Abstract
Objective
Patients with non-alcoholic fatty liver disease (NAFLD) have an increased risk of cardiovascular disease (CVD); however, it is not known if NAFLD contributes to CVD independent of established risk factors. We examined the association between NAFLD and vascular function.
Approach and Results
We conducted a cross-sectional study of 2,284 Framingham Heart Study participants without overt CVD who had liver fat attenuation measured on computed tomography and who had measurements of vascular function and covariates. We evaluated the association between NAFLD and vascular function using multivariable partial correlations adjusting for age, sex, cohort, smoking, diabetes, hyperlipidemia, hypertension, body mass index (BMI) and visceral adipose tissue (VAT). The prevalence of NAFLD in our sample (mean age 52 ± 12 years, 51.4% women) was 15.3%. In age-, sex- and cohort-adjusted analyses, greater liver fat was modestly associated with lower flow-mediated dilation (r = −0.05, P=0.02), lower peripheral arterial tonometry ratio (r = −0.20, P<0.0001), higher carotid-femoral pulse wave velocity (r = 0.13, P<0.0001) and higher mean arterial pressure (r = 0.11, P<0.0001). In multivariable-adjusted models, NAFLD remained associated with higher mean arterial pressure (r=0.06, P=0.005) and lower peripheral arterial tonometry ratio (r= −0.12, P<0.0001). The association between NAFLD and peripheral arterial tonometry ratio persisted after further adjustment for BMI and VAT.
Conclusions
For multiple measures of vascular function, the relation with NAFLD appeared largely determined by shared cardiometabolic risk factors. The persistent relation with reduced peripheral arterial tonometry response beyond established risk factors suggests that NAFLD may contribute to microvascular dysfunction.
Keywords: vascular endothelial dysfunction, computed tomography, microvascular, obesity, risk factors
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver condition in the United States with a general population prevalence of 20-40%.1, 2 NAFLD is associated with insulin resistance, obesity, type 2 diabetes and dyslipidemia.3-5 In addition to the risk for advanced liver disease from non-alcoholic steatohepatitis (NASH), NAFLD confers an increased risk of cardiovascular disease (CVD).6, 7 It is not fully established whether NAFLD increases CVD-related morbidity or mortality independent of known cardiovascular risk factors.8
The mechanisms by which NAFLD may lead to increased CVD are not known.9 NAFLD may lead to increased CVD by modifying traditional risk factors such as dyslipidemia and insulin resistance.10-12 A leading hypothesis is that hepatic steatosis may lead to the production of proinflammatory cytokines, which accelerate atherosclerosis and lead to progressive endothelial dysfunction.6, 13, 14 Indeed, NAFLD is associated with an increased C-reactive protein, a marker of systemic inflammation.15 Vascular endothelial dysfunction occurs early in the atherosclerosis process.16 Non-invasive measures of endothelial function and arterial stiffness such as brachial artery flow-mediated dilation (FMD), fingertip peripheral arterial tonometry (PAT) and arterial tonometry are associated with cardiovascular risk factors and with incident CVD.16-20 There have been several studies on the association of NAFLD and various markers of vascular structure and function.21-33 These studies have been limited by small sample sizes, lack of adequate control for known cardiovascular risk factors, limited evaluation of vascular function and use of clinical samples.22-33
Thus, the goal of our study was to determine the association between NAFLD, as defined by decreased liver attenuation on multi-detector computed tomography (CT), and vascular function as measured by brachial artery FMD, PAT and arterial tonometry in a large, unselected community-based cohort without apparent CVD. Additionally, we explored whether associations remained after adjusting for known cardiovascular risk factors, body mass index (BMI) and visceral adipose tissue (VAT).
Materials and Methods
Materials and methods are available in the online-only Data Supplement.
Results
Study sample characteristics
Participant characteristics and mean values for vascular function measures for those with and without NAFLD are summarized in Table 1. Overall, 15.3% of the sample had a LPR ≤ 0.33, consistent with NAFLD.
Table 1.
Participant characteristics, by presence of non-alcoholic fatty liver disease.
| Clinical characteristics* | NAFLD (LPR ≤ 0.33) (N = 350) |
Non-NAFLD (LPR > 0.33) (N = 1934) |
Total (N = 2284) |
|---|---|---|---|
| Age (years) | 54 ± 12 | 52 ± 12 | 52 ± 12 |
| Women, N (%) | 157 (44.9) | 1017 (52.6) | 1174 (51.4) |
| Offspring, N (%) | 137 (39.1) | 675 (34.9) | 812 (35.6) |
| Current smoking, N (%) | 35 (10.0) | 208 (10.8) | 243 (10.6) |
| Heart rate (beats/min) | 64 ± 10 | 61 ± 10 | 62 ± 10 |
| Total/high density lipoprotein cholesterol | 4.46 ± 1.58 | 3.66 ± 1.19 | 3.78 ± 1.29 |
| Triglycerides (mg/dl) | 170 ± 124 | 107 ± 62 | 116 ± 79 |
| Fasting glucose (mg/dl) | 108 ± 27 | 97 ± 18 | 99 ± 20 |
| Mean arterial pressure (mm Hg) | 94 ± 9 | 90 ± 10 | 90 ± 10 |
| Postmenopausal, N (%) | 100 (63.7) | 516 (50.7) | 616 (52.5) |
| Hormone replacement therapy, N (%) | 14 (8.9) | 96 (9.4) | 110 (9.4) |
| Diabetes, N (%) | 52 (14.9) | 71 (3.7) | 123 (5.4) |
| Hypertension treatment, N (%) | 126 (36.0) | 355 (18.4) | 481 (21.1) |
| Lipid lowering treatment, N (%) | 92 (26.3) | 336 (17.4) | 428 (18.7) |
| C-reactive protein (mg/L)** | 2.57 (1.16,4.76) | 0.98 (0.46,2.25) | 1.12 (0.50,0.27) |
| Alanine Aminotransferase (U/L) | 36.4 ± 27.6 | 24.5 ± 16.6 | 26.3 ± 19.2 |
| Aspartate Aminotransferase (U/L) | 28.6 ± 24.6 | 23.4 ± 9.6 | 24.2 ± 13.2 |
| Adiposity measures | |||
| Body mass index, kg/m2 | 31.0 ± 5.5 | 26.6 ± 4.7 | 27.3 ± 5.1 |
| Waist circumference (cm) | 106 ± 14 | 94 ± 13 | 96 ± 14 |
| Visceral adipose tissue (cm3) | 2488 ± 993 | 1501 ± 876 | 1652 ± 963 |
| Liver phantom ratio | 0.27 ± 0.06 | 0.38 ± 0.02 | 0.36 ± 0.05 |
| Vascular measures | |||
| Brachial artery measures | |||
| Baseline brachial artery diameter (mm) | 4.47 ± 0.86 | 4.14 ± 0.83 | 4.19 ± 0.85 |
| Flow mediated dilation (%) | 4.06 ± 3.65 | 4.81 ± 3.56 | 4.69 ± 3.59 |
| Baseline mean flow velocity (cm/s) | 9.41 ± 5.03 | 7.12 ± 4.28 | 7.49 ± 4.49 |
| Hyperemic mean flow velocity (cm/s) | 56 ± 20 | 59 ± 20 | 58 ± 20 |
| Peripheral Arterial Tonometry measures | |||
| Baseline mean amplitude (unitless) | 6.1 ± 0.8 | 5.6 ± 0.9 | 5.7 ± 0.9 |
| Peripheral arterial tone ratio† (unitless) | 0.53 ± 0.36 | 0.75 ± 0.41 | 0.72 ± 0.41 |
| Arterial Tonometry measures | |||
| Carotid-femoral pulse wave velocity (ms/mm) | 9.0 ± 2.7 | 8.1 ± 2.6 | 8.3 ± 2.6 |
| −1000/Carotid-femoral pulse wave velocity (ms/mm) | −119 ± 28 | −131 ± 29 | −129 ± 30 |
| Forward wave amplitude (mm Hg) | 50 ± 14 | 48 ± 14 | 48 ± 14 |
| Augmentation Index (%) | 11.3 ± 12.8 | 11.6 ± 12.1 | 11.6 ± 12.2 |
| Mean arterial pressure (mm Hg) | 97 ± 10 | 93 ± 11 | 93 ± 11 |
Continuous variables expressed as mean ± sd and categorical variables as n (%) unless noted. NAFLD, Non-alcoholic fatty liver disease; LPR, liver phantom ratio; LFT, liver function test.
Clinical characteristics were assessed for the Offspring participants at examination 7. Adiposity measures were assessed for the Offspring participants between examination 7 and 8. Peripheral arterial tonometry and arterial tonometry variables were assessed for the Offspring participants at examination 8. Third-generation participants had clinical characteristics and all vascular function measurements assessed at examination 1.
Values represent median (Interquartile range)
Peripheral artery tone measures are natural logarithm transformed.
Correlations between liver attenuation and vascular function measures
In minimally adjusted models, liver attenuation was significantly correlated with all vascular function measures, except for hyperemic mean flow velocity and forward wave amplitude (Table 2). With more liver fat (lower LPR), the response to ischemia as measured by FMD was lower, which is consistent with conduit vessel dysfunction. More liver fat was also associated with a lower PAT ratio, which is consistent with small vessel dysfunction. For the measures of arterial stiffness, more liver fat was associated with a higher CFPWV, which is consistent with greater arterial stiffness.
Table 2.
Age-, sex-, cohort-adjusted associations between liver phantom ratio (LPR) and vascular function measures.
| Vascular function measures | −LPR* | ||
|---|---|---|---|
| N | r | P-value | |
| Brachial artery measures | |||
| Flow-mediated dilation (%) | 2061 | −0.05 | 0.02 |
| Hyperemic mean flow velocity (cm/s) | 2061 | −0.03 | 0.19 |
| Peripheral Arterial Tonometry measures | |||
| Peripheral arterial tone ratio† (unitless) | 1393 | −0.20 | <0.0001 |
| Arterial tonometry measures | |||
| −1,000/Carotid-femoral pulse wave velocity (ms/mm) | 2284 | 0.13 | <0.0001 |
| Forward-wave amplitude (mm Hg) | 2284 | 0.02 | 0.27 |
| Mean arterial pressure (mm Hg) | 2284 | 0.1 | <0.0001 |
Data is modeled such that correlations are expressed per lower levels of LPR.
Peripheral artery tonometry measures are natural logarithm transformed.
Multivariable-adjusted partial correlations for NAFLD with vascular function measures
In the multivariable-adjusted model, NAFLD (LPR ≤ 0.33) was positively associated with mean arterial pressure (MAP) (r=0.06, P=0.005) and inversely associated with the PAT ratio (r= −0.12, P<0.0001) (Table 3). After BMI was added to the multivariable model, the correlation between NAFLD (LPR ≤ 0.33) and MAP was no longer statistically significant (r=0.03, P=0.20). NAFLD (LPR ≤ 0.33) was also positively associated with CFPWV in the multivariable adjusted model (r=0.05, P=0.03); however, when this model was also adjusted for MAP the correlation was no longer statistically significant (r=0.02, P=0.29). The correlation between NAFLD (LPR ≤ 0.33) and the PAT ratio remained statistically significant after additionally adjusting for BMI (r=−0.09, P=0.0005) and VAT (r=−0.08, P=0.004) (Table 3). NAFLD (LPR ≤ 0.33) also was associated with higher baseline brachial artery diameter (multivariable model 1 only), baseline brachial artery mean flow velocity and baseline peripheral artery pulse amplitude (Supplementary Table II). When we evaluated LPR as a continuous variable, results were largely similar. (Table 3). The variance inflation factor was < 1.3 for each of these associations, suggesting the lack of severe multi-collinearity.
Table 3.
Multivariable-adjusted* partial correlations for NAFLD as a dichotomous variable (LPR > 0.33 vs. LPR ≤ 0.33) or a continuous variable with vascular function measures.
| Vascular function measures | Model 1: MV* adjusted |
Model 2: Model 1 + BMI adjusted |
Model 3: Model 2 + VAT adjusted |
||||
|---|---|---|---|---|---|---|---|
| N | r | P-Value | r | P-Value | r | P-Value | |
| Dichotomous fatty liver | |||||||
| Brachial artery measures† | |||||||
| Flow-mediated dilation (%) | 2061 | −0.02 | 0.30 | −0.01 | 0.61 | −0.01 | 0.66 |
| Peripheral Arterial Tonometry measures |
|||||||
| Peripheral arterial tone ratio (unitless) | 1393 | −0.12 | <0.0001 | −0.09 | 0.0005 | −0.08 | 0.004 |
| Arterial tonometry measures | |||||||
| −1,000/Carotid-femoral pulse wave velocity (ms/mm) † |
2284 | 0.05 | 0.03 | 0.03 | 0.23 | 0.007 | 0.73 |
| −1,000/Carotid-femoral pulse wave velocity (ms/mm) |
2284 | 0.02 | 0.29 | 0.01 | 0.55 | −0.004 | 0.85 |
| Mean arterial pressure (mm Hg) | 2284 | 0.06 | 0.005 | 0.03 | 0.20 | 0.02 | 0.26 |
| Mean arterial pressure (mm Hg) ‡ | 2284 | 0.05 | 0.03 | 0.02 | 0.37 | 0.02 | 0.28 |
| Continuous fatty liver | |||||||
| Brachial artery measures† | |||||||
| Flow-mediated dilation (%) | 2061 | −0.04 | 0.06 | −0.03 | 0.15 | −0.03 | 0.17 |
| Peripheral Arterial Tonometry measures |
|||||||
| Peripheral arterial tone ratio (unitless) |
1393 | −0.13 | <0.0001 | −0.10 | 0.0001 | −0.08 | 0.002 |
| Arterial tonometry measures | |||||||
| −1,000/Carotid-femoral pulse wave velocity (ms/mm) † |
2284 | 0.03 | 0.12 | 0.01 | 0.52 | −0.009 | 0.66 |
| −1,000/Carotid-femoral pulse wave velocity (ms/mm) |
2284 | 0.02 | 0.31 | 0.01 | 0.57 | −0.01 | 0.68 |
| Mean arterial pressure (mm Hg) | 2284 | 0.02 | 0.28 | −0.01 | 0.68 | −0.01 | 0.51 |
| Mean arterial pressure (mm Hg) ‡ | 2284 | 0.01 | 0.61 | −0.02 | 0.47 | −0.01 | 0.60 |
NAFLD, Non-alcoholic fatty liver disease; BMI, body mass index; VAT, visceral adipose tissue.
Multivariable models adjusted for age, sex, cohort, smoking, mean arterial pressure (not included in models with mean arterial pressure or CFPWV as dependent variable, unless noted), heart rate, walk test (before, only for brachial measures†), total/high density lipoprotein cholesterol, triglycerides, fasting glucose level, menopause, hormone replacement therapy, diabetes, hypertension treatment and lipid lowering treatment.
not adjusted on mean arterial pressure;
additional adjusted on carotid femoral pulse wave velocity.
PAT ratio according to the presence of NAFLD overall and across BMI categories
The least-square means for the PAT ratio according to the presence of NAFLD overall and by BMI category are shown in Figure 1. Overall, the PAT ratio was lower among participants with NAFLD compared to those without NAFLD (p< 0.0001). In the overweight BMI category, the PAT ratio in participants with NAFLD was lower compared to those without NAFLD (p=0.0006), which is consistent with small vessel dysfunction. In the normal weight and obese BMI categories, the findings are in a similar direction; however, not statistically significant (PAT ratio normal weight NAFLD vs non-NAFLD p=0.26; PAT ratio obese NAFLD vs non-NAFLD p=0.06).
Figure 1. Bar chart depicting the multivariable adjusted least square means of peripheral arterial tone (PAT) ratio + standard error (SE) overall and by Body Mass Index (BMI) categories according to presence or absence of NAFLD.
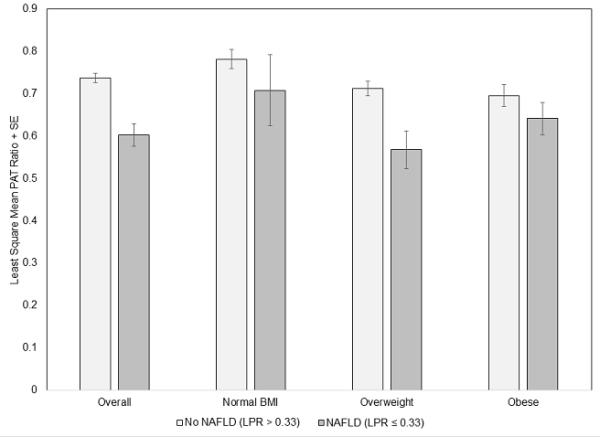
The BMI categories are defined as normal BMI (BMI < 25 kg/m2), Overweight (25 kg/m2 ≤ BMI < 30 kg/m2) and Obese (BMI ≥ 30 kg/m2). No NAFLD represents a normal Liver Phantom Ratio (LPR) (LPR > 0.33) and NAFLD represents an abnormal LPR (LPR ≤ 0.33). Overall, PAT ratio NAFLD vs non-NAFLD p<0.0001;PAT ratio normal weight NAFLD vs non-NAFLD p=0.26; PAT ratio overweight NAFLD vs non-NAFLD p=0.0006; PAT ratio obese NAFLD vs non-NAFLD p=0.06.
PAT ratio according to presence of NAFLD and CRP above and below median
The least-square means for the PAT ratio according to the presence of NAFLD and CRP above and below the median value are shown in Supplementary Figure I. Participants with NAFLD and either CRP < median or CRP ≥ median had a significantly lower PAT ratio compared to those without NAFLD and CRP < median (p=0.008 and p<0.0001 respectively). Among those with NAFLD, there was no difference in the PAT ratio in those with CRP > median vs CRP ≤ median (p=0.99).
PAT ratio according to presence of NAFLD and normal or elevated LFTs
The lease-square means for the PAT ratio according to the presence of NAFLD and elevated LFTs are shown in Supplementary Figure II. Participants with NAFLD and either normal or elevated LFTs had a significantly lower PAT ratio compared to those without NAFLD and normal LFTs (p=0.005 and p=0.0003 respectively). Among those with NAFLD, there was a trend towards a lower PAT ratio among those with elevated LFTs compared to those with normal LFTs; however, this did not meet statistical significance (p=0.41).
Sex interactions
In multivariable models of the main outcome measures, there was no evidence of effect modification by sex (p value range 0.16-0.90).
Analysis limited to the Third-Generation Cohort
In a secondary analysis limited to participants in the Third-Generation Cohort who had all vascular function measurements and the multi-detector CT scans at the same study visit, the results were not substantively changed (Supplementary Table III).
Analysis limited to the participants with all three vascular measures
In a secondary analysis limited to participants who had all three vascular measures (n=1005), the results were not substantively changed (Supplementary Table IV).
Discussion
In this large (n=2284) community-based cohort of participants without apparent CVD, we observed modest associations between lower liver attenuation (more liver fat) and FMD, a measure of conduit artery vasodilator function; PAT ratio, a measure of microvascular function; MAP, a measure of systemic perfusion; and CFPWV, a measure of arterial stiffness. The associations of NAFLD with conduit artery function and arterial stiffness were attenuated and no longer significant after adjusting for CVD risk factors. However, NAFLD remained correlated with measures of microvascular dysfunction including PAT ratio, baseline brachial artery mean flow velocity and baseline peripheral artery pulse amplitude even in models adjusted for CVD risk factors and adiposity measures. These findings are consistent with a potential association between NAFLD and microvascular dysfunction.
We advance the prior literature by evaluating microvascular dysfunction in NAFLD as measured by PAT. Although both markers of vasodilator function, brachial and digital measures of vasodilation evaluate distinct vascular beds and prior work suggests they reflect distinct aspects of vascular function.34-36 We have previously demonstrated differences in the risk factors associated with brachial hyperemia and PAT with low digital vascular function being associated with metabolic risk factors including BMI, cholesterol and the presence of diabetes.34, 37 Relevant to NAFLD, one prior selected study of patients with obstructive sleep apnea identified an association between hepatic steatosis and lower PAT.38 The current investigation demonstrates, in a large community-based sample, an association between PAT ratio and NAFLD after adjusting for cardiovascular and metabolic risk factors. Further, across categories of obesity, the presence of NAFLD was associated with lower PAT hyperemic response. High baseline brachial flow is also gaining appreciation as a metabolic-disease associated vascular alteration.39 In the present study, we observed higher resting flow velocity in participants with NAFLD that may contribute to microvascular damage. These results emphasize the association of NAFLD with abnormalities in the microcirculation.
Previous smaller studies have evaluated the relation of NAFLD with several individual measures of vascular function. In small, clinically selected samples, patients with biopsy proven NAFLD had conduit artery dysfunction, as measured by brachial artery FMD, compared to age- and sex-matched controls.24, 26 The association of clinical NAFLD with reduced FMD persisted when accounting for a limited set of metabolic risk factors.24, 28 NAFLD determined by ultrasound also has been associated with lower endothelium-dependent dilation in clinical samples.22, 25, 31 Similarly, one study showed higher aortic stiffness in patients with biopsy-proven NAFLD.28 In community-based cohorts, an association was observed between sonographically defined NAFLD and higher arterial stiffness measured using a global stiffness measures, brachial-ankle pulse wave velocity, and carotid-femoral pulse wave velocity.28, 29, 32 However, in a study of adolescents with ultrasound defined NAFLD, the association between NAFLD and higher arterial stiffness was limited to participants with high risk metabolic features including greater waist circumference, triglycerides, insulin, systolic blood pressure and lower high-density lipoprotein.27 Thus, whether NAFLD is associated with abnormal vascular function beyond the associated cardiometabolic risk factors remained unclear.
In this study, we had the opportunity to evaluate multiple measures reflecting distinct aspects of vascular health in a community-based sample with a comprehensive assessment of cardiometabolic risk factors. In contrast to prior work, we observed that the NAFLD was no longer associated with conduit artery function or aortic stiffness after adjusting for concurrent risk factors. Thus, our findings suggest that the risk factors that cluster with NAFLD account for the observed vascular dysfunction, particularly in the conduit and large arteries. There are several possible explanations for these apparently discrepant results. First of all, several of the prior studies utilized hospital-based patient samples, which may have a higher prevalence of co-morbid conditions that contribute to conduit artery dysfunction and arterial stiffness compared to our study in a community-based sample. It is also possible that large artery dysfunction and arterial stiffness occur later in the pathogenesis of NAFLD. In addition, the large sample size and detailed assessment of cardiovascular risk factors including adiposity measures allowed for a more complete adjustment for potential confounding in our study. Thus, NAFLD may be associated with vascular dysfunction through processes that also drive the occurrence of traditional risk factors including dyslipidemia, hyperglycemia, insulin resistance, and blood pressure.
Several potential factors may underlie the association of NAFLD with vascular dysfunction. Liver fat accumulation occurs in a complex of metabolic disturbances including abdominal obesity and dyslipidemia.4 Thus, isolating the association of fatty liver from the coexistent metabolic disruptions is not straightforward. Endothelial dysfunction may also contribute to the development of fatty liver.33 It has been proposed that the liver is both exposed to an abnormal metabolic environment and a source of substances that promote vascular damage.13 Fatty liver has been associated with increased pro-inflammatory cytokine production and heightened oxidative stress.13 Thus, reduced vascular function associated with NAFLD may reflect systemic inflammation. Both conduit and small vessel flow-mediated dilation depend on endothelial production of nitric oxide that may be reduced in the setting of inflammation.40 However, it may be that small vessel vasodilator responses have greater susceptibility to metabolic insults, including NAFLD, prior to the development of atherosclerotic disease.17, 34 Further longitudinal studies are needed to define the precise mechanisms linking NAFLD to microvascular damage.
The major strengths of our investigation include our use of a large community-based sample that has not been selected for NAFLD and the detailed assessment of multiple measures of vascular function. In this well-characterized sample with a thorough assessment of covariates using standardized measurements, we are able to add to the current literature by adjusting for several important confounders in exploring the association with endothelial dysfunction and NAFLD in multivariable models.
There are a number of important limitations to our investigation that warrant mention. First, the cross-sectional design of this observational study precludes any inferences on causality or temporality. The FHS is largely Caucasian so results may not be generalizable to individuals of non-European ancestry. Additionally, we defined NAFLD based on CT imaging, which likely underrepresents the burden of NAFLD in the population, which may have led to non-differential misclassification biasing our results to the null.41 Also, CT imaging cannot accurately detect steatohepatitis so we are unable to determine the association of vascular function and NAFLD disease severity. We also lack information about viral hepatitis status and other chronic liver conditions which can cause the appearance of liver fat on CT scan. However, these findings would likely lead to misclassification and would bias our findings towards the null. Thus, they are unlikely to account for our positive results. For the Offspring Cohort participants, there were temporal differences between the vascular measures and CT scans. However, in a sensitivity analysis limited to the Third Generation Cohort participants who had vascular measures and CT scans at the same study visit, our results were similar. Additionally, those participants with available PAT measurements were older and had more cardiometabolic risk factors compared to those with missing data. This may have led to a selection bias away from the null. However, when the analysis was repeated in participants who had all vascular measures, the results were largely unchanged. Overall, while statistically significant, the magnitude of the association between NAFLD and the PAT ratio is modest. Future longitudinal studies are necessary to explore the clinical significance of our findings.
We observed an association between NAFLD and markers of endothelial dysfunction and arterial stiffness. Future longitudinal studies are required to further explore the association between NAFLD and microvascular dysfunction and how this relates to cardiovascular risk.
Supplementary Material
Significance.
Patients with non-alcoholic fatty liver disease (NAFLD) are at risk for cardiovascular disease. We evaluated the association between NAFLD and multiple measures of vascular function in a large (n=2284) community-based cohort of participants without apparent CVD. Overall NAFLD is associated with multiple aspects of vascular function; however, this association is largely attributed to co-existing cardiometabolic risk factors. However, NAFLD remained correlated with measures of microvascular dysfunction in adjusted models. Future longitudinal studies should further explore the association between NAFLD and microvascular dysfunction and how this relates to cardiovascular risk.
Acknowledgements
Sources of Funding: This work was supported by the Boston University School of Medicine and the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract N01-HC-25195), and the Division of Intramural Research of the National Heart, Lung, and Blood Institute. The project was supported by NIH grants R01 AG047645-1, HL102299, 1R01HL60040; HL70100, HL076784, HL077447, HL107385 and the Donald W. Reynolds Foundation. Dr. Long is supported in part by the Boston University Clinical and Translational Science Institute (grant UL1-TR000157). E.K. Speliotes is supported by NIH K23DK08145, The Doris Duke Foundation, Central Society for Clinical Research, and the Department of Internal Medicine and BSSP program at University of Michigan.
Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- CVD
cardiovascular disease
- FMD
flow-mediated dilation
- PAT
peripheral arterial tonometry
- CT
computed tonometry
- BMI
body mass index
- VAT
visceral adipose tissue
- FHS
Framingham Heart Study
- HU
Hounsfield Units
- SAT
subcutaneous adipose tissue
- LPR
liver phantom ratio
- CFPWV
carotid-femoral pulse wave velocity
- MAP
mean arterial pressure
Footnotes
Author contributions: Study concept and design (MTL, RSV, EJB, CSF, NMH); acquisition of data (GFM, JP,RSV,UH,EKS,JAV,EJB,CSF,NMH); Analysis and interpretation of data (MTL, NW, MGL, GFM, RSV, UF, EKS, JAV, EJB, CSF, NMH); drafting of the manuscript (MTL); critical revision of the manuscript for important intellectual content (GFM, JAV, RSV, EJB, CSF, NMH); statistical analysis (NW, MGL); administrative, technical, or material support (UH, FGM, JP); study supervision (CSF,EJB, NMH)
Disclosures: Dr. Mitchell is owner of Cardiovascular Engineering, Inc, a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness.
The other authors report no conflicts.
References
- 1.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the united states from 1988 to 2008. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:524–530. e521. doi: 10.1016/j.cgh.2011.03.020. quiz e560. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Alimentary pharmacology & therapeutics. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Stranges S, Trevisan M, Dorn JM, Dmochowski J, Donahue RP. Body fat distribution, liver enzymes, and risk of hypertension: Evidence from the western new york study. Hypertension. 2005;46:1186–1193. doi: 10.1161/01.HYP.0000185688.81320.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The framingham heart study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arase Y, Suzuki F, Ikeda K, Kumada H, Tsuji H, Kobayashi T. Multivariate analysis of risk factors for the development of type 2 diabetes in nonalcoholic fatty liver disease. Journal of gastroenterology. 2009;44:1064–1070. doi: 10.1007/s00535-009-0091-1. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Lymp JF, St. Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 8.Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, Chim AM, Yu CM, Yu J, Chan FK, Sung JJ, Chan HL. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–1727. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 9.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 10.Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes care. 2006;29:1845–1850. doi: 10.2337/dc06-0455. [DOI] [PubMed] [Google Scholar]
- 11.Adiels M, Taskinen MR, Boren J. Fatty liver, insulin resistance, and dyslipidemia. Curr Diab Rep. 2008;8:60–64. doi: 10.1007/s11892-008-0011-4. [DOI] [PubMed] [Google Scholar]
- 12.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: The multi-ethnic study of atherosclerosis. Atherosclerosis. 2013;227:429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. The New England journal of medicine. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: Has the time come for cardiologists to be hepatologists? Journal of obesity. 2012;2012:483135. doi: 10.1155/2012/483135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndumele CE, Nasir K, Conceicao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1927–1932. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr., Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: The framingham heart study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 17.Kiani S, Aasen JG, Holbrook M, Khemka A, Sharmeen F, LeLeiko RM, Tabit CE, Farber A, Eberhardt RT, Gokce N, Vita JA, Hamburg NM. Peripheral artery disease is associated with severe impairment of vascular function. Vascular medicine. 2013;18:72–78. doi: 10.1177/1358863X13480551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013;2:e000426. doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, Santos RD, Budoff MJ, Nasir K. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 22.Colak Y, Senates E, Yesil A, Yilmaz Y, Ozturk O, Doganay L, Coskunpinar E, Kahraman OT, Mesci B, Ulasoglu C, Tuncer I. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine. 2013;43:100–107. doi: 10.1007/s12020-012-9712-1. [DOI] [PubMed] [Google Scholar]
- 23.Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, Valenti L, Maraschi A, Catapano A, Fargion S. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. The American journal of medicine. 2008;121:72–78. doi: 10.1016/j.amjmed.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 25.Sciacqua A, Perticone M, Miceli S, Laino I, Tassone EJ, Grembiale RD, Andreozzi F, Sesti G, Perticone F. Endothelial dysfunction and non-alcoholic liver steatosis in hypertensive patients. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2011;21:485–491. doi: 10.1016/j.numecd.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Senturk O, Kocaman O, Hulagu S, Sahin T, Aygun C, Konduk T, Celebi A. Endothelial dysfunction in turkish patients with non-alcoholic fatty liver disease. Internal medicine journal. 2008;38:183–189. doi: 10.1111/j.1445-5994.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang RC, Beilin LJ, Ayonrinde O, Mori TA, Olynyk JK, Burrows S, Hands B, Adams LA. Importance of cardiometabolic risk factors in the association between nonalcoholic fatty liver disease and arterial stiffness in adolescents. Hepatology. 2013;58:1306–1314. doi: 10.1002/hep.26495. [DOI] [PubMed] [Google Scholar]
- 28.Vlachopoulos C, Manesis E, Baou K, Papatheodoridis G, Koskinas J, Tiniakos D, Aznaouridis K, Archimandritis A, Stefanadis C. Increased arterial stiffness and impaired endothelial function in nonalcoholic fatty liver disease: A pilot study. American journal of hypertension. 2010;23:1183–1189. doi: 10.1038/ajh.2010.144. [DOI] [PubMed] [Google Scholar]
- 29.Salvi P, Ruffini R, Agnoletti D, Magnani E, Pagliarani G, Comandini G, Pratico A, Borghi C, Benetos A, Pazzi P. Increased arterial stiffness in nonalcoholic fatty liver disease: The cardio-goose study. Journal of hypertension. 2010;28:1699–1707. doi: 10.1097/HJH.0b013e32833a7de6. [DOI] [PubMed] [Google Scholar]
- 30.Yu KJ, Zhang MJ, Li Y, Wang RT. Increased whole blood viscosity is associated with arterial stiffness in patients with non-alcoholic fatty liver disease. Journal of gastroenterology and hepatology. 2013;29:540–544. doi: 10.1111/jgh.12368. [DOI] [PubMed] [Google Scholar]
- 31.Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, Pandey RM, Vikram NK. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in asian indians. Atherosclerosis. 2012;223:507–511. doi: 10.1016/j.atherosclerosis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Lee YJ, Shim JY, Moon BS, Shin YH, Jung DH, Lee JH, Lee HR. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Digestive diseases and sciences. 2012;57:196–203. doi: 10.1007/s10620-011-1819-3. [DOI] [PubMed] [Google Scholar]
- 33.Pasarin M, La Mura V, Gracia-Sancho J, Garcia-Caldero H, Rodriguez-Vilarrupla A, Garcia-Pagan JC, Bosch J, Abraldes JG. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of nafld. PloS one. 2012;7:e32785. doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: The framingham heart study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CR, Bass A, Ellis K, Tran B, Steele S, Caughey M, Stouffer GA, Hinderliter AL. Relation between digital peripheral arterial tonometry and brachial artery ultrasound measures of vascular function in patients with coronary artery disease and in healthy volunteers. The American journal of cardiology. 2012;109:651–657. doi: 10.1016/j.amjcard.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T. Noninvasive vascular function measurement in the community: Cross-sectional relations and comparison of methods. Circulation. Cardiovascular imaging. 2011;4:371–380. doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 37.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the framingham heart study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minville C, Hilleret MN, Tamisier R, Aron-Wisnewsky J, Clement K, Trocme C, Borel JC, Levy P, Zarski JP, Pepin JL. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest. 2014;145:525–533. doi: 10.1378/chest.13-0938. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: The framingham heart study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 40.Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends in cardiovascular medicine. 2006;16:15–20. doi: 10.1016/j.tcm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG, Yu ES, Cho EY. Macrovesicular hepatic steatosis in living liver donors: Use of ct for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


