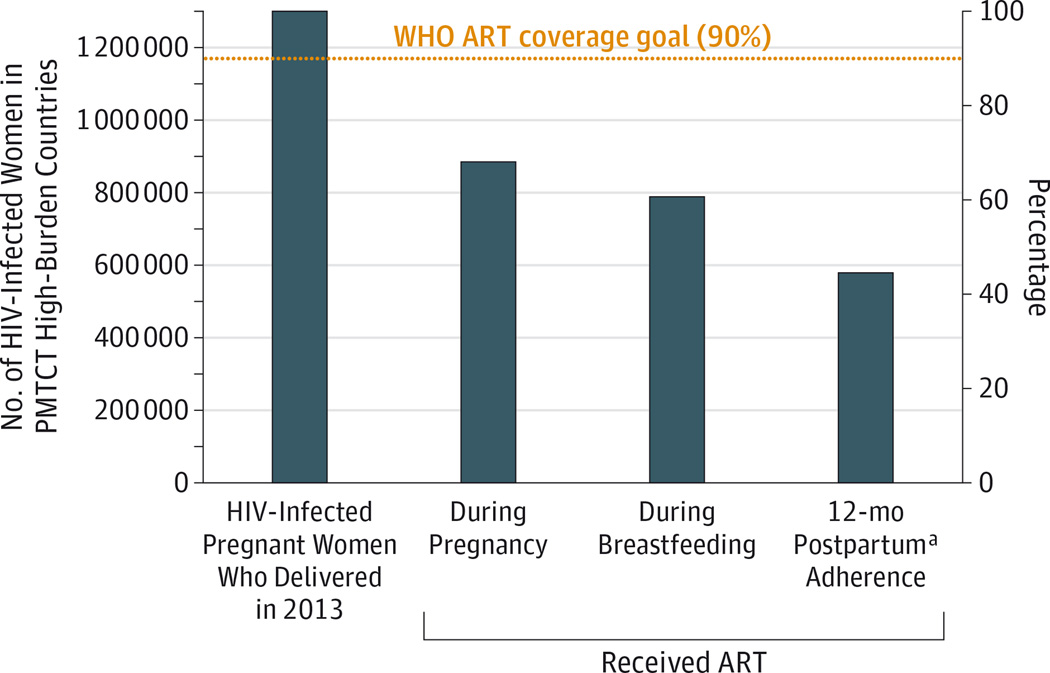
Despite the success of maternal antiretroviral therapy (ART) to prevent mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1), more than 200 000children continue to acquireHIV-1 annually. Infants account for roughly 10% of new HIV-1 infections globally despite making up less than 2%of the world’s population. Moreover, the decline in infant HIV-1 acquisition with implementation of wide-scale maternal-infant ART prophylaxis has not been as steep as predicted, and efforts will not meet the World Health Organization goal to eliminate mother-to-child transmission by 2015. This slow decline in infant HIV-1 infections is partly due to suboptimal coverage at each step of the ART “prevention cascade” (Figure). Yet even if the goal of 90% ART coverage can be achieved, it is estimated that 138 000 infants will still become infected withHIV-1 annually1 because of several situations in which maternal ART prophylaxis is ineffective, including maternal late presentation for care, nonadherence, and acute maternal infection during breastfeeding. In low- and middle-income countries, only half of pregnant women attend therecommended4prenatal visits. Late initiation of ART results in high rates of mother-to-child transmission, with one South African study reporting a tripling of transmission rates in pregnant women who received less than 4 weeks of ART prior to delivery compared with women who received 16 to 32 weeks of treatment.2 Furthermore, a high number of women disengage from care after childbirth (Figure), leaving infants vulnerable to HIV-1 infection. Also of great concern is the increasing incidence ofHIV-1 infection in young women; an estimated 620 000 women aged 15 to 49 years from areas of high HIV-1 prevalence acquired HIV-1 in 2013. Acute HIV-1 infection among mothers accounts for up to 34%of mother-to-child transmission3 and will not be curbed by maternal ART-based strategies. Because of these established gaps in the prevention of mother-to-child transmission, there is a critical need to develop safe, feasible supplemental strategies to be used with the current ART-based strategies, such as a universal infant HIV-1 vaccine.
Figure. Maternal ART Coverage Goal and Currently Achieved ART Coverage in PMTCT High-Burden Countries.
ART indicates antiretroviral therapy; HIV, human immunodeficiency virus; PMTCT, perinatal mother-to-child transmission; WHO, World Health Organization. Because of issues of access and adherence, the maternal ART coverage goal to achieve less than 5%PMTCT (dotted line) well exceeds the currently achieved ART coverage. With this cascade of maternal ART coverage for prevention of PMTCT, 16%of HIV-1–infected women from PMTCT high-burden countries continue to transmit HIV-1 to their infants. Data sources are the UNAIDS 2014 Progress Report on the Global Plan Towards the Elimination of New HIV Infection Among Children by 2015 and Keeping Their Mothers Alive and the WHO Global Update on the Health Sector Response to HIV, 2014.
a Postpartum adherence was estimated using Malawi national PMTCT data for the fourth quarter of 2013, as reported in the Global Update on the Health Sector Response to HIV, 2014.
HIV Vaccination and Sexual HIV-1 Transmission
Despite considerable resources and several phase3clinical trials, an effective vaccine to prevent adult HIV-1 acquisition remains elusive. Investigation of broad and potent virus neutralization in infected individuals has revealed that high levels of B-cell affinity maturation are required to produce antibodies that could neutralize most variants of this highly mutagenic virus. Although these highly somatically mutated, broadly neutralizing antibodies have yet to be induced via vaccination, the adult RV144 pox virus prime and recombinant envelope protein vaccine, which elicited only low-level, non–broadly neutralizing responses, demonstrated some efficacy against HIV-1 acquisition. This vaccine was 31% effective in a heterosexual adult population 3 years after vaccination,4 suggesting that partial protection can be achieved with a vaccine that elicits non–neutralizing antibody response. Infact, IgG responses against the envelope variable loops 1 and 2were associated with a reduced risk of HIV acquisition,5 whereas envelope-specific IgA responses were associated with increased risk of acquisition in vaccinees. Thus, immunologic benchmarks for non–broadly neutralizing HIV-1 vaccine candidates were identified. Importantly, RV144 vaccine efficacy was higher in the first year after vaccination (60%),4 suggesting that the vaccine could be more effective for limited periods of exposure such as during breastfeeding.
Reevaluation of Infant HIV-1 Vaccine Trials
Although HIV-1 vaccines similar to the RV144 vaccine were safe and immunogenic in infant phase 1 trials,6,7 no infant vaccine efficacy trial has progressed because of both limited vaccine candidates and lack of resources focused on pediatric HIV-1 vaccines. However, the efficacy afforded to adults by the RV144 vaccine transformed the outlook for pox prime protein boost HIV-1 vaccine regimens. A recent reanalysis of the antibody responses in HIV-1–vaccinated infants revealed that infants developed robust and durable responses that were similar to or of higher magnitude than in adults who received the moderately effectiveRV144vaccine.8 Moreover, vaccine-elicited IgA responses, which were associated with increased risk of HIV-1 acquisition in adult RV144 vaccinees, were only rarely detected and at very low levels in infants. These studies demonstrated that infant vaccination can elicit high-level and durable antibody responses that have been associated with protection in vaccinated adults, suggesting that new infant HIV vaccine trials be considered.
Potential Benefits and Risks of Infant HIV-1 Vaccines
As for any other new vaccine developed for use in infants, an infant HIV-1 vaccine must be exquisitely safe. Fortunately, in several phase 1 trials, no adverse events have been observed with the current pox vector and protein HIV-1 vaccine candidates, although the number of infants assessed is small.6,7,9,10 The vaccine candidates carry no risk of HIV-1 infection because they are either protein only or contain only a single viral gene. The potential benefit is substantial, eliminating infant HIV-1 acquisition and achieving a generation free of HIV-1 infection. In fact, it is plausible that protection against breast milk HIV-1 transmission will be easier to achieve than that against sexual HIV-1 transmission because of the low-level virus exposure and low efficiency of oral virus transmission. Moreover, vaccination of infants against HIV-1 may have secondary benefits beyond reducing pediatric HIV-1 acquisition. Initiating HIV-1 vaccination in infancy may allow long-term affinity maturation of vaccine-elicited antibody responses over more than a decade, which could then be boosted in adolescence prior to sexual debut. Furthermore, implementation of a multidose HIV-1 vaccine is more likely to be successful in infancy than in adolescence, as demonstrated by the poor uptake of the human papilloma virus vaccine in adolescents vs the highly successful implementation of the multiple-dose hepatitis B vaccine in infants. Therefore, initiation of a multidose HIV-1 vaccine in infancy is likely to be beneficial for both achieving highly mature, broad antibody responses and high vaccine coverage.
Pursuit of Infant HIV-1 Vaccine Clinical Trials in Parallel to Those in Adults
Interventions for children often lag behind those in adults for many reasons, including concerns about initiating studies of novel products in children. Fortunately, these concerns did not impede the development of highly successful vaccines for important pediatric diseases, such as the oral rotavirus vaccine and conjugated vaccines for bacterial infections. Because nearly a quarter million infants become infected with HIV-1 annually, HIV-1 is an important global pediatric pathogen for which the development of additional effective prophylactic measures should not be delayed. Development of infant HIV-1 vaccine strategies should be pursued in parallel to those in adults, rather than waiting for efficacy data in adults, which will delay progress in the quest to eliminate pediatric and potentially other modes of HIV-1 transmission.
Acknowledgments
Funding/Support: Support for this article was provided by the Duke University Center for AIDS Research (grant 5P30 AI064518), the Duke CTSA KL2 career development award (KL2TR001115), and the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (grants UM1AI068632, UM1AI068616, and UM1AI106716), all from the National Institutes of Health.
Role of the Funders/Sponsors: The funders had no role in the preparation, review, or approval of the manuscript or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Contributor Information
Genevieve G. Fouda, Department of Pediatrics, Duke University Medical Center, Durham, North Carolina; and Human Vaccine Institute, Duke University Medical Center, Durham, North Carolina.
Coleen K. Cunningham, Department of Pediatrics, Duke University Medical Center, Durham, North Carolina.
Sallie R. Permar, Department of Pediatrics, Duke University Medical Center, Durham, North Carolina; and Human Vaccine Institute, Duke University Medical Center, Durham, North Carolina.
REFERENCES
- 1.Mahy M, Stover J, Kiragu K, et al. What will it take to achieve virtual elimination of mother-to-child transmission of HIV? an assessment of current progress and future needs. Sex Transm Infect. 2010;86(suppl 2):ii48–ii55. doi: 10.1136/sti.2010.045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman RM, Black V, Technau K, et al. Effects of highly active antiretroviral therapy duration and regimen on risk for mother-to-child transmission of HIV in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2010;54(1):35–41. doi: 10.1097/QAI.0b013e3181cf9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2012;59(4):417–425. doi: 10.1097/QAI.0b013e3182432f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 5.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland EJ, Johnson DC, Muresan P, et al. HIV-1 vaccine induced immune responses in newborns of HIV-1 infected mothers. AIDS. 2006;20(11):1481–1489. doi: 10.1097/01.aids.0000237363.33994.45. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham CK, Wara DW, Kang M, et al. Pediatric AIDS Clinical Trials Group 230 Collaborators. Safety of 2 recombinant human immunodeficiency virus type 1 (HIV-1) envelope vaccines in neonates born to HIV-1-infected women. Clin Infect Dis. 2001;32(5):801–807. doi: 10.1086/319215. [DOI] [PubMed] [Google Scholar]
- 8.Fouda GG, Cunningham CK, McFarland EJ, et al. Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses. J Infect Dis. 2015;211(4):508–517. doi: 10.1093/infdis/jiu444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kintu K, Andrew P, Musoke P, et al. Feasibility and safety of ALVAC-HIV vCP1521 vaccine in HIV-exposed infants in Uganda: results from the first HIV vaccine trial in infants in Africa. J Acquir Immune Defic Syndr. 2013;63(1):1–8. doi: 10.1097/QAI.0b013e31827f1c2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Njuguna IN, Ambler G, Reilly M, et al. PedVacc 002: a phase I/II randomized clinical trial of MVA.HIVA vaccine administered to infants born to human immunodeficiency virus type 1-positive mothers in Nairobi. Vaccine. 2014;32(44):5801–5808. doi: 10.1016/j.vaccine.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]