Abstract
Background
The approval of direct-acting antivirals for Interferon-free treatment revolutionized the therapy of chronic Hepatitis C infection. As of August 2014, two treatment regimens for genotype 1 infection received conditional approval in the European Union: Sofosbuvir and Ribavirin for 24 weeks and Sofosbuvir and Simeprevir with or without Ribavirin for 12 weeks. We aim to analyze the cost-effectiveness of both regimens in Germany.
Methods
We set up a Markov model with a lifetime horizon to simulate immediate treatment success and long-term disease progression for treatment-naive patients. The model analyzes both short-term and long-term costs and benefits from the perspective of the German Statutory Health Insurance. We apply the efficiency frontier method, which was suggested by German Institute for Quality and Efficiency in Health Care for cost-effectiveness analysis in Germany.
Results
The efficiency frontier is defined by dual therapy and first generation direct-acting antiviral Boceprevir, yielding a maximum of € 1,447.69 per additional percentage point of sustained virologic response gained. Even without rebates, Sofosbuvir/Simeprevir is very close with € 1,560.13 per additional percentage point. It is both more effective and less expensive than Sofosbuvir/Ribavirin.
Conclusions
In addition to higher sustained virologic response rates, new direct-acting antivirals save long-term costs by preventing complications such as liver cirrhosis, hepatocellular carcinoma and ultimately liver transplants, thereby offsetting part of higher drug costs. Our findings are in line with the guidance published by German Society for Gastroenterology, Digestive and Metabolic Diseases, which recommends Sofosbuvir/Simeprevir for Interferon ineligible or intolerant patients.
Keywords: Hepatitis C, Interferon-free therapy, Sofosbuvir, Simeprevir, Cost-effectiveness, Germany, Efficiency frontier
Background
Hepatitis C is one of the most common chronic infectious diseases worldwide and in Germany. The total number of patients infected with hepatitis C virus (HCV) is declining in Germany, with an estimated 4,980 new infections in 2013 [1]. In contrast to the declining number of patients, severe long-term complications such as decompensated cirrhosis (DCC), hepatocellular carcinoma (HCC) and ultimately liver transplants (LT) are estimated to be increasing due to the ageing of the existing infected population. Of the HCV-infected patients in Germany, 62 % are infected with HCV genotype 1, which is most difficult to treat [2].
Recently approved direct-acting antiviral agents (DAA) have improved sustained virologic response (SVR) rates compared to the classic combination therapy of PegInterferon (IFN) and Ribavirin (RBV; combination with IFN: PR). Despite higher SVR rates close to 100 %, high treatment costs have stirred a public debate on the pricing of recently approved DAAs [3, 4].
While triple therapy with first generation protease inhibitors (PI) Boceprevir or Telaprevir improved SVR rates compared to PR, IFN was still required with both agents. One of the greatest advantages of new DAAs is the advent of IFN-free treatment regimens. IFN-free therapies provide dramatically increased SVR rates compared to first-generation DAAs and avoid IFN’s side effects like depression, anxiety and fatigue, which in many cases has negative effects on patients’ ability to work [5]. Another advantage of recently approved DAAs are shorter treatment durations, which can contribute to a better compliance.
As of August 2014, two treatment regimens are recommended for IFN-free therapy of HCV genotype 1 infection by German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS) guidelines [6]:
Sofosbuvir (SOF) and Simeprevir (SMV) combination therapy with or without RBV for 12 weeks
SOF and RBV combination therapy for 24 weeks
Since the approval of DAAs, HCV therapy has played a major economic role in the German Statutory Health Insurance (SHI) funds. In 2013, the German SHI funds spent € 48.6 mn on Telaprevir and € 19.0 mn on Boceprevir [7].
In 2011, Germany introduced early benefit assessments and rebate negotiations as a measure of regulating pharmaceutical reimbursement [8]. After approval for the German market, pharmaceutical manufacturers are free to set a price for one year. Within that one year after approval, all prices of newly approved drugs need to be negotiated with the German SHI funds. The outcome of the negotiations is a rebate agreement, which comes into effect one year after approval in Germany [9].
Negotiations for the rebate agreement are based on a 6-month early benefit assessment, which determines additional benefits of the new therapy compared to an existing alternative therapy [10]. Early benefit assessments are conducted by German Federal Joint Committee (GBA). GBA’s assessment in turn relies on a 3-month assessment by German Institute for Quality and Efficiency in Health Care (IQWIG).
The outcomes of cost-effectiveness analyses with the efficiency frontier approach are maximum reimbursable prices. Under current German legislation, no cost-effectiveness analysis is involved in the process of the initial rebate negotiations [11]. However, cost-effectiveness analysis can be requested by either negotiating party as a base for further negotiations, once a first rebate agreement has been reached, i.e., the maximum reimbursable price would serve as a base for a new rebate negotiation between manufacturer and SHI funds. We aim to analyze the cost-effectiveness of DAAs for IFN-free therapies in this context.
Cost-effectiveness analyses are conducted by IQWIG and they are based on the efficiency frontier method [12, 13]. IQWIG’s method has previously been criticized because it does not use quality-adjusted life years (QALYs) as a measure of patient benefits. Contrary to approaches based on QALYs, German IQWIG requires the definition of specific clinical parameters as a measure of patient benefits, i.e., the method is not suitable to define a global threshold per QALY gained, as practiced in England and Wales [14].
Application of IQWIG’s efficiency frontier method requires the assessment of previously existing therapies. The ratio of previous increases in effectiveness and costs determines the efficiency frontier. This was heavily criticized by health economists [14, 15]. The results of efficiency frontier analyses depend on the benefits and costs of previously existing therapies, even though these therapies have never been subject to health technology assessment and no willingness-to-pay can be derived from the prices, which could previously be unilaterally determined by manufacturers.
If the ratio of previous gains in benefits and costs has been relatively expensive (i.e., relatively higher costs per clinical benefit unit), the efficiency frontier approach leads to higher maximum reimbursable prices for new approvals, too. This might not be desired from a regulatory perspective. Another problem of application can arise if multiple efficiency frontiers need to be computed to reflect the relevance of more than one clinical parameter. Naturally, the results of different efficiency frontiers may vary, potentially leading to conflicting results. IQWIG suggested to aggregate specific efficiency frontier results based on patient preferences as found by analytic hierarchy processes or conjoint analyses [16–18]. We aim to analyze whether the problems outlined above prevent useful results if the efficiency frontier method is applied to DAAs for HCV in Germany.
Methods
Both IQWIG and GBA have accepted SVR rates as the most important clinical parameter determining the success of antiviral therapy for patients suffering from chronic HCV infection. In its early benefit assessments of SOF and SMV, German Federal Joint Committee defined both classic dual therapy (PR) and triple therapy with first generation PIs (Boceprevir or Telaprevir) as appropriate comparators. Therefore, we define the efficiency frontier with PR and Boceprevir triple therapy as Boceprevir was the first PI to be approved in Germany.
We model the cost-effectiveness of the two comparators and the two IFN-free therapies with an extended Markov model, which is also used for analysis in the United Kingdom and in Japan [19, 20]. The model simulates the pathways of treatment-naive patients in all possible states of fibrosis (F0-F4). The model has a lifetime time horizon with a cycle length of one year. The cycle length is consistent with previous economic models and reflects the relatively slow progression rate for chronic HCV infection [21, 22].
All modeling characteristics were adapted to reflect clinical practice in Germany. Effectiveness analysis is based on GBA’s assessment of PR and Boceprevir triple therapy as part of early benefit assessments. However, no IFN-free therapy has been assessed by GBA yet. Therefore, benefit analysis of IFN-free regimens is only based on the recommendations published by DGVS [6]. Therefore, the model was extended to incorporate suitable studies to simulate treatment with IFN-free regimens. For SOF/SMV, the COSMOS trial was included in the model, as it reports SVR rates for treatment naive patients after 12 weeks [23]. For SOF/RBV the QUANTUM trial results were used [24].
We conducted a Pubmed search for 'hepatitis c cost germany' to adapt the model to German prices. Out of 65 hits, only 2 studies could be identified as reporting original German cost data for HCV [25, 26]. All costs used in the model are in 2014 Euros and were discounted by 3 %, as suggested by IQWIG. All drug costs reflect German prices as of August 2014 (according to Lauer-Taxe).
The model analyzes two components of health economic interest: The antiviral therapy phase with a follow-up until week 72, and the disease progression with a lifetime perspective (see Fig. 1).
Fig. 1.
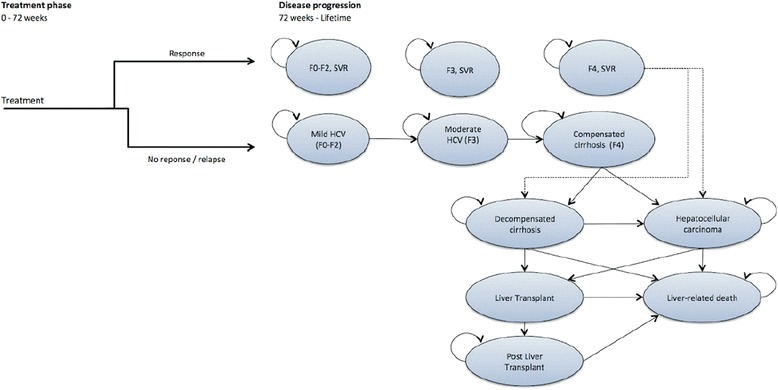
Model structure. The Markov model simulates both immediate treatment success and long-term disease progression. For simplicity, all-cause mortality is not explicitly depicted
The second phase depends on the outcomes of the first phase. The model is not limited to the immediate treatment phase, but takes a lifetime perspective, because long-term complications associated with HCV are very important for a chronic and severe disease like HCV (Table 1). The transition probabilities of the economic model are listed in Table 2. As no specific data for the German context could be found, the model primarily relies on a recent, systematic literature review published as part of an English Health Technology Assessment, which proved to be suitable for the purposes of our model [21].
Table 1.
Costs of adverse events and long-term complications
| On-treatment adverse events | 2014 costs | Source | Probabilistic Sensitivity Analysis | ||
|---|---|---|---|---|---|
| Distribution | Lower | Upper | |||
| Anaemia | 1,821.25 € | [25] | Gamma | 1,457.00 € | 2,185.50 € |
| Neutropenia | 42.54 € | expert opinion | Gamma | 34.03 € | 51.05 € |
| Rash | 26.85 € | [25] | Gamma | 21.48 € | 32.22 € |
| Pruritus | 22.58 € | [25] | Gamma | 18.06 € | 27.10 € |
| Long-term complications | |||||
| Decompensated cirrhosis | 9,656.36 € | [25] | Gamma | 7,725.09 € | 11,587.63 € |
| Hepatocellular carcinoma | 22,762.62 € | [26] | Gamma | 18,210.10 € | 27,315.14 € |
| First year after liver transplant | 155,815.33 € | [26] | Gamma | 124,652.26 € | 186,978.40 € |
| Further years after liver transplant | 22,534.99 € | [26] | Gamma | 18,027.99 € | 27,041.99 € |
Table 2.
Annual transition probabilities in the economic model
| From | To | Mean | SE | Source | Probabilistic Sensitivity Analysis | ||
|---|---|---|---|---|---|---|---|
| Distribution | Lower | Upper | |||||
| F0-F2 | F3 | 0.025 | 0.004 | [21, 32] | Beta | 0.018 | 0.033 |
| F3 | F4 | 0.037 | 0.007 | [21, 32] | Beta | 0.025 | 0.052 |
| F4 | DCC | 0.039 | 0.010 | [21, 33] | Beta | 0.022 | 0.061 |
| HCC | 0.014 | 0.010 | [21, 33] | Beta | 0.002 | 0.039 | |
| SVR F4 | DCC | 0.000 | 0.000 | expert opinion | Beta | 0.000 | 0.000 |
| HCC | 0.005 | 0.001 | [34] | Beta | 0.004 | 0.007 | |
| DCC | HCC | 0.014 | 0.010 | [21, 33] | Beta | 0.002 | 0.039 |
| LT | 0.020 | 0.003 | [21, 35] | Beta | 0.015 | 0.026 | |
| LrD | 0.130 | 0.010 | [21, 33] | Beta | 0.111 | 0.150 | |
| HCC | LT | 0.020 | 0.003 | [32, 35] | Beta | 0.015 | 0.026 |
| LrD | 0.430 | 0.030 | [21, 33] | Beta | 0.372 | 0.489 | |
| LT | LrD | 0.150 | 0.023 | [21, 36] | Beta | 0.109 | 0.197 |
| pLT | LrD | 0.057 | 0.009 | [21, 36] | Beta | 0.041 | 0.075 |
| All-cause mortality | Age/gender specific | Life Tables, German Federal Office of Statistics | |||||
DCC decompensated cirrhosis, HCC hepatocellular carcinoma, SVR sustained virologic response, LT liver transplant, LrD Liver related death, pLT post liver transplant, fibrosis states F0-F4 according to METAVIR
The model is run to simulate the treatment success of the four therapeutic options for our efficiency frontier analysis: PR, Boceprevir + PR, SOF/RBV, SOF/SMV. The most important baseline characteristic of simulated patients is the degree of fibrosis according to the METAVIR scoring system. Each treatment is simulated for the duration as suggested by DGVS guidelines. After each treatment, patients can either have undetectable HCV-RNA and achieve SVR or fail therapy and be assigned to the relapser group. During each treatment course, on-treatment adverse events can appear. Furthermore, all-cause mortality was applied to all possible health states according to German life tables (not shown in Fig. 1 for simplicity). Detailed descriptions of the model's mechanics have been published elsewhere [19, 20].
In order to determine the influence of uncertainty surrounding input parameters, we provide a multivariate probabilistic sensitivity analysis. Parameter estimates were varied within the uncertainty distributions that best reflect the nature of each specific parameter. Uncertainty margins are applied to each input parameter of interest based on corresponding intervals provided in the literature (Table 2) or based on assumptions if information is unavailable. The standard error was assumed to vary 20 % around the mean in case information on variance was not available for a specific parameter (applied to German cost data in Table 1).
We process 3000 simulations, which vary the parameters by random draws from their assumed distributions (as shown in Tables 1 and 2), to represent the uncertainty of model results. We plot the 3000 simulations in a scatterplot in Fig. 3 and compute the percentage of simulations, in which SOF/SMV or SOF/RBV would be above or on the efficiency frontier.
Fig. 3.

Probabilistic sensitivity analysis. Probabilistic Sensitivity Analysis shows 3000 random draws for each of the four therapies. Out of the 3000 draws, SOF/SMV would be above or on the efficiency frontier in 10.0 % of the simulations, while SOF/RBV would never meet the requirement
Results
As of August 2014, one week of SMV treatment costs € 3,829.91, while one week of SOF treatment costs € 4,714.69, i.e. SOF/SMV combination therapy costs € 102,535.14 for 12 weeks, while SOF/RBV costs € 113,152.56 for 24 weeks (DAA costs excluding RBV).
The main efficiency frontier for treatment-naive patients as reported in Fig. 2 is defined by PR and Boceprevir triple therapy. SVR was 46.62 % for treatment with PR for treatment-naive patients. Boceprevir triple therapy improved SVR rates to 58.58 %.
Fig. 2.
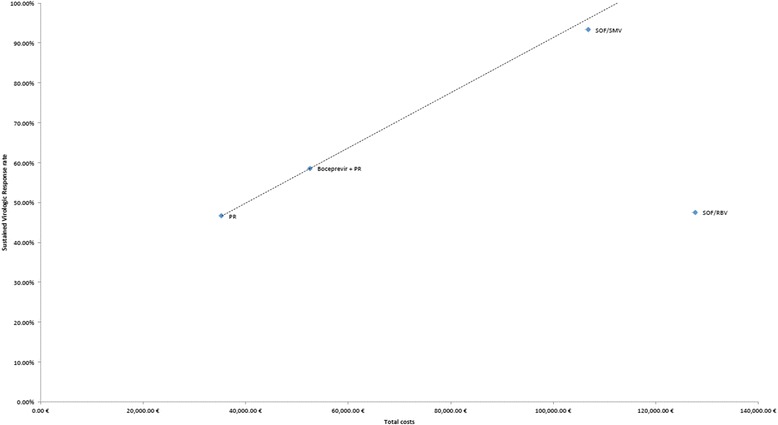
Efficiency frontier. The efficiency frontier plots the required ratio of increases in sustained virologic response rates and costs, as defined by comparators dual therapy (PR) and Boceprevir triple therapy
Boceprevir combination therapy increased costs from € 35,358.93 to € 52,679.70. The increase in total costs is mainly due to € 23,606.10 in PI costs. Along with a better SVR rate, long-term complications and the costs associated with them decreased. The introduction of Boceprevir defined a required marginal efficiency of a maximum of € 1,447.69 per additional SVR percentage point gained. According to IQWIG’s efficiency frontier concept, every new therapy for treating HCV can only cost an additional € 1,447.69 for each additional SVR percentage point, which is gained by the new therapy beyond the rate of 58.58 % which is achievable with Boceprevir (Fig. 2).
In our modeling approach, IFN-free regimen SOF/RBV achieves a rate of 47.37 %, which is below Boceprevir’s SVR rate for treatment-naive patients. Therefore, the required marginal efficiency of SOF/RBV cannot be computed.
Our results show that the combination of SOF/SMV is more effective and cheaper than SOF/RBV (Table 3). SOF/SMV achieves a SVR rate of 93.31 %, i.e., adding 34.73 percentage points to the previously achievable results with Boceprevir. Applying the efficiency frontier cost per additional SVR percentage point, these SVR rates result in maximum costs of € 102,963.08 for SOF/SMV. In our model, SOF/SMV comes very close to this threshold, even though no rebates have been negotiated for SOF or SMV yet. Total costs are € 106,868.76 for SOF/SMV for 12 weeks, i.e., € 3,905.68 or 3.8 % above the threshold defined with the efficiency frontier approach.
Table 3.
Main modeling outcomes
| Treatment naïves population | ||||
|---|---|---|---|---|
| PR | Boceprevir + PR | SOF/RBV | SOF/SMV | |
| Clinical outcomes | ||||
| SVR (24 weeks after tx) | 46.62 % | 58.58 % | 47.37 % | 93.31 % |
| Decompensated cirrhosis | 11.88 % | 10.07 % | 10.64 % | 1.73 % |
| Hepatocellular carcinoma | 5.65 % | 4.97 % | 5.30 % | 2.15 % |
| Liver Transplantation | 1.42 % | 1.22 % | 1.27 % | 0.26 % |
| Liver related death | 13.52 % | 11.71 % | 12.22 % | 3.22 % |
| Costs | ||||
| Total Costs | 35,358.93 € | 52,679.70 € | 127,891.69 € | 106,868.76 € |
| Drug costs | 21,842.68 € | 41,842.96 € | 117,578.96 € | 103,641.74 € |
| Of which PI | - € | 23,606.10 € | 113,152.56 € | 102,535.14 € |
| Of which (P)R | 21,842.68 € | 18,236.86 € | 4,426.40 € | 1,106.60 € |
| Pre-treatment evaluation | 170.99 € | 85.00 € | 85.00 € | 85.00 € |
| Monitoring costs | 1,287.97 € | 1,207.16 € | 1,084.00 € | 878.97 € |
| Adverse event cost | 450.07 € | 923.49 € | 7.00 € | 162.53 € |
| SVR, F0-F2 | 50.75 € | 66.06 € | 46.86 € | 94.11 € |
| SVR, F3 | 25.34 € | 13.18 € | 15.16 € | 28.64 € |
| SVR, F4 | 63.40 € | 27.77 € | 31.94 € | 60.34 € |
| F0-F2 | 703.42 € | 491.51 € | 778.50 € | 72.15 € |
| F3 | 2,881.21 € | 1,109.77 € | 1,268.17 € | 185.75 € |
| F4 | 1,258.16 € | 1,082.28 € | 1,105.84 € | 187.26 € |
| DCC | 2,963.85 € | 2,595.90 € | 2,614.33 € | 450.92 € |
| HCC | 1,459.78 € | 1,307.52 € | 1,348.52 € | 588.77 € |
| LT | 1,133.50 € | 987.14 € | 998.37 € | 219.54 € |
| pLT | 1,067.81 € | 939.97 € | 929.03 € | 213.03 € |
PR PegInterferon and Ribavirin, SOF Sofosbuvir, SMV Simeprevir, SVR sustained virologic response, PI protease inhibitor, fibrosis states F0-F4 according to METAVIR, DCC decompensated cirrhosis, HCC hepatocellular carcinoma, LT, first year after liver transplant, pLT further years after liver transplant
Costs for IFN-free regimens are dominated by DAA costs. For SOF/SMV, DAA costs are € 102,535.14. Along with a much improved SVR rate, SOF/SMV saves € 5,152.67 in costs for long-term complications DCC, HCC and LT.
The maximum cost defined by the efficiency frontier can be met for SOF/SMV by negotiating a rebate of 6.9 % for SOF (lowering SOF’s 28-unit price from € 18,858.76 to € 17,556.86) or by negotiating a rebate of 8.5 % for SMV (lowering SMV’s 28-unit price from € 15,319.62 to € 14,017.72).
Our probabilistic sensitivity analysis shows that even without any rebates, SOF/SMV meets the efficiency frontier requirement in 10.0 % of the cases, while SOF/RBV is never above or on the efficiency frontier at current price levels (Fig. 3).
Discussion
The cost-effectiveness of HCV therapy has been subject to extensive research in Germany and served as a proof of concept to establish the efficiency frontier method [27, 28]. Since the introduction of DAAs, cost-effectiveness analysis of drugs became more important because antiviral treatment became a major cost component in overall HCV treatment costs [29]. Other studies used incremental life years or QALYs as a measure of benefit for DAAs [20, 30, 31].
For the first time in the history of the treatment of chronic HCV, therapy regimens without the requirement for IFN are available. Within few months, two different regimens received conditional approval for the European markets. SOF/RBV and SOF/SMV are the first opportunity for all-oral treatment without patients having to face the side effects caused by IFN therapy. Both regimens are more expensive than first generation DAAs but save long-term costs for HCV complications.
As our application of the efficiency frontier shows, it is a suitable method to plot both SVR rates and associated long-term costs in one diagram. Due to the decisive nature of SVR rates in the measurement of HCV therapy success, no weighting algorithm is needed to aggregate multiple parameters. Since the introduction of pricing negotiations in 2011, no cost-effectiveness analysis has been used yet to renegotiate an initial rebate agreement. Our analysis shows that the efficiency frontier method is suitable to serve such a purpose for DAAs in HCV therapy.
Our model allows to report the immediate short-term effectiveness of the available treatment regimens, while calculating the impact of the treatment success on long-term complications such as DCC, HCC and LT.
Application of the efficiency frontier approach shows that SOF/SMV therapy almost meets the required threshold, even though DAA prices have not been negotiated yet. While our model incorporates long-term benefits in terms of prevented long-term complications, it does not incorporate the cost-savings associated with preventing disease transmissions by untreated HCV patients.
Our study is limited by the lack of head-to-head trials for direct comparison of SOF/SMV and SOF/RBV treatment regimens. Our application of IQWIG’s approach can be criticized for defining an efficiency frontier with treatment regimens, which are not IFN-free and lack a decisive benefit of SOF/RBV and SOF/SMV. However, our results deliver data for the value of additional SVR percentage points gained in the German SHI system.
Our analysis confirms the findings of a recent study conducted for the US context [31]. SOF/SMV is more effective than SOF/RBV and saves costs both in Germany and in the USA.
The new DAAs are more expensive but our modeling approach shows that they prevent expensive long-term complications more effectively than first generation DAAs. While immediate drug costs are high, each case of prevented LT equals savings of more than € 150,000 in the German SHI system.
Our analysis has shown that SOF/SMV is the most cost-effective IFN-free therapy regimen at the moment. Costs are lower for 12 weeks of SOF/SMV than for 24 weeks of SOF/RBV, both in terms of drug costs for the antiviral therapy and in terms of prevented long-term complications.
SOF/SMV requires therapy for only 12 weeks, which could contribute to better patient compliance. The SVR rate of more than 93 % is unprecedented for all-oral, IFN-free HCV therapy and overall costs need to be lowered by less than 4 % to stay within the requirements of the efficiency frontier.
Conclusions
The increase in clinical effectiveness by new DAA approvals is unprecedented in HCV therapy. Contrary to previous incremental improvements, recently approved DAAs SOF and SMV represent a jump in clinical effectiveness. Health economic methods are required to put the jumps in clinical effectiveness and the costs associated with the new therapies into perspective. Decision makers in many jurisdictions need suitable tools for cost-effectiveness analysis of the first IFN-free therapies in order to make reimbursement decisions.
Our analysis has shown that IQWIG's efficiency frontier approach is a suitable method to evaluate recent DAA approvals SOF and SMV for IFN-free therapy. In addition to higher sustained virologic response rates, the evaluated therapies save long-term costs by preventing complications such as liver cirrhosis, hepatocellular carcinoma and ultimately liver transplants, thereby offsetting part of higher drug costs. IQWIG's efficiency frontier approach can also be applied to further new approvals for the treatment of HCV, which are expected in the near future.
Acknowledgements
We thank Kirsten Westerhout, Pharmerit, for contributing to the design and setup of the Probabilistic Sensitivity Analysis.
Abbreviations
- HCV
Hepatitis C virus
- DCC
Decompensated cirrhosis
- HCC
Hepatocellular carcinoma
- LT
Liver transplant
- DAA
Direct-acting antiviral agent
- SVR
Sustained virologic response
- IFN
PegInterferon
- RBV
Ribavirin
- PR
Combination of IFN and RBV
- PI
Protease inhibitor
- DGVS
German Society for Gastroenterology, Digestive and Metabolic Diseases
- SOF
Sofosbuvir
- SMV
Simeprevir
- SHI
Statutory Health Insurance
- GBA
Federal Joint Committee
- IQWIG
Institute for Quality and Efficiency in Health Care
- QALY
Quality-adjusted life year
Footnotes
Competing interests
We disclose the following conflicts of interest/ financial support: This study was funded by Janssen-Cilag GmbH (Johnson&Johnson-Platz 1, 41470 Neuss, Germany, phone +49 2137 9550, www.janssen-deutschland.de). GG and HR received funding for this study by Janssen-Cilag GmbH. JM is employed by Janssen Pharmaceutical K.K., Tokyo, Japan. CG declares that he has no competing interests. The funding source (Janssen-Cilag GmbH) had no influence on the work and writing of the manuscript.
Authors’ contributions
CG conducted the data collection and modeling according to the efficiency frontier approach and drafted the manuscript; GG and JM contributed to the analysis of the data from an economic perspective; HR contributed to the analysis of the data from a medical perspective. All authors contributed to the critical revision of the manuscript and approve of the final version to be published.
Contributor Information
Christian Gissel, Email: cg@phec.de.
Georg Götz, Email: Georg.Goetz@wirtschaft.uni-giessen.de.
Jörg Mahlich, Email: jmahlich@its.jnj.com.
Holger Repp, Phone: +49 641 99 40031, Email: repp@phec.de.
References
- 1.Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J Viral Hepat. 2014;21(Suppl 1):34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 2.Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31(Suppl 2):30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 3.Deuffic-Burban S, Schwarzinger M, Obach D, Mallet V, Pol S, Pageaux GP, et al. Should we await IFN-free regimens to treat HCV genotype 1 treatment-naive patients? A cost-effectiveness analysis (ANRS 95141) J Hepatol. 2014;61:7–14. doi: 10.1016/j.jhep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Reau NS, Jensen DM. Sticker shock and the price of new therapies for hepatitis C: Is it worth it? Hepatology. 2014;59:1246–9. doi: 10.1002/hep.27039. [DOI] [PubMed] [Google Scholar]
- 5.Huckans M, Fuller B, Wheaton V, Jaehnert S, Ellis C, Kolessar M, et al. A longitudinal study evaluating the effects of interferon-alpha therapy on cognitive and psychiatric function in adults with chronic hepatitis C. J Psychosom Res. 2015;78:184–92. doi: 10.1016/j.jpsychores.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarrazin C, Berg T, Buggisch P, Dollinger M, Hinrichsen H, Hüppe D, et al. Aktuelle Empfehlung der DGVS und des bng zur Therapie der chronischen Hepatitis C. 09.06.2014. German Society for Gastroenterology, Digestive and Metabolic Diseases. 2014. http://www.dgvs.de/fileadmin/user_upload/Leitlinien/Empfehlung-Therapie-HepatitisC-DGVS-bng-09.06.2014.pdf. Accessed 16 Mar 2015.
- 7.Schwabe U, Paffrath D. Arzneiverordnungs-Report 2014: Aktuelle Daten, Kosten, Trends und Kommentare. Berlin: Springer; 2014. [Google Scholar]
- 8.Gerber A, Stock S, Dintsios CM. Reflections on the changing face of German pharmaceutical policy: how far is Germany from value-based pricing? Pharmacoeconomics. 2011;29:549–53. doi: 10.2165/11592580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Henschke C, Sundmacher L, Busse R. Structural changes in the German pharmaceutical market: price setting mechanisms based on the early benefit evaluation. Health Policy. 2013;109:263–9. doi: 10.1016/j.healthpol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Ruof J, Schwartz FW, Schulenburg JM, Dintsios CM. Early benefit assessment (EBA) in Germany: analysing decisions 18 months after introducing the new AMNOG legislation. Eur J Health Econ. 2014;15:577–89. doi: 10.1007/s10198-013-0495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivandic V. Requirements for benefit assessment in Germany and England - overview and comparison. Health Econ Rev. 2014;4:12. doi: 10.1186/s13561-014-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute for Quality and Efficiency in Health Care. General methods for the assessment of the relation of benefits to costs. Version 1.0. 2009. https://www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf. Accessed 16 Mar 2015.
- 13.Caro JJ, Nord E, Siebert U, McGuire A, McGregor M, Henry D, et al. The efficiency frontier approach to economic evaluation of health-care interventions. Health Econ. 2010;19:1117–27. doi: 10.1002/hec.1629. [DOI] [PubMed] [Google Scholar]
- 14.Sculpher M, Claxton K. Sins of omission and obfuscation: IQWIG's guidelines on economic evaluation methods. Health Econ. 2010;19:1132–6. doi: 10.1002/hec.1645. [DOI] [PubMed] [Google Scholar]
- 15.Brouwer WB, Rutten FF. The efficiency frontier approach to economic evaluation: will it help German policy making? Health Econ. 2010;19:1128–31. doi: 10.1002/hec.1644. [DOI] [PubMed] [Google Scholar]
- 16.Danner M, Hummel JM, Volz F, van Manen JG, Wiegard B, Dintsios CM, et al. Integrating patients’ views into health technology assessment: Analytic hierarchy process (AHP) as a method to elicit patient preferences. Int J Technol Assess Health Care. 2011;4:369–75. doi: 10.1017/S0266462311000523. [DOI] [PubMed] [Google Scholar]
- 17.Institute for Quality and Efficiency in Health Care. Choice-based Conjoint Analysis – pilot project to identify, weight, and prioritize multiple attributes in the indication “hepatitis C”. Version 1.1. 2014. https://www.iqwig.de/download/GA10-03_Executive-summary-of-working-paper-1.1_Conjoint-Analysis.pdf. Accessed 16 Mar 2015. [PubMed]
- 18.Institute for Quality and Efficiency in Health Care. Analytic Hierarchy Process (AHP) – pilot project to elicit patient preferences in the indication “depression”. Version 1.0. 2013. https://www.iqwig.de/download/Executive-summary-of-working-paper_Analytic-Hierarchy-Process-pilot-project.pdf. Accessed 16 Mar 2015. [PubMed]
- 19.Westerhout K, Treur M, Mehnert A, Pascoe K, Ladha I, Belsey J. Cost utility analysis of simeprevir used with peginterferon + ribavirin (SMV/PR) in the management of genotype 1 (G1) and 4 (G4) hepatitis C virus (HCV) infection; from the perspective of the UK National Health Service (NHS) Value Health. 2014;17:A679. doi: 10.1016/j.jval.2014.08.2527. [DOI] [PubMed] [Google Scholar]
- 20.Kuwabara H, Westerhout K, Treur M, Cerri K, Mahlich J, Yatsuhashi H. Cost-effectiveness analysis of simeprevir in combination with peginterferon and ribavirin for treatment-naïve chronic hepatitis C genotype 1 patients in Japan. J Med Econ. 2015;12:1–36. doi: 10.3111/13696998.2015.1029492. [DOI] [PubMed] [Google Scholar]
- 21.Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1–210. doi: 10.3310/hta15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–205. doi: 10.3310/hta11110. [DOI] [PubMed] [Google Scholar]
- 23.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–65. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 24.Lalezari JP, Nelson DR, Hyland RH, Lin M, Rossi SJ, Symonds WT, et al. Once Daily Sofosbuvir Plus Ribavirin for 12 and 24 Weeks in Treatment-Naive Patients with HCV Infection: the QUANTUM Study. J Hepatol. 2013;58:S346. doi: 10.1016/S0168-8278(13)60847-8. [DOI] [Google Scholar]
- 25.Stahmeyer JT, Schauer S, Rossol S, Wedemeyer HH, Wirth D, Bianic F, et al. Cost-effectiveness of Triple Therapy with Telaprevir for Chronic Hepatitis C Virus Patients in Germany. J Health Econ Outcomes Res. 2013;1:239–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Wasem J, Sroczynski G, Aidelsburger P, Buchberger B, Hessel F, Conrads-Frank A, et al. Health economics of chronic infectious diseases: the example of hepatitis C. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2006;49:57–63. doi: 10.1007/s00103-005-1190-7. [DOI] [PubMed] [Google Scholar]
- 27.Siebert U, Sroczynski G, German Hepatitis C Model (GEHMO) Group, HTA Expert Panel on Hepatitis C. Antiviral combination therapy with interferon/peginterferon plus ribavirin for patients with chronic hepatitis C in Germany: a health technology assessment commissioned by the German Agency for Health Technology Assessment. German Med Sci. 2003;1:Doc07, 1–18. [PMC free article] [PubMed]
- 28.Siebert U, Sroczynski G, Aidelsburger P, Rossol S, Wasem J, Manns MP, et al. Clinical effectiveness and cost effectiveness of tailoring chronic hepatitis C treatment with peginterferon alpha-2b plus ribavirin to HCV genotype and early viral response: a decision analysis based on German guidelines. Pharmacoeconomics. 2009;27:341–54. doi: 10.2165/00019053-200927040-00006. [DOI] [PubMed] [Google Scholar]
- 29.Stahmeyer JT, Rossol S, Bert F, Antoni C, Demir M, Hinrichsen H, et al. Cost of treating hepatitis C in Germany: a retrospective multicenter analysis. Eur J Gastroenterol Hepatol. 2014;26:1278–85. doi: 10.1097/MEG.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 30.Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60:530–7. doi: 10.1016/j.jhep.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60:37–45. doi: 10.1002/hep.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10:1–113. doi: 10.3310/hta10210. [DOI] [PubMed] [Google Scholar]
- 33.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 34.Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–7. doi: 10.1016/j.jhep.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52:425–32. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinical Effectiveness Unit Royal College of Surgeons of England . UK & Ireland liver transplant audit, 2003. London: Royal College of Surgeans; 2003. [Google Scholar]


