Abstract
Developmental bisphenol A (BPA) exposure increases adulthood hepatic steatosis with reduced mitochondrial function. To investigate potential epigenetic mechanisms behind developmental BPA-induced hepatic steatosis, pregnant Sprague-Dawley rats were dosed with vehicle (oil) or BPA (100 μg/kg/day) from gestational day 6 until postnatal day (PND) 21. After weaning, offspring were either challenged with a high-fat (HF; 45% fat) or remained on a control (C) diet until PND110. From PND60 to 90, both BPA and HF diet increased the fat/lean ratio in males only, and the combination of BPA and HF diet appeared to cause the highest ratio. On PND110, Oil-HF, BPA-C, and BPA-HF males had higher hepatic lipid accumulation than Oil-C, with microvesicular steatosis being marked in the BPA-HF group. Furthermore, on PND1, BPA increased and modified hepatic triglycerides (TG) and free fatty acid (FFA) composition in males only. In PND1 males, BPA increased hepatic expression of FFA uptake gene Fat/Cd36, and decreased the expression of TG synthesis- and β-oxidation-related genes (Dgat, Agpat6, Cebpα, Cebpβ, Pck1, Acox1, Cpt1a, Cybb). BPA altered DNA methylation, histone marks (H3Ac, H4Ac, H3Me2K4, H3Me3K36), and decreased the binding of several transcription factors (Pol II, C/EBPβ, SREBP1) within the male Cpt1a gene, the key β-oxidation enzyme. In PND1 females, BPA only increased the expression of genes involved in FFA uptake and TG synthesis (Lpl, Fasn, and Dgat). These data suggest that developmental BPA exposure alters and reprograms hepatic β-oxidation capacity in males, potentially thorough the epigenetic regulation of genes, and further alters the response to a HF diet.
Keywords: Bisphenol A (BPA), adiposity, endocrine disrupting chemical (EDC), NAFLD, methylation, histones
INTRODUCTION
Various dietary, behavioral, and genetic factors contribute to the increased incidence of metabolic syndrome worldwide, and recent data suggest that environmental endocrine disrupting chemicals (EDCs) may also be important factors in the growing epidemic of obesity and metabolic disorders. Although a number of EDCs have been shown to disrupt normal reproductive outcomes (Unuvar and Buyukgebiz, 2012), more recently several EDCs, including the ubiquitous plasticizer bisphenol A (BPA), were shown to alter metabolic processes and have been associated with obesity and adulthood chronic diseases (Olsen et al., 2012; Trasande et al., 2012).
The Developmental Origins of Health and Disease Hypothesis suggests that exposure to in utero dietary or environmental stressors can program the adulthood phenotype, and this has been confirmed in humans and experimental animal models (Barker, 2004; Hanley et al., 2010; Tamashiro and Moran, 2010). In animals, maternal dietary manipulations during pregnancy have been shown to contribute to the development of non-alcoholic fatty liver disease (NAFLD) in offspring (Aagaard-Tillery et al., 2008; McCurdy et al., 2009), and recent studies suggest that prenatal low-dose BPA exposure can also modify markers of hepatic energy metabolism at weaning (Somm et al., 2009) and lead to steatosis in adult animals (Jiang et al., 2014). While the precise mechanisms behind these observations are not completely understood, in cell models, BPA’s effect on hepatocyte metabolism has been proposed to occur through endoplasmic reticulum stress (Asahi et al., 2010), the production of reactive oxygen species (Huc et al., 2012), and decreased fatty acid β-oxidation (Grasselli et al., 2013).
Carnitine palmitoyltransferase 1a (CPT1A) in the liver is the regulatory enzyme for the transport of long-chain fatty acids into the mitochondria for subsequent β-oxidation (Nakamura et al., 2014). In humans, CPT1A deficiency causes hepatomegaly, hepatic steatosis, and metabolic perturbations (Bennett and Santani, 1993), while in rats, Cpt1a expression was shown to be down-regulated during steatosis in male rats consuming a high-fat diet (Xie et al., 2010), while a moderate overexpression of Cpt1a in obese male rats increased hepatic fatty acid β-oxidation and decreased hepatic triglyceride accumulation (Stefanovic-Racic et al., 2008). Cpt1a expression has been shown to be regulated by the binding of transcription factors (including C/EBPβ and SREBP1 (Attia et al., 2011; Thakran et al., 2013) in cell culture, as well as with altered DNA methylation in humans (Frazier-Wood et al., 2014; Irvin et al., 2014). However, despite the critical regulatory role of this gene in hepatic energy metabolism and lipid accumulation, and the proposed role of BPA exposure in NAFLD development, no studies have specifically assessed the gene’s potential epigenetic regulation in response to developmental BPA exposure. Therefore, the present study first determined the effect of prenatal exposure to BPA, with or without a post-weaning HF dietary challenge, on adult liver pathophysiology. We then investigated whether the adulthood phenotypes observed were associated with neonatal changes in hepatic lipid metabolism and fatty acid homeostasis – at the biochemical, genetic, and epigenetic levels, with a special focus on Cpt1a. Furthermore, because BPA is known to affect males and females differently, and because NAFLD development has been shown to be sex-specific (Lazo et al., 2013), we also considered offspring’s sex in our analyses.
METHODS
Dosing of BPA and Animal Experimental Design during Gestation and Weaning
Timed-pregnant Sprague-Dawley rats (n=6/treatment group) were obtained from Charles River Laboratories (Wilmington, MA, USA) on gestational day 2, individually housed in ventilated cages in a temperature-controlled environment, and fed a modified AIN-93G diet and water ad libitum. Beginning on gestational day 6 until postnatal day (PND) 21, dams were randomized into two groups, and daily orally dosed using a 1 mL Eppendorf pipette with either vehicle control (tocopherol-stripped corn oil) or BPA (100 μg/kg/day BW). This dose has been shown to affect metabolism (including increased body weight and altered glucose homeostasis) and reproductive outcomes (including altered estrous cyclicity and hormone levels) in previous rodent models of perinatal exposure (Rubin et al., 2001; Liu et al., 2013). A relatively similar perinatal BPA exposure dose (50 μg/kg/day BW) led to steatosis in adult offspring (Jiang et al., 2014; Wei et al., 2014), and adult exposure to BPA at 50 and 500 μg/kg/day BW increased hepatic triglycerides in mice (Marmugi et al., 2012). Immediately after birth, litter sex distributions and offspring body weight were recorded, male and female pups (n=6, one of each sex from each dam) were removed for sampling, and the remainder of the pups were completely mixed within each treatment group, and 4 male and 4 female pups were randomly selected and placed back with each dam within the respective control or BPA-treated groups for cross-fostering. PND1 livers were snap-frozen in liquid nitrogen and stored at −80°C for all subsequent biochemical and molecular analyses.
Animal Experimental Design after Weaning Using a High-fat Dietary Challenge
On PND22, male and female pups were once again randomized into new cages (within treatment groups), with 2–3 male or female offspring per cage, and separated into two dietary groups: control (C: 64% carbohydrate, 20% protein, and 16% fat) or high-fat diet (HF: 35% carbohydrate, 20% protein, and 45% fat) (Table 1), which yielded 4 experimental post-weaning groups: Oil-C, Oil-HF, BPA-C, and BPA-HF. All animals were single-housed after the onset of puberty until the end of the study (PND110), when tissues were collected and either snap-frozen in liquid nitrogen and stored at −80°C or fixed in 10% formalin for histopathology analysis.
Table 1.
Diet Composition.*
|
|
Control (C)
|
High-Fat (HF)
|
||
|---|---|---|---|---|
| %
|
Gram
|
kcal
|
Gram
|
kcal
|
| Protein | 20 | 20 | 24 | 20 |
| Carbohydrate | 64 | 64 | 41 | 35 |
| Fat | 7 | 16 | 24 | 45 |
| Kcal/g | 4.0 | 4.8 | ||
|
| ||||
|
Ingredients
| ||||
| Casein | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn Starch | 397.5 | 1590 | 105 | 420 |
| Maltodextrin | 132 | 528 | 132 | 528 |
| Sucrose | 100 | 400 | 100 | 400 |
| Cellulose | 50 | 0 | 50 | 0 |
| Soybean Oil | 70 | 630 | 70 | 630 |
| Lard | 0 | 0 | 130 | 1170 |
| Mineral Mix | 35 | 0 | 35 | 0 |
| Vitamin Mix | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2.5 | 0 | 2.5 | 0 |
Research Diets, Inc, New Brunswick, NJ
Body Weight, Cumulative Energy Intake, and Body Composition throughout the Study
Body weight and food intake were measured every 4 days beginning on PND22. Energy intake after weaning was calculated by weighing pellets remaining on the fourth day, subtracting from initial food supplied, and multiplying daily calculated intake by the caloric composition of each diet (4.0 kcal/g in control and 4.8 kcal/g in HF). Body composition was measured on PND1, at weaning on PND21, after the onset of puberty on PND60, and on PND90 using the EchoMRI-700 Body Composition Analyzer (Echo Medical Systems, Houston, TX), which allows for measurement of fat and lean mass in conscious and unrestrained animals. On PND1 and PND21, male and female pups were scanned as a litter, and on PND60 and PND90 offspring were scanned individually.
Liver Histopathology and Staining in Adult Offspring
At the end of the study (PND110), liver samples from adult animals were fixed in 10% formalin, embedded in paraffin, and 3 μm histological slides were prepared and stained with hematoxylin and eosin. Formalin-fixed tissues were embedded in OCT, and slides were prepared with a cryostat and stained with Oil-Red-O. Histopathological evaluation was performed by a board-certified pathologist. Samples were first assigned a score for overall hepatic vacuolation severity, and then independently graded for either macro- or micro-vacuolation. Pathological findings were graded from 1 (minimal) to 5 (severe). For all analyses, the pathologist was blind to the treatment, and reported all information using a numerical code.
Hepatic Triglyceride (TG) Content, Free Fatty Acid (FFA) Content, and FFA Composition in PND1 Offspring
Hepatic lipids were extracted and purified from frozen livers from 6 male and 6 female PND1 pups from each maternal treatment group (oil or BPA) using the Bligh & Dyer method (Bligh and Dyer, 1959). A portion of the resulting chloroform layer was used for TG quantification. Briefly, this chloroform aliquot was dried under nitrogen and re-suspended in a 1:1:3 solution of TritonX100-Methanol-Tert-Butanol. This dilution of lipids was analyzed for TG content as previously reported (Miao et al., 2004).
The remainder of the chloroform phase was utilized for FFA gas chromatographic (GC) analysis. Fatty acid methyl esters (FAMEs) were prepared by derivatization of chloroform extracts in 3 N methanolic HCl (95°C, 40 min), using 17:0 (heptadecanoic acid) as the internal standard. Samples were dried under nitrogen, re-suspended in hexane, and resulting FAMEs were injected onto a 30 m × 0.32 mm DB-225 column with 0.25 μm film thickness, with helium as the carrier gas and using a 5890 series II gas chromatograph (Hewlett-Packard, Palo Alto, CA) equipped with a flame ionization detector. GC was programmed with starting temperature of 180°C (5 min), ramping at 6°C/min increments to final temperature 220°C (held 15 min). Peaksimple software (SRI Instruments, Torrance, CA) was used for peak integration and analysis. Total FFAs were calculated as the sum of all identified peaks using several commercially available FAME standards (PUFA-2, Cat# 47015-U; PUFA-3, Cat# 47085-U; and Low Erucic Rapeseed Oil, Cat# O7756, Sigma-Aldrich, St. Louis, MO). Long-chain FFAs were defined as those having tails with 14–21 carbons, and very long-chain FFAs as those having tails with 22–26 carbons.
Hepatic RNA Isolation and mRNA Expression Analysis in PND1 Offspring
To investigate the mRNA expression of hepatic lipid metabolism-associated genes, frozen livers from 6 male and 6 female PND1 pups from each maternal treatment group (oil or BPA; same as those used for FFA analysis) was ground in liquid nitrogen with mortar and pestle. Total RNA isolation, cDNA synthesis and real-time PCR were performed as previously described (Strakovsky and Pan, 2011). A serial dilution was used to create a standard curve for quantification and a dissociation curve was analyzed following each reaction. All primers for real-time PCR analysis (Table 2) were designed using the VectorNTIR® software (Life Technologies™, Grand Island, NY), analyzed using BLAST, and synthesized by IDT (Coralville, IA). All mRNA data were normalized to the housekeeping gene encoding ribosomal protein L7a (rpl7a), the expression of which did not differ by treatment group.
Table 2.
Primer Information
| Gene for mRNA Analysis | Forward Sequence and Transcript Location | Reverse Sequence and Transcript Location | Transcript ID |
|---|---|---|---|
| Acox1 | GCCTTTGTTGTCCCTATCCGT (+433) | ACCGATATCCCCGACAGTGAT (+504) | ENSRNOT00000042372 |
| Agpat6 | TCTGCCACTCAGGATTGCTC (+1449) | GCAGGTATCCAACCACGGTA (+1525) | ENSRNOT00000024392 |
| Ash1l | GGGGCGAAAACCAAAAACTGTC (+3404) | GCCGTTTGTTCAAGCACAGCAA (+3481) | ENSRNOT00000027629 |
| Cebpa | AGTCGGTGGATAAGAACAGCAACG (+821) | GCTGTTTGGCTTTATCTCGGCTC (+910) | ENSRNOT00000014517 |
| Cebpb | AGAACGAGCGGCTGCAGAAGA (+1220) | GAACAAGTTCCGCAGCGTGC (+1287) | ENSRNOT00000072673 |
| Cpt1a | GAGCGACTCTTCAATACTTCCC (+1027) | TGTGCCTGCTGTCCTTGATA (+1102) | ENSRNOT00000019652 |
| Cyba | GGA CTC CCA TTG AGC CTA (+120) | GTT GGT AGG TGG CTG CTT (+190) | ENSRNOT00000017564 |
| Cybb | GTG TGT CGG AAT CTC CTC (+211) | GGT TCC TGT CCA GTT GTC (+292) | ENSRNOT00000038994 |
| Dgat1 | TCAATCTGTGGTGCCGCCAG (+708) | CCCACTGACCTTCTTCCCTGCA (+775) | ENSRNOT00000039795 |
| Dnmt1 | TCCTACGCCATGCCCAGTTTG (+1713) | GAAGATGGGCGTCTCATCATC G (+1792) | ENSRNOT00000064932 |
| Dnmt3a | GCCCATTCGATCTGGTGATTG (+2255) | TCGTAAAGTCCCTTGCGGGC (+2333) | ENSRNOT00000047210 |
| Dnmt3b | AATGCGCTGGGTACAGTGGTTTG (+1197) | AACAGACCCAGAGCCACCAGCT (+1274) | ENSRNOT00000015482 |
| Fabp1 | GAGCCAAGAGAACTTTGAGCCCTT (+77F) | CCTTGATGTCCTTCCCTTTCTGGA (+156) | ENSRNOT00000008840 |
| Fasn | CTTTGTGAGCCTCACCGCCAT (+1747) | ATGCCATCAGGTTTCAGCCCC (+1811) | NM_017332.1 |
| Fat/Cd36 | AGTGCTCTCCCTTGATTCTGC (+151) | GAGCCCACAGTTCAGATCACA (+213) | ENSRNOT00000061687 |
| Hdac1 | GGAGGTGGCTATACCATC (+916) | AGGGATCTCTGTGTCCAG (+993) | ENSRNOT00000012854 |
| Hdac3 | CGTGGCGTATTTCTACGACCCC (+12) | GTTTCATCGGGTGTCCAGCTCC (+76) | ENSRNOT00000060417 |
| Kmt2b | CTAGAATCAGGTCAGGGTCGTGGTC (+961) | GGTCCCCTTTCCTGTTCATCTCC (+1049) | ENSRNOT00000046359 |
| Kmt2c | ACACACCGAACACCGTGAACATG (+1847) | CTGCTGCTGTTGCTGGAGAATGA (+1941) | ENSRNOT00000071915 |
| L7a | GAGGCCAAAAAGGTGGTCAATCC (+64) | CCTGCCCAATGCCGAAGTTCT (+127) | ENSRNOT00000006754 |
| Lpl | GCCACTTCAACCACAGCAGCAA (+376) | GGGCACCCAACTCTCATACATTCC (+455) | ENSRNOT00000016543 |
| Pck1 | AGGAGGAAGAAAGGTGGCACCAG (+90) | GGCAGAGAAGTCCAGACCATTATGC (+182) | ENSRNOT00000031586 |
| Cpt1a MSP Analysis | Forward Sequence and Transcript Location | Reverse Sequence and Transcript Location | Transcript ID |
|---|---|---|---|
| Methylated | AAGGTTCGCGGTTAAGGTTAC (−2815) | AACGTCCTACTTTACATCCGTC (−2736) | ENSRNOT00000019652 |
| Unmethylated | GGTTTGTGGTTAAGGTTATGG (−2813) | AACATCCTACTTTACATCCATC (−2736) | |
|
| |||
| Methylated | GTTGGTATTATTTTTGGGAGCG (−1148) | ATACCCTCTACTTCTCCAATACGC (−1061) | |
| Unmethylated | GTTGGTATTATTTTTGGGAGTGT (−1148) | AAATACCCTCTACTTCTCCAATACA (−1059) | |
|
| |||
| Methylated | TTAGAGATTGTTTAAGGTCGCGT (−557) | TCACTCAAACCGAATCACGTA (−484) | |
| Unmethylated | TGTTTAGAGATTGTTTAAGGTTGTG (−560) | TTCACTCAAACCAAATCACATA (−483) | |
|
| |||
| Methylated | TTTCGGGTTAATTTTCGTTC (−297) | CCTTAAACAACTATCTAAATACACG (−238) | |
| Unmethylated | ATTTTGGGTTAATTTTTGTTTGG (−298) | CCTTAAACAACTATCTAAATACACA (−238) | |
|
| |||
| Methylated | TTTTTAGTCGGTCGATTTCGA (−4) | CGAAACAAACACTATCCTCCG (+71) | |
| Unmethylated | TTTTTTAGTTGGTTGATTTTGA (−5) | AAAACAAACACTATCCTCCACA (+70) | |
|
| |||
| Methylated | AAATTTTACGATTCGAGTTCGA (+1478) | AATTAAATACACGCTATACCTCGTA (+1564) | |
| Unmethylated | TTAAATTTTATGATTTGAGTTTGA (1476) | TATTTATAATTAAATACACACTATACCTCA (+1571) | |
| Cpt1a ChIP Analysis | Forward Sequence and Transcript Location | Reverse Sequence and Transcript Location | Transcript ID |
|---|---|---|---|
| AAGCAGGACGCCAAGAAAAGAGG (−2747) | TCCCTAAAACCCCATCCTAACCC (−2681) | ENSRNOT00000019652 | |
| ACCACGGCTCTTGATGTC (−1050) | GAGCATCCTTCACTGGGT (−989) | ||
| ACCAGGCTAGATACAATCAGG (−401) | ATGCACGGTACAGAAAGGA (−345) | ||
| TACAATCAGGCTCAGGACTCTC (−321) | GAATGCACGGTACAGAAAGG (−254) | ||
| CAGCAAGCATACATCACCACC (−31) | GTCCTCTGCTTTAGGTCCTCACT (+43) | ||
| GCAAAGATACATCCTCAGCTCAGG (+1315) | TTTAAGCCTAGTAGGCGGGTCA (+1393) |
Genomic DNA Isolation and Methylation Analysis of Cpt1a Using Methylation Sensitive PCR (MSP) in PND1 Offspring
To determine whether BPA affected the methylation status of Cpt1a, the gene encoding the regulatory and rate-limiting step of hepatic β-oxidation, MSP was performed as previously reported (Strakovsky et al., 2014a) to assess the methylation status of six sites surrounding and spanning the Cpt1a promoter (approximately 3 kB upstream and 2 kB downstream of the transcription start site). MSP analysis provides a valid and reliable approach for comparing relative changes in DNA methylation between treatment groups within relatively targeted regions of the DNA. Briefly, frozen livers from 6 male and 6 female PND1 pups from each maternal treatment group (oil or BPA; same as those used for FFA and mRNA analyses) were ground in liquid nitrogen with mortar and pestle. Genomic DNA (gDNA) was isolated using the GenElute Mammalian Genomic DNA Purification Kit (Sigma-Aldrich) per manufacturer’s instruction with an additional initial spin-down to remove the lipid layer. gDNA (20 ng/μl in a 20 μL reaction volume) was bisulfite converted using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA), following the manufacturer’s instructions. Real-time PCR was performed using 20 ng gDNA as the template, SYBR Green PCR Master Mix (Quanta Biosciences, Gaithersburg, MD), and 5 μM of each forward and reverse primer (Table 2) designed using the methprimer website (http://www.urogene.org/methprimer/index.html) (Li and Dahiya, 2002) to specifically amplify either the methylated or unmethylated templates in the 7300 Real-Time PCR System (Applied Biosystem) as for mRNA analysis, but using 45 cycles. A serial dilution from a reaction combining one sample from each experimental group was used to create an internal standard curve for quantification. Data are presented as the ratio of methylated values divided by the sum of methylated and unmethylated values.
Hepatic Chromatin Immunoprecipitation (ChIP) Analysis of the Cpt1a gene in PND1 Offspring
To investigate whether BPA had an effect on histone marks and transcription factor binding within the Cpt1a gene, ChIP analysis was performed following a modified protocol (Chen et al., 2004) as previously described (Strakovsky et al., 2011), but using magnetic beads in the current study. ChIP was performed using frozen livers from 6 male and 6 female PND1 pups from each maternal treatment group (oil or BPA; same as those used for FFA, mRNA, and MSP analyses), and examined the regions approximately corresponding to those regions also assessed by MSP. Briefly, 100 mg of frozen liver samples were ground using a mortar and pestle with liquid nitrogen and washed with PBS. The samples were resuspended in PBS and cross-linked in 1% formaldehyde for 10 min at room temperature. After centrifugation, the pellets were resuspended in nuclei swelling buffer containing protease inhibitor and phosphorylation inhibitor. The nuclei were lysed in SDS buffer containing protease and phosphorylation inhibitors. The chromatin was sonicated (Fisher Scientific model 100 Sonic Dismembrator, Pittsburgh, PA) on ice with 5 bursts for 40 s at power setting 5 with 2 min cooling intervals between each burst. After removal of the cell debris by centrifugation at 13000 rpm in 4 °C for 10 min, sheared chromatin was diluted to 10 mL in ChIP Dilution Buffer. Then, 1 mL of the diluted lysate was incubated overnight on a hematology mixer (Model 346, Fisher Scientific) with 2 μg of each primary antibody at 4 °C (Table 3). Each antibody was first bound to Dynabeads® Protein G beads (Life Technologies) by incubating 1 μg of antibody with 450 μg beads (15 mL of the suspension provided by the manufacturer) at room temperature in PBS-T (PBS with 0.02% Tween 20) for 1 hr and washed to remove any unbound antibody, and 750 μL of sheared chromatin was added to the beads and incubated at room temperature for 2 h to form the chromatin-antibody-bead complex. Normal rabbit IgG was used as the negative control, and supernatant from the normal rabbit IgG was also saved as the input for normalization of the data. Pellets containing the immunoprecipitated complexes were washed sequentially with 1 mL of low salt solution, high salt solution, and LiCl solution, and twice with TE (pH 8.0). Antibody/protein/DNA complexes were eluted from the magnetic beads by adding twice 250 μL of elution buffer followed by shaking at 37°C for 15 min at 300 rpm and flash spinning at room temperature. The combined supernatants were incubated at 65°C for 5 hr with 20 μL 5 mol/L NaCl and 1 μg of RNase A (Qiagen, Hilden, Germany) to reverse the formaldehyde cross-linking and release the DNA fragments. Samples were then treated with proteinase K (Sigma) at 37°C for 1 hr to remove any protein. DNA was purified with a DNA miniprep system (Qiagen). 5 μL of immunoprecipitated DNA was used for the real-time PCR reaction (primers are listed in Table 2).
Table 3.
Antibody Information
| Antibody
|
Species
|
Source
|
Catalog Number
|
|---|---|---|---|
| IgG | Rabbit | Santa Cruz | sc-2027 |
| Pol II (CTD4H8) | Mouse | Millipore/Upstate | 05-623 |
| H3Ac | Rabbit | Millipore | 06-599 |
| H4Ac | Rabbit | Millipore/Upstate | 06-866 |
| H3K4Me2 | Rabbit | Millipore/Upstate | 07-030 |
| H3K9Me3 | Rabbit | Millipore/Upstate | 07-442 |
| H3K27Me3 | Rabbit | Millipore/Upstate | 07-449 |
| H3K36Me3 | Rabbit | Abcam | ab9050 |
| C/EBPβ | Rabbit | Santa Cruz | sc-150 |
| SREBP-1 | Rabbit | Santa Cruz | sc-366 |
We evaluated several histone modifications and the binding of hepatic lipid metabolism-related transcription factors (C/EBPβ and SREBP1) within regions approximately corresponding to regions of the DNA also evaluated for changes to the methylation status of Cpt1a. Specifically, we assessed the acetylation status of both histone H3 and histone H4 (H3Ac and H4Ac), the di-methylation of histone H3, lysine 4 (H3Me2K4), and the tri-methylation of histone H3, lysine 36 (H3Me3K27). We also evaluated the tri-methylation of histone H3, lysine 9 and 27 (H3Me3K9 and H3Me3K27), but did not observe significant binding (when compared to IgG; data not shown). The binding of RNA Polymerase II (CTD4H8; Pol II) was assessed as a marker of active gene transcription.
Statistical Analysis
Adulthood body weight, energy intake, and hepatic vacuolation were analyzed using two-way analysis of variance (ANOVA) with interactions to determine the overall effects of post-weaning diet, prenatal BPA exposure, and the interaction between the two main factors. When an interaction was present, a post-hoc Tukey test was used to further determine differences between groups in n=5 male or female animals per group. Daily energy intake was summed to show overall intake from PND69-97. The PND60-90 fat/lean mass ratio was analyzed using two-way repeated-measures ANOVA to determine the overall effects of post-weaning diet, prenatal BPA exposure, and the interaction between the two main factors. When an interaction was present, a post-hoc Tukey test was used to determine differences between groups. The fat/lean mass ratio at birth and weaning (n=5 litters), hepatic TG content, FFA content, and FFA distribution, as well as gene expression, MSP results, and ChIP results in PND1 livers (n=6 male or female pups) was analyzed using Student t-test in Excel. All other analyses were performed in SAS (Chicago, IL). Differences were considered significant at P<0.05.
RESULTS
Body Weight and Energy Intake in Adult Offspring and Body Composition throughout the Study
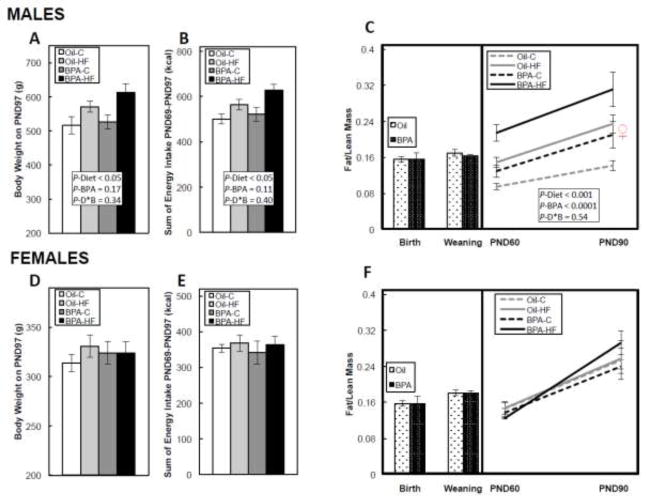
BPA did not significantly alter maternal weight gain, maternal body composition at delivery or at weaning, litter size, sex distribution, or birth weights in either male or female offspring (data not shown). In males, HF diet significantly (P<0.05) increased both body weight (Figure 1A), and cumulative energy intake (Figure 1B) from PND69-97, while BPA only trended to increase body weight (P=0.17) and energy intake (P=0.11). BPA had no effect on body composition (fat/lean mass ratio) at birth and weaning in males, but during the post-weaning to adulthood period, both HF diet (P<0.001) and BPA (P<0.0001) increased the fat/lean mass ratio. While there was no statistically significant interaction between the factors, the BPA-HF group appeared to have the highest fat/lean mass ratio when compared to all other groups (Figure 1C). In females, neither BPA nor a HF diet affected body weight (Figure 1D), cumulative energy intake (Figure 1E), or body composition throughout the study (Figure 1F).
FIGURE 1. Body weight on postnatal day (PND) 97 in male (A) and female (D) offspring, cumulative energy intake (kcal) from PND 69-97 in male (B) and female (E) offspring, and the fat/lean mass ratio at birth, weaning, and from PND60-90 in male (C) and female (F) offspring.
Dams were dosed with BPA (100 μg/kg/day BW) or vehicle (oil) from gestational d6 until PND21, and offspring then consumed a post-weaning control (C) or high-fat (HF) diet until PND110. The ♀ symbol in Figure 1C is shown for comparison, and represents the mean fat/lean mass ratio in females on PND90. Values are means ± SEM. n=6 litters for birth and weaning body composition, and n=5 male or female offspring in puberty and adulthood. Bars with different letters differ at P<0.05 if an interaction was present between the Diet and BPA factors using 2-way ANOVA.
Liver Histopathology and Lipid Accumulation in Adult Offspring
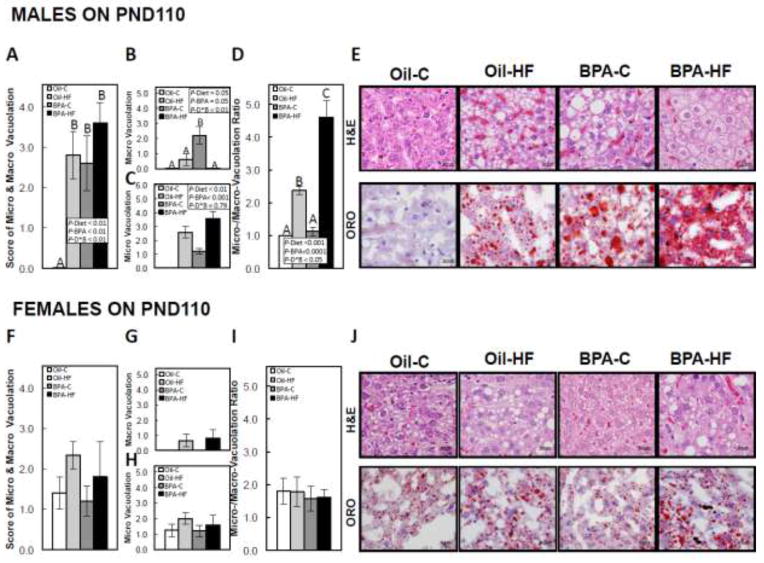
In PND110 males, both HF diet (P<0.01) and BPA (P<0.01) increased overall hepatocellular vacuolation, and there was a significant interaction between the factors (P<0.01), where Oil-C males had no vacuolation, and overall vacuolation was increased by a HF diet in Oil-dosed animals, whereas all BPA-exposed animals had increased vacuolation, regardless of diet, when compared to Oil-C (Figure 2A and representative images of H&E and ORO in Figure 2E). In males, both HF diet (P<0.05) and BPA (P<0.05) increased macro-vacuolation, and there was a significant interaction between the factors (P<0.01), where macro-vacuolation was more prominent in BPA-C males when compared to Oil-HF and almost absent in the BPA-HF group (Figure 2B and representative images in Figure 2E). Both HF diet (P<0.01) and BPA (P<0.001) increased micro-vacuolation, with no interaction between the factors (Figure 2C and representative images in Figure 2E). Also, in males, both HF diet (P<0.001) and BPA (P<0.0001) increased the ratio of micro/macro-vacuolation, and there was a significant interaction between the factors (P<0.05), where the ratio was increased in Oil-HF and BPA-HF males when compared with Oil-C, but BPA-HF males had the highest ratio when compared to all groups (Figure 2D and representative images in Figure 2E). In females, neither BPA nor the HF diet had a significant effect on overall vacuolation (Figure 2F), macro-vacuolation (Figure 2G), micro-vacuolation (Figure 2H), or the ratio of micro/macro-vacuolation (Figure 2I, with representative images in Figure 2J).
FIGURE 2. Postnatal day (PND) 110 hepatic vacuolation scores in male (A) and female (F), macro-vacuolation in male (B) and female (G), micro-vacuolation in male (C) and female (H), ratio of micro/macro-vacuolation in male (D) and female (I) and representative images of vacuolation using hematoxylin & eosin (H&E) staining (top panels) and lipid accumulation using Oil-Red-O (ORO) staining (lower panels) in male (E) and female (F) offspring.
Dams were dosed with BPA (100 μg/kg/day BW) or vehicle (oil) from gestational d6 until PND21, and offspring then consumed a post-weaning control (C) or high-fat (HF) diet until PND110. Findings were graded from 1 (minimal) to 5 (severe). Values are means ± SEM. n=5 male or female offspring. Bars with different letters differ at P<0.05 if an interaction was present between the Diet and BPA factors using 2-way ANOVA.
Hepatic TG and FFA Amount, and FFA Composition in PND1 Offspring
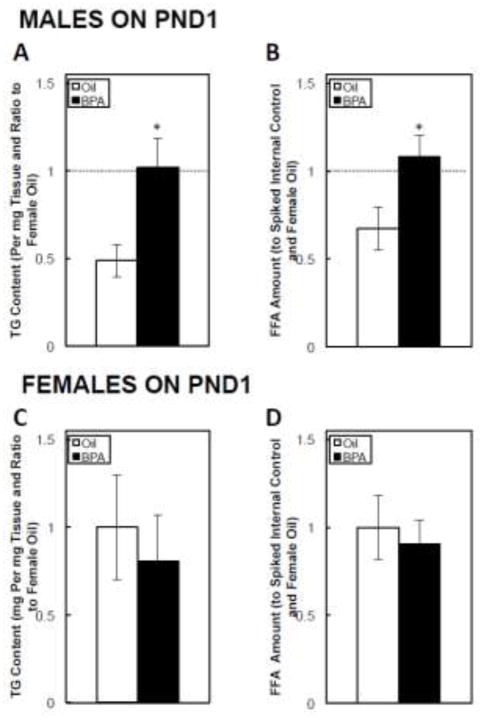
On PND1, BPA significantly increased the amount of both hepatic TG (Figure 3A) and FFAs (Figure 3B) in males. In contrast, BPA had no effect on either TGs (Figure 3C) or FFAs (Figure 3D) in females.
FIGURE 3. Postnatal day (PND) 1 hepatic triglyceride (TG) content in male (A) and female (C) offspring, and free fatty acid (FFA) content in male (B) and female (D) offspring.
Dams were dosed with BPA (100 μg/kg/day BW) or vehicle (oil) from gestational d6 until PND21. The data are shown relative to the PND1 female Oil group. The dashed lines in Figures 3A and 3B are shown for comparison, and represents the mean hepatic triglyceride or free fatty acid content in females on PND1. Values are means ± SEM. n=6 pups, with male or female offspring. *P<0.05.
Also on PND1, as a % of total FFAs (which was increased in males), BPA significantly increased the % of monounsaturated long-chain, and decreased the % of very-long chain FFAs, as well as the ratio of very-long chain/long-chain FFAs (Table 4, *P<0.05 or **P<0.01). BPA had no significant effect on FFA composition or distribution in PND1 female liver (Table 4).
Table 4.
Fatty Acid Composition in Postnatal Day 1 Offspring Liver
| Male
|
Female
|
|||
|---|---|---|---|---|
| Oil-Control | BPA | Oil-Control | BPA | |
| Fatty Acid Composition (% of Total) | ||||
|
| ||||
| Saturated | 39.79 ± 1.75 | 39.13 ± 1.88 | 39.24 ± 1.23 | 41.16 ± 1.96 |
| Monounsaturated | 21.05 ± 0.29 | 23.69 ± 1.21* | 21.91 ± 0.82 | 22.82 ± 2.02 |
| Polyunsaturated | 39.16 ± 1.65 | 37.18 ± 2.29 | 38.85 ± 0.87 | 36.02 ± 2.68 |
| Long-chain | 85.92 ± 0.44 | 88.75 ± 0.43** | 87.67 ± 1.01 | 90.18 ± 1.07 |
| Very long-chain | 14.08 ± 0.44 | 11.25 ± 0.43* | 12.33 ± 1.01 | 9.82 ± 1.07 |
| Very long-chain/Long-chain | 0.16 ± 0.01 | 0.13 ± 0.01** | 0.13 ± 0.01 | 0.11 ± 0.01 |
P<0.05.
P<0.0;
Long chain: tails with 14–21 carbons, Very long-chain: tails with 22–26 carbons.
Hepatic mRNA Expression in PND1 Offspring
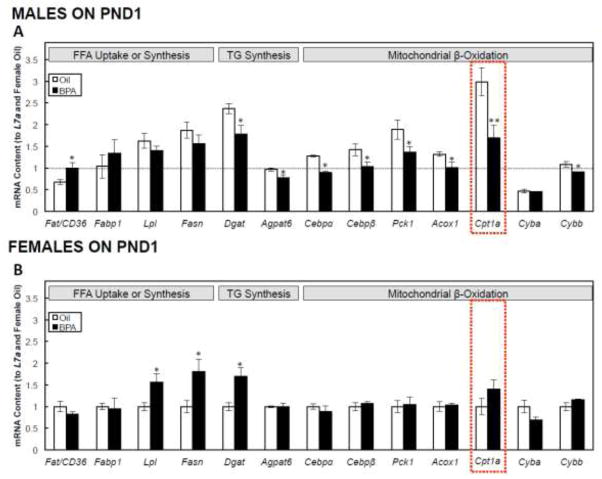
In PND1 males, BPA increased the expression of Fat/Cd36, decreased the expression of TG synthesis-related genes, Dgat and Agpat6 (P<0.05), but had no effect on Fabp1, Lpl, or Fasn. BPA in males also decreased the expression of genes directly or indirectly associated with hepatic mitochondrial β-oxidation, including Cebpα, Cebpβ, Pck1, Acox1, Cybb (P<0.05), and Cpt1a (P<0.01), the gene encoding the rate-limiting step of hepatic fatty acid β-oxidation, but had no effect on Cyba (Figure 4A). In females, BPA increased the expression of fatty acid uptake and synthesis genes, including Lpl, Fasn, and Dgat (P<0.05), but had no effect on any hepatic β-oxidation-related genes (Figure 4B).
FIGURE 4. Postnatal day (PND) 1 hepatic metabolism-related mRNA expression in male (A) and female (B) offspring.
Dams were dosed with BPA (100 μg/kg/day BW) or vehicle (oil) from gestational d6 until PND21. All data were first normalized to the L7a housekeeping gene, and are shown relative to the PND1 female oil group. The dashed line in Figures 4A is shown for comparison, and represents the mean hepatic mRNA expression in females on PND1. Values are means ± SEM. n=6 pups, with male or female offspring. *P<0.05 and **P<0.01.
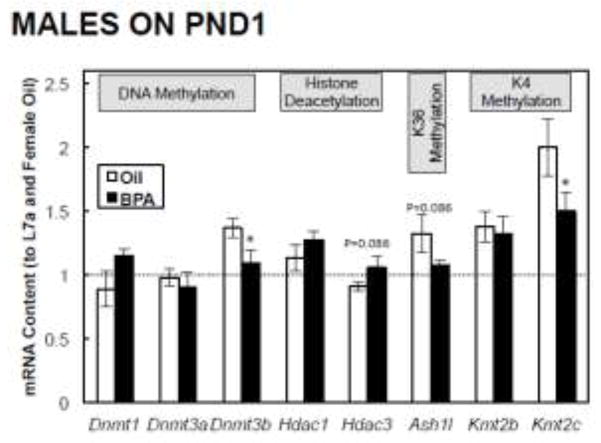
We also analyzed several key DNA and histone modifiers to examine how their mRNA expression correlates with DNA methylation or histone modifications. In PND1 males, BPA had no effect on the expression of Dnmt1, Dnmt3a, Hdac1, Km2b, trended to increase Hdac3 (P=0.086) and decrease Ash1l (P=0.086), and significantly decreased the expression of Dnmt3b and Kmt2c (P<0.05; Figure 5). In females, BPA had no effect on the expression of Dnmt1, Dnmt3a, Dnmt3b, Hdac1, Hdac3, Ash1l, Kmt2b, and significantly increased the expression of Kmt2c (P<0.05; Supplementary Figure 1).
FIGURE 5. Postnatal day (PND) 1 epigenetic modifier-related mRNA expression in male offspring.
Dams were dosed with BPA (100 μg/kg/day BW) or vehicle (oil) from gestational d6 until PND21. All data were first normalized to the L7a housekeeping gene, and are shown relative to the PND1 female oil group. The dashed line is shown for comparison, and represents the mean hepatic mRNA expression in females on PND1. Values are means ± SEM. n=6 male pups. *P<0.05.
Hepatic Methylation of the Cpt1a Gene in PND1 Offspring
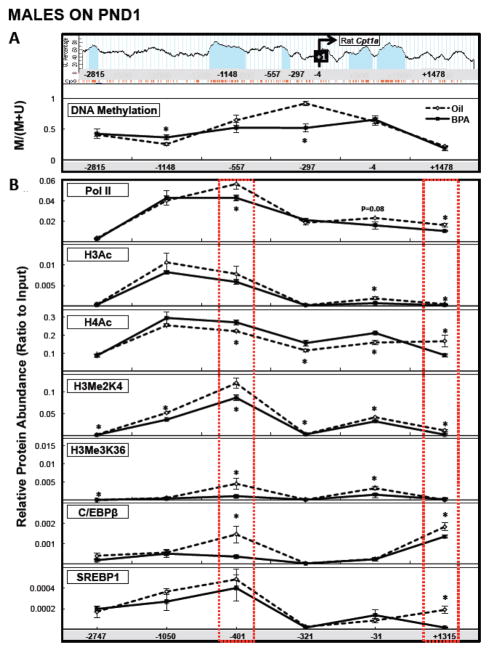
We focused our methylation analysis within approximately 5 kB spanning the Cpt1a transcription start site (TSS) (Figure 6A or Supplementary Figure 2A, upper panels). In PND1 males, BPA increased methylation at a region approximately 1 kB upstream of the TSS (−1148; P<0.05), and decreased methylation in a region approximately 250 bp upstream of the TSS (−297) (P<0.005; Figure 6A, lower panel). In females, BPA had no effect on DNA methylation within any of the regions tested (Supplementary Figure 2A, lower panel).
FIGURE 6. Postnatal day (PND) 1 hepatic Cpt1a methylation analysis (A; bottom panel) and chromatin immunoprecipitation analysis (B) of approximately 5 kB of the rat Cpt1a gene in male offspring.
Six regions within the Cpt1a gene were selected for Methylation Sensitive PCR analysis (A, top panel, with CpG islands signified by light blue columns and individual CpGs by orange lines below), and six regions approximately corresponding to those selected for methylation analysis were selected for chromatin immunoprecipitation analysis. Red dashed lines represent regions of DNA where the pattern of histone marks and transcription factor binding reflects heterochromatin and potential regulatory regions for Cpt1a transcriptional inhibition. Dams were dosed with BPA (100 μg/kg/day BW) or vehicle (oil) from gestational d6 until PND21. Values are means ± SEM. n=6 male pups. *P<0.05 for the ratio of (Methylated) to (Methylated+Unmethylated) for methylation analysis after normalization to an internal standard (representing the amount of methylation relative to total DNA input), and for the ratio of each immunoprecipitated protein after normalization to Input.
Histone Modifications and Transcription Factor Binding within the Cpt1a Gene in PND1 Offspring
In PND1 males, BPA decreased H3Me2K4 and H3Me3K36 within the region approximately 2.7 kB upstream of the TSS (−2747). At approximately 1 kB upstream of the TSS (−1050), BPA decreased H3Me2K4. Within the region approximately 400 bp upstream of the TSS (−401), BPA decreased Pol II, H3Me2K4, H3Me3K36, and C/EBPβ, but increased H4Ac (P<0.05), and within the region approximately 300 bp upstream of the TSS (−321), BPA decreased H3Me2K4 and increased H4Ac (P<0.05). Within the region spanning the TSS (−31), BPA decreased Pol II (trend, P=0.08), H3Ac, H3Me2K4, and H3Me3K36, and increased H4Ac (P<0.05). BPA decreased Pol II, H3Ac, H4ac, H3Me2K4, C/EBPβ, and SREBP1 within the region approximately 1.3 kB downstream of the TSS (P<0.05; Figure 6B).
In females, BPA decreased H3Ac at two of the regions tested (−1050 and −401; P<0.05) and also decreased H3Me3K36 at two of the regions tested (−401 and −31; P<0.05). However, it had no effect on H3Me2K4, or the binding of Pol II, C/EBPβ, and SREBP1 (Supplementary Figure 2B).
DISCUSSION
The current study demonstrates that early-life BPA exposure is associated with the sex-specific epigenetic regulation of Cpt1a, the key enzyme in hepatic fatty acid β-oxidation. We observed that the inhibition of hepatic Cpt1a and other fatty acid β-oxidation genes at birth was associated with steatosis in adult males. Furthermore, early-life BPA exposure disrupted hepatic lipid handling and appeared to further exacerbate the negative long-term consequences of a post-weaning HF diet, thus contributing to the adulthood NAFLD phenotype. These effects were associated with changes to DNA methylation as well as with covalent changes to histone tails within the Cpt1a gene.
Sex differences in adiposity and NAFLD development in response to BPA
In the current study, we observed metabolic and hepatic dysregulations in response to BPA and a HF diet in male, but not female offspring. The baseline measurements for numerous outcomes (including hepatic mRNA expression, fat/lean mass ratio, and hepatic TG/FFA content) in the current study were different between males and females. The prevalence of NAFLD in humans has been shown to be sex-specific, with higher rates in males than females in the 1988–1994 NHANES survey (Lazo et al., 2013). There has also been a larger increase in prevalence in males than in females from the 1988–1994 survey to the 2007–2010 survey (Welsh et al., 2013). These sex differences have been attributed to innate differences in hepatic metabolism between males and females, which likely include differences in hepatic clearance of toxic substances. A study in BPA-treated rats showed that females had lower serum BPA concentrations than males, and a higher activity of UGT2B1, an isoform of a UDP-glucuronosyltransferase involved in BPA glucuronidation and clearance (Takeuchi et al., 2004). In the current study, males, but not females, were sensitive to the effects of prenatal BPA exposure, which could potentially be related to differences in BPA clearance during the exposure window. Although BPA is a known endocrine-disruptor, potentially acting through estrogen receptors (ERs), whether these outcomes are related to ER signaling in liver is not clear. A study in a hepatic cell line not expressing ERα and having very low expression of ERβ showed that BPA was able to induce lipid accumulation and decrease lipid secretion, potentially through PI3K/Akt signaling (Grasselli et al., 2013). Additional studies will be needed to establish the precise signaling pathways and mechanistic basis behind these observations in cell culture and our in vivo observations in males in response to BPA.
BPA exposure is associated with disrupted hepatic lipid metabolism
A previous study in Sprague-Dawley rats demonstrated that females perinatally exposed to BPA in the drinking water had increased adiposity on PND21 and an increase in hepatic expression of Fasn, Acc, and Srebp1c (Somm et al., 2009). In our study, we also observed that females had increased Fasn expression on PND1, without evidence of TG or FFA accumulation at birth, and without hepatic steatosis in adulthood. Hepatic lipids were not measured or examined in the previous study, making it difficult to compare their study and ours. Additionally, both males and females in the previous study had increased body weights at birth, which was not observed in our study. This may be due to differences in exposure mode (in drinking water vs. as an oral gavage) or dose (100 μg/kg/day in our study vs. approximated 70 μg/kg/day in the previous study). Furthermore, a recent study in male Wistar rats showed that gestational and lactational BPA exposure (40 μg/kg/day) increased adulthood steatosis, together with reduced mitochondrial function, and was associated with increased TG and reactive oxygen species, and reduced ATP production in adults (15 and 26 weeks). NAFLD is known to be associated with mitochondrial damage, which has been shown to be both the upstream cause (by decreasing fatty acid β-oxidation capacity) and downstream consequence (through excessive ROS production) of steatosis. In the previous manuscript, the authors concluded that because the decrease in several mitochondrial respiratory complexes at postnatal week 3 preceded hepatic lipid accumulation at postnatal weeks 15 and 26, prenatal BPA’s effect on mitochondrial function likely contributed to the increased steatosis observed in adult animals (Jiang et al., 2014). While we observed the increase in both TG and FFA at birth with a concurrent decrease in genes associated with hepatic β-oxidation in response to BPA, based on the previous study by Jiang et al, and the numerous reports of drug- and toxin-induced hepatic steatosis (mediated through the inhibition of β-oxidation), our observations support the notion that developmental BPA exposure targets mitochondrial β-oxidation, which contributes to lipid accumulation at birth and long-term steatosis. Furthermore, while we did not observe changes in maternal body composition or body weight in response to BPA, additional extensive studies are warranted to determine whether BPA-induced outcomes in neonatal liver are due to changes in maternal physiology or to the direct body burden of BPA on fetal tissues.
Interestingly, in addition to modifying overall TG and FFA content in PND1 male offspring, BPA also modified the types and classes of FFAs in liver. Specifically, BPA-exposed males had an increased amount of monounsaturated fatty acids and a decreased ratio of very long-chain to long-chain FFAs, a process partly regulated by β-oxidation, since the final step in the synthesis of 22:6 (docosahexaenoic acid, DHA) requires one round of peroxisomal β-oxidation (Barcelo-Coblijn and Murphy, 2009). Whether this occurred in response to the direct action of BPA on fetal FFA supply, or whether these changes in β-oxidation resulted in altered FFA metabolism will need to be further investigated.
Perinatal exposure to BPA programs the hepatic response to a post-weaning HF diet in male offspring
The concept of “developmental programming” suggests that numerous in utero factors can program hepatic development and function (McMillen et al., 2009), and these factors – including BPA exposure – could then govern the response to postnatal lifestyle factors, such as diet. In the current study, while all treatment groups had increased hepatic vacuolation and lipid accumulation when compared to Oil-C males, BPA-HF males had the highest ratio of micro/macro-vacuolation. Microvesicular steatosis is a hallmark sign of hepatic toxicity, associated with mitochondrial damage and decreased mitochondrial β-oxidation capacity (Fromenty and Pessayre, 1995), and is also the histological hallmark of acute metabolic perturbation in liver (Jaeschke, 2011). This appears to occur because hepatic β-oxidation capacity becomes overloaded, leading to a decrease in FFA esterification to TG, and this sudden increase in small vesicles prevents the standard coalescence of lipid droplets into storage macrovacuoles (Fromenty and Pessayre, 1995), which likely explains the observed “worst case scenario” observed in the males in our study who were first exposed to BPA in utero and then challenged with a HF diet. Recently, a study was published using Wistar rats exposed prenatally to BPA at 50 μg/kg/day, and consuming a post-weaning HF diet. Male rats (female data were not shown) consuming a HF diet presented with BPA-induced hepatic steatosis at 27 weeks (Wei et al., 2014), and this was associated with a decrease in mitochondrial respiratory capacity and ATP production in the same model (Jiang et al., 2014). The study by Wei et al. showed that in adult animals that had steatosis, Cpt1a was actually increased following developmental BPA exposures, and we also observed that BPA-exposed males in the current study had increased Cpt1a mRNA expression in adulthood (data not shown). Wei et al. proposed that this increase in adults was likely a compensatory mechanism, and that the early-life “first hit” in the progression of NAFLD would likely be associated with a decrease in Cpt1a, and therefore a decrease in fatty acid β-oxidation. We have confirmed this proposed “first hit” by showing that developmental BPA exposure was associated with a significant downregulation of Cpt1a expression in males at birth, together with a decrease in the expression of other fatty acid β-oxidation-related genes. As previously discussed, CPT1A inhibition is related to metabolic perturbation, so it is likely that both in our adult animals and in the study by Wei et al, the compensatory response was still quite robust in young adults, but would decrease with age and as animals progressed into fibrosis or further into NAFLD. Together, these studies using 2 different strains of rats suggest that exposure to BPA during a critical developmental window programs a dysfunction in hepatic lipid metabolism and homeostasis (in males only, as shown in our study), which prevents the appropriate storage and processing of FFAs in response to a long-term diet high in lipids, likely favoring the development of NAFLD.
Cpt1a, the key regulatory enzyme of mitochondrial β-oxidation is epigenetically regulated in PND1 male offspring
Developmental programming of chronic diseases has been proposed to occur through epigenetic modification of genes (Maunakea et al., 2010). Specifically, it is thought that epigenetics serve as an adaptive mechanism during sensitive windows of development, and these early alterations in epigenotype and genotype increase the adulthood susceptibility to diseases, potentially by altering the response to postnatal cues. While several types of epigenetic modifications have been shown to interact to regulate gene expression, DNA methylation and the covalent modification of histone tails remain the most widely studied epigenetic regulators (Portela and Esteller, 2010). We and others have previously shown that maternal diet can alter the hepatic epigenome and metabolic phenotype in offspring (Strakovsky et al., 2011; Strakovsky et al., 2014b; Suter et al., 2014), and a study in human fetal liver (Nahar et al., 2014) and in mice (Anderson et al., 2012) suggests that gestational BPA exposure is also associated with altered DNA methylation in offspring. However, no studies have specifically addressed the question of whether BPA exposure also alters the histone code in livers of neonates, and how these marks interact with DNA hypo- or hyper-methylation to regulate transcription.
In the current study, we observed several differentially methylated regions in male PND1 offspring within a ~5 kB region of the Cpt1a gene spanning the TSS. Promoter DNA methylation within CG-rich regions (CpG Islands) has been shown to be associated with programmed transcriptional regulation (Straussman et al., 2009), and we observed that BPA decreased Cpt1a expression, and this was associated with CpG Island DNA hypermethylation within one of the regions tested in males. We also observed that H3Me2K4, a marker of transcriptional activation (Rivera et al., 2014), was decreased within all regions tested in males, but not affected in females. Furthermore, while H3Me3K36, also a marker of actively transcribed genes, was decreased within several regions in males, it was also decreased in females – but without a concurrent change in the expression of Cpt1a in females. Our mRNA analysis of several DNA methylation-, histone acetylation- and histone methylation-related genes showed a significant decrease in the male mRNA expression of Kmt2c, which methylates and activates H3Me2K4, and this corresponded to a decrease in H3Me2K4 and decreased Cpt1a expression. In females, this gene was significantly upregulated, without a concurrent consequence for either its target histone modification or for Cpt1a expression. These data suggest that K4 dimethylation at histone H3 may be a unique regulatory mechanism for the transcription of Cpt1a in response to BPA.
Increasing evidence is beginning to suggest that the active transcriptional regulation of genes is a result of the interaction of numerous epigenetic modifications (Murr, 2010). In the current study, we focused our analyses on DNA methylation and histone modifications within specific regions of the chromatin, thus allowing us to assess any potential interactions between these events within the Cpt1a gene. While we did observe that BPA hypermethylated the DNA within one CpG island, which would typically be considered a marker of heterochromatin and transcriptional inhibition, we also observed significant hypomethylation of the DNA by BPA within a region located just outside of a CpG island (sometimes referred to as a “shore”), as well as a coordinated decrease in the mRNA expression of Dnmt3b, which is responsible for de novo DNA methylation. However, and perhaps more significantly, we assessed the potential interaction of DNA methylation within these regions with histone modifications. Interestingly, the substantial hypomethylation at the island “shore” was accompanied by relatively low binding (compared to other regions) of H3Ac, H3Me2K4, H3Me3K36, C/EBPβ, and SREBP1. Furthermore, this region of seemingly “naked chromatin” was surrounded by sites of BPA-induced decreases of histone marks that represent active transcription. The Cpt1a gene has been shown to have a TATA-less promoter (Rivera et al., 2014) that is highly dependent on the binding of transcriptional regulators on the DNA (especially in CCAAT boxes located throughout the promoter and the remainder of the genome) (Mantovani, 1998; Steffen et al., 1999), thus chromatin structure (through histones and methyl groups) likely drives and regulates the assembly of necessary transcriptional machinery. While we did not specifically address the precise mechanisms that regulate Cpt1a transcription, we report that the transcriptional inhibition of this critical gene by BPA in males is associated with changes to both DNA methylation, as well as the histone code. Furthermore, these marks were associated with the decreased binding of Pol II within several of the regions tested, as well as of both C/EBPβ, and SREBP1 (which have been previously shown to regulate Cpt1a transcription in vitro (Attia et al., 2011; Thakran et al., 2013)) at the promoter, confirming the important role of epigenetics in establishing chromatin conformation and thus directing the binding of transcription factors.
In conclusion, we have demonstrated that the downregulation in Cpt1a mRNA expression in male, but not female offspring at birth in response to developmental BPA exposure is associated with susceptibility for adulthood hepatic steatosis, especially in response to a post-weaning HF diet. We also showed that this potentially occurs through the epigenetic regulation of the gene, with both DNA methylation and histone modifications likely playing an important role in regulating chromatin structure and gene expression. Future studies are critical to determine the basis for the observed sex differences in the hepatic response to BPA as well as to determine what role maternal physiology plays in the observed adulthood hepatic phenotype. Furthermore, more research in the field is needed to address the question of whether additional epigenetic mechanisms, other that DNA methylation and histone modifications, govern the dysregulated transcription of Cpt1 (and other developmental genes) in response to early-life BPA exposure.
Supplementary Material
Highlights.
Developmental BPA exposure exacerbates HF-diet induced steatosis in adult males
Gestational BPA exposure increases hepatic lipid accumulation in neonatal males
BPA decreases Cpt1a and other hepatic β-oxidation genes in neonatal males
BPA alters neonatal male Cpt1a DNA methylation, histones, and transcription factors
Acknowledgments
R. S. Strakovsky was supported by the National Institute of Environmental Health Sciences (NIEHS) training grant T32 ES007326. This work was also supported by the College of ACES pilot grant (to Y-X.P.), NIH R01ES019178 (JAF), NIH P01 ES022848 (JAF), and EPA RD-83459301 (JAF). This publication was made possible by U.S. Environmental Protection Agency (US EPA) grant RD83543401 and National Institute for Environmental Health Sciences (NIEHS) grant ES022848. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The authors thank Dr. Anna Dilger for the EchoMri use and training and Dr. Hong Chen for her technical expertise.
Abbreviations Used
- Acox1
acyl-CoA oxidase 1, palmitoyl
- Agpat6
1-acylglycerol-3-phosphate O-acyltransferase 6
- Ash1l
absent, small, or homeotic-like 1l
- BPA
Bisphenol A
- BW
Body weight
- Cebpα
CCAAT/enhancer binding protein (C/EBP), alpha
- Cebpβ or C/EBPβ
CCAAT/enhancer binding protein (C/EBP) beta
- Cpt1a
carnitine palmitoyltransferase 1a
- Cyba
cytochrome b-245, alpha polypeptide
- Cybb
cytochrome b-245, beta polypeptide
- Dgat1
diacylglycerol O-acyltransferase 1
- Dnmt1, Dnmt3a, or Dnmt3b
DNA methyltransferase1, 3a, or 3b
- EDC
endocrine disrupting chemical
- Fabp1
fatty acid binding protein 1
- Fasn
fatty acid synthase
- Fat/Cd36
fatty acid translocase/cluster determinant 36
- FFA
free fatty acids
- H3Ac or H4Ac
acetylated histone H3 or H4
- H3Me2K4
di-methylated histone H3 at lysine residue 4
- H3Me3K9 H3Me3K27, or H3Me3K36
tri-methylated histone H3 at lysine residue 9, 27, or 36
- HF
high-fat
- Hdac1 or Hdac3
histone deacetylase 1 or 3
- Kmt2b or Kmt2c
lysine(K)-specific methyltransferase 2b or 2c
- Lpl
lipoprotein lipase
- NAFLD
non-alcoholic fatty liver disease
- Pck1
phosphoenolpyruvate carboxykinase 1 (soluble)
- PND
postnatal day
- SREBP1
sterol regulatory element binding protein 1
- TG
triglycerides
- TSS
transcription start site
Footnotes
Statement of Ethics: The authors declare no financial conflict of interest. We certify that all applicable institutional and governmental regulations regarding the ethical use of animals were followed during this research (University of Illinois Institutional Animal Care and Use Committee approval #11191).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41:91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, Weinhouse C, Rozek LS, Dolinoy DC. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environmental and molecular mutagenesis. 2012;53:334–342. doi: 10.1002/em.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi J, Kamo H, Baba R, Doi Y, Yamashita A, Murakami D, Hanada A, Hirano T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010;87:431–438. doi: 10.1016/j.lfs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Attia RR, Sharma P, Janssen RC, Friedman JE, Deng X, Lee JS, Elam MB, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by CCAAT/enhancer-binding protein beta (C/EBPbeta) J Biol Chem. 2011;286:23799–23807. doi: 10.1074/jbc.M111.246389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res. 2009;48:355–374. doi: 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58:114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Santani AB. Carnitine Palmitoyltransferase 1A Deficiency. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem. 2004;279:50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- Frazier-Wood AC, Aslibekyan S, Absher DM, Hopkins PN, Sha J, Tsai MY, Tiwari HK, Waite LL, Zhi D, Arnett DK. Methylation at CPT1A locus is associated with lipoprotein subfraction profiles. J Lipid Res. 2014;55:1324–1330. doi: 10.1194/jlr.M048504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacology & therapeutics. 1995;67:101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- Grasselli E, Cortese K, Voci A, Vergani L, Fabbri R, Barmo C, Gallo G, Canesi L. Direct effects of Bisphenol A on lipid homeostasis in rat hepatoma cells. Chemosphere. 2013;91:1123–1129. doi: 10.1016/j.chemosphere.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Hanley B, Dijane J, Fewtrell M, Grynberg A, Hummel S, Junien C, Koletzko B, Lewis S, Renz H, Symonds M, Gros M, Harthoorn L, Mace K, Samuels F, van Der Beek EM. Metabolic imprinting, programming and epigenetics - a review of present priorities and future opportunities. Br J Nutr. 2010;104(Suppl 1):S1–25. doi: 10.1017/S0007114510003338. [DOI] [PubMed] [Google Scholar]
- Huc L, Lemarie A, Gueraud F, Helies-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicology in vitro : an international journal published in association with BIBRA. 2012;26:709–717. doi: 10.1016/j.tiv.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Irvin MR, Zhi D, Joehanes R, Mendelson M, Aslibekyan S, Claas SA, Thibeault KS, Patel N, Day K, Jones LW, Liang L, Chen BH, Yao C, Tiwari HK, Ordovas JM, Levy D, Absher D, Arnett DK. Epigenome-wide association study of fasting blood lipids in the Genetics of Lipid-lowering Drugs and Diet Network study. Circulation. 2014;130:565–572. doi: 10.1161/CIRCULATIONAHA.114.009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Xia W, Zhu Y, Li X, Wang D, Liu J, Chang H, Li G, Xu B, Chen X, Li Y, Xu S. Mitochondrial dysfunction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicology letters. 2014;228:85–92. doi: 10.1016/j.toxlet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. American journal of epidemiology. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu P, Qian W, Li Y, Zhao J, Huan F, Wang J, Xiao H. Perinatal bisphenol A exposure and adult glucose homeostasis: identifying critical windows of exposure. PLoS One. 2013;8:e64143. doi: 10.1371/journal.pone.0064143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R. A survey of 178 NF-Y binding CCAAT boxes. Nucleic Acids Res. 1998;26:1135–1143. doi: 10.1093/nar/26.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, Bertrand-Michel J, Pineau T, Guillou H, Martin PG, Mselli-Lakhal L. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology. 2012;55:395–407. doi: 10.1002/hep.24685. [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Chepelev I, Zhao K. Epigenome mapping in normal and disease States. Circ Res. 2010;107:327–339. doi: 10.1161/CIRCRESAHA.110.222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Rattanatray L, Duffield JA, Morrison JL, MacLaughlin SM, Gentili S, Muhlhausler BS. The early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol. 2009;646:71–81. doi: 10.1007/978-1-4020-9173-5_8. [DOI] [PubMed] [Google Scholar]
- Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, Billheimer J, Mukherjee R. Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J Lipid Res. 2004;45:1410–1417. doi: 10.1194/jlr.M300450-JLR200. [DOI] [PubMed] [Google Scholar]
- Murr R. Interplay between different epigenetic modifications and mechanisms. Adv Genet. 2010;70:101–141. doi: 10.1016/B978-0-12-380866-0.60005-8. [DOI] [PubMed] [Google Scholar]
- Nahar MS, Kim JH, Sartor MA, Dolinoy DC. Bisphenol A-associated alterations in the expression and epigenetic regulation of genes encoding xenobiotic metabolizing enzymes in human fetal liver. Environmental and molecular mutagenesis. 2014;55:184–195. doi: 10.1002/em.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–144. doi: 10.1016/j.plipres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Olsen L, Lind L, Lind PM. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol Environ Saf. 2012;80:179–183. doi: 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Rivera C, Gurard-Levin ZA, Almouzni G, Loyola A. Histone lysine methylation and chromatin replication. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagrm.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Huppi PS. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009;117:1549–1555. doi: 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O’Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab. 2008;294:E969–977. doi: 10.1152/ajpendo.00497.2007. [DOI] [PubMed] [Google Scholar]
- Steffen ML, Harrison WR, Elder FF, Cook GA, Park EA. Expression of the rat liver carnitine palmitoyltransferase I (CPT-Ialpha) gene is regulated by Sp1 and nuclear factor Y: chromosomal localization and promoter characterization. Biochem J. 1999;340 (Pt 2):425–432. [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Lezmi S, Flaws JA, Schantz SL, Pan YX, Helferich WG. Genistein exposure during the early postnatal period favors the development of obesity in female, but not male rats. Toxicol Sci. 2014a;138:161–174. doi: 10.1093/toxsci/kft331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Pan Y-X. A Decrease in DKK1, a WNT Inhibitor, Contributes to Placental Lipid Accumulation in an Obesity-Prone Rat Model. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Zhang X, Zhou D, Pan YX. Gestational High Fat Diet Programs Hepatic Phosphoenolpyruvate Carboxykinase (Pck) Expression and Histone Modification in Neonatal Offspring Rats. J Physiol. 2011 doi: 10.1113/jphysiol.2010.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Zhang X, Zhou D, Pan YX. The regulation of hepatic Pon1 by a maternal high-fat diet is gender specific and may occur through promoter histone modifications in neonatal rats. J Nutr Biochem. 2014b;25:170–176. doi: 10.1016/j.jnutbio.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straussman R, Nejman D, Roberts D, Steinfeld I, Blum B, Benvenisty N, Simon I, Yakhini Z, Cedar H. Developmental programming of CpG island methylation profiles in the human genome. Nat Struct Mol Biol. 2009;16:564–571. doi: 10.1038/nsmb.1594. [DOI] [PubMed] [Google Scholar]
- Suter MA, Ma J, Vuguin PM, Hartil K, Fiallo A, Harris RA, Charron MJ, Aagaard KM. In utero exposure to a maternal high-fat diet alters the epigenetic histone code in a murine model. Am J Obstet Gynecol. 2014;210:463.e461–463 e411. doi: 10.1016/j.ajog.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Tsutsumi O, Nakamura N, Ikezuki Y, Takai Y, Yano T, Taketani Y. Gender difference in serum bisphenol A levels may be caused by liver UDP-glucuronosyltransferase activity in rats. Biochem Biophys Res Commun. 2004;325:549–554. doi: 10.1016/j.bbrc.2004.10.073. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Moran TH. Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav. 2010;100:560–566. doi: 10.1016/j.physbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakran S, Sharma P, Attia RR, Hori RT, Deng X, Elam MB, Park EA. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J Biol Chem. 2013;288:807–818. doi: 10.1074/jbc.M112.437970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- Unuvar T, Buyukgebiz A. Fetal and neonatal endocrine disruptors. J Clin Res Pediatr Endocrinol. 2012;4:51–60. doi: 10.4274/Jcrpe.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Sun X, Chen Y, Li Y, Song L, Zhou Z, Xu B, Lin Y, Xu S. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J Endocrinol. 2014;222:313–325. doi: 10.1530/JOE-14-0356. [DOI] [PubMed] [Google Scholar]
- Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:496–500. e491. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Li H, Wang K, Lin J, Wang Q, Zhao G, Jia W, Zhang Q. Analysis of transcriptome and metabolome profiles alterations in fatty liver induced by high-fat diet in rat. Metabolism. 2010;59:554–560. doi: 10.1016/j.metabol.2009.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.