Abstract
Atrial disease or myopathy forms the substrate for atrial fibrillation (AF) and underlies the potential for atrial thrombus formation and subsequent stroke. Current diagnostic approaches in patients with AF focus on identifying clinical predictors with evaluation of left atrial size by echocardiography serving as the sole measure specifically evaluating the atrium. Although the atrial substrate underlying AF is likely developing for years prior to the onset of AF, there is no current evaluation to identify the pre-clinical atrial myopathy. Atrial fibrosis is one component of the atrial substrate that has garnered recent attention based on newer MRI techniques that have been applied to visualize atrial fibrosis in humans with prognostic implications regarding success of treatment. Advanced ECG signal processing, echocardiographic techniques, and MRI imaging of fibrosis and flow provide up-to-date approaches to evaluate the atrial myopathy underlying AF. While thromboembolic risk is currently defined by clinical scores, their predictive value is mediocre. Evaluation of stasis via imaging and biomarkers associated with thrombogenesis may provide enhanced approaches to assess risk for stroke in patients with AF. Better delineation of the atrial myopathy that serves as the substrate for AF and thromboembolic complications might improve treatment outcomes. Furthermore, better delineation of the pathophysiologic mechanisms underlying the development of the atrial substrate for AF, particularly in its earlier stages, could help identify blood and imaging biomarkers that could be useful to assess risk for developing new onset AF and suggest specific pathways that could be targeted for prevention.
Keywords: arrhythmia, cardiovascular imaging, electrophysiology, embolic stroke, clinical electrophysiology, drugs, cardiovascular imaging agents/Techniques
Atrial fibrillation (AF) affects >2 million Americans1–3 with projections that it will affect 8–12 million people in the US by 2050.1, 4 The incremental cost related to AF in the US is estimated at $6–26 billion per year.5 It is associated with reduced quality of life6–8 and increased risk of stroke9 and death.10 Despite this overwhelming and growing health burden, it is striking that for decades, there has been essentially no change in the recommended initial clinical evaluation of patients with AF. The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society (AHA/ACC/HRS) Guideline for the Management of Patients With Atrial Fibrillation recommends that the initial evaluation includes history and physical examination, ECG, transthoracic echocardiogram, and blood tests of thyroid, renal, and hepatic function.11 This initial evaluation focuses on identifying clinical factors that predispose to or may precipitate AF. Typical cardiovascular causes include hypertension, valvular heart disease, heart failure, and coronary artery disease, but these diagnoses do not indicate the degree of involvement of the heart, or more specifically the left atrium (LA) in the disease process. With the exception of LA size, no other LA features are evaluated. To date, LA size has not proven to be useful for clinical decision making.
Once AF is diagnosed, treatment addresses the precipitating cause (if identified), restoration/maintenance of sinus rhythm (versus rate control), and prevention of thromboembolic complications. The risk models used to identify candidate patients for anticoagulation include the commonly used CHADS2 and CHA2DS2-VASc scores (defined in Table 1). They demonstrate only mediocre predictive values with C statistics ranging from 0.55–0.64.12 In another large cohort of patients, the CHA2DS2-VASc score was noted to have a C statistic of only 0.6713 but it is currently recommended by guidelines.11 Similarly, rhythm control treatment options have only mediocre success. A meta-analysis14 reported antiarrhythmic drug efficacy of 52% compared to 25% for placebo and catheter ablation success rate of 71% after multiple procedures. A recent report15 noted a 5 year arrhythmia-free survival of 63% after the last catheter ablation procedure. Thus, for both rhythm management and stroke prevention there are opportunities to improve our understanding of the underlying disease process in the atrium with the aim of improving treatment outcomes. The need for better characterization of the atrial substrate has been promoted by the European Heart Rhythm Association16 and the AHA/ACC/HRS.11 As there may be significant overlap among the pathophysiologic determinants of response to rhythm control treatments and thrombus formation in patients with AF, this report will summarize some of the newer approaches that are available to advance our understanding of the underlying atrial myopathy that forms the substrate for AF.
Table 1.
Calculation of CHADS2 and CHA2DS2-VASc scores. The indicated number of points are added up for each condition that exists.
| CHADS2 | CHA2DS2-VASc | |
|---|---|---|
| Congestive heart failure | 1 | 1 |
| Hypertension | 1 | 1 |
| Age | ||
| 65–74 years | 1 | 1 |
| ≥75 years | 1 | 2 |
| Diabetes mellitus | 1 | 1 |
| Stroke or TIA | 2 | 2 |
| Vascular disease | 1 | |
| Sex category = female | 1 |
Pathogenesis of AF
There is increasing recognition that one of the contributing abnormalities to the development of AF is atrial fibrosis.17–24 Importantly, it has recently been demonstrated that the degree of fibrosis has therapeutic implications.25 The DECAAF study26 established that the degree of atrial fibrosis is (inversely) associated with the successful outcome of catheter ablation for treatment of AF. As LA fibrosis is one of the pathogenic pathways for the development of AF, and it likely develops over some period of time prior to the clinical appearance of AF, delineation of the various stages of disease progression may help develop better diagnostic and therapeutic approaches to AF. Just as breakthroughs in the prevention and treatment of coronary artery disease and acute myocardial infarction resulted directly from ascertaining the role of coronary atherosclerosis, especially in its pre-clinical phases, and atherosclerotic plaque rupture in the pathogenesis of myocardial infarction, longitudinal assessment of the development of the substrate for AF could alter the diagnostic and therapeutic landscape.
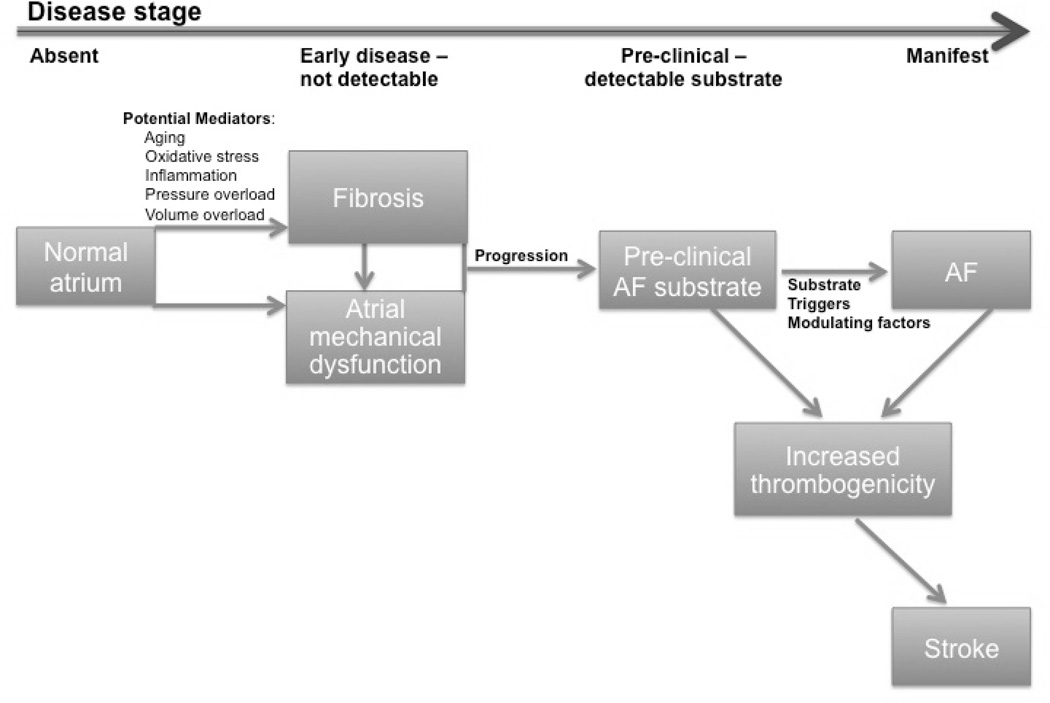
Figure 1 provides a schematic for a disease progression model that focuses on the development of atrial fibrosis. Four disease stages are shown – no disease, early disease that is not detectable, detectable substrate without AF (i.e. pre-clinical substrate), and manifest AF. Potential mediators known to promote fibrosis in the atrium include pressure and volume overload, aging,27 atrial stretch,19 inflammation,28, 29 and oxidative stress.28, 30 As the atrial myopathy progresses, it can lead to atrial dysfunction that may be detectable by currently available diagnostic tests. The role of atrial dysfunction in the pathogenesis of stroke in patients with atrial myopathy without AF is also of interest.
Figure 1.
Schematic of the progression of disease in patients who are developing the substrate for AF.
What is the atrial substrate underlying AF?
AF may be associated with structural as well as electrophysiological remodeling. Some of these remodeling changes may be detectable prior to the onset of clinical AF. Structural remodeling may incorporate changes in LA size and the development of atrial fibrosis. Various reports have highlighted the role of fibrosis17–24 in the pathogenesis of AF. Fibrosis may contribute to the substrate for reentry by increasing heterogeneity of conduction in the atria. Oakes et al25 introduced delayed enhancement MR imaging of the LA to detect fibrosis, demonstrating that areas of delayed enhancement corresponded to areas of low voltage during intracardiac mapping. Others have applied this technique as well to evaluate LA fibrosis.31–33
Electrophysiologic remodeling has multiple manifestations. The concept that “AF begets AF”34 reflects the shortening of atrial refractory periods that occurs with repeated bouts of pacing or AF that further promotes AF. Changes in ion channel function, calcium loading, and conduction are components of atrial electrical remodeling that are usually noted after the development of AF.35 However, electrical changes may also precede the onset of AF. In the ASSERT trial,36 serial noninvasive programmed atrial stimulation was performed in 458 patients with implanted pacemakers and demonstrated no change in refractory periods in those who went on to develop atrial tachyarrhythmias while P wave prolongation and increased vulnerability to induction of AF was noted. Conduction slowing and increased vulnerability to AF have also been noted in patients with hypertension without AF.37
An important contributor to electrophysiological remodeling is the autonomic nervous system. Autonomic remodeling occurs in a variety of disease processes and can serve as an important substrate for AF, with both the parasympathetic and sympathetic nervous system playing roles in the genesis and maintenance of AF.38–40 The parasympathetic nervous system may have a more dominant contribution to AF substrate – primarily by leading to shortening and heterogeneity of refractoriness in the atria – with the sympathetic nervous system playing a more modulatory role. The pulmonary veins, often involved in the pathogenesis of AF, are much more densely innervated - with both parasympathetic and sympathetic nerves – than the rest of the LA.41 While catheter ablation to achieve denervation in the region of the pulmonary veins42 has been shown to improve outcomes, this finding has not been consistent. Significant interest remains in the role of catheter ablation of the autonomic ganglia.43
Identifying the pre-clinical atrial myopathy
Though not currently part of clinical practice, it is conceivable that identification of the early stages of atrial myopathy might allow for early preventive treatments to prevent progression of atrial disease and AF. A variety of treatments have in fact been evaluated. Emerging data show that there are a number of upstream therapies – ACE inhibitors/angiotensin receptor blockers,44 aldosterone blockers,45 statins,46–49 and omega-3 polyunsaturated fatty acids50, 51 – that might prevent AF in susceptible populations, but few prospective studies have been performed, the data are not consistent, and the effect of these therapies on the atrium have not been evaluated in humans.52–57 Statins and aldosterone blockers appear to be the most promising agents. Statins may prevent early atrial electrical remodeling,56 even if they may not be effective in the treatment of established AF.57 In a meta-analysis49 of 20 studies with 23577 patients evaluating both primary and secondary prevention of AF, statin use was associated with a reduced incidence of AF (5.72% versus 7.38% for placebo, odds ratio 0.49, 95% CI 0.37–0.65); a larger effect was noted for secondary versus primary prevention. Aldosterone has been shown to cause atrial fibrosis.58 Spironolactone has been shown to reduce LA fibrosis (and P wave duration) in a post-myocardial infarction rat model of heart failure.59 The EMPHASIS-HF study reported a substantial reduction in new onset AF in patients with heart failure who were treated with eplerenone versus placebo (2.7% versus 4.5%, hazard ratio 0.58, 95%CI 0.35–0.96).45 While not specifically evaluated in this study, it is likely that at entry the bulk of the patient population had some substrate for AF,60 as they were older (mean age 68–70 years) and all had heart failure, 2/3 had hypertension, and 1/3 had diabetes. If one accepts the notion that these patients had pre-clinical substrate, including LA fibrosis, the ability of eplerenone to reduce new onset AF is impressive and suggests that there is a role for this therapy even when the atrial substrate for AF is already present. Thus, to enable trials of targeted treatment for early atrial myopathy, better tools are needed to identify this substrate and track treatment effects.
A number of previous observational studies (Table 2)61–65 have assessed risk factors for new AF in people without clinical history of prior AF. These studies have focused on clinical factors associated with AF (not anatomic findings nor direct assessment of potential mechanisms) and all prior studies have been limited to clinically-detected AF, leaving a gap in our understanding of the pathogenesis of asymptomatic AF, an increasingly recognized important health problem. Several multivariable quantitative risk models have been validated in external cohorts. The Framingham Heart Study (FHS) model66 was validated in 2 cohorts [The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study and Cardiovascular Health Study]. Validation confirmed that risk of incident AF in community-dwelling whites and African-Americans can be assessed by clinical variables and accounted for up to 64% of the overall AF risk.67 In the Atherosclerosis Risk in Communities (ARIC) study,68 the same risk factors were studied to determine population attributable risk. During 17 years of follow-up, 1,520 incident AF cases were identified. Overall, 57% of AF cases could be accounted for by having ≥1 borderline or elevated risk factors. Attributable risks in these ranges suggest there could be a role for additional mechanistic factors. An additional study was conducted by Alonso et al69 (CHARGE-AF consortium). They collected individual-level data from 3 US cohorts (ARIC; CHS; FHS), including 18,556 men and women aged 46 to 94 years (19% African Americans, 81% whites) to derive predictive models for AF using clinical variables. Validation of the derived models was performed in 7,672 participants from AGES and the Rotterdam Study. A 5-year predictive model including age, race, height, weight, systolic and diastolic BP, smoking, use of blood pressure medication, diabetes, and history of MI and heart failure had good discrimination (C-statistic, 0.765; 95% CI, 0.748 to 0.781). Adding standard variables from the resting ECG (i.e., PR interval and ECG-LVH) did not improve the overall model discrimination.
Table 2.
Previous epidemiologic studies of risk factors for incident atrial fibrillation.
| Author (Ref No.) |
Study Name | Number in Study |
Ages (years) |
Follow- up length |
Detection Methods | Primary Risk Factors |
|---|---|---|---|---|---|---|
| Psaty (61) | Cardiovascular Health Study (CHS) |
4844 | 65+ | 3 years | Self-report (SR); Exam ECG; Hospital Discharge Info |
Age; male sex; diuretic use; history of CHD or valve disease; SBP; height; glucose; LA size (Echo) |
| Schnabel (66) | Framingham Heart Study (FHS) |
4764 | 45–95 | 10 years | Exam ECG; physician records; Hospital discharge data |
Age; male sex; BMI; SBP; treatment for hypertension; ECG PR-interval; clinically significant cardiac murmur; heart failure. Echocardiogram findings not additive. |
| Chamberlain (62) | Atherosclerosis Risk in Communities Study (ARIC) |
14546 | 45–64 | 10 years | Exam ECG; Hospital discharge data; Death Certificates |
Age; race; height; smoking; SBP, BP medication use; precordial murmur; ECG LVH; LA enlargement (by ECG); diabetes; CHD; heart failure. |
| Heeringa (63–65) | Rotterdam Study | 6808 | 55+ | 7–8 years | Exam ECG; Medical records; National registration system of all hospital discharges |
Age; gender; BMI; hypertension; SBP; cholesterol; diabetes; LVH by ECG; MI; heart failure; cigarette smoking; asymptomatic TSH levels in the “normal range”; carotid intima-media thickness. |
SBP=systolic blood pressure; LA=left atrial; ECG=electrocardiogram; BMI=body mass index; LVH=left ventricular hypertrophy; CHD=coronary heart disease; MI=myocardial infarction; TSH=thyroid stimulating hormone
Obesity is a particularly relevant risk factor for development of AF given the growing obesity epidemic. In the Framingham cohort, obesity was associated with a 4% increased risk in incident AF per unit increase in BMI in both men and women.70 Obesity encompasses a heterogeneous set of risk factors that may predispose to developing AF, including the associated medical conditions that are intimately linked to risk of AF, such as hypertension and diabetes. Obesity may have direct effects on the LA substrate mediated by increases in inflammation, oxidative stress, and left atrial volume.71–73 Epicardial fat, in particular, is associated with increased prevalence of AF74 or AF burden.75 The role of obesity in the pathogenesis of AF was demonstrated in the ARREST-AF study76 in which a cohort of patients underwent a structured risk factor management program following catheter ablation for treatment of AF. Compared to a control group who did not participate in the program, these patients experienced a 13% decrease in BMI with significant declines in blood pressure and LA volume index. Over a two-year follow-up, there was substantially higher freedom from AF in the risk factor management group (multivariable hazard ratio 4.8, 95%CI 2.04–11,4, p<0.001). Given the potentially large impact of risk factor management in the secondary prevention of AF, it is possible that better delineation of the multiple pathophysiologic pathways that are responsible for the development of the pre-clinical atrial substrate could form the basis for a more informed strategy for addressing these modifiable risk factors for primary prevention of AF, in addition to the other cardiovascular benefits.
Very few studies have examined the physiologic or detailed anatomic mechanisms that might underlie AF development in previously healthy people. One such study was reported in 2012 based on an analysis of echocardiographic diastolic parameters from the CHS.77 AF was ascertained by self-report, annual study ECG for the first 9 years of CHS, and reports from hospitalizations. For the first time, this study found that atrial factors beyond LA size were associated with new onset of AF. In addition to LA diameter, the most significant predictors of incident AF were Doppler peak E-wave velocity (HR 1.5 (CI 1.3–1.9) for highest vs. lowest quintile), and Doppler A-wave velocity time integral, which displayed a U-shaped relationship with risk of AF (HR 0.7 (CI 0.6–0.9) for middle vs. lowest quintile).
Techniques for evaluating atrial myopathy - substrate for AF
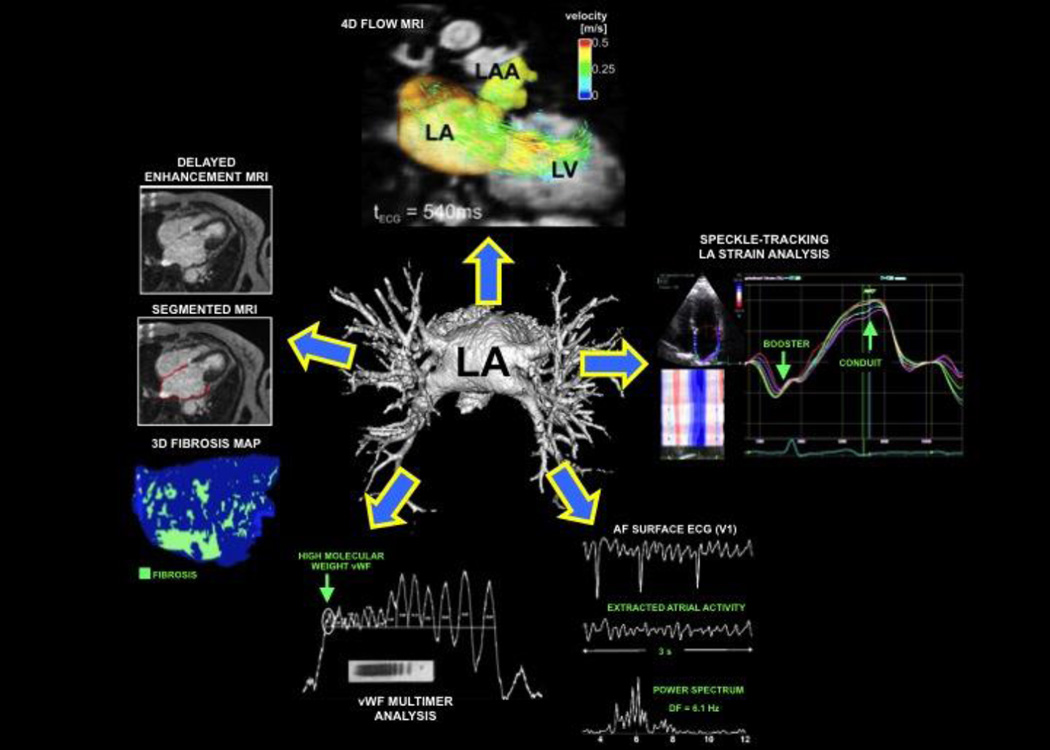
There are several techniques for evaluating the atrial myopathy, as shown in Figure 2. These include echocardiography, cardiac MRI, ECG waveform analysis, and biomarker analyses. Echocardiography has been the imaging technique of choice for evaluating the LA because of its widespread availability and ease of use. LA dimension/volume has been consistently shown to be a predictor of incident78 and recurrent AF.79–81 Yet, the LA size or volume has not been used clinically for treatment decisions. There are other echocardiographic techniques that could better delineate the presence of atrial myopathy; these include assessment of LA chamber function (e.g., LA ejection fraction, LA function index [LAFI]); tissue Doppler imaging (i.e., a’ velocity) for evaluation of the ability of LA contraction to affect the longitudinal velocity of the basal LV in late diastole; speckle-tracking strain analysis (for the evaluation of LA mechanics); and 3D echocardiography (for the evaluation of LA size, shape, and function). Echocardiographic assessment of LA function provides novel insight into LA pathophysiology in the setting of AF. The LAFI, a rhythm-independent marker of LA function that incorporates LA size and the left ventricular outflow tract velocity-time integral (a marker of left ventricular stroke volume), is associated with adverse outcomes in patients with coronary disease, and provides a measure of LA function independent of underlying rhythm.82, 83 The tissue Doppler a’ velocity, which is measured at the septal and lateral mitral annulus, conveys information on both the LA contractile function and the stiffness of the LV at ventricular end-diastole.
Figure 2.
Multiple noninvasive techniques to evaluate the left atrium in patients with atrial fibrillation.
Measurement of LA strain is a novel technique that quantifies segmental and global LA myocardial mechanics in both sinus rhythm and AF. LA strain evaluates the longitudinal shortening and lengthening of segments of the LA myocardium throughout the cardiac cycle. The resultant strain curves quantify the LA conduit function (peak positive LA strain), booster function (peak negative strain [in patients with coordinated atrial contraction]), and the reservoir function (total LA strain). Decreased LA strain (indicative of worse LA mechanical function) has been associated with the development of AF. In addition, LA strain has been used to predict AF recurrence after AF ablation and future stroke risk in the setting of AF, and worse LA strain parameters correlate with reduced exercise capacity, higher CHADS2 score, and increased risk of adverse cardiovascular events.84–90
As noted above, delayed enhancement MRI has been used to detect atrial fibrosis in vivo. The technical challenges of this technique to image fibrosis in the thin walled LA are well appreciated. T1 mapping has been developed to identify diffuse myocardial fibrosis and has recently been applied to the LA91, 92 with good correlation to intracardiac LA electrogram voltage. 4D flow MRI (3D flow velocity measured over time) is a novel technique for the comprehensive assessment of cardiovascular hemodynamics in the heart and great vessels.93–97 4D flow MRI is uniquely suited for evaluation of LA flow velocities (an index of atrial function) as it can provide blood velocity in 3 orthogonal directions with full volumetric coverage of the LA throughout the cardiac cycle with a single, free-breathing 10–15 minute acquisition.94, 97–99 The 3D segmentation of LA volume enables quantitative analysis of 3-directional velocities inside the entire LA over all time frames of the cardiac cycle.99 Recent studies demonstrated low inter-observer variability and good test-retest reliability for the quantification of blood flow velocities and other hemodynamic parameters.100–102 We have demonstrated the potential for this technique to identify different flow patterns in patients with AF, even for those not in sinus rhythm during imaging.99, 103
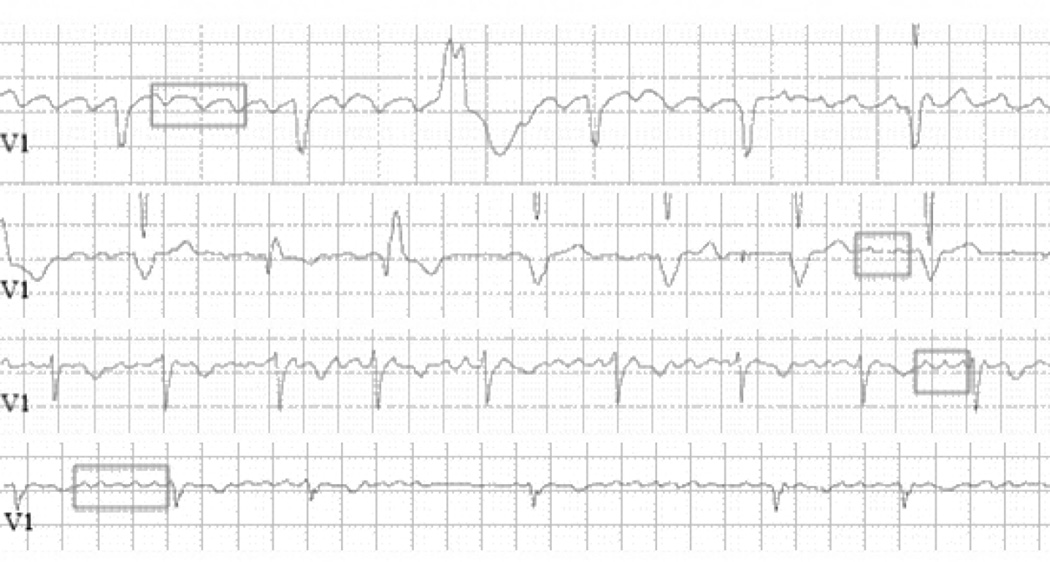
Even the standard ECG recorded during AF is an underutilized tool. It is typically used only to make a diagnosis of AF with no further analysis of the waveform. There is, in fact, significant electrical information in the f waves of the AF ECG. As shown in figure 3, close examination of the ECG can clearly identify differences in rate and other features. Frequency analysis of the f waves can be done to quantify features such as rate. It has been demonstrated that f wave analyses are associated with clinical AF outcomes such as the response to anti-arrhythmic drugs,104 catheter ablation,105, 106 and maintenance of sinus rhythm after cardioversion.107, 108 Dofetilide drug effect – slowing of the dominant frequency – can be detected on the standard 12 lead ECG.109 After the first dose of dofetilide, the AF dominant frequency was reduced (6.46±0.87 vs 4.92±0.62 Hz, p<0.0001). Importantly, drug effect on the atrium was not correlated with its effect on the ventricle as measured by the rate corrected QT interval. In addition, many current approaches to evaluate the atrial substrate focus on intracardiac electrogram properties such as amplitude, frequency, and complexity37, 110–113 as surrogates of the actual anatomic substrate. These parameters are also likely reflected in the surface ECG, but standardized approaches are needed to define optimal techniques, leads for analysis, and measures that provide clinically useful information.
Figure 3.
ECG V1 lead recordings from 4 different patients with AF showing f waves. Although f waves in AF are variable throughout a recording, they are sometimes well-visualized, allowing the variation in their rate and amplitude to be characterized. Differences in f wave rate can be appreciated in some parts of these recordings. In the 1st and 4th tracings, the same size box encompasses 3 and 5 F waves, respectively; in the 2nd and 3rd tracings, the same size box encompasses 2 and 3 F waves, respectively. Other characteristics differ, as well – F wave amplitude and consistency of activation.
Advanced ECG, echocardiographic, and MRI techniques are available to characterize atrial abnormalities that could be important factors affecting treatment efficacy. None of these advanced techniques has yet become a routine part of clinical practice. An understanding of how the results of these tests can affect therapeutic results, as recently demonstrated for fibrosis detected by MRI and its effect on ablation outcomes,26 is needed.
Atrial myopathy and stroke risk
While several AF studies have shown an association between atrial fibrosis and either stroke or predictors of thrombus formation/stroke,114–116 a causal link has yet to be demonstrated. The factors implicated in thrombogenesis are described by the classic Virchow’s triad and consist of endothelial/endocardial damage or dysfunction, stasis, and hypercoagulability which describes the major factors conducive to thrombogenesis. There is evidence supporting each of these factors in AF.117 Further studies may elucidate the link between these abnormalities and the atrial myopathy of AF. It is noteworthy that in addition to the relationship between markers of coagulation and stroke in AF, there is also a relationship to other clinical outcomes in AF.
Stasis, as noted by the echocardiographic display of smoke or diminished LA appendage flow velocities by echocardiography,118–122 has been shown to be associated with stroke in patients with AF. Studies utilizing transesophageal echocardiography (TEE) have shown that decreased flow in the LA and particularly LA appendage (LAA), which is the typical site of thrombus formation, are independent risk factors for stroke in AF.120, 122, 123 Furthermore, the presence of spontaneous echo contrast, a marker of blood stasis, has been shown to be an independent predictor for thromboembolism.118, 124 Specifically, a prior TEE study found that peak LAA emptying velocity <0.2m/s was associated with increased risk for atrial thrombus formation and embolic stroke.120 Multivariate analysis including clinical risk factors found that peak LAA emptying velocity <0.2m/s was independently associated with LAA thrombus (relative risk 2.6, p = 0.02). While the velocities were significantly lower in patients with AF versus sinus rhythm at the time of imaging (0.33±0.20 m/s versus 0.61±0.35 m/s, p<0.001), there was significant overlap in the distribution of velocities, indicating that even patients with prior AF but in sinus rhythm at the time of imaging may have significantly depressed LAA emptying velocity. Other studies have shown that LA size and LAA geometry may be additional parameters influencing thromboembolic risk.125–127
Stasis can also be detected by the novel 4D flow MRI technique.99 This technique can noninvasively display complex 3D blood flow patterns in-vivo and has facilitated insight into complex normal and altered cardiovascular hemodynamics previously limited by other imaging strategies.128–130 Preliminary findings demonstrate that 4D flow MRI can detect individual physiologic changes in LA flow velocities in patients with AF that are not conveyed by the standard CHA2DS2-VASc clinical risk score.131–133 We have demonstrated an inverse relationship between flow and the CHA2DS2-VASc score. As with TEE, flow velocities in patients imaged during sinus rhythm were higher than those imaged in AF, but were significantly depressed compared to a similarly aged cohort without a history of AF. Almost by definition, the atrial myopathy underlying AF is likely responsible for the diminished flow observed in patients with AF imaged when in sinus rhythm. In addition, there may be a dynamic change in LA flow velocities in sinus rhythm depending on degree of atrial myopathy, frequency and duration of AF episodes, and time from the most recent episode of AF. In addition, other factors such as LA geometry may affect the tendency for areas of stasis and thrombus formation. In a study127 evaluating the LA appendage by CT and MRI, stroke risk differed depending on anatomic details of the LA appendage, but AF occurrence was not reported. The relationship of stasis to other atrial substrate parameters and to clinical outcomes requires further study.
Despite a large body of work demonstrating an increase in prothrombotic factors in patients with AF, these are usually not incorporated in the clinical diagnostic evaluation of stroke risk. Hemostasis occurs in three general phases: initiation, propagation, and termination.134 Initiation is characterized by platelet adherence and aggregation at the site of vascular injury and trace thrombin formation. Larger amounts of thrombin are formed during the propagation phase, and thrombin formation is curtailed by inhibitors during the termination phase, which includes activation and inhibition of fibrinolysis.
Von Willebrand factor (VWF) is a multimeric plasma glycoprotein that plays a central role in hemostasis and participates in angiogenesis, cell proliferation, and inflammation135 and is active during the initiation phase of hemostasis. The highest molecular weight (HMW) multimers are the most thrombogenic.136 Within minutes of the onset of AF, atrial blood shows evidence of platelet and endothelial cell activation.137 The activated endothelium, under shear, extrudes VWF from its Weibel-Palade bodies. The hemostatic potential of the VWF increases with its size (extent of multimerization) and the magnitude of the applied hydrodynamic shear.138 The HMW multimers become anchored to the cell surface where they unravel and bind platelets, initiating thrombus formation.139 The unraveling of the multimers exposes a cleavage site between aminoacids 1605 and 1606 (tyrosine/methionine) that is attacked by the protease, ADAMTS13 (a disintegrin-like and metalloprotease domain with thrombospondin type-1 motif, number 13). There are conditions that result in resistance to proteolysis; for example, alterations in glycosylation140 and oxidative stress.141 The latter might be particularly relevant to patients with AF, in whom oxidative stress secondary to disturbed atrial circulation could result in resistance of VWF to ADAMTS13, an increase in HMW multimers, and thrombosis. In healthy persons, the ratio of VWF antigen to ADAMTS13 is unity, reflecting the balance between VWF and its protease. Ratios are significantly higher in patients with chronic AF than in those with paroxysmal AF (P<0.01) or controls (P<0.0001).142 In addition, there are significant correlations between the ratio and the LA diameter (P=0.0002) and LA appendage flow velocity (P=0.002). A high ratio of VWF:ADAMTS13 independently predicts major adverse cardiovascular events in patients with AF (hazard ratio 2.17, P=0.007).143 After cardioversion, the ratio was an independent predictor of recurrent AF (HR 1.88, P=0.03).144
VWF145–148 is increased in patients with nonvalvular AF as compared to those in sinus rhythm, irrespective of a history of stroke. In the ARIC Study, VWF was associated with AF independent of other CV risk factors.149 In multivariable Cox models, the hazard ratio for incident AF associated with a 1-standard deviation increase in VWF was 1.17 (95% CI 1.11–1.23). Conway et al150 and Krishnamoorthy et al151 reported that raised VWF levels in patients with AF predicted stroke and vascular events, and Roldan et al152 found that high VWF levels are an independent risk factor for adverse events in AF patients on anticoagulant therapy. The concentrations of VWF in LA blood are increased in patients with persistent AF and are higher than in the LA of those with paroxysmal AF or controls.153 They are also associated with spontaneous echocardiographic contrast, and are higher in AF patients with than without LA thrombi (200 vs 155 IU/dl, P=0.0006).154 The level of VWF is also associated with the extent of periatrial epicardial fat, but not body mass index or epicardial adipose tissue, suggesting local adiposity affects VWF concentrations.155
A variety of other prothrombotic abnormalities have been noted in patients with AF. These include alterations in D-dimer, platelet factor-4, thrombin-antithrombin complexes, and plasminogen activator inhibitor-1 (PAI-1). D-dimer has been evaluated most extensively; significant associations between D-dimer levels and the risk of stroke, cardiovascular death, and major bleeding outcomes were reported in the ARISTOTLE trial.156 In those with new onset AF, D-dimer levels increase above the normal range within 12 hours, and reach a plateau at the 18th hour.157 In patients with chronic AF, the range of D-dimer increase is characteristic for each patient and is stable over time.158 In patients with chronic AF, elevated levels of platelet factor-4 are observed.145, 147, 148, 159, 160 There is evidence of thrombin generation in AF, as detected by increases in thrombin-antithrombin complexes and prothrombin fragment 1+2.161, 162
Why do thrombi occur more often in the left than right atrium? There may be greater tendency to stasis and/or procoagulant factors in the LA. Activated protein C is one of the most potent natural anticoagulants; protein C binds to the endothelial protein C receptor where it is activated by a complex of thrombin and thrombomodulin. Cervero et al163 reported a significant underexpression of thrombomodulin by the left as compared to the right atrial endocardium, and less than half the ability to activate protein C. In pigs with induced AF, PAI-1 protein expression was increased in the LA but not the right atrium, and the LA increase was accompanied by a decrease in nitric oxide.164 In patients with chronic AF, thrombin generation is increased in the LA compared to the periphery.165 Platelet and leukocyte activation, and leukocyte platelet aggregates were observed in the LA blood of patients with spontaneous echocardiographic contrast.166
It is evident that procoagulant factors in AF are associated with AF and atrial disease. VWF, ADAMTS13, thrombomodulin, and PAI-1 are all either synthesized or released from the endothelium, and are affected by diseases that attack the endothelium. There is considerable evidence of endothelial dysfunction in AF; for example, nitric oxide synthase and NO bioavailability are decreased164 and levels of MMP-2 and sVCAM-1, markers of endothelial remodeling, are increased.167 The complex interplay of hemostatic and anatomic factors in the LA may underly a prothrombotic state more localized to the LA.
Recent data raise the possibility that there is a complex relationship between thromboembolic risk and AF. The RACE trial168 noted that “patients with risk factors may have a stroke after cessation of anticoagulant therapy, despite maintenance of sinus rhythm”. The TRENDS study169 showed that most of the 40 patients with AF and implanted devices who experienced cerebrovascular events or systemic emboli did not have AF episodes proximal to the event. Additionally, a retrospective analysis of 568 continuously monitored patients demonstrated an interaction between AF duration and CHADS2 score, such that patients with scores≥3 were at increased stroke risk regardless of AF duration, those with scores of zero were at low risk regardless of AF duration, and those in between had risk profiles that depended on the duration of AF episodes.170 Data from the Multi-Ethnic Study of Atherosclerosis171 and the Cardiovascular Health Study172 have identified an association between P wave terminal force in V1 (left atrial abnormality) and stroke that is independent of AF. These observations support the conjecture that the atrial myopathy underlying the development of AF may also directly affect the risk of thrombosis via effects on atrial flow and/or the hemostatic profile, thereby increasing the risk for thromboembolism, even in the absence of AF (this potential pathway is illustrated in Figure 1). In addition, the disease processes underlying the development of the atrial myopathy may also be associated with stroke etiology unrelated to emboli from the left atrium. More detailed understanding of the atrial myopathy, specifically its impact on atrial flow velocities or stasis, as well as the prothombotic factors present in individual patients might enhance risk stratification currently assessed by the clinically-based CHA2DS2-VASc score.
Other biomarkers in AF
While biomarkers are generally not specific to atrial myocardial disease, various biomarkers (in addition to the coagulation biomarkers described above) spanning myocyte injury/stress, inflammation, and fibrosis have been linked to outcomes in AF173 and have been reported to be elevated in AF or predictive of development of AF.174 The natriuretic peptides, troponin, CRP, IL-6, among others, have been related to outcomes in AF.173, 175–182 It is not yet known which of these markers is most predictive of treatment outcomes, but there is strong evidence for the role of inflammation in AF28, 29 and emerging evidence for the prognostic importance of the natriuretic peptides and troponin.174, 175, 178, 183–185 Persistent elevation of troponin and NT-proBNP in the RELY trial was associated with adverse outcomes.186 Serial changes in biomarkers of inflammation (CRP, IL-6), natriuretic peptides (ANP, BNP), and collagen turnover (MMP-2, TIMP-2) have been reported after ablation187 or medical therapy.57 Thus, baseline evaluations of these biomarkers could indicate the involvement of the specific pathways and serial study could provide evidence of salutary effects of medical therapy on atrial substrate.
Conclusion
A clear delineation of the atrial myopathy that serves as the substrate for AF might improve treatment outcomes. Stroke and major bleeding complications associated with anticoagulation remain significant concerns in patients with AF. Better assessment of stroke risk can improve the risk-benefit ratio of current and future therapies. Current pharmacological treatments for rhythm control of AF have only modest efficacy and suffer from potentially life-threatening side effects. While the AF guidelines do provide an algorithm for choosing anti-arrhythmic drugs, this is based on the presence and severity of a patient’s underlying heart disease, and designed to limit proarrhythmic complications rather than matching specific treatments to the underlying atrial substrate for AF. Current ablative and surgical approaches to AF, though somewhat more successful, use an anatomic, ‘one-size fits all’ strategy (with some minor variations), focusing on pulmonary vein isolation, that does not address the specific mechanisms underlying this complex arrhythmia. Moreover, in the STAR AF II randomized clinical trial,188 there was no significant benefit to adding either ablation of complex fractionated electrograms or additional LA linear lesion sets compared to pulmonary vein isolation alone in patients with persistent AF. Better delineation of the atrial myopathic substrate and its relationship to arrhythmia mechanisms in individual patients could potentially substantially improve the results of catheter ablation, as defining the primary arrhythmia substrate for other types of supraventricular tachycardias has been associated with success rates exceeding 95%.189 As noted by the European Heart Rhythm Association consensus conference, it therefore is likely that “a new taxonomy of AF may be needed based on the pathophysiological type of AF to allow personalized management of AF to come to full fruition.” Further evaluation will be required to confirm that treatment outcomes improve if a comprehensive evaluation of the atrial substrate is used to guide management decisions.
Finally, better delineation of the pathophysiologic mechanisms underlying the development of the atrial substrate for AF, particularly in its earlier stages, could help identify blood and imaging biomarkers that could be useful to assess risk for developing new onset AF. Primary prevention strategies could potentially target specific mechanisms and novel treatments could be tested in appropriate higher risk groups. In addition, these blood and imaging biomarkers might serve as surrogate endpoints for early assessment of novel interventions designed for the primary prevention of AF.
Acknowledgments
Funding Sources: Dr. Goldberger has been supported by grant #R21-HL113895 from the National Heart, Lung, and Blood Institute. Dr. Arora has been supported by grant # R01-HL09340 from the National Heart, Lung, and Blood Institute. Dr. Greenland has been supported by grant #s HHSN268201300027C and N01-HC-95164 from the National Institutes of Health. Dr. Markl has been supported by grant #12GRNT12080032 from the American Heart Association. Dr. Ng has been supported by grant #12GRNT12070241 from the American Heart Association and grant # R21-HL110041 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Disclosures: None
REFERENCES
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 5.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins LS, Brodsky M, Schron E, Chung M, Rocco T, Lader E, Constantine M, Sheppard R, Holmes D, Mateski D, Floden L, Prasun M, Greene HL, Shemanski L. Quality of life in atrial fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:112–120. doi: 10.1016/j.ahj.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 7.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448. doi: 10.1016/j.amjmed.2005.10.057. e1–19. [DOI] [PubMed] [Google Scholar]
- 8.Perret-Guillaume C, Briancon S, Wahl D, Guillemin F, Empereur F. Quality of Life in elderly inpatients with atrial fibrillation as compared with controlled subjects. J Nutr Health Aging. 2010;14:161–166. doi: 10.1007/s12603-009-0188-5. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 11.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Van Staa TP, Setakis E, Di Tanna GL, Lane DA, Lip GY. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost. 2011;9:39–348. doi: 10.1111/j.1538-7836.2010.04085.x. [DOI] [PubMed] [Google Scholar]
- 14.Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 15.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A, Bailleul C, Bax J, Benninger G, Blomstrom-Lundqvist C, Boersma L, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, Casadei B, Clemens A, Crijns H, Derwand R, Dobrev D, Ezekowitz M, Fetsch T, Gerth A, Gillis A, Gulizia M, Hack G, Haegeli L, Hatem S, Georg Hausler K, Heidbuchel H, Hernandez-Brichis J, Jais P, Kappenberger L, Kautzner J, Kim S, Kuck KH, Lane D, Leute A, Lewalter T, Meyer R, Mont L, Moses G, Mueller M, Munzel F, Nabauer M, Nielsen JC, Oeff M, Oto A, Pieske B, Pisters R, Potpara T, Rasmussen L, Ravens U, Reiffel J, Richard-Lordereau I, Schafer H, Schotten U, Stegink W, Stein K, Steinbeck G, Szumowski L, Tavazzi L, Themistoclakis S, Thomitzek K, Van Gelder IC, von Stritzky B, Vincent A, Werring D, Willems S, Lip GY, Camm AJ. Personalized management of atrial fibrillation: Proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2013;15:1540–1556. doi: 10.1093/europace/eut232. [DOI] [PubMed] [Google Scholar]
- 17.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, Kottkamp H, Dhein S. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett TH, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4:S24–27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 20.Chimenti C, Russo MA, Carpi A, Frustaci A. Histological substrate of human atrial fibrillation. Biomed Pharmacother. 2010;64:177–183. doi: 10.1016/j.biopha.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Choisy SC, Barman P, Zhang H, Hancox JC, Jones SA, James AF. Atrial remodeling and the substrate for atrial fibrillation in rat hearts with elevated afterload. Circ Arrhythm Electrophysiol. 2011;4:761–769. doi: 10.1161/CIRCEP.111.964783. [DOI] [PubMed] [Google Scholar]
- 22.Tan AY, Zimetbaum P. Atrial fibrillation and atrial fibrosis. J Cardiovasc Pharmacol. 2011;57:625–629. doi: 10.1097/FJC.0b013e3182073c78. [DOI] [PubMed] [Google Scholar]
- 23.Koduri H, Ng J, Cokic I, Aistrup GL, Gordon D, Wasserstrom JA, Kadish AH, Lee R, Passman R, Knight BP, Goldberger JJ, Arora R. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. 2012;5:640–649. doi: 10.1161/CIRCEP.111.970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kottkamp H. Atrial fibrillation substrate: the “unknown species”-- from lone atrial fibrillation to fibrotic atrial cardiomyopathy. Heart Rhythm. 2012;9:481–482. doi: 10.1016/j.hrthm.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 27.Luo T, Chang CX, Zhou X, Gu SK, Jiang TM, Li YM. Characterization of atrial histopathological and electrophysiological changes in a mouse model of aging. Int J Mol Med. 2013;31:138–146. doi: 10.3892/ijmm.2012.1174. [DOI] [PubMed] [Google Scholar]
- 28.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 29.Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med. 2011;17:556–563. doi: 10.1016/j.molmed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Youn JY, Zhang J, Zhang Y, Chen H, Liu D, Ping P, Weiss JN, Cai H. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters DC, Wylie JV, Hauser TH, Nezafat R, Han Y, Woo JJ, Taclas J, Kissinger KV, Goddu B, Josephson ME, Manning WJ. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging. 2009;2:308–316. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seitz J, Horvilleur J, Lacotte JDOHI, Mouhoub Y, Maltret A, Salerno F, Mylotte D, Monchi M, Garot J. Correlation between AF substrate ablation difficulty and left atrial fibrosis quantified by delayed-enhancement cardiac magnetic resonance. Pacing Clin Electrophysiol. 2011;34:1267–1277. doi: 10.1111/j.1540-8159.2011.03148.x. [DOI] [PubMed] [Google Scholar]
- 33.Jadidi AS, Cochet H, Shah AJ, Kim SJ, Duncan E, Miyazaki S, Sermesant M, Lehrmann H, Lederlin M, Linton N, Forclaz A, Nault I, Rivard L, Wright M, Liu X, Scherr D, Wilton SB, Roten L, Pascale P, Derval N, Sacher F, Knecht S, Keyl C, Hocini M, Montaudon M, Laurent F, Haissaguerre M, Jais P. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J Am Coll Cardiol. 2013;62:802–812. doi: 10.1016/j.jacc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 34.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 35.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 36.Healey JS, Israel CW, Connolly SJ, Hohnloser SH, Nair GM, Divakaramenon S, Capucci A, Van Gelder IC, Lau CP, Gold MR, Carlson M, Themeles E, Morillo CA. Relevance of electrical remodeling in human atrial fibrillation: results of the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial mechanisms of atrial fibrillation study. Circ Arrhythm Electrophysiol. 2012;5:626–631. doi: 10.1161/CIRCEP.112.970442. [DOI] [PubMed] [Google Scholar]
- 37.Medi C, Kalman JM, Spence SJ, Teh AW, Lee G, Bader I, Kaye DM, Kistler PM. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:1317–1324. doi: 10.1111/j.1540-8167.2011.02125.x. [DOI] [PubMed] [Google Scholar]
- 38.Olshansky B. Interrelationships between the autonomic nervous system and atrial fibrillation. Prog Cardiovasc Dis. 2005;48:57–78. doi: 10.1016/j.pcad.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4:S61–64. doi: 10.1016/j.hrthm.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora R. Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: implications for therapies targeting the atrial autonomic nervous system. Circ Arrhythm Electrophysiol. 2012;5:850–859. doi: 10.1161/CIRCEP.112.972273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 42.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 43.Katritsis D, Merchant FM, Mela T, Singh JP, Heist EK, Armoundas AA. Catheter ablation of atrial fibrillation the search for substrate-driven end points. J Am Coll Cardiol. 2010;55:2293–2298. doi: 10.1016/j.jacc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Zhang P, Mu Y, Gao M, Wang JR, Wang Y, Su LQ, Hou YL. The role of renin-angiotensin system blockade therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Clin Pharmacol Ther. 2010;88:521–531. doi: 10.1038/clpt.2010.123. [DOI] [PubMed] [Google Scholar]
- 45.Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H, Vincent J, Pitt B EMPHASIS-HF Investigators. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Zhang Y, Gao M, Wang J, Wang Q, Wang X, Su L, Hou Y. Statin therapy for the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Pharmacotherapy. 2011;31:1051–1062. doi: 10.1592/phco.31.11.1051. [DOI] [PubMed] [Google Scholar]
- 47.Hung CY, Lin CH, Loh el W, Ting CT, Wu TJ. CHADS(2) score, statin therapy, and risks of atrial fibrillation. Am J Med. 2013;126:133–140. doi: 10.1016/j.amjmed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 48.Pinho-Gomes AC, Reilly S, Brandes RP, Casadei B. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibition with statins. Antioxid Redox Signal. 2014;20:1268–1285. doi: 10.1089/ars.2013.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang WT, Li HJ, Zhang H, Jiang S. The role of statin therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Br J Clin Pharmacol. 2012;74:744–756. doi: 10.1111/j.1365-2125.2012.04258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakabe M, Shiroshita-Takeshita A, Maguy A, Dumesnil C, Nigam A, Leung TK, Nattel S. Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation. 2007;116:2101–2109. doi: 10.1161/CIRCULATIONAHA.107.704759. [DOI] [PubMed] [Google Scholar]
- 51.Mayyas F, Sakurai S, Ram R, Rennison JH, Hwang ES, Castel L, Lovano B, Brennan ML, Bibus D, Lands B, Barnard J, Chung MK, Van Wagoner DR. Dietary omega3 fatty acids modulate the substrate for post-operative atrial fibrillation in a canine cardiac surgery model. Cardiovasc Res. 2011;89:852–861. doi: 10.1093/cvr/cvq380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–2372. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 53.Rahimi K, Emberson J, McGale P, Majoni W, Merhi A, Asselbergs FW, Krane V Macfarlane PW and PROSPER Executive. Effect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. BMJ. 2011;342:d1250. doi: 10.1136/bmj.d1250. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz GG, Chaitman BR, Goldberger JJ, Messig M. High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Am Heart J. 2011;161:993–999. doi: 10.1016/j.ahj.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Ramadeen A, Connelly KA, Leong-Poi H, Hu X, Fujii H, Van Krieken R, Laurent G, Holub BJ, Bazinet RP, Dorian P. N-3 polyunsaturated fatty acid supplementation does not reduce vulnerability to atrial fibrillation in remodeling atria. Heart Rhythm. 2012;9:1115–1122. doi: 10.1016/j.hrthm.2012.02.013. e4. [DOI] [PubMed] [Google Scholar]
- 56.Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–2319. doi: 10.1161/01.CIR.0000145163.56529.D1. [DOI] [PubMed] [Google Scholar]
- 57.Negi S, Shukrullah I, Veledar E, Bloom HL, Jones DP, Dudley SC. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial) J Cardiovasc Electrophysiol. 2011;22:414–419. doi: 10.1111/j.1540-8167.2010.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 59.Milliez P, Deangelis N, Rucker-Martin C, Leenhardt A, Vicaut E, Robidel E, Beaufils P, Delcayre C, Hatem SN. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26:2193–2199. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 60.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 61.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 62.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heeringa J, Hoogendoorn EH, van der Deure WM, Hofman A, Peeters RP, Hop WC, den Heijer M, Visser TJ, Witteman JC. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam Study. Arch Intern Med. 2008;168:2219–2224. doi: 10.1001/archinte.168.20.2219. [DOI] [PubMed] [Google Scholar]
- 64.Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–1169. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Rooij FJ, Lip GY, Witteman JC. Subclinical atherosclerosis and risk of atrial fibrillation: the Rotterdam Study. Arch Intern Med. 2007;167:382–387. doi: 10.1001/archinte.167.4.382. [DOI] [PubMed] [Google Scholar]
- 66.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy-Dicey A, Harris TB, Pencina MJ, D’Agostino RB, Levy D, Kannel WB, Wang TJ, Kronmal RA, Wolf PA, Burke GL, Launer LJ, Vasan RS, Psaty BM, Benjamin EJ, Gudnason V, Heckbert SR. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang TJ, Parise H, Levy D, D’Agostino RB, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 71.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 72.Magnani JW, Hylek EM, Apovian CM. Obesity begets atrial fibrillation: a contemporary summary. Circulation. 2013;128:401–405. doi: 10.1161/CIRCULATIONAHA.113.001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abed HS, Wittert GA. Obesity and atrial fibrillation. Obes Rev. 2013;14:929–938. doi: 10.1111/obr.12056. [DOI] [PubMed] [Google Scholar]
- 74.Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, Tchou PJ, Chung MK. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:230–236. doi: 10.1161/CIRCEP.110.957241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33:904–912. doi: 10.1093/eurheartj/ehr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 79.Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, Brodsky M, Barrell P, Greene HL. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026–2033. doi: 10.1016/j.jacc.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 80.Marchese P, Bursi F, Delle Donne G, Malavasi V, Casali E, Barbieri A, Melandri F, Modena MG. Indexed left atrial volume predicts the recurrence of non-valvular atrial fibrillation after successful cardioversion. Eur J Echocardiogr. 2011;12:214–221. doi: 10.1093/ejechocard/jeq176. [DOI] [PubMed] [Google Scholar]
- 81.Marchese P, Malavasi V, Rossi L, Nikolskaya N, Donne GD, Becirovic M, Colantoni A, Luciani A, Modena MG. Indexed left atrial volume is superior to left atrial diameter in predicting nonvalvular atrial fibrillation recurrence after successful cardioversion: a prospective study. Echocardiography. 2012;29:276–284. doi: 10.1111/j.1540-8175.2011.01580.x. [DOI] [PubMed] [Google Scholar]
- 82.Thomas L, Hoy M, Byth K, Schiller NB. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr. 2008;9:356–362. doi: 10.1016/j.euje.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. 2012;59:673–680. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 85.Saha SK, Anderson PL, Caracciolo G, Kiotsekoglou A, Wilansky S, Govind S, Mori N, Sengupta PP. Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation. J Am Soc Echocardiogr. 2011;24:506–512. doi: 10.1016/j.echo.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 86.Shih JY, Tsai WC, Huang YY, Liu YW, Lin CC, Huang YS, Tsai LM, Lin LJ. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr. 2011;24:513–519. doi: 10.1016/j.echo.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 87.Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, Mondillo S. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264–269. doi: 10.1016/j.amjcard.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 88.Hong J, Gu X, An P, Luo T, Lv Q, Kang J, He Y, Hu R, Liu X, Ma C. Left atrial functional remodeling in lone atrial fibrillation: a two-dimensional speckle tracking echocardiographic study. Echocardiography. 2013;30:1051–1060. doi: 10.1111/echo.12200. [DOI] [PubMed] [Google Scholar]
- 89.Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart. 2012;98:1311–1317. doi: 10.1136/heartjnl-2012-302007. [DOI] [PubMed] [Google Scholar]
- 90.Morris DA, Parwani A, Huemer M, Wutzler A, Bekfani T, Attanasio P, Friedrich K, Kuhnle Y, Haverkamp W, Boldt LH. Clinical significance of the assessment of the systolic and diastolic myocardial function of the left atrium in patients with paroxysmal atrial fibrillation and low CHADS(2) index treated with catheter ablation therapy. Am J Cardiol. 2013;111:1002–1011. doi: 10.1016/j.amjcard.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 91.Beinart R, Khurram IM, Liu S, Yarmohammadi H, Halperin HR, Bluemke DA, Gai N, van der Geest RJ, Lima JA, Calkins H, Zimmerman SL, Nazarian S. Cardiac magnetic resonance T1 mapping of left atrial myocardium. Heart Rhythm. 2013;10:1325–1331. doi: 10.1016/j.hrthm.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ling LH, McLellan AJ, Taylor AJ, Iles LM, Ellims AH, Kumar S, Teh A, Lee G, Wong MC, Azzopardi S, Sellenger MA, Morton JB, Kalman JM, Kistler PM. Magnetic resonance post-contrast T1 mapping in the human atrium: validation and impact on clinical outcome after catheter ablation for atrial fibrillation. Heart Rhythm. 2014;11:1551–1559. doi: 10.1016/j.hrthm.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 93.Barker AJ, Markl M, Burk J, Lorenz R, Bock J, Bauer S, Schulz-Menger J, von Knobelsdorff-Brenkenhoff F. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5:457–466. doi: 10.1161/CIRCIMAGING.112.973370. [DOI] [PubMed] [Google Scholar]
- 94.Markl M, Kilner PJ, Ebbers T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:7. doi: 10.1186/1532-429X-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frydrychowicz A, Markl M, Hirtler D, Harloff A, Schlensak C, Geiger J, Stiller B, Arnold R. Aortic hemodynamics in patients with and without repair of aortic coarctation: in vivo analysis by 4D flow-sensitive magnetic resonance imaging. Invest Radiol. 2011;46:317–325. doi: 10.1097/RLI.0b013e3182034fc2. [DOI] [PubMed] [Google Scholar]
- 96.Harloff A, Simon J, Brendecke S, Assefa D, Helbing T, Frydrychowicz A, Weber J, Olschewski M, Strecker C, Hennig J, Weiller C, Markl M. Complex plaques in the proximal descending aorta: an underestimated embolic source of stroke. Stroke. 2010;41:1145–1150. doi: 10.1161/STROKEAHA.109.577775. [DOI] [PubMed] [Google Scholar]
- 97.Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E, Langer M, Hennig J, Frydrychowicz A. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging. 2007;25:824–831. doi: 10.1002/jmri.20871. [DOI] [PubMed] [Google Scholar]
- 98.Markl M, Geiger J, Kilner PJ, Foll D, Stiller B, Beyersdorf F, Arnold R, Frydrychowicz A. Time-resolved three-dimensional magnetic resonance velocity mapping of cardiovascular flow paths in volunteers and patients with Fontan circulation. Eur J Cardiothorac Surg. 2011;39:206–212. doi: 10.1016/j.ejcts.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 99.Fluckiger JU, Goldberger JJ, Lee DC, Ng J, Lee R, Goyal A, Markl M. Left atrial flow velocity distribution and flow coherence using four-dimensional FLOW MRI: a pilot study investigating the impact of age and Pre- and Postintervention atrial fibrillation on atrial hemodynamics. J Magn Reson Imaging. 2013;38:580–587. doi: 10.1002/jmri.23994. [DOI] [PubMed] [Google Scholar]
- 100.Markl M, Wallis W, Harloff A. Reproducibility of flow and wall shear stress analysis using flow-sensitive four-dimensional MRI. J Magn Reson Imaging. 2011;33:988–994. doi: 10.1002/jmri.22519. [DOI] [PubMed] [Google Scholar]
- 101.Geiger J, Markl M, Herzer L, Hirtler D, Loeffelbein F, Stiller B, Langer M, Arnold R. Aortic flow patterns in patients with Marfan syndrome assessed by flow-sensitive four-dimensional MRI. J Magn Reson Imaging. 2012;35:594–600. doi: 10.1002/jmri.23500. [DOI] [PubMed] [Google Scholar]
- 102.Markl M, Wegent F, Zech T, Bauer S, Strecker C, Schumacher M, Weiller C, Hennig J, Harloff A. In vivo wall shear stress distribution in the carotid artery: effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ Cardiovasc Imaging. 2010;3:647–655. doi: 10.1161/CIRCIMAGING.110.958504. [DOI] [PubMed] [Google Scholar]
- 103.Markl M, Fluckiger JU, Lee DC, Ng J, Goldberger J. Velocity quantification by electrocardiography-gated phase contrast magnetic resonance imaging in patients with cardiac arrhythmia: A simulation study based on real time transesophageal echocardiography data in atrial fibrillation. J Comput Assist Tomogr. 2015;39:422–427. doi: 10.1097/RCT.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bollmann A, Kanuru NK, McTeague KK, Walter PF, DeLurgio DB, Langberg JJ. Frequency analysis of human atrial fibrillation using the surface electrocardiogram and its response to ibutilide. Am J Cardiol. 1998;81:1439–1445. doi: 10.1016/s0002-9149(98)00210-0. [DOI] [PubMed] [Google Scholar]
- 105.Meo M, Zarzoso V, Meste O, Latcu DG, Saoudi N. Non-invasive prediction of catheter ablation outcome in persistent atrial fibrillation by exploiting the spatial diversity of surface ECG. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:5531–5534. doi: 10.1109/IEMBS.2011.6091411. [DOI] [PubMed] [Google Scholar]
- 106.Meo M, Zarzoso V, Meste O, Latcu DG, Saoudi N. Catheter ablation outcome prediction in persistent atrial fibrillation based on spatio-temporal complexity measures of the surface ECG. Computing in Cardiology. 2011;2011:261–264. [Google Scholar]
- 107.Bollmann A, Husser D, Steinert R, Stridh M, Soernmo L, Olsson SB, Polywka D, Molling J, Geller C, Klein HU. Echocardiographic and electrocardiographic predictors for atrial fibrillation recurrence following cardioversion. J Cardiovasc Electrophysiol. 2003;14:S162–165. doi: 10.1046/j.1540.8167.90306.x. [DOI] [PubMed] [Google Scholar]
- 108.Holmqvist F, Stridh M, Waktare JE, Sornmo L, Olsson SB, Meurling CJ. Atrial fibrillatory rate and sinus rhythm maintenance in patients undergoing cardioversion of persistent atrial fibrillation. Eur Heart J. 2006;27:2201–2207. doi: 10.1093/eurheartj/ehl098. [DOI] [PubMed] [Google Scholar]
- 109.Raygor V, Ng J, Goldberger J. Surface ECG f wave analysis of dofetilide drug effect in the atrium. J Cardiovasc Electrophysiol. 2015;26:644–648. doi: 10.1111/jce.12645. [DOI] [PubMed] [Google Scholar]
- 110.Chao TF, Cheng CC, Lin WS, Tsao HM, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Liu SH, Hartono B, Wu TJ, Chen SA. Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2011;8:1155–1159. doi: 10.1016/j.hrthm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 111.Suenari K, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Huang SY, Tai CT, Nakano Y, Kihara Y, Tsao HM, Wu TJ, Chen SA. Relationship between arrhythmogenic pulmonary veins and the surrounding atrial substrate in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:405–410. doi: 10.1111/j.1540-8167.2010.01932.x. [DOI] [PubMed] [Google Scholar]
- 112.Hartono B, Lo LW, Cheng CC, Lin YJ, Chang SL, Hu YF, Suenari K, Li CH, Chao TF, Liu SH, Niu YL, Chang HY, Ambrose K, Yu WC, Hsu TL, Chen SA. A novel finding of the atrial substrate properties and long-term results of catheter ablation in chronic atrial fibrillation patients with left atrial spontaneous echo contrast. J Cardiovasc Electrophysiol. 2012;23:239–246. doi: 10.1111/j.1540-8167.2011.02170.x. [DOI] [PubMed] [Google Scholar]
- 113.Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, Morton JB, Sanders P, Kalman JM. Long-term effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm. 2012;9:473–480. doi: 10.1016/j.hrthm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 114.Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–838. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Akoum N, Fernandez G, Wilson B, McGann C, Kholmovski E, Marrouche N. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:1104–1109. doi: 10.1111/jce.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scridon A, Tabib A, Barres C, Julien C, Chevalier P. Left atrial endocardial fibrosis and intra-atrial thrombosis - landmarks of left atrial remodeling in rats with spontaneous atrial tachyarrhythmias. Rom J Morphol Embryol. 2013;54:405–411. [PubMed] [Google Scholar]
- 117.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373:155–166. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 118.Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med. 1998;128:639–647. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 119.Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961–969. doi: 10.1016/0735-1097(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 120.Goldman ME, Pearce LA, Hart RG, Zabalgoitia M, Asinger RW, Safford R, Halperin JL. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study) J Am Soc Echocardiogr. 1999;12:1080–1087. doi: 10.1016/s0894-7317(99)70105-7. [DOI] [PubMed] [Google Scholar]
- 121.Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994;24:755–762. doi: 10.1016/0735-1097(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 122.Handke M, Harloff A, Hetzel A, Olschewski M, Bode C, Geibel A. Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation--a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J Am Soc Echocardiogr. 2005;18:1366–1372. doi: 10.1016/j.echo.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Pollick C, Taylor D. Assessment of left atrial appendage function by transesophageal echocardiography. Implications for the development of thrombus. Circulation. 1991;84:223–231. doi: 10.1161/01.cir.84.1.223. [DOI] [PubMed] [Google Scholar]
- 124.Asinger RW, Koehler J, Pearce LA, Zabalgoitia M, Blackshear JL, Fenster PE, Strauss R, Hess D, Pennock GD, Rothbart RM, Halperin JL. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: II. Dense spontaneous echocardiographic contrast (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study) J Am Soc Echocardiogr. 1999;12:1088–1096. doi: 10.1016/s0894-7317(99)70106-9. [DOI] [PubMed] [Google Scholar]
- 125.Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:6–12. doi: 10.7326/0003-4819-116-1-6. [DOI] [PubMed] [Google Scholar]
- 126.Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. 1998;158:1316–1320. doi: 10.1001/archinte.158.12.1316. [DOI] [PubMed] [Google Scholar]
- 127.Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 128.Harloff A, Strecker C, Frydrychowicz AP, Dudler P, Hetzel A, Geibel A, Kollum M, Weiller C, Hennig J, Markl M. Plaques in the descending aorta: a new risk factor for stroke? Visualization of potential embolization pathways by 4D MRI. J Magn Reson Imaging. 2007;26:1651–1655. doi: 10.1002/jmri.21126. [DOI] [PubMed] [Google Scholar]
- 129.Markl M, Draney MT, Hope MD, Levin JM, Chan FP, Alley MT, Pelc NJ, Herfkens RJ. Time-resolved 3-dimensional velocity mapping in the thoracic aorta: visualization of 3-directional blood flow patterns in healthy volunteers and patients. J Comput Assist Tomogr. 2004;28:459–468. doi: 10.1097/00004728-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 130.Foll D, Taeger S, Bode C, Jung B, Markl M. Age, gender, blood pressure, and ventricular geometry influence normal 3D blood flow characteristics in the left heart. Eur Heart J Cardiovasc Imaging. 2013;14:366–373. doi: 10.1093/ehjci/jes196. [DOI] [PubMed] [Google Scholar]
- 131.Lee D, Markl M, Ng J, Carr M, Benefiled B, Carr J, Goldberger J. Atrial fibrillation is associated with altered left atrial 3D hemodynamics and increased stasis. Circulation. 2014;130:A14026. [Google Scholar]
- 132.Lee D, Goldberger J, Fluckiger J, Ng J, Carr J, Collins J, Markl M. Analysis of left atrial flow velocity distribution by 4D flow MRI in patients with atrial fibrillation. Circulation. 2013;128:A17900. [Google Scholar]
- 133.Goldberger J, Fluckiger J, Lee D, Ng J, Olsen AB, Carr J, Markl M. Left atrial flow velocity distribution in atrial fibrillation by 4D flow MRI: A new marker for risk of stroke? Heart Rhythm. 2013;10:S384. [Google Scholar]
- 134.Mann KG. Thrombin generation in hemorrhage control and vascular occlusion. Circulation. 2011;124:225–235. doi: 10.1161/CIRCULATIONAHA.110.952648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428–2437. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
- 136.Stegner D, Nieswandt B. Size does matter: VWF in MI. Blood. 2012;120:5096–5097. doi: 10.1182/blood-2012-09-452813. [DOI] [PubMed] [Google Scholar]
- 137.Akar JG, Jeske W, Wilber DJ. Acute onset human atrial fibrillation is associated with local cardiac platelet activation and endothelial dysfunction. J Am Coll Cardiol. 2008;51:1790–1793. doi: 10.1016/j.jacc.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 138.Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986;78:1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121:270–277. doi: 10.1182/blood-2012-07-442285. [DOI] [PubMed] [Google Scholar]
- 140.McGrath RT, van den Biggelaar M, Byrne B, O’Sullivan JM, Rawley O, O’Kennedy R, Voorberg J, Preston RJ, O’Donnell JS. Altered glycosylation of platelet-derived von Willebrand factor confers resistance to ADAMTS13 proteolysis. Blood. 2013;122:4107–4110. doi: 10.1182/blood-2013-04-496851. [DOI] [PubMed] [Google Scholar]
- 141.Fu X, Chen J, Gallagher R, Zheng Y, Chung DW, Lopez JA. Shear stress-induced unfolding of VWF accelerates oxidation of key methionine residues in the A1A2A3 region. Blood. 2011;118:5283–5291. doi: 10.1182/blood-2011-01-331074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uemura T, Kaikita K, Yamabe H, Soejima K, Matsukawa M, Fuchigami S, Tanaka Y, Morihisa K, Enomoto K, Sumida H, Sugiyama S, Ogawa H. Changes in plasma von Willebrand factor and ADAMTS13 levels associated with left atrial remodeling in atrial fibrillation. Thromb Res. 2009;124:28–32. doi: 10.1016/j.thromres.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 143.Freynhofer MK, Gruber SC, Bruno V, Hochtl T, Farhan S, Zaller V, Wojta J, Huber K. Prognostic value of plasma von Willebrand factor and its cleaving protease ADAMTS13 in patients with atrial fibrillation. Int J Cardiol. 2013;168:317–325. doi: 10.1016/j.ijcard.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 144.Freynhofer MK, Bruno V, Jarai R, Gruber S, Hochtl T, Brozovic I, Farhan S, Wojta J, Huber K. Levels of von Willebrand factor and ADAMTS13 determine clinical outcome after cardioversion for atrial fibrillation. Thromb Haemost. 2011;105:435–443. doi: 10.1160/TH10-09-0615. [DOI] [PubMed] [Google Scholar]
- 145.Gustafsson C, Blomback M, Britton M, Hamsten A, Svensson J. Coagulation factors and the increased risk of stroke in nonvalvular atrial fibrillation. Stroke. 1990;21:47–51. doi: 10.1161/01.str.21.1.47. [DOI] [PubMed] [Google Scholar]
- 146.Li-Saw-Hee FL, Blann AD, Lip GY. A cross-sectional and diurnal study of thrombogenesis among patients with chronic atrial fibrillation. J Am Coll Cardiol. 2000;35:1926–1931. doi: 10.1016/s0735-1097(00)00627-6. [DOI] [PubMed] [Google Scholar]
- 147.Hatzinikolaou-Kotsakou E, Kartasis Z, Tziakas D, Hotidis A, Stakos D, Tsatalas K, Bourikas G, Kotsakou ME, Hatseras DI. Atrial fibrillation and hypercoagulability: dependent on clinical factors or/and on genetic alterations? J Thromb Thrombolysis. 2003;16:155–161. doi: 10.1023/B:THRO.0000024053.45693.fc. [DOI] [PubMed] [Google Scholar]
- 148.Freestone B, Chong AY, Lim HS, Blann A, Lip GY. Angiogenic factors in atrial fibrillation: a possible role in thrombogenesis? Ann Med. 2005;37:365–372. doi: 10.1080/07853890510037392. [DOI] [PubMed] [Google Scholar]
- 149.Alonso A, Tang W, Agarwal SK, Soliman EZ, Chamberlain AM, Folsom AR. Hemostatic markers are associated with the risk and prognosis of atrial fibrillation: the ARIC study. Int J Cardiol. 2012;155:217–222. doi: 10.1016/j.ijcard.2010.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation. 2003;107:3141–3145. doi: 10.1161/01.CIR.0000077912.12202.FC. [DOI] [PubMed] [Google Scholar]
- 151.Krishnamoorthy S, Khoo CW, Lim HS, Lane DA, Pignatelli P, Basili S, Violi F, Lip GY. Prognostic role of plasma von Willebrand factor and soluble E-selectin levels for future cardiovascular events in a ‘real-world’ community cohort of patients with atrial fibrillation. Eur J Clin Invest. 2013;43:1032–1038. doi: 10.1111/eci.12140. [DOI] [PubMed] [Google Scholar]
- 152.Roldan V, Marin F, Muina B, Torregrosa JM, Hernandez-Romero D, Valdes M, Vicente V, Lip GY. Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 2011;57:2496–2504. doi: 10.1016/j.jacc.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 153.Scridon A, Girerd N, Rugeri L, Nonin-Babary E, Chevalier P. Progressive endothelial damage revealed by multilevel von Willebrand factor plasma concentrations in atrial fibrillation patients. Europace. 2013;15:1562–1566. doi: 10.1093/europace/eut121. [DOI] [PubMed] [Google Scholar]
- 154.Ammash N, Konik EA, McBane RD, Chen D, Tange JI, Grill DE, Herges RM, McLeod TG, Friedman PA, Wysokinski WE. Left atrial blood stasis and Von Willebrand factor-ADAMTS13 homeostasis in atrial fibrillation. Arterioscler Thromb Vasc Biol. 2011;31:2760–2766. doi: 10.1161/ATVBAHA.111.232991. [DOI] [PubMed] [Google Scholar]
- 155.Girerd N, Scridon A, Bessiere F, Chauveau S, Geloen A, Boussel L, Morel E, Chevalier P. Periatrial epicardial fat is associated with markers of endothelial dysfunction in patients with atrial fibrillation. PLoS One. 2013;8:e77167. doi: 10.1371/journal.pone.0077167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Christersson C, Wallentin L, Andersson U, Alexander JH, Ansell J, De Caterina R, Gersh BJ, Granger CB, Hanna M, Horowitz JD, Huber K, Husted S, Hylek EM, Lopes RD, Siegbahn A. D-dimer and risk of thromboembolic and bleeding events in patients with atrial fibrillation--observations from the ARISTOTLE trial. J Thromb Haemost. 2014;12:1401–1412. doi: 10.1111/jth.12638. [DOI] [PubMed] [Google Scholar]
- 157.Wang TL, Hung CR, Chang H. Evolution of plasma D-dimer and fibrinogen in witnessed onset of paroxysmal atrial fibrillation. Cardiology. 2004;102:115–118. doi: 10.1159/000078410. [DOI] [PubMed] [Google Scholar]
- 158.Mahe I, Drouet L, Chassany O, Mazoyer E, Simoneau G, Knellwolf AL, Caulin C, Bergmann JF. D-dimer: a characteristic of the coagulation state of each patient with chronic atrial fibrillation. Thromb Res. 2002;107:1–6. doi: 10.1016/s0049-3848(02)00184-6. [DOI] [PubMed] [Google Scholar]
- 159.Kamath S, Chin BS, Blann AD, Lip GY. A study of platelet activation in paroxysmal, persistent and permanent atrial fibrillation. Blood Coagul Fibrinolysis. 2002;13:627–636. doi: 10.1097/00001721-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 160.Inoue H, Nozawa T, Okumura K, Jong-Dae L, Shimizu A, Yano K. Prothrombotic activity is increased in patients with nonvalvular atrial fibrillation and risk factors for embolism. Chest. 2004;126:687–692. doi: 10.1378/chest.126.3.687. [DOI] [PubMed] [Google Scholar]
- 161.Vene N, Mavri A, Kosmelj K, Stegnar M. High D-dimer levels predict cardiovascular events in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost. 2003;90:1163–1172. doi: 10.1160/TH03-06-0363. [DOI] [PubMed] [Google Scholar]
- 162.Ohara K, Inoue H, Nozawa T, Hirai T, Iwasa A, Okumura K, Lee JD, Shimizu A, Hayano M, Yano K. Accumulation of risk factors enhances the prothrombotic state in atrial fibrillation. Int J Cardiol. 2008;126:316–321. doi: 10.1016/j.ijcard.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 163.Cervero J, Montes R, Espana F, Esmon CT, Hermida J. Limited ability to activate protein C confers left atrial endocardium a thrombogenic phenotype: a role in cardioembolic stroke? Stroke. 2011;42:2622–2624. doi: 10.1161/STROKEAHA.111.614420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, Wilcox JN, Dudley SC, Harrison DG, Langberg JJ. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 165.Lim HS, Willoughby SR, Schultz C, Gan C, Alasady M, Lau DH, Leong DP, Brooks AG, Young GD, Kistler PM, Kalman JM, Worthley MI, Sanders P. Effect of atrial fibrillation on atrial thrombogenesis in humans: impact of rate and rhythm. J Am Coll Cardiol. 2013;61:852–860. doi: 10.1016/j.jacc.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 166.Zotz RJ, Muller M, Genth-Zotz S, Darius H. Spontaneous echo contrast caused by platelet and leukocyte aggregates? Stroke. 2001;32:1127–1133. doi: 10.1161/01.str.32.5.1127. [DOI] [PubMed] [Google Scholar]
- 167.Ehrlich JR, Kaluzny M, Baumann S, Lehmann R, Hohnloser SH. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol. 2011;100:1029–1036. doi: 10.1007/s00392-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 168.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 169.Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, Ziegler PD TRENDS Investigators. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011;8:1416–1423. doi: 10.1016/j.hrthm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 170.Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, Favale S, Molon G, Ricci R, Biffi M, Russo G, Vimercati M, Corbucci G, Boriani G. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–248. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 171.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Nazarian S, Okin PM. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. doi: 10.1161/STROKEAHA.114.006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kamel H, Bartz TM, Longstreth WT, Okin PM, Thacker EL, Patton KK, Stein PK, Gottesman RF, Heckbert SR, Kronmal RA, Elkind MS, Soliman EZ. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke. 2015;46:711–716. doi: 10.1161/STROKEAHA.114.007762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–1480. doi: 10.1093/eurheartj/eht024. [DOI] [PubMed] [Google Scholar]
- 174.Smith JG, Newton-Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, Platonov PG, Hedblad B, Engstrom G, Wang TJ, Melander O. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–1719. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Latini R, Masson S, Pirelli S, Barlera S, Pulitano G, Carbonieri E, Gulizia M, Vago T, Favero C, Zdunek D, Struck J, Staszewsky L, Maggioni AP, Franzosi MG, Disertori M GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med. 2011;269:160–171. doi: 10.1111/j.1365-2796.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- 176.Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED, Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of Get With the Guidelines-Heart Failure. Circ Heart Fail. 2012;5:191–201. doi: 10.1161/CIRCHEARTFAILURE.111.965681. [DOI] [PubMed] [Google Scholar]
- 177.Dernellis J, Panaretou M. Effect of C-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J. 2005;150:1064. doi: 10.1016/j.ahj.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 178.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA, Magnani JW, Ellinor PT, Wang TJ, Levy D, Vasan RS, Benjamin EJ. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 181.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 182.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 183.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mohanty S, Mohanty P, Di Biase L, Rong B, Burkhardt D, Gallinghouse JG, Horton R, Sanchez JE, Bailey S, Zagrodzky J, Natale A. Baseline B-type natriuretic peptide: a gender-specific predictor of procedure-outcome in atrial fibrillation patients undergoing catheter ablation. J Cardiovasc Electrophysiol. 2011;22:858–865. doi: 10.1111/j.1540-8167.2011.02036.x. [DOI] [PubMed] [Google Scholar]
- 185.den Uijl DW, Delgado V, Tops LF, Ng AC, Boersma E, Trines SA, Zeppenfeld K, Schalij MJ, van der Laarse A, Bax JJ. Natriuretic peptide levels predict recurrence of atrial fibrillation after radiofrequency catheter ablation. Am Heart J. 2011;161:197–203. doi: 10.1016/j.ahj.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 186.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Siegbahn A, Yusuf S, Wallentin L. Importance of persistent elevation of cardiac biomarkers in atrial fibrillation: a RE-LY substudy. Heart. 2014;100:1193–1200. doi: 10.1136/heartjnl-2013-304872. [DOI] [PubMed] [Google Scholar]
- 187.Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, Kofune M, Nagashima K, Mano H, Sonoda K, Kasamaki Y, Hirayama A. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22:987–993. doi: 10.1111/j.1540-8167.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 188.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 189.Giedrimas E, Goldberger JJ. Catheter ablation for supraventricular tachycardias: contemporary issues. Future Cardiol. 2013;9:581–596. doi: 10.2217/fca.13.26. [DOI] [PubMed] [Google Scholar]