Abstract
Purpose of review
To review our current understanding of the relationship between absorption of nutrients and intestinal inflammatory response.
Recent findings
There is increasing evidence linking gut local inflammatory events with the intake of nutrients. Our recent studies, using the conscious lymph fistula rat model, demonstrate that fat absorption activates the intestinal mucosal mast cells. This is accompanied by a dramatic increase in the lymphatic release of mast cell mediators including histamine, rat mucosal mast cell protease II (RMCPII), as well as the lipid mediator prostaglandin D2 (PGD2). Clinical studies suggest that increased consumption of animal fat may play a role in the pathogenesis of inflammatory bowel disease. This impact of dietary fat may not be restricted to the gut but may extend to the whole body. There is evidence linking a high-fat diet-induced metabolic syndrome, with a low-grade chronic inflammatory state. In this review, we hope to convince the readers that fat absorption can have far reaching physiological and pathophysiological consequences.
Summary
Understanding the relationship between nutrient absorption and intestinal inflammation is important. We need a better understanding of the interaction between enterocytes and the intestinal immune cells in nutrient absorption and the gut inflammatory responses.
Keywords: fatty acids, inflammatory bowel disease, intestinal immune cells, mesenteric lymph, metabolic syndrome, mucosal mast cells
Introduction
The gastrointestinal tract carries out divergent roles which include the absorption of nutrients and also defending the body from harmful pathogens, for example bacteria and viruses. The absorptive process involves the gut epithelium and is highly effective. However, it also involves processes that have the potential to compromise the integrity of the gut epithelium. Therefore the gut immune system, the largest immune system in our body, must respond to pathogens while at the same time remaining relatively unresponsive to the food ingested and the trillions of commensal gut microbiota that reside in the human gastrointestinal tract [1••,2]. The unique role played by the gut in the digestion and absorption of nutrients as well as host defense requires the close interaction and coordination of both the epithelial cells located in the gut epithelium and many immune cells situated in the lamina propria [3••]. Regulation of this homeostasis involves central and peripheral nervous system, gastrointestinal hormones and peptides, cytokines and chemokines secreted by enterocytes, as well as the intestinal immune cells [4,5]. Although the underlying mechanisms involved are complex and not well understood, the prevailing notion is that failure to maintain the balance between the host and its microbiota (as well as food antigens) has adverse consequences on both the health and well-being of the intestine and the body. Considerable information has been reported regarding the gut inflammation induced by commensal microbiota (please see reviews) [1••,2]. However, how this balance is influenced by daily events such as the digestion or absorption of nutrients especially the absorption of fat with the coincident assembly and transport of chylomicrons remains relatively poorly understood, with the exception of a few isolated studies [6,7]. A number of excellent studies have demonstrated a close link between dietary fat absorption and the intestinal immune system [7–10]. For instance, it has been reported that acute duodenal feeding or long-term consumption of fat (e.g. olive oil or saturated butter fat) increases lymphocyte trafficking in rat intestinal lymph [7], and increased production of cytokines like tumor necrosis factor-α (TNF-α), interferon or interleukin 6 (IL-6) by macrophages [8], intraepithelial lymphocyte [9], and intestinal epithelial cells [10]. These observations are further extended by our recent findings that a duodenal infusion of liposyn II, a lipid emulsion containing mainly polyunsaturated fatty acids (PUFAs) activated intestinal mucosal mast cells (MMC) resulting in the release of various mast cell mediators such as histamine, protease [rat mast cell protease II (RMCPII)], as well as the lipid mediator prostaglandin D2 (PGD2) into intestinal lymph (unpublished data). Clinical studies have also suggested that dietary fat intake not only plays a significant role in the pathogenesis of inflammatory bowel diseases (IBDs) [11,12], but it may also be involved in the systemic low-grade inflammation associated with obesity, type II diabetes, hyperlipidemia, fatty liver and cardiovascular diseases [13–15]. Both human and animal models raise the interesting and obviously important question of whether the consumption of fat in general or a certain type of fat specifically, can induce intestinal inflammatory reaction. How does the small intestine sense different types of nutrients, and how does the absorption of some nutrients, for example fat induce an inflammatory response, whereas others do not? We believe that a better understanding of the mechanisms involved will provide new insights in improving human health and in the clinical management of IBD and metabolic diseases such as diabetes, fatty liver storage and cardiovascular diseases. Therefore, in this review we will focus on recent ongoing research on fat-induced intestinal inflammation.
Events associated with fat absorption in the small intestine
Intestinal absorption of nutrients is associated with a marked increase in intestinal blood flow, as much as doubling of fasting blood flow and this phenomenon is called postprandial hyperemia [16]. Long-chain fatty acids are the most potent nutrients that can initiate hyperemia in the presence of bile salt, but glucose, proteins, and peptides can also induce hyperemia [16]. In addition to the increased blood flow in gut, there is an increase in vascular permeability in response to fat absorption. As a result of the increase in mesenteric blood flow and in vascular permeability, gastrointestinal lymphatic flow and composition (such as an increase in protein concentration) also change dramatically during feeding [17,18]. In addition to the prevention of edema, the gastrointestinal lymphatic system is also coupled with other gastrointestinal function (transport of chylomicrons) [19].
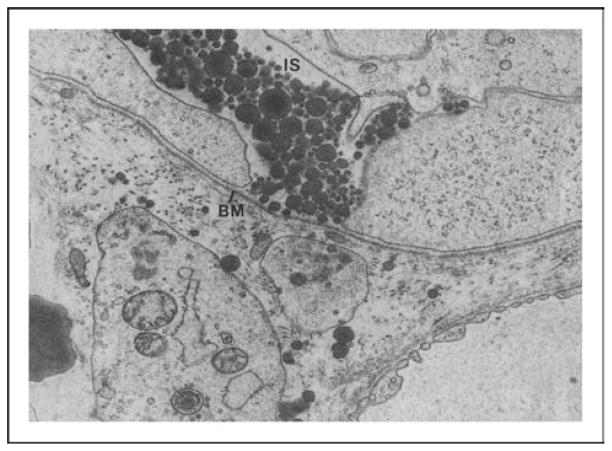
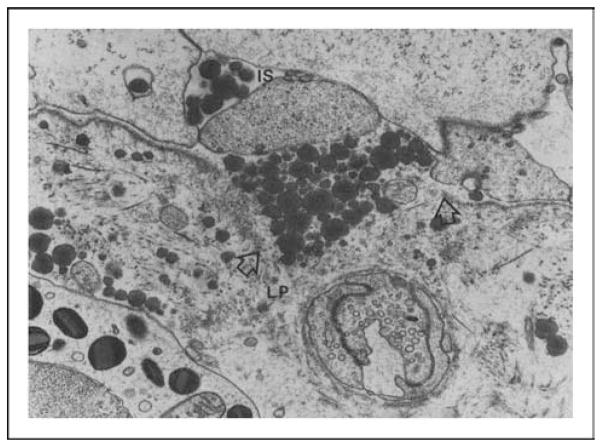
Dietary fats are digested and absorbed by the enterocytes and packaged into chylomicrons. Chylomicrons are packaged by the enterocytes and secreted into the intercellular space by exocytosis [19]. The journey of the chylomicrons from the intercellular space to the lamina propria is hampered by the basement membrane (as indicated by the arrows in Fig. 1) and thus, they accumulate in the intercellular space. As a result, the intercellular space becomes greatly distended, which probably loosens the junctional complex between the enterocytes. The junctional complexes are comprised of tight junctions, adherens junctions, gap junctions, and desmosomes, which are dynamic structures that restrict the passage of macromolecules larger than 50 A° [20••]. To allow the chylomicrons to cross the basement membrane, one possible mechanism is that there is breakage of the basement membrane (as indicated by the two arrows in Fig. 2). Both chylomicrons, containing long-chain fatty acids, and lymphocytes are transported in the mesenteric lymph [19], and lymphatic flow, protein flux, and lymphocyte trafficking all increase dramatically during fat absorption [7,17,18]. Fat absorption also stimulates intestinal immune cells including macrophages and intraepithelial lymphocytes and enterocytes to release cytokines [7–10].
Figure 1. Basal portion of jejunal absorptive cells and basement membrane from a rat absorbing lipid.
Note large intracellular spaces (ISs) between absorptive cells and chylomicrons contained within it. Continuous basement membrane is applied closely to basal plasma membrane of absorptive cells (magnification ×27 600). BM, basement membrane. Used with permission from American Physiological Society.
Figure 2. This electron micrograph demonstrates a break at basement membrane (arrows) and chylomicrons can be seen moving from intercellular space to lamina propria (magnification ×27 600).
IS, intercellular space; LP, lamina propria. Used with permission from American Physiological Society.
Lymphatic release of histamine and diamine oxidase during fat absorption
We recently demonstrated that the release of histamine into intestinal lymph increased dramatically after intra-duodenal infusion of fat emulsion liposyn II (20%) [21]. Our data suggest that the increased histamine release during fat absorption may contribute to the increased lymph flow and vascular permeability. This increase in the hydration (expansion) of the interstitial matrix in the lamina propria probably facilitates the movement of the chylomicron particles to the central lacteals [19]. Therefore, the release of histamine during fat absorption may facilitate the diffusive movement of chylomicron particles through the lamina propria.
Excessive histamine is, however, detrimental and can lead to a number of pathophysiological conditions such as anaphylaxis, food allergy, and IBD [22,23]. Enzymatic inactivation of circulating histamine is mediated by the enzyme diamine oxidase (EC 1.4.3.6, DAO) [24]. DAO is found in high concentrations in the small intestine, kidney and placenta of humans and other mammals [25]. Intestinal DAO is synthesized continuously by mature enterocytes and stored in plasma membrane-associated vesicular structures in the mucosal epithelial cells. DAO is secreted into the circulation upon stimulation and acts as a rate-limiting enzyme in the terminal catabolism of histamine and polyamines in vivo [24–26]. In the gut, DAO is considered to be a physiological barrier against luminal histamine, putrescine and cadaverine, which originate from ingested food and intestinal bacteria.
Studies in our laboratory have shown that DAO secretion into intestinal lymph significantly increases during active fat absorption [21,27]. Lymphatic histamine and DAO both peak at the same time during fat absorption, suggesting a close relationship between the two. It is tempting to speculate that DAO is released during fat absorption to safeguard against the deleterious effect of the excessive histamine secretion during fat absorption. The increased release of histamine and DAO is specific to fat feeding and is not shared by carbohydrate or protein feeding [27].
Activation of mucosal mast cells and release of mast cell mediators during fat absorption
Release of histamine is considered a hallmark of mast cell activation [28]. Is the increase in lymph histamine during fat absorption originating from the mucosal mast cells? Utilizing the rat mucosal mast cell protease II (RMCPII), a specific marker of mucosal mast cell (MMC), we showed that there was a dramatic (~20-fold) increase in lymphatic concentration during active fat absorption (unpublished data).
Mucosal mast cells are resident cells of hematopoietic lineage which are derived from CD13+CD34+KIT (CD117) bone marrow progenitors [28]. As part of the intestinal immune system, large numbers of MMCs (2–3% of lamina propria cells) are present mainly in the lamina propria [28], located beneath the basement membrane. MMCs not only regulate epithelial function but also crosstalk with the other mucosal immune cells through the release of a wide range of mediators [29]. In addition, there is a close bidirectional connection between MMCs and enteric nerves that is of vital importance in the regulation of gastrointestinal functions [30]. Activated mast cells release a wide variety of bioactive mediators including preformed mediators stored in granules (e.g. histamine and proteases) as well as newly synthesized mediators (e.g. prostaglandins, leukotrienes and cytokines) [31]. The physiological significance of the activated MMCs during fat absorption is unknown. However, the MMC activation will likely have an impact on fat absorption through the release of the proinflammatory mediators which in turn could stimulate the nerves and/or other intestinal immune cells. MMCs have been shown to affect intestinal motility, increase intestinal hyperpermeability and serve as an important element in several physiological and immunological functions of the gastrointestinal tract [29,31,32].
Activation or recruitment of other inflammatory cells during fat absorption
Although the main role of epithelial enterocytes is absorption of nutrients, it has been shown that they serve the critical function of epithelial barrier by interacting with luminal antigens, commensal microbiota, and crosstalking with mucosal immune cells through cytokines (such as IL-6, TNF-α) or chemokines [1••,3••,29]. In addition, the other types of cells located in the gut epithelial layer, including M (microfold) cells, enteroendocrine cells, intraepithelial lymphocytes, and Paneth cells can sample the intestinal contents and provide information about microbiota or luminal nutrients to the intestinal immune cells localized in the inductive sites (Peyer’s patches, mesenteric lymph nodes, lymphoid follicles, and colonic patches) and the effector sites in which dendritic cells, T lymphocytes, B lymphocytes, macrophage, and MMCs are located [1••,2]. The function of these immune cells in the regulation of intestine immunity has been ably reviewed (please see the related reviews on dendritic cells [33], T lymphocytes [34], T-regulatory cells [35], and macrophage [36]). The activation of enterocytes and intestinal immune cells including lymphocytes, macrophages, dendritic cells and intraepithelial lymphocytes during fat absorption has been reported [7–10,37]. Considering the important role of MMCs in the enteric neuro-endocrine-immune network [30,38] and the significant role they play in the development of IBD [23,32], it is reasonable to emphasize the importance of targeting mast cells in the treatment of gastrointestinal disorders [39].
Regulation of immune function by different types of fatty acids
Studies have shown that it is long-chain fatty acids (LCFAs) (C14 : 0–C24 : 0), rather than medium-chain fatty acids (MCFAs) (C6 : 0–C12 : 0), that activate intestinal lymphocytes, intraepithelial lymphocytes, dendritic cells and intestinal epithelial cells to secrete inflammatory cytokines [7–10,37]. In our studies, we also found that active absorption of the long-chain triglyceride (trilinolein, C18 : 2) but not the medium-chain triglyceride (trycaprylin, C8) activated the MMCs (unpublished data). These findings clearly support the notion that LCFAs act differently from MCFAs on the intestinal immune cells and the inflammatory responses. How does the gut sense different types of fatty acid and respond differently? Over the past few years, several G-protein-coupled receptors (GPRs), a large and diverse family of proteins, located in the gastrointestinal tract have been reported to respond to nutrients and their breakdown products, that is fatty acids, sugars, amino acids and proteolytic products (please see reviews [40,41]). For instance, luminal contents activate GPRs in the enteroendocrine cell membranes, transduce extracellular stimuli into intracellular signals, leading to increased intracellular Ca2+ levels and the release of peptide hormones [41]. Of these receptors, a variety of long-chain free fatty acids (FFAs) have been identified as ligands for the FFA1 (previously termed GPR40, GRP84 and GRP120 receptors, whereas short-chain FFAs activate FFA2 and FFA3 (previously termed GPR43 and GPR41, respectively) [40]. It is not completely understood how the GPRs sense different types of fatty acids and either activate or suppress the intestinal immune reaction during fat absorption. However, nuclear factors such as nuclear factor kappa B (NF-κB) or peroxisome proliferator-activated receptor gamma (PPAR-γ) (please see the related reviews [42,43]) have been shown to either activate or inhibit intestinal inflammatory reaction and we need to better understand how they are involved in the interplay between absorption of nutrients and the gut epithelial and immune cells in health and disease. A recent exciting study has shown that GPR 120 is a functional n-3 fatty acid receptor/sensor and mediates potent anti-inflammatory and insulin-sensitizing effects in vivo by suppressing macrophage-induced tissue inflammation [44].
Significance of the nutrient-induced intestinal inflammation
The observations of the activation of intestinal immune cells by LCFAs raise important questions, why the intestinal immune cells are activated during the normal process of fat absorption? Do they play a role in fat absorption or whether their activation is merely corollary to this process?
Mucosal mast cell activation and gut permeability
As mentioned earlier, we believe that in order for the chylomicron particles to pass through the basement membrane and gain access to the central lacteals it probably involves the breakage of the basement membrane (Fig. 2) [19]. This notion is further supported by a previous study demonstrating that during fat absorption, the jejunal epithelium is temporarily injured and the ‘injury’ is rapidly repaired (by epithelial restitution) within 50 min after the termination of lipid infusion [6]. There is considerable evidence showing that intestinal transcellular, and/or paracellular permeability are affected by mast cell mediators including interferon-γ, TNF-α, IL-1β, IL-4 and IL-13, tryptase via protease activation receptor-2 [45••]. In-vitro studies also showed that RMCPII directly increases epithelial permeability by decreasing the expression of the tight junction associated protein occludin and ZO-1 [46]. In addition, RMCPII can selectively attack type IV collagen, which is found in intestinal basement membranes [47]. Therefore, it is tempting to speculate that the role of the transient activation of the MMCs might be related to the perforation of the basement membrane coupled with the loosening of the junctional complex as a result of the accumulation of a large number of chylomicron particles during the normal process of fat absorption. Further experiments are needed to understand the role of MMC activation on the transport of chylomicrons and fat absorption.
Fat absorption, mucosal mast cell activation and inflammatory bowel disease
Diet, in particular dietary fat is thought to have an important role in the pathogenesis and treatment of IBD (Crohn’s disease and ulcerative colitis) [11,12,48]. As a result of a breakdown of the balance between absorbing essential nutrients and preventing the entry as well as responding to harmful luminal contents, the intestinal barrier is disrupted and leads to permeability defects in IBD [20••,49]. Although the pathogenesis is only partially understood, increased permeability via both the transcellular and paracellular routes, and increased apoptosis of epithelial cells are major characteristics of IBD [20••,49]. On the basis of our previous findings [6,19], if the acute consumption of a lipid meal can result in the temporary ‘injury’ of the intestinal epithelium, what would happen if the gastrointestinal tract is exposed chronically to a high-fat diet? It is entirely possible that the chronic consumption of a high-fat diet can compromise the integrity of the intestinal barrier by disrupting the balance of epithelial injury and restitution [50], due to inappropriate and exaggerated mucosal immune responses that can potentially result in increased entry of pathogens or microorganisms into the body from the intestinal lumen. The integrity of the tight junction is compromised by the chronic consumption of a high-fat diet [51,52]. Therefore, pathological conditions of the gut such as IBD may develop. The fact that the proinflammatory factors such as histamine, TNF-α and interleukins (e.g. IL-8, IL-13) that are found to be produced in excessive amounts in IBD [49] are also produced during active fat absorption [8,9] raises the possibility that chronic ingestion of a high-fat diet may initiate the release of these proinflammatory factors by mast cells as well as by other immune cells. Thus, it is not surprising to find numerous reports linking chronic consumption of high fat and the increased incidence of IBD [11,12,48,49]. The importance of disrupted intestinal barrier in IBD [20••] and the role of mast cells and their major secretory products in IBD have been reviewed [23,32].
Mucosal mast cell activation by fat absorption and metabolic syndrome
Is the small intestine an important contributor of circulating inflammatory mediators? Could the gut-derived inflammatory factors contribute to the pathogenesis of metabolic syndrome (obesity, type II diabetes, insulin resistance) that is associated with chronic low-grade inflammation, characterized by the increased circulating levels of proinflammatory cytokines [53–55]? Our recent finding that circulating RMCPII is increased during lipid infusion (unpublished data) provides evidence that activation of MMC and the release of MMC mediators by fat absorption can definitely contribute to the circulating inflammatory mediators. What about the other MMC mediators like TNF-α which is mainly from the gut or IL-6, an important proinflammatory factor found in metabolic syndrome [53–55]? A further study of the circulating proinflammatory factors including cytokines and chemokines that originate from the gut may provide new insight to our understanding of the physiological origin and significance of the systemic low-grade inflammatory syndrome. In recent years, much attention has concentrated on the role of macrophages in adipose tissues and the release of adipokines in the pathogenesis of metabolic syndrome [55,56]. Considering the fact that dietary fat is first exposed to the intestine and has the potential to induce inflammatory reaction of the gastrointestinal tract [7–10]. This chain of events not only occurs daily, but is also influenced by the chronic feeding of a high-fat diet and also the presence of microbiota in the gastrointestinal tract [57] and therefore may represent a major source of the circulating proinflammatory factors. This notion is further supported by the recent finding that high-fat diet promotes intestinal inflammation [58], which precedes and correlates with obesity and insulin resistance in mice [57]. Therefore, we propose that the intestinal MMC (activated by fat absorption) may also be an important source of the circulating proinflammatory mediators. This notion is further supported by the recent and very exciting finding that genetic deficiency as well as pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice [59].
Conclusion
In conclusion, fat absorption involves not only the participation of a single layer of epithelial cells, but also the participation of intestinal immune cells as well. In addition, the impact of fat absorption may not only be restricted to the gut, but may also have profound impact on the systemic circulating proinflammatory mediators and the associated metabolic consequences. To better understand if the inflammatory reaction is required for the normal process of fat absorption, how the gut senses different types of fatty acids and induces proinflammatory or anti-inflammatory reactions, and how gastrointestinal homeostasis is maintained to allow the efficient absorption and transport lipids with minimal activation of the mast cells and other inflammatory cells are all important questions that need to be examined. A better understanding of the processes involved may offer new therapeutic approaches for the treatment of IBD and diet-induced metabolic syndrome.
Key points.
Fat absorption and intestinal inflammatory reaction.
Activation of intestine mucosal mast cells and release of mast cell mediators by long-chain fatty acids.
The role of intestinal inflammatory reaction in chylomicron transport.
Dietary fat and inflammatory bowel disease and diet-induced metabolic syndrome.
Acknowledgments
Funding received for this work is from National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-56863 (P. Tso), DK-59360 (P. Tso).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 413).
- 1••.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. This is an outstanding review about how the intestinal homeostasis is maintained by the intestine immune system to allow the host and gut microbiota to coexist and disease states that when this balance fails. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. Review. [DOI] [PubMed] [Google Scholar]
- 3••.Chorny A, Puga I, Cerutti A. Innate signaling networks in mucosal IgA class switching. Adv Immunol. 2010;107:31–69. doi: 10.1016/B978-0-12-381300-8.00002-2. This is an excellent review discussing recent advances on the mechanisms by which intestinal bacteria, epithelial cells, dendritic cells, and macrophages crosstalk with intestinal T cells and B cells to induce immunoglobulin A class switching and production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kvietys PR, Specian RD, Grisham MB, Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol. 1991;261:G384–G391. doi: 10.1152/ajpgi.1991.261.3.G384. [DOI] [PubMed] [Google Scholar]
- 7.Miura S, TsuzukiI Y, Hokari R, Issii H. Modulation of intestinal immune system by dietary fat intake: relevance to Crohn’s disease. J Gastroenterol Hepatol. 1998;13:1183–1190. [PubMed] [Google Scholar]
- 8.Fujiyama Y, Hokari R, Miura S, et al. Butter feeding enhances TNF-alpha production from macrophages and lymphocyte adherence in murine small intestinal microvessels. J Gastroenterol Hepatol. 2007;22:1838–1845. doi: 10.1111/j.1440-1746.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- 9.Hara Y, Miura S, Komoto S, et al. Exposure to fatty acids modulates interferon production by intraepithelial lymphocytes. Immunol Lett. 2003;86:139–148. doi: 10.1016/s0165-2478(03)00007-5. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida H, Miura S, Kishikawa H, et al. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr. 2001;131:2943–2950. doi: 10.1093/jn/131.11.2943. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52:885–897. doi: 10.1002/mnfr.200700289. Review. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Nakahigashi M, Saniabadi AR. Review article: diet and inflammatory bowel disease: epidemiology and treatment. Aliment Pharmacol Ther. 2009;30:99–112. doi: 10.1111/j.1365-2036.2009.04035.x. [DOI] [PubMed] [Google Scholar]
- 13.Das UN. Obesity: genes, brain, gut, and environment. Nutrition. 2010;26:459–473. doi: 10.1016/j.nut.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008;24:320–327. doi: 10.1097/MOG.0b013e3282fbccf2. Review. [DOI] [PubMed] [Google Scholar]
- 15.Ohman MK, Wright AP, Wickenheiser KJ, et al. Visceral adipose tissue and atherosclerosis. Curr Vasc Pharmacol. 2009;7:169–179. doi: 10.2174/157016109787455680. [DOI] [PubMed] [Google Scholar]
- 16.Eduardo J. The role of macronutrients in gastrointestinal blood flow. Curr Opin Clin Nutr Metab Care. 2005;8:552–556. doi: 10.1097/01.mco.0000170755.32996.1d. [DOI] [PubMed] [Google Scholar]
- 17.Lu WJ, Yang Q, Sun W, et al. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol. 2008;294:G1130–G1138. doi: 10.1152/ajpgi.00400.2007. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Li X, Ji Y, et al. Effect of ezetimibe on incretin secretion in response to the intestinal absorption of a mixed meal. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1003–G1011. doi: 10.1152/ajpgi.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–G726. doi: 10.1152/ajpgi.1986.250.6.G715. [DOI] [PubMed] [Google Scholar]
- 20••.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. This is an outstanding review discussing recent understanding of the intestinal permeability and the underlying mechanism of the IBD. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Li XM, Yang Q, et al. Intestinal fat absorption is associated with the release of histamine and its degrading enzyme diamine oxidase. Gastroenterol. 2009;136 (Suppl 1):A729. [Google Scholar]
- 22.Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 23.He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309–318. doi: 10.3748/wjg.v10.i3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones BL, Kearns GL. Histamine: new thoughts about a familiar mediator. Clin Pharmacol Ther. 2011;89:189–197. doi: 10.1038/clpt.2010.256. [DOI] [PubMed] [Google Scholar]
- 25.Kitanaka J, Kitanaka N, Tsujimura T, et al. Expression of diamine oxidase (histaminase) in guinea-pig tissues. Eur J Pharmacol. 2002;437:179–185. doi: 10.1016/s0014-2999(02)01302-x. [DOI] [PubMed] [Google Scholar]
- 26.Wolvekamp MC, de Bruin RW. Diamine oxidase: an overview of historical, biochemical and functional aspects. Dig Dis. 1994;12:2–14. doi: 10.1159/000171432. [DOI] [PubMed] [Google Scholar]
- 27.Wollin A, Wang XL, Tso P. Nutrients regulate diamine oxidase release from intestinal mucosa. Am J Physiol. 1998;275:R969–R975. doi: 10.1152/ajpregu.1998.275.4.R969. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol. 2009;31:185–205. doi: 10.1007/s00281-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, Sharma A. Mast cells: emerging sentinel innate immune cells with diverse role in immunity. Mol Immunol. 2010;48:14–25. doi: 10.1016/j.molimm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Stead RH, Colley EC, Wang B, et al. Vagal influences over mast cells. Auton Neurosci. 2006;125:53–61. doi: 10.1016/j.autneu.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Söderholm JD. Mast cells and mastocytosis. Dig Dis. 2009;27 (Suppl 1):129–136. doi: 10.1159/000268133. [DOI] [PubMed] [Google Scholar]
- 32.Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J Gastroenterol. 2007;13:3027–3030. doi: 10.3748/wjg.v13.i22.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang SY, Kweon MN. Langerin-expressing dendritic cells in gut-associated lymphoid tissues. Immunol Rev. 2010;234:233–246. doi: 10.1111/j.0105-2896.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 34.van Wijk F, Cheroutre H. Intestinal T cells: facing the mucosal immune dilemma with synergy and diversity. Semin Immunol. 2009;21:130–138. doi: 10.1016/j.smim.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Platt AM, Mowat AM. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett. 2008;119:22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Tsuzuki Y, Miyazaki J, Matsuzaki K, et al. Differential modulation in the functions of intestinal dendritic cells by long- and medium-chain fatty acids. J Gastroenterol. 2006;41:209–216. doi: 10.1007/s00535-005-1747-0. [DOI] [PubMed] [Google Scholar]
- 38.Van Nassauw L, Adriaensen D, Timmermans JP. The bidirectional communication between neurons and mast cells within the gastrointestinal tract. Auton Neurosci. 2007;133:91–103. doi: 10.1016/j.autneu.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Santos J, Alonso C, Guilarte M, et al. Targeting mast cells in the treatment of functional gastrointestinal disorders. Curr Opin Pharmacol. 2006;6:541–546. doi: 10.1016/j.coph.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Geraedts MC, Troost FJ, Saris WH. Gastrointestinal targets to modulate satiety and food intake. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 41.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 43.Marion-Letellier R, Déchelotte P, Iacucci M, Ghosh S. Dietary modulation of peroxisome proliferator-activated receptor gamma. Gut. 2009;58:586–593. doi: 10.1136/gut.2008.162859. [DOI] [PubMed] [Google Scholar]
- 44.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. This is an excellent review discussing recent understanding of the intestinal permeability and its regulation by neuroimmune factors. [DOI] [PubMed] [Google Scholar]
- 46.Scudamore CL, Jepson MA, Hirst BH, Miller HR. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occluding. Eur J Cell Biol. 1998;75:321–330. doi: 10.1016/s0171-9335(98)80065-4. [DOI] [PubMed] [Google Scholar]
- 47.Patrick MK, Dunn IJ, Buret A, et al. Mast cell protease release and mucosal ultrastructure during intestinal anaphylaxis in the rat. Gastroenterology. 1988;94:1–9. doi: 10.1016/0016-5085(88)90603-8. [DOI] [PubMed] [Google Scholar]
- 48.Bassaganya-Riera J, Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2010;13:569–573. doi: 10.1097/MCO.0b013e32833b648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rakoff-Nahoum S, Bousvaros A. Innate and adaptive immune connections in inflammatory bowel diseases. Curr Opin Gastroenterol. 2010;26:572–577. doi: 10.1097/MOG.0b013e32833f126d. [DOI] [PubMed] [Google Scholar]
- 50.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de La Serre CB, Ellis CL, Lee J, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab (Lond) 2010;7:19. doi: 10.1186/1743-7075-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 54.Pfützner A, Weber MM, Forst T. A biomarker concept for assessment of insulin resistance, beta-cell function and chronic systemic inflammation in type 2 diabetes mellitus. Clin Lab. 2008;54:485–490. Review. [PubMed] [Google Scholar]
- 55.Sell H, Eckel J. Adipose tissue inflammation: novel insight into the role of macrophages and lymphocytes. Curr Opin Clin Nutr Metab Care. 2010;13:366–370. doi: 10.1097/MCO.0b013e32833aab7f. [DOI] [PubMed] [Google Scholar]
- 56.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding S, Chi MM, Scull BP, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]