Abstract
Most organs are composed of tubes of differing cellular architectures, including intracellular, “seamless” tubes. Two studies examining the morphogenesis of the C. elegans excretory canal cell seamless tubes reveal a previously unappreciated role for osmoregulation of tubulogenesis: hyperosmotic shock recruits canalicular vesicles to the lumenal membrane to promote seamless tube growth.
There are a finite number of possible tube architectures (multi-cellular, autocellular, and seamless; Figure 1a), but cells have revealed a surprisingly diverse repertoire of mechanisms for making them. Both multi-cellular and auto-cellular tubes are composed of polarized cells, organized such that the tube lumens are lined by apical membranes sealed together by epithelial adherens and tight junctions (or septate junctions in invertebrates). So-called “seamless tubes” lack junctional seams and instead possess a lumenal space surrounded by a continuous intracellular apical membrane. Although not as richly documented in the literature as larger seamed tubes, seamless tubes have been described in the vertebrate vascular system, the fly respiratory system, and the nematode renal system3–5.
Figure 1. Formation of seamless tubes.
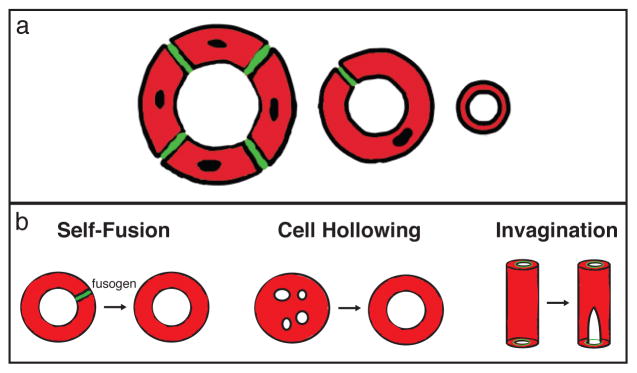
Tube architectures defined by the presence and number of cell junctions are schematized in cross-section (a). Multicellular tubes (left) are comprised of 2 or more cells surrounding a lumenal space and connected by intercellular junctions (green). Auto-cellular tubes (middle) are single cells wrapped around a lumenal space and sealed into a tube by self-junctions (green). Seamless tubes (right) have an intracellular lumen bounded by an internal apical membrane. In (b), three mechanisms of seamless tube formation are illustrated: (left) in self-fusion, the junctional seam of an autocellular tube is removed in a fusogen-dependent step; (middle) cell hollowing, in which large membrane-bound bodies (“vacuoles”) coalesce and fuse in the middle of the cell; (right) invagination, where a small apical domain demarcated by inter-cellular junctions is extended internally
A series of recent papers have revealed differing mechanisms by which seamless tubes may form and grow 4, 6–8. The cell-hollowing model proposes that membrane vesicles coalesce into larger “vacuoles” in the middle of cells that fuse to each other to generate patent seamless tubes (Figure 1b). This model owes its origin to early work in the vertebrate vascular system, but has since been supported by EM analysis of the C. elegans canal cell, antibody staining of developing tracheal terminal cells, and live imaging studies in zebrafish 5, 7, 9, 10. However, studies in zebrafish and fly propose a different mechanism, based on apical membrane invagination, in which a patch of apical membrane defined by a ring of intercellular adherens junctions is extended internally and elaborated upon (Figure 1b) 4, 8. A third mechanism has been identified in the duct cell of the C. elegans excretory system, in which it forms an auto-cellular tube that initially contains junctions which are removed through self-fusion steps 11 (Figure 1b)
Seamless tube forming cells play critical roles in various organs in response to changes in physiological conditions. For example, hypoxia induces the sprouting of vascular tip cells (many of which later form seamless tubes) that lead the outgrowth of new vessels into under-vascularized tissue. Likewise, local hypoxia stimulates the terminal cells of the Drosophila tracheal system to branch, with each new branch containing a seamless tube12. Two studies in this issue of Nature Cell Biology show that the canal cell of the C. elegans excretory system responds to osmotic challenge by changing the rate of seamless tube growth1,2. In addition, Khan et al. suggest that flux through a water channel provides a lumen-expanding mechanical force2 while Kolotuev et al bring insight into the origins of the lumenal membrane by proposing that canal cell canaliculi act as a “membrane reservoir” for lumen lengthening during seamless tube growth1
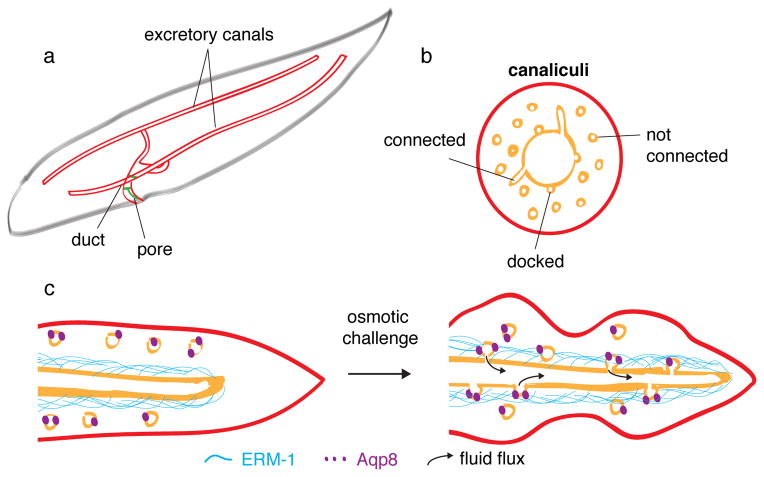
The excretory system regulates the osmolarity of the nematode, and is comprised of four cells: the duct, pore, canal and gland cells. Of these, the canal cell is the largest – indeed, the largest cell in the entire worm – and generates an H-shaped unicellular tube that spans the entire length of the animal (Figure 2a). The branched seamless tube of this remarkable cell has been reported to form by vacuole fusion10. Kolotuev and colleagues show that the four cellular appendages of the H-shaped cell are first elaborated by growth cone-like processes, and then converted into tubes by the extension of intracellular lumen into each of them 2.
Figure 2. Canaliculi: membrane reservoir or flux capacitor?
In (a), the three tube forming cells of the excretory system of the nematode are drawn (cell outlines, red; junctions, green; apical membrane, gold); seamless tubes form in the large H-shaped canal cell. In (b) a cross sectional view of one arm of a canal cell tube is illustrated, with the lumen and canalicular channels and vesicles indicated. In (c), the effect of osmotic challenge on the canalicular network and lumen size is schematized.
Building an intracellular apical membrane would seem likely to require extensive contribution from the cell cytoskeleton, a hypothesis that has garnered increasing attention in recent years. Both microfilaments and microtubules have been shown to play structural and instructive roles during seamless tubulogenesis. Actin regulators have been implicated in lumen initiation and maintenance in cultured endothelial cells during seamless capillary morphogenesis13. In the seamless tubes of Drosophila terminal cells, apical actin and the microtubule-organizing center protein, γ-tubulin, are both tightly associated with the lumenal membrane 7,8 and mutant analyses demonstrate a requirement for the microtubule associated dynein motor complex in seamless tube formation 7. Khan et al. 1 and Kolotuev et al.2 now provide genetic support for a role of microfilaments in seamless tube formation in canal cells, and for intermediate filaments in seamless tube maintenance. Khan et al show a definitive requirement for the sole C. elegans Ezrin-Radixin-Moesin family member, ERM-1, in seamless tube formation and outgrowth in the nematode excretory canal1. ERM-1 is believed to bind and stabilize actin, and in its absence, canal cells accumulate discontinous vacuoles, with varying degrees of apical identity, at the expense of an intracellular lumen. These data offer strong support for the vacuole fusion model, in which large intermediates with partial apical character coalesce to form a seamless tube. Live imaging of the process would be definitive, but is technically challenging in this system. Kolotuev et al. report that knockdown of the apical-localized intermediate filament protein, ifb-1, results in cystic canals, and at times a complete collapse of the lumen2.
Where does membrane added to the growing seamless tube come from? Myriad small membrane channels, termed canaliculi, are found in the canal cell cytoplasm and intersect with the excretory canal tubes (Figure 2b) 3. Historically these canaliculi were thought to be extensions of the tube apical membrane, but Kolotuev et al. now convincingly show through 3D tomographic reconstruction that only one-third of canaliculi are continuous with the tube lumen2. Instead, the canaliculi appear to constitute a dynamic tubulo-vesicular network that forms transient connections to the canal lumen. Interestingly, the number of connections between the lumenal membrane and canaliculi increased significantly when worms were exposed to osmotic challenge. Increased connectivity promoted tube growth, as the rate of canal extension nearly doubled upon repeated exposure to high salt. Conversely, mutations that compromised the ability of the worm to respond to osmotic stress resulted in severely shortened excretory canal tubes, leading Kolotuev and colleagues to conclude that osmoregulation is critical for tube growth.
But how are canalicular/lumenal contacts regulated by osmotic stress? This question is illuminated by the studies of Khan et al.1. They show that ERM-1, which is enriched at the lumenal membrane of canal cells, physically interacts with Aquaporin8 (Aqp8). Aqp8 is a member of a large family of water-transporting channels required in many organ systems for fluid homeostasis, and under isotonic conditions is primarily localized to canaliculi and absent from the lumenal membrane. During periods of osmotic shock, Aqp8 becomes enriched in “varicosities” (also referred to as “pearls” and “peri-lumenal cuffs”) that overlap significantly with apical ERM-1. Thus, physiological changes in osmolarity may drive seamless tube growth by promoting a transient increase in canalicular-lumen membrane connection that is coupled to an aquaporin-induced fluid flux (Figure 2c). The resultant force exerted on the canalicular membrane is proposed by Khan et al to drive lumen expansion1. While Kolotuev and colleagues suggest that fluid flux might drive addition of canalicular membrane to the lumenal membrane, Khan and colleagues find that Aqp8 is not redistributed onto the lumenal membrane, thus leaving the precise mechanism by which fluid flux increases lumenal membrane to be resolved in future studies. Nevertheless, it is clear that Aqp8-mediated fluid flux is important for canal cell seamless tube morphogenesis, since the wide cystic lumens induced by over-expression of the Aqp8 water channel, or its interacting partner ERM-1, were suppressed by the Aqp8 channel inhibitor, mercury. This role for hydrodynamic force in canal cell lumen formation is a striking parallel to the requirement for blood flow in the lumenization of seamless tubes in the zebrafish vascular system4.
Kolotuev et al. also examine the role of the homeobox protein Prox1 in canal cell extension and lumen formation2. The authors report that the C. elegans Prox1 affects the transcription, both directly and indirectly, of osmoregulatory and cytoskeletal genes required for canal cell morphogenesis. Intriguingly, vertebrate Prox1 is required for specification of lymphatic fates; Prox1-positive endothelial cells sprout from the cardinal vein and intersegmental vessels in order to form the lymphatic network of tubes. In the absence of prox1, endothelial cells normally destined to be lymphatic are unable to exit the vascular epithelium and thus cannot adopt lymphatic identity14. In contrast, prox1 mutant canal cells are specified correctly, but morphogenesis of the excretory organ is dramatically affected. It is worth noting that prox1 is also expressed in the vertebrate renal system, although expression initiates after organ differentiation; morpholino-mediated knockdown of prox1 in zebrafish does not affect specification or early morphogenesis of the pronephros but rather is thought to affect later steps of kidney development15. As both lymphatic vessels and renal organs mediate fluid drainage, Kolotuev et al speculate on the possibility of a common ancestral drainage organ regulated by a Prox1-like transcription factor.
The complementary studies from the Gobel and Labouesse labs highlight the contribution of cytoskeletal elements to both building and maintaining intracellular apical membranes, and reveal the canaliculli as a tubulovesicular membrane reservoir that may be tapped for lumen expansion in response to osmotic changes. As the canal cell develops, normal fluctuations in osmolarity promote Aqp8 interactions with apical ERM-1, bringing canalicular and lumenal membranes into close apposition, and thus promoting tube growth. Although canaliculi have not been described in other cell types that form seamless tubes, it will be interesting to determine whether analogous tubulo-vesicular compartments may act as “membrane reservoirs” for tube expansion in other systems.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Khan LA, et al. Nature Cell Biology. 2013 [Google Scholar]
- 2.Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse MA. Nature Cell Biology. 2013 doi: 10.1038/ncb2662. [DOI] [PubMed] [Google Scholar]
- 3.Buechner M. Trends Cell Biol. 2002;12:479–484. doi: 10.1016/s0962-8924(02)02364-4. [DOI] [PubMed] [Google Scholar]
- 4.Herwig L, et al. Curr Biol. 2011;21:1942–1948. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Samakovlis C, et al. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 6.Gervais L, Lebreton G, Casanova J. Dev Biol. 2012;362:187–193. doi: 10.1016/j.ydbio.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Schottenfeld-Roames J, Ghabrial AS. Nat Cell Biol. 2012;14:386–393. doi: 10.1038/ncb2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gervais L, Casanova J. Curr Biol. 2010;20:359–366. doi: 10.1016/j.cub.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 9.Kamei M, et al. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 10.Berry KL, Bulow HE, Hall DH, Hobert OA. Science. 2003;302:2134–2137. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- 11.Stone CE, Hall DH, Sundaram MV. Dev Biol. 2009;329:201–211. doi: 10.1016/j.ydbio.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarecki J, Johnson E, Krasnow MA. Cell. 1999;99:211–220. doi: 10.1016/s0092-8674(00)81652-9. [DOI] [PubMed] [Google Scholar]
- 13.Sacharidou A, Stratman AN, Davis GE. Cells Tissues Organs. 2012;195:122–143. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, et al. Blood. 2012;120:2340–2348. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YW, Gao W, Teh HL, Tan JH, Chan WK. Mol Cell Biol. 2003;23:7243–7255. doi: 10.1128/MCB.23.20.7243-7255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]