Abstract
Gaucher disease (GD), a prototype lysosomal storage disorder, results from inherited deficiency of lysosomal glucocerebrosidase due to biallelic mutations in GBA. The result is widespread accumulation of macrophages engorged with predominantly lysosomal glucocerebroside. A complex multisystem phenotype arises involving the liver, spleen, bone marrow and occasionally the lungs in type 1 Gaucher disease; in neuronopathic fulminant type 2 and chronic type 3 disease there is in addition progressive neurodegenerative disease. Manifestations of Gaucher disease type 1 (GD1) include hepatosplenomegaly, cytopenia, a complex pattern of bone involvement with avascular osteonecrosis (AVN), osteoporosis, fractures and lytic lesions. Enzyme replacement therapy became the standard of care in 1991, and this has transformed the natural history of GD1. This article reviews the clinical phenotypes of GD, diagnosis, pathophysiology and its natural history. A subsequent chapter discusses the treatment options.
Keywords: Gaucher disease, Lysosomal storage disorder, GBA, neuronopathic Gaucher disease, Ashkenazi Jews, bone disease, enzyme therapy
Introduction
Gaucher disease (GD) is one of the most frequent lysosomal disorders with a prevalence of 1:50,000 in the general population, but as high as 1 in 850 in the Ashkenazi Jewish (AJ) population.(1,2) Philippe Charles Ernest Gaucher’s MD thesis of 1882 annotated a previously unreported disorder in which he described abnormal histiocytes in the tissues of a 32 year-old woman who died of cachexia and massive hepatosplenomegaly. (3) Gaucher concluded that his patient suffered from a neoplasm of the spleen. To this day, malignancy is often the first diagnosis considered when a patient presents with the classic clinical signs of Gaucher disease.(4)
The eponym “Gaucher Disease” was introduced by Mandlebaum.(5) It was recognized as a multi-systemic chronic disease involving the liver, spleen, bone marrow and lymph nodes with familial aggregation. A similar systemic disease associated with rapidly progressive neurodegenerative disease (Gaucher disease Type 2) was first described in 1927, (6) followed, in 1959, by a report of a less rapidly progressive neurodegenerative disease (Gaucher disease type 3) in the Norrbottnian region of Northern Sweden.(7) In 1965 Roscoe Brady and his colleagues at the NIH unraveled the metabolic defect in Gaucher disease as an inherited deficiency of lysosomal glucocerebrosidase,(8) followed more than a quarter of a century later by the ultimate realization of enzyme replacement therapy by the same group.(9)
Three forms of the disease have been recognized based on age of onset, clinical signs and presence and rate of progression or absence of neurological disease. These are classified as Gaucher disease type 1 (GD1), which is characterized by splenomegaly, blood disorders, orthopedic complications and lack of neurological symptoms; Gaucher disease type 2 (GD2), characterized by hepatosplenomegaly and central nervous system involvement within the first year of life, and Gaucher disease type 3 (GD3), with nervous system involvement in childhood. These three forms of the disease share the same defect in the enzyme glucocerebrosidase and present a continuum of clinical picture; however, the distinct subtypes help in treatment decision-making.(10,11) It also became apparent that GD1 is especially prevalent in people of Ashkenazi Jewish (AJ) ancestry.(12)
The Pathogenetic Basis of GD
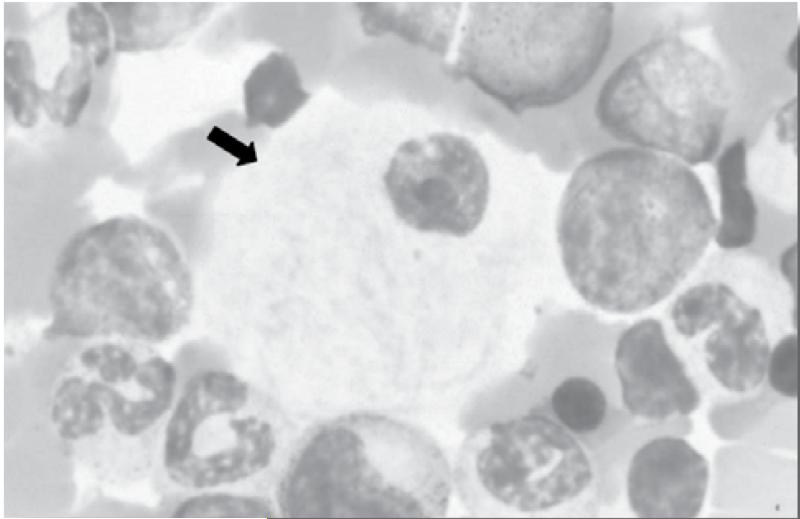
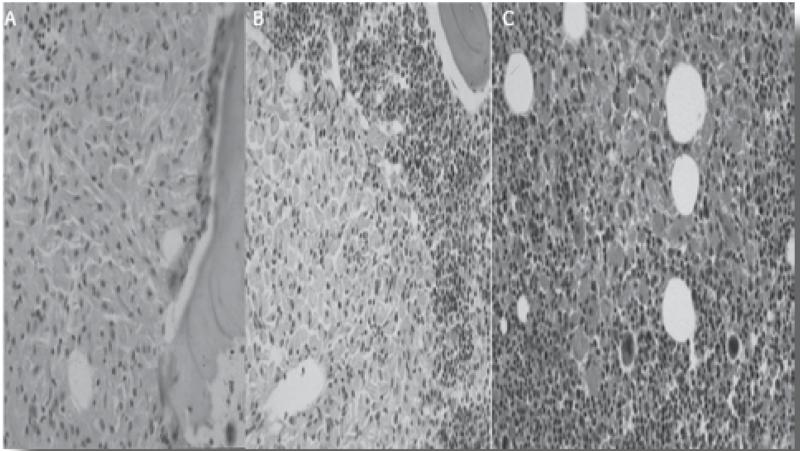
The eponymous Gaucher cells (foamy macrophages) seen on bone marrow aspirate and in other tissues represent macrophages engorged with glucosylceramide (GL1)-laden lysosomes.(13,14) They are characteristic of, but not exclusive to, Gaucher disease since many lipid-laden macrophages have a similar appearance that can sometimes be misleading in making the diagnosis. Gaucher cells have the appearance of signet rings with ‘wrinkled tissue paper’ because the nucleus is pushed to the periphery of the cells by the massive accumulation of glucocerebroside structures in the lysosomal system.(Figure 1) Widespread accumulation of these storage cells underlies the multisystemic manifestations of Gaucher disease involving the liver, spleen, bone marrow, and occasionally the lungs.(15) Lysosomal lipids spill over into the cytoplasm. By pathways that are not fully understood, but which may involve ceramidase, glucosylceramide is converted into glucosylsphingosine (Lyso GL1). Serum levels of glucosylceramide are elevated some 5 to 10 -fold, but there is massive elevation of glucosylsphingosine to >100 fold and lyso GL1 is by far the most prevalent plasma sphingolipid. There is accumulating evidence that Lyso GL1 and its downstream lipids mediate the widespread cellular dysfunction seen in Gaucher disease.(16) Massive generation of these bioactive lipids from the macrophage system engages other cell types in the pathophysiology; in particular, there is prominent involvement of the immune system indicated by hypercytokinemia, reflecting both the innate and adaptive immune systems as well as osteoblast dysfunction that may contribute to osteopenia by osteoblast-osteoclast regulation uncoupling.(17) Mesenchymal stem cells in Gaucher disease have a decreased capacity to differentiate into osteoclasts.(18) Although the Gaucher cell is the epicenter of disease pathophysiology and generator of bioactive lipids, the amount of GL1 that accumulates in massively enlarged organs such as the spleen of GD patients is <1% by weight, underscoring system-wide involvement in GD beyond the macrophage system. In contrast, cellular injury in the brain in neuronopathic Gaucher disease is mediated by direct toxic effects of bioactive lipids in neuronal cells, although Gaucher cells have been described in the perivascular space.
Figure 1. A typical Gaucher cell seen on a bone marrow aspiration slide (arrow), containing ‘wrinkled tissue paper’. The nucleus is pushed to the periphery of the cells by the massive accumulation of glucocerebroside structures in the lysosomal system.
Clinical Presentation of GD: GD1
Visceral and Hematologic Disease
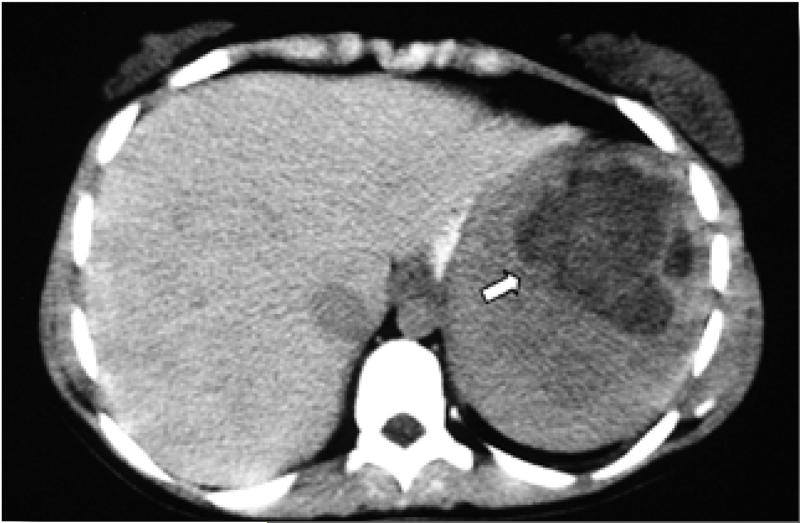
Accumulation of GL1-laden macrophages in the liver and spleen results in massive hepatosplenomegaly with abdominal distention, and such accumulation in the bone marrow together with hypersplenism results in cytopenia.(19) Thrombocytopenia may present as childhood epistaxis, easy bruising or overt bleeding, particularly with trauma, surgery, or pregnancy; however, in addition, a thrombocytopathy, yet to be fully delineated, may also exist.(20) Leukopenia is commonly encountered and deficient neutrophil function has also been reported. With increasing splenomegaly - up to 85 times normal - there is increasing prevalence of focal defects, representing areas of infarction, focal massive accumulation of Gaucher cells and/or extramedullary hematopoiesis,(Figure 2) which may be misdiagnosed as malignancy. In patients with intact spleens, hepatomegaly is almost universally present but is usually not massive unless massive splenomegaly is also present. In a subset of patients, who underwent splenectomy in the pre-enzyme therapy era, advanced liver disease with portal hypertension and hepatopulmonary syndrome may occur. A frequent hepatobiliary manifestation of Gaucher disease is cholesterol cholelithiasis. Additional hematologic manifestations include acquired coagulopathy, vitamin B12 deficiency and hyperferritinemia. There is a substantial phenotypic variability in GD1 including individuals with very mild disease or even totally asymptomatic disease to patients with severe disease burden.(21)
Figure 2. Abdominal CT showing a “Gaucheroma” (arrow) a focal massive accumulation of Gaucher cells and/or extramedullary hematopoesis.
Bone Disease
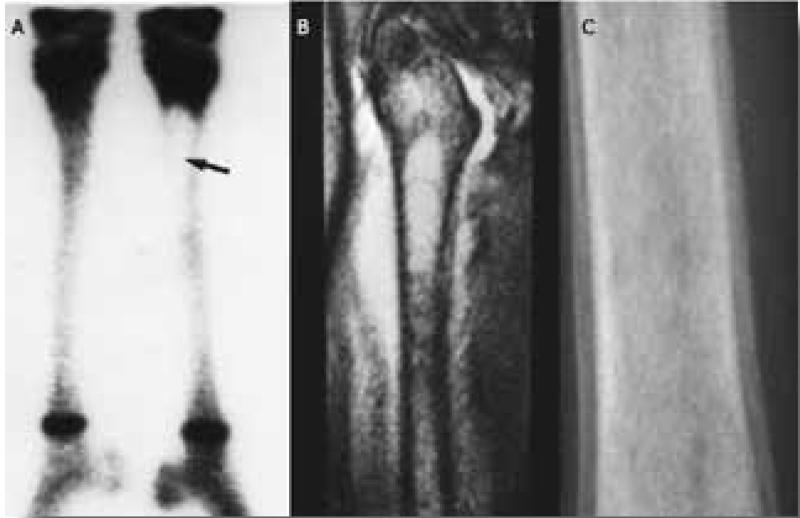
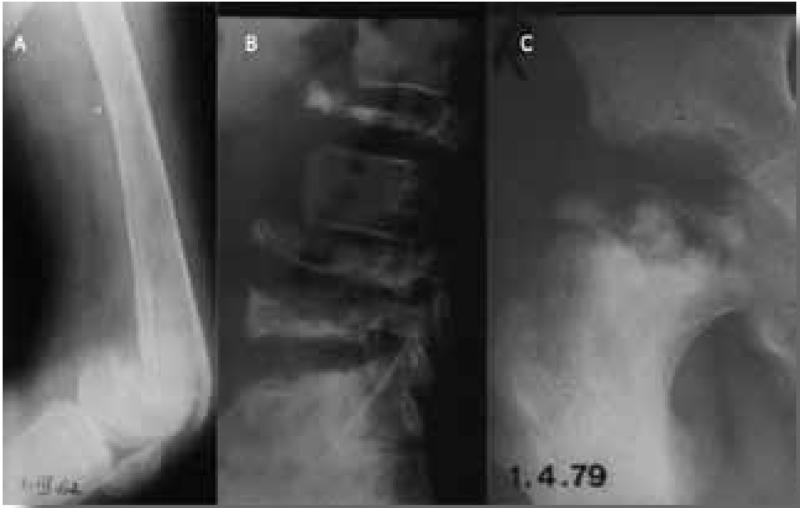
Skeletal manifestations of GD result in the most debilitating and potentially irreversible complications and can lead to long-term disability.(22) Bone involvement in Gaucher disease is highly complex and multidimensional. Primary involvement of the bone marrow compartment in the form of Gaucher cell infiltration is likely associated with marrow fibrosis and impaired hematopoesis. Bioactive lipids originating from Gaucher cells engage other bone marrow cell types, such as osteoblasts and possibly endothelial cells, setting the stage for avascular osteonecrosis and osteopenia.(23) The most dramatic manifestation of bone disease is the acute bone crisis. This presents as sudden onset of excruciating pain, often resistant to morphine, with swelling and erythema, fever and leukocytosis, but with negative blood cultures and it is usually confined to one bone.(24, 25) Imaging typically reveals lack of uptake on radionuclide bone scan in the acute phase (Figure 3A) and increased uptake six weeks later.(26) MRI in acute bone crises reveals localized edema of the bone and soft tissues with T-weighted signal at the site of the crisis.(Figure 3B) (27) Several weeks after the bone crisis has subsided, plain radiology typically shows periosteal elevation (Figure 3C) after the acute crisis in cases not treated with high dose steroids.(28) The bone is often severely damaged by the ensuing avascular necrosis and a fracture may follow. (Figure 4A) Fractures of weight-bearing bones, such as vertebrae, (Figure 4B) the femoral head (Figure 4C) or calcaneus, can occur without previous overt bone crisis or pain. Secondary degenerative osteoarthritis may follow such episodes. Release of cytokines such as IL1 and TNF, known to be present in the Gaucher cells and to be involved in local subperiosteal edema, have been suggested as the cause of the bone crises. This would explain the immediate response to high dose steroids that block the edema and pain and prevent the periosteal elevation.(24) Other manifestations of bone disease including medullary infarction, osteopenia, osteoporosis, cystic changes, osteolytic lesions, and growth failure in children, can result in significant morbidity, disability and reduction in quality of life (27,29-31) Asymptomatic modulation of the bone, especially of the distal femur (Ehrlenmeyer flask deformity), is commonly seen. The vast majority of patients have evidence of skeletal manifestations on imaging even though they may be entirely asymptomatic.(27, 32)
Figure 3.
A. Technicium bone scan shows lack of uptake in the acute phase of an acute bone crisis. B. MRI in acute bone crises reveals localized edema of the bone and soft tissues with T-weighted signal at the site of the crisis. C. Several weeks after the bone crisis has subsided, plain radiology typically shows periosteal elevation
Figure 4.
A. Pathological fracture of distal femur at site of previous bone crisis. The patient did not come to hospital since she considered it a less painful “mild bone crisis”. B. Multiple collapsed vertebrae with no prior bone crisis. C. Destruction of femoral head following its collapse. No previous bone crisis at this site.
Neurological Manifestations in GD1
Although there is no primary CNS disturbance in GD1, peripheral neuropathy is more common than in the general population.(33-36) The association of GD1 and GBA mutations with Parkinson’s disease and dementia has been extensively studied in the last decade. Between 5% and 7% of patients with GD1 may develop Parkinsonism before age 70 years, and 9 to 12% before age 80 years, which is an approximately 26-fold higher life-time risk of developing Parkinson’s disease compared to the general population.(37) GBA mutations confer a risk of Parkinson’s disease in the biallelic form as well as in the heterozygote carrier state. Among patients with Parkinson’s disease the odds ratio for the presence of GBA mutations is 5.4; a pathophysiological basis for the synucleopathy with GBA mutations is under intense investigation.(38-40)
Pulmonary Involvement
With time, the majority of GD2 and GD3 patients develop infiltrative lung disease due to the accumulation of Gaucher cells in the lung parenchyma and, especially in GD2, in the alveolar spaces (‘lipid pneumonia’). In GD1, pulmonary involvement is rarely seen in the form of interstitial lung disease. Although rare, pulmonary hypertension may occur in GD1 asplenic patients. Fortunately splenectomy is now almost never performed.(41)
Immunologic Abnormalities
The important role of immune cells in the pathophysiology of Gaucher disease is underscored by the high prevalence of polyclonal gammopathy in children and adults;(42) there is an increased risk of monoclonal gammopathy of unknown significance (MGUS) and multiple myeloma (43) and a higher prevalence of autoimmune hemolytic anemia and thrombocytopenia. The incidence of other autoimmune diseases is also likely increased in GD1 and has been studied in humans and in mice.(44-47)
Malignancy
Numerous studies have shown an increased risk of multiple myeloma and non-myeloma hematological malignancies in GD1, even among homozygous N370S patients who often have clinically mild disease.(48) The relative risk of multiple myeloma was found to be 5.9 (95% confidence interval [95% CI]: 2.8, 10.8). The relative risk of cancer overall was 0.79 (95% CI: 0.67, 0.94) (49), but no correlation except for splenectomy exists between the severity of the disease and the risk of malignancy. (50) It is unclear if there is an elevated risk for non-hematological malignancies in GD; apart from hepatocellular carcinoma, renal cell carcinoma and melanoma. (49,50)
Neuronopathic GD: GD2 and GD3
Although GD has a continuum range of symptoms, and GD3 can be considered as the milder form of GD2,(11) the formal distinction between the types is important clinically as enzyme treatment is futile in GD2. Clinical assignment into GD2 or GD3 can be challenging in very young infants. Since both GD2 and GD3 can arise in patients homozygous for the mutation L444P, this is not helpful in differentiating between them. Generally GD2 presents early in infancy with severe visceral disease and some neurological signs; neurological disease progresses rapidly leading to death by age 2 years.(51) Neurological signs in GD2 include hypotonia, progressively impaired cognition, impaired hearing, ocular involvement including impaired vision, ocular apraxia, strabismus and ophthalmoparesis, and bulbar and pyramidal signs. Abnormal brain stem auditory evoked response (BAER) testing, abnormal visual evoked response (VER) and mild cerebral atrophy on brain MRI have been reported in GD2. These patients should receive only supportive care since enzyme replacement therapy (ERT) does not alter the devastating natural history.(52-54) Such families should be offered genetic counseling and prenatal diagnosis in future pregnancies.
GD3 is the chronic neuronopathic form and its prevalence is 1:50,000 and might be even higher in the Far East and Egypt. The disease manifests in childhood, but is slowly progressive and the life span may extend as far as the 5th or even the 6th decade. GD3 is subclassified into three subtypes: GD3a is characterized by dominant neurological manifestations and mild visceral disease, GD3b is characterized by massive visceral disease and skeletal manifestations but mild neurological signs, and GD3c is unique in having a phenotype of visceral disease in association with cardiac involvement consisting of calcification of the aorta and calcification of heart valves.(55) GD3c is also uniquely associated with a perfect genotype/phenotype correlation across several ethnicities with homozygosity for the mutation D409H. The neurological symptoms in GD3 include oculomotor apraxia, seizures, progressive myoclonic epilepsy, and later dementia and ataxia, although some patients seem to have no manifestations beyond the abnormal saccadic eye movements. The Norbottnian-Polish GD3 variant is characterized by early onset with massive visceral involvement, progressive kyphoscoliosis and mild cognitive deficits. (56) GD3 patients can benefit from ERT, although since it does not cross the blood brain barrier, it does not improve the neurological aspects of the disease.(54)
Perinatal-lethal GD2
The perinatal-lethal form of GD2 manifests pre- or perinatally with hepatosplenomegaly, pancytopenia, and skin changes consistent with ichthyosiform or collodion skin abnormalities, or as nonimmune hydrops fetalis.(57) Arthrogryposis and distinctive facial features are seen in 35-43% of patients.(58) This form is characterized by rapid neurological deterioration and death in utero or within three months of birth and is usually associated with complex alleles.
Diagnosis of GD
Clinical suspicion
GD is suspected when a patient presents with at least two of the following findings: hepatosplenomegaly, thrombocytopenia +/− anemia, characteristic bone lesions, or signs of CNS involvement in GD2 or GD3.(4) Suspicion should be higher when the patient is of AJ background. Clinical findings alone are not diagnostic; GD biomarkers can aid in confirming clinical suspicion, but are not diagnostic.
Diagnostic Tests
Bone Marrow Biopsy
Historically, patients with GD were diagnosed by bone marrow examination since the combination of anemia, thrombocytopenia, and/or splenomegaly suggested a hematological malignancy.(59) Gaucher cells can be seen in a bone marrow biopsy of patients with GD, and stain positively with periodic acid-Schiff (PAS) reagent. However, bone marrow biopsy is now only indicated when a hematological co-morbidity is suspected.
β-glucosylceramidase Enzyme Activity
Enzyme activity, the gold standard for GD diagnosis, is usually measured in peripheral blood leukocytes using the substrate 4-methylumbelliferyl-β-D-glucopyranoside in a fluorometric assay. In GD patients the glucosylceramidase enzyme activity in peripheral blood leukocytes is 0%-15% of normal activity. No correlation exists between the level of the enzyme activity and the severity or type of GD. Carriers have an enzyme activity intermediate between the normal and the GD affected levels. Enzyme activity is an unreliable test for carrier detection because of the overlap in enzyme activity levels between carriers and non-carriers and between carriers and affected individuals.
Molecular Genetic Testing
Deficiency of acid β-glucosidase in GD arises from biallelic mutations in GBA except for rare individuals with Sapocin C deficiency. In 99% of GD patients full sequencing of GBA will reveal their two causative mutations. Over 300 mutations are known to cause GD.(60) In AJ, four mutations - N370S, L444P, 84GG and IVS2+1 - account for the condition in 94% of the patients and carriers, enabling the use of targeted mutation analysis for diagnosis,(61) but account for only 50-60% of the cases in non AJ GD patients. When GD is suspected blood leukocyte acid β-glucosidase enzyme activity should be measured; molecular genetic testing gives prognostic information and facilitates family screening.
Genotype-phenotype Correlation
The genotype-phenotype correlation in GD is not absolute, but can aid in the counseling and management of GD patients. N370S is the most common and the mildest GBA mutation. The presence of at least one N370S mutation accurately predicts GD1, while homozygosity for the mutation L444P is accurately predictive of neuronopathic GD, although it is not possible to establish whether the patient has GD2 or GD3.(60) The mutation N370S accounts for about 80% of disease alleles in either a homozygous state (N370S/N370S) or in a compound heterozygous state in the AJ patients.(62) Patients who are homozygous for N370S usually have a milder disease; however, some patients may be severely symptomatic.(63) Patients with cardiovascular involvement are invariably homozygous for the mutation D409H. Homozygosity for the mutations 84GG or IVS2+1 are presumed to be lethal. The mutations 84GG and V394L are unique to AJ GD patients.
Disease Monitoring
Evaluation of overall severity of GD requires comprehensive evaluation of all the organ systems involved in the disease process. These are summarized in therapeutic goals for GD and are discussed at length by Pastores et al.,(64) and in the revised recommendations in children by Kaplan et al. (65) Consideration should be given to the importance of designated GD clinics in which the physicians following these patients are knowledgeable about the disease and the treatment options. In places where the disease is rare and the distances are great, the patient should be monitored closely by a local physician and have a consultation with a GD expert once a year or if complications develop.
Physical examination and a review of the medical history should be performed every 6-12 months with special attention to bleeding episodes, bone pain, hepatosplenomegaly, growth in children, and neurological evaluation especially in the neurological GD types. Complete blood count is necessary to look for anemia and thrombocytopenia. Assessment of liver and spleen volume is recommended using MRI. Abdominal US can also be used when MRI is not readily available, but is an inadequate tool for volume measurement. Classically, plain x-rays of the distal femur can demonstrate the Erlenmeyer flask deformity. Follow-up of the skeleton with spine and femur MRI reveals the extent of marrow involvement and the presence of fibrosis and/or infarction.(66) The bone marrow burden (BMB) score can be used to follow up the patient’s bone marrow involvement using MRI and is a reproducible semiquantitative scoring system.(67,68) Dual-energy X-ray absorptiometry (DEXA) of the lumbar spine and hips is used to assess the bone mineral density.(69)
Serum Biomarkers of GD
Biomarkers evaluated every 6-12 months can assess the clinical progression or stabilization of the patient’s disease. Elevated serum concentrations of tartrate-resistant acid phosphatase, ferritin, plasma activity of chitotriosidase (a macrophage-derived chitin-fragmenting hydrolase), angiotensin converting enzyme (ACE), and plasma concentration of PARC/CCL18 are followed. Serum concentrations of total cholesterol and HDL are usually low.(70) In the 4% of affected individuals of European origin who are homozygous for a common null mutation of the chitotriosidase gene, other markers can be used.(71) Marker levels are thought to be correlated with the disease burden. The patient is usually his own control and the levels over time help to monitor his treatment and usually normalize with adequate treatment.
Natural History of Gaucher Disease
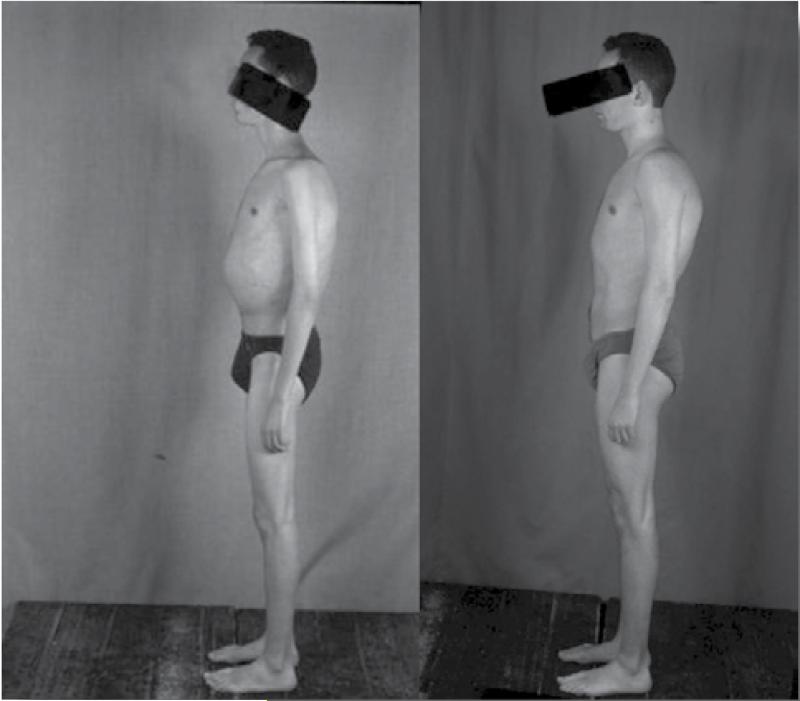
The improvement in the health status of patients with Gaucher disease given adequate enzyme replacement therapy has been truly remarkable. Those patients who have not suffered irreversible damage become normal healthy individuals. Although repeat bone marrow examination is not generally needed or recommended as part of serial follow up, if this is done, an initial bone marrow replacement can be seen on bone marrow biopsy,(Figure 5A) improves over time and infiltration by hemopoetic cells (Figure 5B) becomes dominant.(Figure 5C) The patients gain a normal body shape (Figure 6) and bone cortex improves markedly (Figure 7 - narrow arrows). Irreversible changes, however, are not improved.(Figure 7 - broad arrows)
Figure 5.
Bone marrow biopsy of posterior. iliac crest. A. Replacement of marrow cavity by Gaucher cells prior to enzyme replacement therapy (1990). B. Partial infiltration by hematopoietic cells after treatment with enzyme replacement therapy (1997). C. Continued improvement of bone marrow cavity following continued enzyme replacement therapy (2003).
Figure 6. Clinical image of a patient prior to and following several years of enzyme replacement therapy.
Figure 7.
A. Severe osteoporosis of cortex of upper femur (narrow arrows) (1987). B. Cortical recalcification following 20 years of enzyme replacement therapy (2013). Note the lack of improvement of the collapsed femoral head joint (broad arrows).
As investigative efforts to develop enzyme replacement therapy started to yield sporadic tantalizing results, interest in defining the clinical spectrum of Gaucher disease and its natural history gained momentum. The first Registry of 395 Gaucher disease patients compiled by Dr. Robert Lee in 1982 (72) depicted a uniformly devastating disease with premature deaths from bleeding complications, bone marrow failure, liver failure, crippling skeletal disease and a high incidence of malignancies in GD1. In neuronopathic forms of the disease, in addition, pulmonary involvement was highlighted. A significant proportion of patients who underwent splenectomy developed accelerated skeletal disease.(72,73)
Much progress has been made in the era of enzyme therapy to delineate the natural history in diverse patient populations. In general GD is a chronic progressive disease. Stratification of disease manifestations and natural course by GBA genotype is important to assess the impact of mutations on phenotype, although genotype does not accurately predict prognosis in individual patients. Thus although N370S homozygous patients, compared to N370S compound heterozygous patients, have the mildest overall disease, there is extraordinary heterogeneity in disease severity as well as in the pattern of organ involvement. An ICGG Registry compared 798 N370S homozygotes with 1,278 N370S compound heterozygotes and showed that 32% and 65%, respectively, were diagnosed before the age of 20 years. (62) At diagnosis, however, even N370S homozygous patients had a significant disease burden evidenced by irreversible skeletal lesions (17% versus 26%); anemia (18% versus 29%); thrombocytopenia (52% versus 62%); hepatomegaly (44% versus 72%); splenomegaly (73% versus 91%); and osteopenia or osteoporosis (48.6% versus 51%). A subset of N370S homozygous patients exhibited more severe clinical manifestations - 9% had severe thrombocytopenia, 3% had severe hepatomegaly, 11% had severe splenomegaly, 7% reported bone crises and 11% had osteoporosis. Large single center studies showed that they have progressive skeletal disease but mild visceral and hematologic disease.(74,75)
Thus, individuals may present with type 1 Gaucher disease at any time from infancy to late adulthood with widely differing manifestations that include variable combinations of massive splenomegaly, hepatomegaly, growth retardation and destructive bone disease in childhood to mild hepatosplenomegaly presenting as late as the seventh or eighth decade of life.(28,76,77) Early onset disease in childhood generally heralds a rapidly progressive natural history of the disease.(78) However, disease manifestations in different organs may progress at different rates; for example, bone disease may occur without significant hematologic disease and vice versa (28) and severity of disease in one organ does not reliably predict disease in other organs or overall disease severity. Hematologic and visceral manifestations tend to occur early when the disease presents in childhood or early adulthood, and for this reason, Gaucher disease is often considered a blood disorder. Indeed, most Gaucher patients are diagnosed and followed up by hematologists.(4) The increasing recognition of progressive bone involvement in Gaucher patients has increasingly shaped monitoring guidelines and treatment decisions due to high associated morbidity. (26,28-30) Similarly, osteopenia occurs in young children and adolescents as well as in adults (30,74,79) and this, together with other evidence of osteoblast-osteoclast uncoupling mediated by the accumulation of bioactive lipids (22) has lead to the concept that the fundamental defect underlying osteopenia in Gaucher disease is failure to achieve peak bone mass. This underlies the rationale for recommendations to monitor bone density in children to assess response to enzyme therapy, (74) although some have doubted its significance.(80)
Developing a model for personalized therapy in GD1 requires accurate prediction of the risk of disabling skeletal complications such as fractures and AVN. While there are more such complications in N370S compound heterozygotes compared to N370S homozygous patients, it is impossible to accurately predict the likelihood of their occurrence in individual patients. Contra intuitively the risk factors for fractures and AVN were found to be unrelated to standard measures of disease severity, such as visceral involvement or even genotype.(69) The only risk factor for fractures was lumbar spine bone density. The only risk factors for AVN were splenectomy (see below) and anemia: patients with AVN were 1.6 times more likely to be anemic compared to matched controls. Decisions to start enzyme therapy should not be made solely on the severity of visceral or hematologic disease without adequately evaluating the disease burden in the skeletal compartment.
Does splenectomy exacerbate bone disease?(28,72,73) Approximately 28% of patients in the Registry are splenectomized.(28) Multiple logistic regression to identify predictors for AVN found splenectomy to be the strongest risk factor conferring a more than 2-fold increased risk.(81) There are compelling data on the temporal sequence of increased incidence of AVN following splenectomy. (82) Other data suggest no link between splenectomy and osteopenia, as children and young adults with the most strikingly reduced bone mineral density had the lowest prevalence of splenectomy.(30) Splenectomy may also predispose patients to an increased risk of malignancy,(83) portal hypertension, hepatopulmonary syndrome and pulmonary hypertension (40,84) as well as increasing the risk of infection. (73) In addition, there is an increased risk of symptomatic cholesterol cholelithiasis.(74,85) A reduced life expectancy by nine years compared to the normal population has been described (associated with splenectomy).(86) Whether a shortened lifespan is the result of worsened hepatic, skeletal and pulmonary complications, or is a consequence of more severe overall disease that may influence longevity independent of spleen status, is unknown.(86) With the availability of effective treatment, the need for splenectomy has declined dramatically but rare indications still exist such as splenic rupture, ITP and autoimmune hemolytic anemia resistant to other therapies as well as life-threatening pressure effects such as hydronephrosis.(74)
Acknowledgments
The authors thank Dr. Gabrielle J. Halpern for her editorial assistance.
Footnotes
Disclosure
The authors declare no conflict of interest.
Hagit N. Baris
Ian J. Cohen
Pramod K. Mistry
References
- 1.Cox TM, Schofield JP. Gaucher’s disease: clinical features and natural history. Baillieres Clin Haematol. 1997;10:657–689. doi: 10.1016/s0950-3536(97)80033-9. [DOI] [PubMed] [Google Scholar]
- 2.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 3.Petsko GA, Grabowski GA, Kolodny EH. Gaucher disease. In: Scriver CM, Beudet AL, Sly WS, Valle D, editors. Molecular Bases of Inherited disease. McGraw Hill; 2010. [Google Scholar]
- 4.Mistry PK, Cappellini MD, Lukina E, Ozsan H, Mach Pascual S, Rosenbaum H, Helena Solano M, Spigelman Z, Villarrubia J, Watman NP, Massenkeil G. A reappraisal of Gaucher disease-diagnosis and disease management algorithms. Am J Hematol. 2011;86:110–115. doi: 10.1002/ajh.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandlebaum FS. A contribution to the pathology of primary splenomegaly (Gaucher type), with the report of an autopsy on a male child four and one half years of age. J Exp Med. 1912;16:797–821. doi: 10.1084/jem.16.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberling C, Woringer P. La maladie de Gaucher chez la nourrisson. Rev Fr Pédiatr. 1927;3:475–532. [Google Scholar]
- 7.Svennerholm L, Dreborg S, Erikson A, Groth CG, Hillborg PO, Håkansson G, Nilsson O, Tibblin E. Gaucher disease of the Norrbottnian type (type III). Phenotypic manifestations. Prog Clin Biol Res. 1982;95:67–94. [PubMed] [Google Scholar]
- 8.Brady RO, Kanfer J, Shapiro D. The metabolism of glucocerebrosides. I. Purification and properties of a glucocerebroside-cleaving enzyme from spleen tissue. J Biol Chem. 1965;240:39–43. [PubMed] [Google Scholar]
- 9.Brady RO. Benefits from unearthing “a biochemical Rosetta Stone”. J Biol Chem. 2010;285:41216–41221. doi: 10.1074/jbc.X110.197954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodson P, Goldblatt J, Beighton P. Non-neuropathic Gaucher disease presenting in infancy. Arch Dis Child. 1979;54:707–709. doi: 10.1136/adc.54.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goker-Alpan O, Schiffmann R, Park JK, Stubblefield BK, Tayebi N, Sidransky E. Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. J Pediatr. 2003;143:273–276. doi: 10.1067/S0022-3476(03)00302-0. [DOI] [PubMed] [Google Scholar]
- 12.Matoth Y, Fried K. Chronic Gaucher’s disease; clinical observations on 34 patients. Isr J Med Sci. 1965;1:521–530. [PubMed] [Google Scholar]
- 13.Grabowski GA. Gaucher disease and other storage disorders. Hematology Am Soc Hematol Educ Program. 2012;2012:13–18. doi: 10.1182/asheducation-2012.1.13. [DOI] [PubMed] [Google Scholar]
- 14.Cox TM, Cachón-González MB. The cellular pathology of lysosomal diseases. J Pathol. 2012;226:241–254. doi: 10.1002/path.3021. [DOI] [PubMed] [Google Scholar]
- 15.Pastores GM. Gaucher’s disease. Pathological features. Baillieres Clin Haematol. 1997;10:739–749. doi: 10.1016/s0950-3536(97)80037-6. [DOI] [PubMed] [Google Scholar]
- 16.Ferraz MJ, Kallemeijn WW, Mirzaian M, Herrera Moro D, Marques A, Wisse P, Boot RG, Willems LI, Overkleeft HS, Aerts JM. Gaucher disease and Fabry disease: New markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim Biophys Acta. 2014;1841:811–825. doi: 10.1016/j.bbalip.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Reed M, Baker RJ, Mehta AB, Hughes DA. Enhanced differentiation of osteoclasts from mononuclear precursors in patients with Gaucher disease. Blood Cells Mol Dis. 2013;51:185–194. doi: 10.1016/j.bcmd.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Lecourt S, Mouly E, Freida D, Cras A, Ceccaldi R, Heraoui D, Chomienne C, Marolleau JP, Amulf B, Porcher R, Caillaud C, Vanneaux V, Belmatoug N, Larghero J. A prospective study of bone marrow hematopoietic and mesenchymal stem cells in type 1 Gaucher disease patients. PLoS One. 2013;8:e69293. doi: 10.1371/journal.pone.0069293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimran A, Altarescu G, Rudensky B, Abrahamov A, Elstein D. Survey of hematological aspects of Gaucher disease. Hematology. 2005;10:151–156. doi: 10.1080/10245330500067181. [DOI] [PubMed] [Google Scholar]
- 20.Gillis S, Hyam E, Abrahamov A, Elstein D, Zimran A. Platelet function abnormalities in Gaucher disease patients. Am J Hematol. 1999;61:103–106. doi: 10.1002/(sici)1096-8652(199906)61:2<103::aid-ajh5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Sibille A, Eng CM, Kim SJ, Pastores G, Grabowski GA. Phenotype/genotype correlations in Gaucher disease type 1: clinical and therapeutic implications. Am J Hum Genet. 1993;52:1094–1101. [PMC free article] [PubMed] [Google Scholar]
- 22.Grabowski G, Kolodny EH, Weinreb N. Gaucher disease: phenotypic and genetic variation. In: Scriver CR, Beaudet AL, Valle D, et al., editors. The Online Metabolic and Molecular Basis of Inherited Metabolic Disease. McGraw-Hill Companies; New York: 2010. Available at: http://genetics.accessmedicine.com/mmbid/public/co_contents/toc_part16.html. [Google Scholar]
- 23.Mistry PK, Liu J, Yang M, Nottoli T, McGrath J, Jain D, Zhang K, Keutzer J, Chuang WL, Mehal WZ, Zhao H, Lin A, Mane S, Liu X, Peng YZ, Li JH, Agrawal M, Zhu LL, Blair HC, Robinson LJ, Iqbal J, Sun L, Zaidi M. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc Natl Acad Sci U S A. 2010;107:19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen IJ, Kornreich L, Mekhmandarov S, Katz K, Zaizov R. Effective treatment of painful bone crises in type 1 Gaucher’s disease with high dose prednisolone. Arch Dis Child. 1996;75:218–222. doi: 10.1136/adc.75.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen IJ. Bone crises in Gaucher disease. Isr Med Assoc J. 2003;5:838–839. [PubMed] [Google Scholar]
- 26.Katz K, Mechlis-Frish S, Cohen IJ, Horev G, Zaizov R, Lubin E. Bone scans in the diagnosis of bone crisis in patients who have Gaucher disease. J Bone Joint Surg Am. 1991;73:513–517. [PubMed] [Google Scholar]
- 27.Wenstrup RJ, Roca-Espiau M, Weinreb NJ, Bembi B. Skeletal aspects of Gaucher disease: a review. Br J Radiol. 2002;75(Suppl 1):A2–12. doi: 10.1259/bjr.75.suppl_1.750002. [DOI] [PubMed] [Google Scholar]
- 28.Pastores GM, Meere PA. Musculoskeletal complications associated with lysosomal storage disorders: Gaucher disease and Hurler-Scheie syndrome (mucopolysaccharidosis type I) Curr Opin Rheumatol. 2005;17:70–78. doi: 10.1097/01.bor.0000147283.40529.13. [DOI] [PubMed] [Google Scholar]
- 29.Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, Pastores G, Rosenbloom BE, Scott CR, Wappner RS, Weinreb NJ, Zimran A. The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- 30.Poll LW, Maas M, Terk MR, Roca-Espiau M, Bembi B, Ciana G, Weinreb NJ. Response of Gaucher bone disease to enzyme replacement therapy. Br J Radiol. 2002;75(Suppl 1):A25–36. doi: 10.1259/bjr.75.suppl_1.750025. [DOI] [PubMed] [Google Scholar]
- 31.Mistry PK, Weinreb NJ, Kaplan P, Cole JA, Gwosdow AR, Hangartner T. Osteopenia in Gaucher disease develops early in life: response to imiglucerase enzyme therapy in children, adolescents and adults. Blood Cells Mol Dis. 2011;46:66–72. doi: 10.1016/j.bcmd.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastores GM, Patel MJ, Firooznia H. Bone and joint complications related to Gaucher disease. Curr Rheumatol Rep. 2000;2:175–180. doi: 10.1007/s11926-000-0059-x. [DOI] [PubMed] [Google Scholar]
- 33.Capablo JL, Saenz de Cabezón A, Fraile J, Alfonso P, Pocovi M, Giraldo P, Spanish Group on Gaucher Disease Neurological evaluation of patients with Gaucher disease diagnosed as type 1. J Neurol Neurosurg Psychiatry. 2008;79:219–222. doi: 10.1136/jnnp.2006.111518. [DOI] [PubMed] [Google Scholar]
- 34.Halperin A, Elstein D, Zimran A. Are symptoms of peripheral neuropathy more prevalent in patients with Gaucher disease? Acta Neurol Scand. 2007;115:275–278. doi: 10.1111/j.1600-0404.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 35.Biegstraaten M, Mengel E, Maródi L, Petakov M, Niederau C, Giraldo P, Hughes D, Mrsic M, Mehta A, Hollak CE, van Schaik IN. Peripheral neuropathy in adult type 1 Gaucher disease: a 2-year prospective observational study. Brain. 2010;133:2909–2919. doi: 10.1093/brain/awq198. [DOI] [PubMed] [Google Scholar]
- 36.Chérin P, Rose C, de Roux-Serratrice C, Tardy D, Dobbelaere D, Grosbois B, Hachulla E, Jaussaud R, Javier RM, Noël E, Clerson P, Hartmann A. The neurological manifestations of Gaucher disease type 1: the French Observatoire on Gaucher disease (FROG) J Inherit Metab Dis. 2010;33:331–338. doi: 10.1007/s10545-010-9095-5. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbloom B, Balwani M, Bronstein JM, Kolodny E, Sathe S, Gwosdow AR, Taylor JS, Cole JA, Zimran A, Weinreb NJ. The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cells Mol Dis. 2011;46:95–102. doi: 10.1016/j.bcmd.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis. 2006;36:426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Sidransky E. Gaucher disease: insights from a rare Mendelian disorder. Discov Med. 2012;14:273–281. [PMC free article] [PubMed] [Google Scholar]
- 40.Maor G, Filocamo M, Horowitz M. ITCH regulates degradation of mutant glucocerebrosidase: implications to Gaucher disease. Hum Mol Genet. 2013;22:1316–1327. doi: 10.1093/hmg/dds535. [DOI] [PubMed] [Google Scholar]
- 41.Mistry PK, Sirrs S, Chan A, Pritzker MR, Duffy TP, Grace ME, Meeker DP, Goldman ME. Pulmonary hypertension in type 1 Gaucher’s disease: genetic and epigenetic determinants of phenotype and response to therapy. Mol Genet Metab. 2002;77:91–98. doi: 10.1016/s1096-7192(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 42.Wine E, Yaniv I, Cohen IJ. Hyperimmunoglobulinemia in pediatric-onset type 1 Gaucher disease and effects of enzyme replacement therapy. J Pediatr Hematol Oncol. 2007;29:451–457. doi: 10.1097/MPH.0b013e31806451d3. [DOI] [PubMed] [Google Scholar]
- 43.Brautbar A, Elstein D, Pines G, Abrahamov A, Zimran A. Effect of enzyme replacement therapy on gammopathies in Gaucher disease. Blood Cells Mol Dis. 2004;32:214–217. doi: 10.1016/j.bcmd.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Haratz D, Manny N, Raz I. Autoimmune hemolytic anemia in Gaucher’s disease. Klin Wochenschr. 1990;68:94–95. doi: 10.1007/BF01646850. [DOI] [PubMed] [Google Scholar]
- 45.Shoenfeld Y, Beresovski A, Zharhary D, Tomer Y, Swissa M, Sela E, Zimran A, Zevin S, Gilburd B, Blank M. Natural autoantibodies in sera of patients with Gaucher’s disease. J Clin Immunol. 1995;15:363–372. doi: 10.1007/BF01541326. [DOI] [PubMed] [Google Scholar]
- 46.Pandey MK, Grabowski GA. Immunological cells and functions in Gaucher disease. Crit Rev Oncog. 2013;18:197–220. doi: 10.1615/critrevoncog.2013004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Halene S, Yang M, Iqbal J, Yang R, Mehal WZ, Chuang WL, Jain D, Yuen T, Sun L, Zaidi M, Mistry PK. Gaucher disease gene GBA functions in immune regulation. Proc Natl Acad Sci U S A. 2012;109:10018–10023. doi: 10.1073/pnas.1200941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes DA, Pastores GM. Haematological manifestations and complications of Gaucher disease. Curr Opin Hematol. 2013;20:41–47. doi: 10.1097/MOH.0b013e32835a9148. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbloom BE, Weinreb NJ, Zimran A, Kacena KA, Charrow J, Ward E. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood. 2005;105:4569–4572. doi: 10.1182/blood-2004-12-4672. [DOI] [PubMed] [Google Scholar]
- 50.Mistry PK, Taddei T, vom Dahl S, Rosenbloom BE. Gaucher disease and malignancy: a model for cancer pathogenesis in an inborn error of metabolism. Crit Rev Oncog. 2013;18:235–246. doi: 10.1615/critrevoncog.2013006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta N, Oppenheim IM, Kauvar EF, Tayebi N, Sidransky E. Type 2 Gaucher disease: phenotypic variation and genotypic heterogeneity. Blood Cells Mol Dis. 2011;46:75–84. doi: 10.1016/j.bcmd.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bove KE, Daugherty C, Grabowski GA. Pathological findings in Gaucher disease type 2 patients following enzyme therapy. Hum Pathol. 1995;26:1040–1045. doi: 10.1016/0046-8177(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 53.Prows CA, Sanchez N, Daugherty C, Grabowski GA. Gaucher disease: enzyme therapy in the acute neuronopathic variant. Am J Med Genet. 1997;71:16–21. [PubMed] [Google Scholar]
- 54.Vellodi A, Tylki-Szymanska A, Davies EH, Kolodny E, Bembi B, Collin-Histed T, Mengel E, Erikson A, Schiffmann R, European Working Group on Gaucher Disease Management of neuronopathic Gaucher disease: revised recommendations. J Inherit Metab Dis. 2009;32:660–664. doi: 10.1007/s10545-009-1164-2. [DOI] [PubMed] [Google Scholar]
- 55.Abrahamov A, Elstein D, Gross-Tsur V, Farber B, Glaser Y, Hadas-Halpern I, Ronen S, Tafakjdi M, Horowitz M, Zimran A. Gaucher’s disease variant characterised by progressive calcification of heart valves and unique genotype. Lancet. 1995;346(8981):1000–1003. doi: 10.1016/s0140-6736(95)91688-1. [DOI] [PubMed] [Google Scholar]
- 56.Tylki-Szymañska A, Keddache M, Grabowski GA. Characterization of neuronopathic Gaucher disease among ethnic Poles. Genet Med. 2006;8:8–15. doi: 10.1097/01.gim.0000196443.42899.25. [DOI] [PubMed] [Google Scholar]
- 57.Orvisky E, Park JK, LaMarca ME, Ginns EI, Martin BM, Tayebi N, Sidransky E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol Genet Metab. 2002;76:262–270. doi: 10.1016/s1096-7192(02)00117-8. [DOI] [PubMed] [Google Scholar]
- 58.Mignot C, Gelot A, Bessières B, Daffos F, Voyer M, Menez F, Fallet Bianco C, Odent S, Le Duff D, Loget P, Fargier P, Costil J, Josset P, Roume J, Vanier MT, Maire I, Billette de Villemeur T. Perinatal-lethal Gaucher disease. Am J Med Genet A. 2003;120A:338–344. doi: 10.1002/ajmg.a.20117. [DOI] [PubMed] [Google Scholar]
- 59.Beutler E. Gaucher disease: multiple lessons from a single gene disorder. Acta Paediatr Suppl. 2006;95(451):103–109. doi: 10.1111/j.1651-2227.2006.tb02398.x. [DOI] [PubMed] [Google Scholar]
- 60.Koprivica V, Stone DL, Park JK, Callahan M, Frisch A, Cohen IJ, Tayebi N, Sidransky E. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66:1777–1786. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenbloom BE, Weinreb NJ. Gaucher disease: a comprehensive review. Crit Rev Oncog. 2013;18:163–175. doi: 10.1615/critrevoncog.2013006060. [DOI] [PubMed] [Google Scholar]
- 62.Pastores GM, Hughes DA. Gaucher disease. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews™ [Internet] University of Washington; Seattle (WA): Jul 27, 2000. Seattle; 1993-2013. [updated 2013 Sep 19] [PubMed] [Google Scholar]
- 63.Fairley C, Zimran A, Phillips M, Cizmarik M, Yee J, Weinreb N, Packman S. Phenotypic heterogeneity of N370S homozygotes with type I Gaucher disease: An analysis of 798 patients from the ICGG Gaucher Registry. J Inherit Metab Dis. 2008;31:738–744. doi: 10.1007/s10545-008-0868-z. [DOI] [PubMed] [Google Scholar]
- 64.Pastores GM, Weinreb NJ, Aerts H, Andria G, Cox TM, Giralt M, Grabowski GA, Mistry PK, Tylki-Szymańska A. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41(4 Suppl 5):4–14. doi: 10.1053/j.seminhematol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan P, Baris H, De Meirleir L, Di Rocco M, El-Beshlawy A, Huemer M, Martins AM, Nascu I, Rohrbach M, Steinbach L, Cohen IJ. Revised recommendations for the management of Gaucher disease in children. Eur J Pediatr. 2013;172:447–458. doi: 10.1007/s00431-012-1771-z. [DOI] [PubMed] [Google Scholar]
- 66.Maas M, Poll LW, Terk MR. Imaging and quantifying skeletal involvement in Gaucher disease. Br J Radiol. 2002;75(Suppl 1):A13–24. doi: 10.1259/bjr.75.suppl_1.750013. [DOI] [PubMed] [Google Scholar]
- 67.Robertson PL, Maas M, Goldblatt J. Semiquantitative assessment of skeletal response to enzyme replacement therapy for Gaucher’s disease using the bone marrow burden score. AJR Am J Roentgenol. 2007;188:1521–1528. doi: 10.2214/AJR.06.1410. [DOI] [PubMed] [Google Scholar]
- 68.Roca M, Mota J, Alfonso P, Pocoví M, Giraldo P. S-MRI score: A simple method for assessing bone marrow involvement in Gaucher disease. Eur J Radiol. 2007;62:132–137. doi: 10.1016/j.ejrad.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 69.Wenstrup RJ, Kacena KA, Kaplan P, Pastores GM, Prakash-Cheng A, Zimran A, Hangartner TN. Effect of enzyme replacement therapy with imiglucerase on BMD in type 1 Gaucher disease. J Bone Miner Res. 2007;22:119–126. doi: 10.1359/jbmr.061004. [DOI] [PubMed] [Google Scholar]
- 70.Cohen IJ, Yaniv I, Baris H. Diagnosis of severe type 1 Gaucher’s disease before irreversible damage occurs: is HDL cholesterol the answer? Br J Haematol. 2010;150:118–119. doi: 10.1111/j.1365-2141.2010.08167.x. [DOI] [PubMed] [Google Scholar]
- 71.Grace ME, Balwani M, Nazarenko I, Prakash-Cheng A, Desnick RJ. Type 1 Gaucher disease: null and hypomorphic novel chitotriosidase mutations - implications for diagnosis and therapeutic monitoring. Hum Mutat. 2007;28:866–873. doi: 10.1002/humu.20524. [DOI] [PubMed] [Google Scholar]
- 72.Lee RE. The pathology of Gaucher disease. Prog Clin Biol Res. 1982;95:177–217. [PubMed] [Google Scholar]
- 73.Cox TM, Aerts JM, Belmatoug N, Cappellini MD, vom Dahl S, Goldblatt J, Grabowski GA, Hollak CE, Hwu P, Maas M, Martins AM, Mistry PK, Pastores GM, Tylki-Szymanska A, Yee J, Weinreb N. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. J Inherit Metab Dis. 2008;31:319–336. doi: 10.1007/s10545-008-0779-z. [DOI] [PubMed] [Google Scholar]
- 74.Taddei TH, Dziura J, Chen S, Yang R, Hyogo H, Sullards C, Cohen DE, Pastores G, Mistry PK. High incidence of cholesterol gallstone disease in type 1 Gaucher disease: characterizing the biliary phenotype of type 1 Gaucher disease. J Inherit Metab Dis. 2010;33:291–300. doi: 10.1007/s10545-010-9070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balwani M, Fuerstman L, Kornreich R, Edelmann L, Desnick RJ. Type 1 Gaucher disease: significant disease manifestations in “asymptomatic” homozygotes. Arch Intern Med. 2010;170:1463–1469. doi: 10.1001/archinternmed.2010.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaplan P, Andersson HC, Kacena KA, Yee JD. The clinical and demographic characteristics of nonneuronopathic Gaucher disease in 887 children at diagnosis. Arch Pediatr Adolesc Med. 2006;160:603–608. doi: 10.1001/archpedi.160.6.603. [DOI] [PubMed] [Google Scholar]
- 77.Kaplan P, Mazur A, Manor O, Charrow J, Esplin J, Gribble TJ, Wappner RS, Wisch JS, Weinreb NJ. Acceleration of retarded growth in children with Gaucher disease after treatment with alglucerase. J Pediatr. 1996;129:149–153. doi: 10.1016/s0022-3476(96)70203-2. [DOI] [PubMed] [Google Scholar]
- 78.Baldellou A, Andria G, Campbell PE, Charrow J, Cohen IJ, Grabowski GA, Harris CM, Kaplan P, McHugh K, Mengel E, Vellodi A. Paediatric non-neuronopathic Gaucher disease: recommendations for treatment and monitoring. Eur J Pediatr. 2004;163:67–75. doi: 10.1007/s00431-003-1363-z. [DOI] [PubMed] [Google Scholar]
- 79.Mistry PK, Deegan P, Vellodi A, Cole JA, Yeh M, Weinreb NJ. Timing of initiation of enzyme replacement therapy after diagnosis of type 1 Gaucher disease: effect on incidence of avascular necrosis. Br J Haematol. 2009;147:561–570. doi: 10.1111/j.1365-2141.2009.07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ciana G, Deroma L, Franzil AM, Dardis A, Bembi B. Long-term bone mineral density response to enzyme replacement therapy in a retrospective pediatric cohort of Gaucher patients. J Inherit Metab Dis. 2012;35:1101–1106. doi: 10.1007/s10545-012-9476-z. [DOI] [PubMed] [Google Scholar]
- 81.Khan A, Hangartner T, Weinreb NJ, Taylor JS, Mistry PK. Risk factors for fractures and avascular osteonecrosis in type 1 Gaucher disease: a study from the International Collaborative Gaucher Group (ICGG) Gaucher Registry. J Bone Miner Res. 2012;27:1839–1848. doi: 10.1002/jbmr.1680. [DOI] [PubMed] [Google Scholar]
- 82.Deegan PB, Pavlova E, Tindall J, Stein PE, Bearcroft P, Mehta A, Hughes D, Wraith JE, Cox TM. Osseous manifestations of adult Gaucher disease in the era of enzyme replacement therapy. Medicine (Baltimore) 2011;90:52–60. doi: 10.1097/MD.0b013e3182057be4. [DOI] [PubMed] [Google Scholar]
- 83.Lo SM, Stein P, Mullaly S, Bar M, Jain D, Pastores GM, Mistry PK. Expanding spectrum of the association between Type 1 Gaucher disease and cancers: a series of patients with up to 3 sequential cancers of multiple types - correlation with genotype and phenotype. Am J Hematol. 2010;85:340–345. doi: 10.1002/ajh.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lo SM, Liu J, Chen F, Pastores GM, Knowles J, Boxer M, Aleck K, Mistry PK. Pulmonary vascular disease in Gaucher disease: clinical spectrum, determinants of phenotype and long-term outcomes of therapy. J Inherit Metab Dis. 2011;34:643–650. doi: 10.1007/s10545-011-9313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenbaum H, Sidransky E. Cholelithiasis in patients with Gaucher disease. Blood Cells Mol Dis. 2002;28:21–27. doi: 10.1006/bcmd.2001.0480. [DOI] [PubMed] [Google Scholar]
- 86.Weinreb NJ, Deegan P, Kacena KA, Mistry P, Pastores GM, Velentgas P, vom Dahl S. Life expectancy in Gaucher disease type 1. Am J Hematol. 2008;83:896–900. doi: 10.1002/ajh.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]