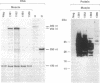
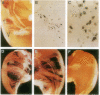
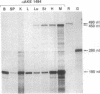
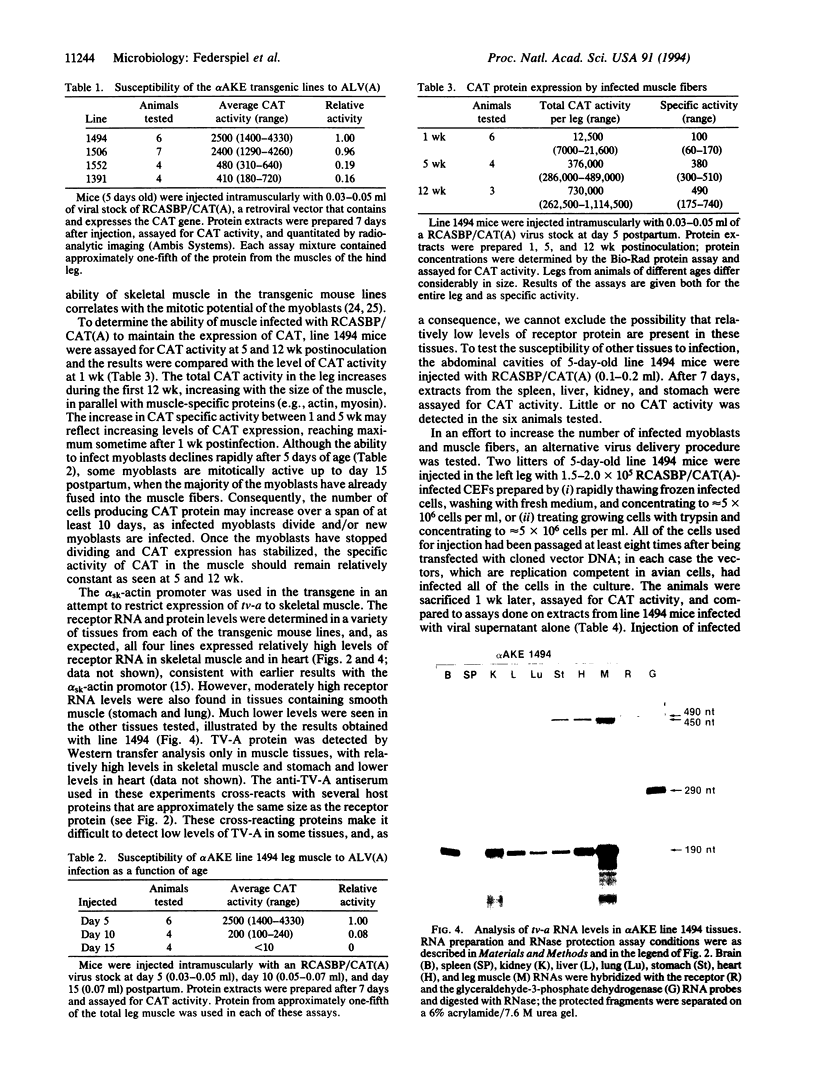
Abstract
Avian leukosis viruses (ALVs) have been used extensively as genetic vectors in avian systems, but their utility in mammals or mammalian cell lines is compromised by inefficient viral entry. We have overcome this limitation by generating transgenic mice that express the receptor for the subgroup A ALV under the control of the chicken alpha sk-actin promoter. The skeletal muscles of these transgenic animals are susceptible to efficient infection by subgroup A ALV. Because infection is restricted to cell lineages that express the transgene, the method has utility for studies of development and oncogenesis and will provide models for tissue-specific gene therapy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Bates P., Young J. A., Varmus H. E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993 Sep 24;74(6):1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- Berberich S. L., Macias M., Zhang L., Turek L. P., Stoltzfus C. M. Comparison of Rous sarcoma virus RNA processing in chicken and mouse fibroblasts: evidence for double-spliced RNA in nonpermissive mouse cells. J Virol. 1990 Sep;64(9):4313–4320. doi: 10.1128/jvi.64.9.4313-4320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerkoel C. F., Federspiel M. J., Salter D. W., Payne W., Crittenden L. B., Kung H. J., Hughes S. H. A new defective retroviral vector system based on the Bryan strain of Rous sarcoma virus. Virology. 1993 Aug;195(2):669–679. doi: 10.1006/viro.1993.1418. [DOI] [PubMed] [Google Scholar]
- Connolly L., Zingler K., Young J. A. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J Virol. 1994 Apr;68(4):2760–2764. doi: 10.1128/jvi.68.4.2760-2764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete D. M., Cepko C. L. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993 Apr;13(4):2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields-Berry S. C., Halliday A. L., Cepko C. L. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Temin H. M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977 Nov;24(2):461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse J. J., Petropoulos C. J., Crittenden L. B., Hughes S. H. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J Virol. 1988 Dec;62(12):4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. M., Blau H. M. Migration of myoblasts across basal lamina during skeletal muscle development. Nature. 1990 May 24;345(6273):350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Glover C., Reichmann M. E. Rous sarcoma virus infection of synchronized cells establishes provirus integration during S-phase DNA synthesis prior to cellular division. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2601–2605. doi: 10.1073/pnas.78.4.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. M. Satellite cells and myofiber growth in the rat soleus and extensor digitorum longus muscles. Dev Biol. 1978 Jul;65(1):1–10. doi: 10.1016/0012-1606(78)90174-4. [DOI] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Petropoulos C. J., Hughes S. H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991 Jul;65(7):3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C. J., Payne W., Salter D. W., Hughes S. H. Appropriate in vivo expression of a muscle-specific promoter by using avian retroviral vectors for gene transfer [corrected]. J Virol. 1992 Jun;66(6):3391–3397. doi: 10.1128/jvi.66.6.3391-3397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C. J., Rosenberg M. P., Jenkins N. A., Copeland N. G., Hughes S. H. The chicken skeletal muscle alpha-actin promoter is tissue specific in transgenic mice. Mol Cell Biol. 1989 Sep;9(9):3785–3792. doi: 10.1128/mcb.9.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A., Bates P., Varmus H. E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993 Apr;67(4):1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]