significantly different between women with and without BV, but not between women with and without H2O2-producing lactobacilli within those groups.
Conclusions
The presence of H2O2-producing lactobacilli is associated with lower levels of some vaginal pro-inflammatory cytokines, even in women with BV.
Keywords: Hydrogen peroxide producing Lactobacillus, vaginal cytokines, bacterial vaginosis
Introduction
The human vagina is normally colonized by billions of bacteria. In many healthy women, the dominant bacterial genus is Lactobacillus, whose members produce lactic acid and maintain a low vaginal pH.(1, 2) Colonization with Lactobacillus species that produce hydrogen peroxide (H2O2) has been associated with lower rates of bacterial vaginosis,(3) preterm birth,(4) HIV acquisition(5) and higher rates of pregnancy implantation after in vitro fertilization.(6) However, studies have not demonstrated the same benefit for non- H2O2 -producing Lactobacillus species.
Bacterial vaginosis (BV) is a syndrome characterized both by an absence of lactobacilli and an increase in the diversity of the vaginal microbial community.(7) Women with BV have an increased risk for preterm birth,(8) late miscarriage,(9) and HIV acquisition.(5) Given the strong inverse correlation between Lactobacillus colonization and BV, it is difficult to determine whether lactobacilli are protective or whether BV is harmful. Is the presence of Lactobacillus species good, or simply a marker for the absence of bad?
Many of the complications associated with BV are thought to be related to the inflammatory response to the BV-associated bacterial species.(10) Higher vaginal fluid levels of interleukin (IL)-6, IL-8, and IL-1β have been associated with increased risk of HIV acquisition,(11, 12) as well as short cervix and preterm birth.(13, 14) Vaginal fluid human beta defensing (HBD2) (15) and secretory leukocyte protease inhibitor (SLPI) (12) levels correlate with anti-HIV activity. BV is associated with elevated IL-1β, IL-6, and IL-8, and decreased HBD2 and SLPI.(16) One possible mechanism for the beneficial effect of vaginal H2O2 -producing lactobacilli is that they alter the mucosal immune response to negative stimuli.(17) In vitro and in models of colitis, colonization with Lactobacillus species has been associated with decreased inflammation.(17, 18)
We hypothesized that in women with BV, the presence of vaginal H2O2 -producing lactobacilli would be associated with lower levels of IL-1β, IL-6, and IL-8 and higher levels of HBD2 and SLPI.
Material and Methods
Clinical cohort
This was a secondary analysis of samples and data collected from non-pregnant women enrolled in a prospective cohort of racial disparities in pre-term birth in Washington State. For the primary study, women who had delivered an early preterm infant (20–34weeks) or a term infant (≥37 weeks) and who were US-born, King County Washington residents with no history of hypertensive complications in the preceding pregnancy were enrolled and underwent a pelvic exam. All participants signed informed consent for participation, and the study was approved by the University of Washington Institutional Review Board. Vaginal flora pattern was characterized by Gram stain using the Nugent criteria: a score of 0–3 indicated normal flora, and 7–10 indicated BV. Women were tested for Neisseria gonorrhea and Chlamydia trachomatis using a combined nucleic acid amplification test (Aptima™ Combo 2, Gen-Probe, San Diego, CA) of vaginal fluid or urine. Trichomonas vaginalis was diagnosed by culture (In-Pouch™ TV, Biomed Diagnostics, White City, OR). Vaginal swabs were collected for bacterial culture and placed directly into Port-a-Cul system for transport to the lab, where bacteria were cultured and identified using standard techniques.(19) An additional Dacron swab was saturated with vaginal fluid from the posterior fornix, placed in a sterile cryovial, eluted in 0.9 mL phosphate-buffered saline and stored at −80C until assayed.
For this substudy, we selected samples from women with either BV or normal vaginal microbiota by Nugent score, who also had data on presence of H2O2-producing Lactobacillus by culture. Due to funding limitations, cultures were not performed during the whole duration of the primary study so only part of the primary cohort was eligible for this substudy. Women with intermediate flora (Nugent score 4–6) were excluded from this analysis. Four women with a positive culture for T. vaginalis were also excluded from analysis. Some of the samples selected for this substudy had already been used for previous analyses in the cohort and were not available.
Laboratory methods
Vaginal swabs were thawed, vortexed for 1 minute, and then centrifuged at 14,000 × g for 10 minutes. The pro-inflammatory cytokines IL-6, IL-8, and IL-1β, the mucosal defense molecule SLPI and the antimicrobial peptide HBD2 were measured in swab supernatant using standard enzyme-linked immunosorbent assay (ELISA) as previously described.(20) (21) Values that fell below the lower limit of detection for all cytokines were assigned values of half the lower limit of detection, and included in the analysis.
Eluted fluid from vaginal swabs was thawed, mixed by vortex shaker for 1 minute and 100 uL underwent DNA extraction with the MoBio Bacteremia DNA Isolation Kit (MoBio, Carlsbad, CA). All extracted DNA was tested in a quantitative PCR using primers targeting the human 18S rRNA gene to validate that successful DNA extraction occurred. An internal amplification control PCR using exogenous DNA from a jellyfish gene was used to test for presence of PCR inhibitors. DNA was then subjected to ten separate taxon-directed 16S rRNA gene quantitative PCR assays for the detection and quantification of individual bacteria as previously described.(22, 23) Negative assays were assigned a value of half the lower limit of detection for that assay, and were included in all analyses, including the calculation of mean bacterial concentrations.
Statistical analysis
Comparisons of demographic factors and immune markers between the four groups were made using ANOVA, Kruskal-Wallis or chi-square. When the Kruskal-Wallis test was significant it was followed by the Dunn test for pairwise comparisons, with Bonferroni correction for multiple comparisons. Linear regression was performed with each individual immune marker as an outcome measure, using robust standard errors and dummy variables for each of the four groups, with BV-negative, H2O2-negative as the reference category. Additional regression analyses were performed to evaluate the effect of the quantity of Lactobacillus species by qPCR on the quantity of each immune marker. We made an a priori decision to adjust for race, as previous studies have shown differences in vaginal cytokines and vaginal microbiota between African American and Caucasian women.(24, 25) All analyses were performed using Stata v10.
Results
Demographic characteristics
Of 311 women enrolled in the original cohort who had a Nugent score of 0–3 or 7–10, only 92/203(45%) with a normal Nugent score and 62/108(59%) with BV had culture results available and were eligible for this secondary analysis. The participants with samples remaining for this analysis included 26/32 BV negative, H2O2-producing Lactobacillus negative (H2O2−), 47/60 BV-negative, H2O2+, 27/51 BV positive, H2O2− and 10/13 BV positive, H2O2+. These four groups were overall demographically similar, with the exception that the BV negative, H2O2− group had a higher proportion of white participants and a lower proportion of African American participants than the other groups. (Table 1) The subpopulation with available samples was not significantly different than the group of all eligible women (i.e. BV− or BV+, with culture results available)(data not shown).
Table 1.
Demographic characteristics of the participants included in this analysis from each of four groups: with or without bacterial vaginosis (BV) by Nugent score, further stratified by detection of hydrogen-peroxide (H2O2) producing Lactobacillus species by culture.
| BV−, H2O2− | BV−, H2O2+ | BV+, H2O2− | BV+, H2O2+ | p value | |
|---|---|---|---|---|---|
| n | 26 | 47 | 27** | 10 | |
|
| |||||
| Age (mean ± SD) | 27 ± 7 | 30 ± 6 | 29 ± 7 | 30 ± 5 | 0.42 |
| Ethnicity (n (%)) | |||||
| White | 18 (69%) | 18 (38%) | 7 (26%) | 2 (20%) | 0.02 |
| African American | 5 (19%) | 21 (45%) | 17 (63%) | 7 (70%) | |
| Native American | 3 (12%) | 3 (6%) | 1 (4%) | 0 (0%) | |
| More than one | 0 (0%) | 5 (11%) | 2 (7%) | 1 (10%) | |
| Marital status | |||||
| Single | 4 (15%) | 13 (28%) | 8 (30%) | 3 (30%) | 0.30 |
| Married/partnered | 21 (81%) | 30 (64%) | 13 (48%) | 6 (60%) | |
| Other | 1 (4%) | 3 (6%) | 6 (22%) | 1 (10%) | |
| Index delivery* | |||||
| Preterm | 2 (8%) | 8 (17%) | 1 (4%) | 1 (10%) | 0.32 |
| Term | 24 (92%) | 39 (83%) | 26 (96%) | 9 (90%) | |
| Obstetric History | |||||
| Gravidity (median, IQR) | 2 (1,4) | 3 (2,4) | 3 (2,5) | 3 (2,4) | 0.12 |
| Parity (median, IQR) | 1 (1,2) | 2 (1,2) | 2 (1,4) | 2.5 (1, 3) | 0.05 |
Delivery in the year prior to study enrollment
Excluded from this group were 6 women with a positive test for Trichomonas vaginalis
Detection of BV-associated species differed by BV diagnosis, but not by presence of H2O2–producing Lactobacillus
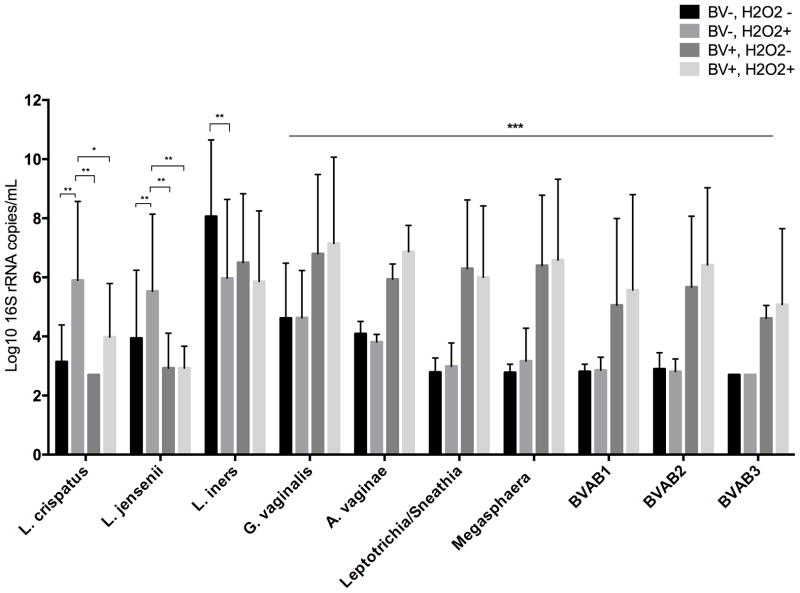
L. crispatus and L. jensenii were detected significantly more often in BV negative women, while Megasphaera, Leptotrichia/Sneathia, BVAB1, BVAB2 and BVAB3 were detected significantly more often in BV positive women. There were no differences in detection of L. iners, G. vaginalis, or A. vaginae between the four groups. (Table 2) When comparing BV negative women, L. crispatus and L. jensenii were more common when culture detected H2O2-producing Lactobacillus. Among BV positive women, only L. crispatus was significantly more common when H2O2-producing Lactobacillus were detected; L. jensenii was detected in only two BV positive women, 1 in each group. Within BV diagnosis categories there were no significant differences in detection of BV-associated species between women with and without H2O2-producing Lactobacillus by culture, though there was a trend to lower detection of Megasphaera and Leptotrichia/Sneathia when H2O2-producing lactobacilli were present.
Table 2.
Comparison of detection of ten bacterial species by qPCR in each of four groups: women with or without bacterial vaginosis (BV) by Nugent score, and with or without hydrogen-peroxide (H2O2) producing Lactobacillus species detected by culture. P value is for chi square comparison between all four groups. Significant differences between BV+ or BV− women with and without H2O2-producing Lactobacillus detected are noted with symbols.
| BV−, H2O2− | BV−, H2O2+ | BV+, H2O2− | BV+, H2O2+ | p value | |
|---|---|---|---|---|---|
| n | 26 | 47 | 27 | 10 | |
|
| |||||
| L. crispatus | 4 (15%) | 30 (64%)* | 0 | 4 (40%)† | < 0.001 |
| L. jensenii | 8 (31%) | 29 (62%)* | 1 (4%) | 1 (10%) | < 0.001 |
| L. iners | 23 (88%) | 36 (77%) | 21 (78%) | 8 (80%) | 0.66 |
| G. vaginalis | 17 (65%) | 35 (74%) | 21 (78%) | 8 (80%) | 0.68 |
| A. vaginae | 11 (42%) | 22 (47%) | 19 (70%) | 8 (80%) | 0.05 |
| Leptotrichia/Sneathia | 1 (4%) | 8 (17%) | 21 (78%) | 7 (70%) | < 0.001 |
| Megasphaera | 2 (8%) | 11 (23%) | 22 (81%) | 7 (70%) | < 0.001 |
| BVAB1 | 5 (19%) | 8 (17%) | 15 (56%) | 5 (50%) | 0.001 |
| BVAB2 | 4 (15%) | 3 (6%) | 18 (67%) | 7 (70%) | < 0.001 |
| BVAB3 | 0 | 0 | 12 (44%) | 5 (50%) | < 0.001 |
p < 0.01 for comparison between BV− women with and without H2O2-producing lactobacilli
p < 0.01 for comparison between BV+ women with and without H2O2-producing lactobacilli
Quantity of BV-associated species varied by BV status, but not by presence of H2O2–producing Lactobacillus
In all comparisons, the quantity of bacteria was significantly different between the four groups, with p < 0.01. After pairwise comparisons, L. crispatus and L. jensenii were present in significantly higher quantity in the BV−, H2O2+ group compared to the other three groups (p ≤ 0.01 for all comparisons)(Figure 1). L. iners was present in significantly higher quantity in the BV−, H2O2− group compared to the BV−, H2O2+ group (p = 0.006), but was not significantly different from the BV+ groups. For all of the BV-associated species, the BV+ groups had significantly higher concentrations than the BV− groups, while comparisons within BV diagnosis category were not significantly different.
Figure 1.
Comparison of quantity of bacterial species by qPCR assay between BV positive and negative women with and without H2O2-producing Lactobacillus detected by culture. All species show significantly different quantities between the 4 groups with p < 0.01 (Kruskal-Wallis). * adjusted p < 0.05, ** p ≤ 0.01, *** For all of these species, pairwise comparison between the BV+ women and both BV− groups was significant, with p < 0.01, while comparison within BV diagnosis was not significantly different. (Dunn test, with Bonferroni correction). If not noted, the comparison is not statistically significant.
In unadjusted analysis, vaginal fluid immune markers differed between women with and without BV, as well as between women with and without H2O2-producing Lactobacillus
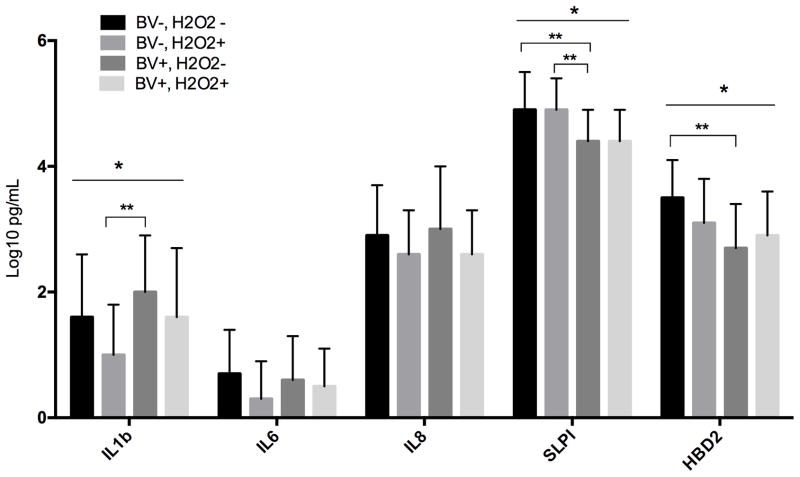
When comparing vaginal fluid immune response markers IL1β, SLPI and HBD2 were significantly different between the four groups.(Figure 2) In women with and without BV, IL1β was lower when H2O2–producing lactobacilli were detected. The biggest difference was between the BV−, H2O2+ group compared to the BV+, H2O2− group (p = 0.003), with a trend to a significantly difference with the BV−, H2O2− group as well (p = 0.07). Women with BV had lower levels of SLPI than women without BV whether H2O2+ (p = 0.002) or H2O2− (p = 0.02).. For HBD2 the lowest value was in the BV+, H2O2− and the highest in the BV−, H2O2− group. IL6 and IL8 did not differ between the groups.
Figure 2.
Comparison of quantity of cytokines, chemokines and antimicrobial peptides in vaginal fluid between between BV positive and negative women with and without H2O2-producing Lactobacillus detected by culture. * indicates a significant difference (p < 0.05) within BV positive or negative women between those with and without H2O2-producing lactobacilli. ** indicates a significant difference between all 4 groups (p < 0.05).
In adjusted analysis, colonization with H2O2-producing Lactobacillus was associated with differences in IL1β, SLPI and HBD2
In regression analysis, adjusting for race and using BV negative women without H2O2-producing Lactobacillus detected as a reference category, the only analyte that was significantly different for each of the other three groups was HBD2, which was lowest in the BV positive women without H2O2-producing Lactobacillus detected. (Table 3) SLPI was lower in BV+ women without H2O2-producing Lactobacillus detected, and IL1β in BV− women with H2O2-producing Lactobacillus compared to the reference group.
Table 3.
Association between Lactobacillus colonization and quantity and quantity of vaginal fluid immune markers, as measured by linear regression analysis.
| IL1β | IL6 | IL8 | SLPI | HBD2 | |
|---|---|---|---|---|---|
| Study group (adjusted for race)*
| |||||
| BV−, H2O2 − | Ref | Ref | Ref | Ref | Ref |
| BV−, H2O2+ | −0.50 (−0.98, −0.02) | −0.30 (−0.63, 0.04) | −0.22 (−0.66, −.21) | −0.1 (−.3, 0.2) | −0.55 (−0.88, −0.22) |
| BV+, H2O2 − | 0.55 (−0.001, 1.09) | 0.03 (−0.36, 0.41) | 0.20 (−0.29, 0.69) | −0.6 (−0.9, −0.3) | −1.00 (−1.38, −0.62) |
| BV+, H2O2+ | 0.15 (−0.58, 0.87) | −0.16 (−0.67, 0.34) | −0.27 (−0.92, 0.38) | −0.3 (−0.6, 0.1) | −0.83 (−1.33, −0.33) |
|
| |||||
| Quantity of Lactobacillus (adjusted for race)*
| |||||
| L. crispatus | −0.16 (−0.23, −0.08) | −0.03 (−0.08, 0.03) | −0.07 (−0.14, 0.001) | 0.05 (0.01, 0.09) | 0.001 (−0.06, 0.06) |
| L. jensenii | −0.06 (−0.15, 0.02) | −0.01 (−0.07, 0.04) | −0.03 (−0.1, 0.04) | 0.05 (0.01, 0.10) | .08 (0.03, 0.14) |
| L. iners | 0.03 (−0.04, 0.11) | −0.01 (−0.05, 0.04) | 0.01 (−0.05, 0.07) | 0.01 (−0.03, 0.05) | 0.04 (−.01, .09) |
|
| |||||
| Quantity of Lactobacillus (adjusted for race and BV diagnosis)*
| |||||
| L. crispatus | −0.11 (−0.2, −0.03) | −0.01 (−0.07, 0.05) | −0.06 (−0.13, 0.02) | 0.02 (−0.02, 0.06) | −0.05 (−0.11, 0.01) |
| L. jensenii | −0.003 (−0.09, 0.08) | 0.002 (−0.06, 0.06) | −0.01 (−0.09, 0.07) | 0.03 (−0.02, 0.07) | 0.05 (−0.01, 0.11) |
| L. iners | 0.05 (−0.02, 0.12) | −0.001 (−0.05, 0.05) | 0.01 (−0.05, 0.07) | −0.001 (−0.04, 0.04) | 0.03 (−0.02, 0.08) |
All values are regression coefficients (95% confidence intervals). The analyses for Lactobacillus quantity show the association between a 1 log10 increase in Lactobacillus concentration and the log10-transformed immune marker concentration.
Bold values are statistically significant
In a regression analysis adjusted for race, higher quantities of L. crispatus were associated with significantly lower concentrations of IL1β and higher concentrations of SLPI but no difference in HBD2.(Table 3) Higher quantities of L. jensenii were associated with higher concentrations of SLPI and HBD2. When the analysis was also adjusted for BV diagnosis, the only association that remained significant was that between quantities of L. crispatus and IL1β. Quantity of L. iners was not associated with any of the analytes measured in either analysis.
Discussion
Overall our results suggest that H2O2-producing lactobacilli have an immunomodulatory effect in the vagina, primarily through their impact on IL1β. Additionally, it seems that this effect is independent of BV diagnosis, though BV clearly has a strong immunostimulatory effect on vaginal mucosa. Quantity of L. crispatus and L. jensenii, common H2O2 producing species, showed a correlation between bacterial quantity and inflammatory markers, while quantity of L. iners (a non- H2O2-producing species) showed no association with cytokine levels, suggesting that H2O2 production may be a marker for these immunomodulatory effects.
For many years the dogma in the field suggested that Lactobacillus were beneficial because the H2O2 produced by some species killed the BV-associated bacterial species, (26, 27) or inactivated viruses like HIV.(28) However, more recent work has demonstrated that the amount of H2O2 produced in the anaerobic environment of the vagina (as opposed to aerobic culture conditions in the lab) is minimal or easily neutralized,(29) and unlikely to be able to have the effects attributed to the beneficial Lactobacillus species.(30) Some have argued that lactic acid is the true effector molecule, and O’Hanlon et al showed that an acid pH induced by lactic acid, but not acetic acid, is inhibitory to BV-associated bacterial species.(31) However, as all Lactobacillus species make lactic acid, this does not explain the clinical differences seen in epidemiologic studies between women with and without H2O2-producing species. An alternative explanation is that H2O2-production is a marker for species with some other characteristic that provides the reproductive health benefits. Our results demonstrate that this may be immunomodulation, possibly to decrease mucosal inflammation.
Data on the effects of H2O2-producing Lactobacillus species specifically on vaginal markers of mucosal immunity are limited. Anderson et al measured IL1β, IL6 and SLPI (as well as 5 other analytes) in 47 women without BV, and characterized the vaginal microbiota by culture-based methods. After adjusting for race and BMI, no differences in any cytokine markers were seen between women with and without H2O2-producing Lactobacillus species detected.(32) In a small study of 42 Indian women with normal Nugent scores, use of a vaginal probiotic tablet containing L. brevis, L. salivarius and L. plantarum for 8 days was associated with a decrease in vaginal IL1β and IL6 on day 9, which was not seen in women randomized to a placebo tablet.(33) In vitro, Rose et al showed that adding L. crispatus or L. jensenii to cultured vaginal epithelial cells in the presence of the toll-like receptor agonists PIC and FSL-1 decreased levels of IL6, IL8 and/or TNF-α compared to the agonist alone. This effect varied by strain: a laboratory isolate of L. jensenii had less of an effect than a clinical isolate.(17) Our results showed no impact of lactobacilli on IL6 or IL8, but did show a decrease in IL1β (classically pro-inflammatory), and an increase in SLPI (classically anti-inflammatory), which is consistent with our hypothesis. However, the results for HBD2 demonstrate the complexity of the immune response: the highest levels of this antimicrobial peptide were seen in women who were BV negative and had no H2O2-producing lactobacilli, while the lowest were in women who were BV+, H2O2−. This may suggest that there is an optimal range for HBD2, and that too high or too low is undesirable.
The classic teaching in the field is that Lactobacillus species control the growth of BV-associated species.(34, 35) The only interventional study to examine the effect of an H2O2-producing Lactobacillus on individual vaginal BV-associated bacterial species was the Phase 2A randomized trial of the H2O2-producing probiotic candidate L. crispatus CTV-05. Presence and quantity of BV-associated bacterial species were measured before and after treatment using the same assays described in our study. In women who established colonization with the probiotic there was a significant drop in the quantity of G. vaginalis, A. vaginae and BVAB2, which was not seen among women who did not establish colonization with the CTV-05 strain.(36) Cervicovaginal fluid from healthy women has been shown to inhibit growth of E. coli in vitro, and this inhibitory activity has been linked to the presence of proteins from L. crispatus and/or L. jensenii.(37) Our data show no difference in quantities of BV-associated species between women with BV with and without H2O2-producing Lactobacillus species. However, women with BV and H2O2-producing Lactobacillus species detected had lower quantities of the Lactobacillus species we measured, suggesting that perhaps BV-associated species have a negative impact on the lactobacilli.
Taken together with the broader literature, our results suggest that there is an immunomodulatory effect of some H2O2-producing Lactobacillus species that is not simply due to the absence of BV-associated species. However, our results also suggest that this effect may not be robust for all markers, especially in the face of the strong inflammatory response generated by bacterial vaginosis. In addition, our data suggest that the presence of H2O2-producing Lactobacillus alone does not have a suppressive effect on BV-associated species. Both of these associations may be more dependent on quantity of the Lactobacillus species, as suggested by the association we saw between quantity of L. crispatus and L. jensenii with vaginal immune marker concentrations. A study of the L. crispatus CTV-05 probiotic for prevention of recurrent urinary tract infections only saw a beneficial effect in women who established high-quantity vaginal colonization (> 106 16S rRNA gene copies/swab).(38)
Our study has several limitations, including its cross-sectional design, which limits our ability to infer causal relationships. We have limited data on potential confounders that might be present such as douching or recent sexual activity. This was a secondary analysis of the remaining biologic samples from a larger cohort; this could introduce some selection bias. However, the participants with samples remaining were not significantly different than the larger cohort. We used species-specific PCR to characterize several BV-associated species, but this does not comprehensively evaluate the vaginal microbiota. Some of the differences seen may be related to species that were not tested for in this study.
Our results suggest that H2O2-producing Lactobacillus can have an immunomodulatory effect in the vagina, which is not simply due to the absence of pro-inflammatory bacterial species. However, the protective role of these species and their utility as probiotics may not be a simple story, as the anti-inflammatory potential is attenuated in the presence of BV-associated species and the presence of H2O2-producing Lactobacillus is not associated with lower quantities of pathogenic BV-associated species.
Acknowledgments
The data in this paper were derived from a study supported by NIH grant HD-41682 (JH). Dr. Mitchell is supported by a K08 from NIAID (1K08AI087969) and a Clinical Scientist Development Award from the Doris Duke Foundation.
Footnotes
Conflict of interest: The authors report no conflicts of interest
References
- 1.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2010;108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 2013;8(11):e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallor AC, Antonio MA, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis. 2001;184(11):1431–6. doi: 10.1086/324445. [DOI] [PubMed] [Google Scholar]
- 4.Martius J, Krohn MA, Hillier SL, Stamm WE, Holmes KK, Eschenbach DA. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol. 1988;71(1):89–95. [PubMed] [Google Scholar]
- 5.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180(6):1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 6.Moore DE, Soules MR, Klein NA, Fujimoto VY, Agnew KJ, Eschenbach DA. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril. 2000;74(6):1118–24. doi: 10.1016/s0015-0282(00)01624-1. [DOI] [PubMed] [Google Scholar]
- 7.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333(26):1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 9.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. Bmj. 1994;308(6924):295–8. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mlisana K, Naicker N, Werner L, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. 2012;206(1):6–14. doi: 10.1093/infdis/jis298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison C, Fichorova R, Mauck C, et al. Cervical Inflammation and Immunity Associated with Hormonal Contraception, Pregnancy and HIV-1 Seroconversion. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 13.Raiche E, Ouellet A, Berthiaume M, Rousseau E, Pasquier JC. Short and inflamed cervix predicts spontaneous preterm birth (COLIBRI study) J Matern Fetal Neonatal Med. 2014;27(10):1015–9. doi: 10.3109/14767058.2013.847917. [DOI] [PubMed] [Google Scholar]
- 14.Taylor BD, Holzman CB, Fichorova RN, et al. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum Reprod. 2013;28(4):942–52. doi: 10.1093/humrep/det019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh M, Fahey JV, Shen Z, et al. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One. 2010;5(6):e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell C, Marrazzo J. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol. 2014;71(6):555–63. doi: 10.1111/aji.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose WA, 2nd, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One. 2012;7(3):e32728. doi: 10.1371/journal.pone.0032728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackos AR, Eubank TD, Parry NM, Bailey MT. Probiotic Lactobacillus reuteri attenuates the stressor-enhanced severity of Citrobacter rodentium infection. Infect Immun. 2013;81(9):3253–63. doi: 10.1128/IAI.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eschenbach DA, Patton DL, Meier A, et al. Effects of oral contraceptive pill use on vaginal flora and vaginal epithelium. Contraception. 2000;62(3):107–12. doi: 10.1016/s0010-7824(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell C, Balkus J, Agnew K, Lawler R, Hitti J. Changes in the vaginal microenvironment with metronidazole treatment for bacterial vaginosis in early pregnancy. J Womens Health (Larchmt) 2009;18(11):1817–24. doi: 10.1089/jwh.2009.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell C, Gottsch ML, Liu C, Fredricks DN, Nelson DB. Associations between vaginal bacteria and levels of vaginal defensins in pregnant women. Am J Obstet Gynecol. 2013;208(2):132 e1–7. doi: 10.1016/j.ajog.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in Vaginal Bacterial Concentrations with Intravaginal Metronidazole Therapy for Bacterial Vaginosis as Assessed by Quantitative PCR. J Clin Microbiol. 2009 doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryckman KK, Williams SM, Krohn MA, Simhan HN. Racial differences in cervical cytokine concentrations between pregnant women with and without bacterial vaginosis. J Reprod Immunol. 2008;78(2):166–71. doi: 10.1016/j.jri.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1(2):121–33. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 26.Patterson JL, Girerd PH, Karjane NW, Jefferson KK. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol. 2007;197(2):170 e1–7. doi: 10.1016/j.ajog.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eschenbach DA, Davick PR, Williams BL, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27(2):251–6. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klebanoff SJ, Kazazi F. Inactivation of human immunodeficiency virus type 1 by the amine oxidase-peroxidase system. J Clin Microbiol. 1995;33(8):2054–7. doi: 10.1128/jcm.33.8.2054-2057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Hanlon DE, Lanier BR, Moench TR, Cone RA. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis. 2010;10:120. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muench DF, Kuch DJ, Wu H, et al. Hydrogen peroxide-producing lactobacilli inhibit gonococci in vitro but not during experimental genital tract infection. J Infect Dis. 2009;199(9):1369–78. doi: 10.1086/597390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BL, Cu-Uvin S, Raker CA, Fitzsimmons C, Hillier SL. Subtle perturbations of genital microflora alter mucosal immunity among low-risk pregnant women. Acta Obstet Gynecol Scand. 2011;90(5):510–5. doi: 10.1111/j.1600-0412.2011.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemalatha R, Mastromarino P, Ramalaxmi BA, Balakrishna NV, Sesikeran B. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur J Clin Microbiol Infect Dis. 2012;31(11):3097–105. doi: 10.1007/s10096-012-1671-1. [DOI] [PubMed] [Google Scholar]
- 34.Atassi F, Brassart D, Grob P, Graf F, Servin AL. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol. 2006;48(3):424–32. doi: 10.1111/j.1574-695X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 35.Matu MN, Orinda GO, Njagi EN, Cohen CR, Bukusi EA. In vitro inhibitory activity of human vaginal lactobacilli against pathogenic bacteria associated with bacterial vaginosis in Kenyan women. Anaerobe. 2010;16(3):210–5. doi: 10.1016/j.anaerobe.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Ngugi BM, Hemmerling A, Bukusi EA, et al. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex Transm Dis. 2011;38(11):1020–7. doi: 10.1097/OLQ.0b013e3182267ac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalyoussef S, Nieves E, Dinerman E, et al. Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions. PLoS One. 2012;7(11):e49506. doi: 10.1371/journal.pone.0049506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–7. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]