Abstract
Renal fibrosis, particularly tubulointerstitial fibrosis, is the common final outcome of almost all progressive chronic kidney diseases. Renal fibrosis is also a reliable predictor of prognosis and a major determinant of renal insufficiency. Irrespective of the initial causes, renal fibrogenesis is a dynamic and converging process that consists of four overlapping phases: priming, activation, execution and progression. Nonresolving inflammation after a sustained injury sets up the fibrogenic stage (priming) and triggers the activation and expansion of matrix-producing cells from multiple sources through diverse mechanisms, including activation of interstitial fibroblasts and pericytes, phenotypic conversion of tubular epithelial and endothelial cells and recruitment of circulating fibrocytes. Upon activation, matrix-producing cells assemble a multicomponent, integrin-associated protein complex that integrates input from various fibrogenic signals and orchestrates the production of matrix components and their extracellular assembly. Multiple cellular and molecular events, such as tubular atrophy, microvascular rarefaction and tissue hypoxia, promote scar formation and ensure a vicious progression to end-stage kidney failure. This Review outlines our current understanding of the cellular and molecular mechanisms of renal fibrosis, which could offer novel insights into the development of new therapeutic strategies.
Introduction
An estimated 13% of the adult population in the USA has some degree of chronic kidney disease (CKD),1,2 and a considerable proportion of cases eventually progress to end-stage kidney failure, a devastating condition that requires lifelong dialysis or kidney transplantation. Numerous epidemiological studies indicate that the prevalence of patients with end-stage renal disease is increasing worldwide.1,3,4 As a result, CKD has become a major public health problem on a global scale, which imposes enormous socioeconomic burdens on the affected individuals, families and societies. As the population of patients with diabetes mellitus and obesity continues to grow, it is highly probable that this trend of increasing prevalence of CKD will not come to an end in the foreseeable future. Despite the enormity of this problem, current therapeutic options for CKD in the clinical setting are scarce and often ineffective. An approved treatment specifically targeted to renal fibrosis is almost nonexistent. In this context, an improved understanding of the cellular and molecular mechanisms of renal fibrosis is paramount and essential, not only for gaining novel insights into the pathogenesis of the process, but also for developing rational strategies to treat patients with fibrotic kidney disorders.
Renal fibrogenesis is considered, by and large, to be a failed wound healing process that occurs after the initial insults of various injuries.5,6 In this regard, the cellular and molecular responses in the injured kidneys are largely dictated by evolutionarily conserved defense programs of wound healing in an attempt to repair and recover from damage. Almost all the cell types in the kidneys, including fibroblasts, tubular epithelial cells, pericytes, endothelial cells, vascular smooth muscle cells, mesangial cells and podocytes, as well as the infiltrated cells such as lymphocytes, macrophages and fibrocytes, are involved and participate in some way in the pathogenesis of renal fibrosis, which illustrates the immense complexity of this process.7,8 The scope of this Review is limited to cellular and molecular mechanisms of tubulointerstitial fibrosis, which is the final outcome of all progressive kidney diseases. This focus precludes any detailed discussion of the pathogenesis of glomerulosclerosis. However, key events in tubulointerstitial fibrosis also take place in the glomeruli after injury, which include glomerular infiltration of inflammatory cells, myofibroblastic activation of the mesangial cells and epithelial–mesenchymal transition (EMT) of the podocytes.9–11 Therefore, in many aspects, major fibrogenic mechanisms are common and shared by different tissue compartments in the kidneys.
Major cellular events in tubulointerstitial fibrosis include: infiltration of inflammatory cells; fibroblast activation and expansion from various sources; production and deposition of a large amount of extracellular matrix (ECM) components; and tubular atrophy and microvascular rarefaction (Figure 1). Conceivably, each and every one of these pathologic features could contribute to the relentless progression of fibrosis in its own unique way. Together, they constitute a core set of fibrogenic events that result in the ultimate destruction of renal parenchyma and loss of kidney function. On the basis of the sequence of these destructive events, the pathogenesis of renal fibrosis can be artificially divided into four overlapping phases: priming, activation, execution and progression.5,12 However, such a division is arbitrary, as in reality renal fibrogenesis is a dynamic process in which many of these events occur concomitantly.
Figure 1.
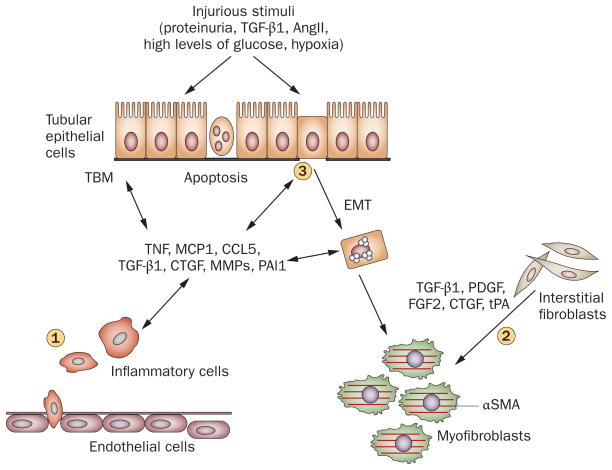
Major events in renal interstitial fibrogenesis. (1) Peritubular infiltration of inflammatory cells, particularly T cells and macrophages, is an early event that sets up a fibrogenic stage. (2) Myofibroblast activation and expansion from various sources. The majority of the matrix-producing myofibroblasts are probably generated from local activation of interstitial fibroblasts. (3) Tubular cell apoptosis and EMT, leading to tubular atrophy. Abbreviations: αSMA, α smooth muscle actin; AngII, angiotensin II; CCL5, chemokine (C–C motif) ligand 5; CTGF, connective tissue growth factor; EMT, epithelial–mesenchymal transition; FGF2, basic fibroblast growth factor; MCP1, monocyte chemotactic protein 1; MMPs, matrix metalloproteinases; PAI1, plasminogen activator inhibitor 1; PDGF, platelet-derived growth factor; TBM, tubular basement membrane; TGF-β1, transforming growth factor β1; TNF, tumor necrosis factor; tPA, tissue-type plasminogen activator.
Priming
Tissue injury provokes inflammation by creating pro-inflammatory niches in which the concentration gradients of chemotactic cytokines provide a directional signal for guiding the infiltration of inflammatory cells to the injured sites.13 Renal fibrosis is almost always preceded by the infiltration of inflammatory cells, including lymphocytes, monocytes/macrophages, dendritic cells and mast cells. Although inflammation is an integral part of the host defense mechanisms in response to injury, nonresolving inflammation is a major driving force in the development of fibrotic disease.14,15 Following injury, infiltrated inflammatory cells become activated, produce molecules that damage tissues such as reactive oxygen species (ROS), and induce the production of fibrogenic cytokines and growth factors.16–19 This series of events builds up sustained profibrotic cytokine pressure within the local microenvironment and primes fibroblasts and tubular epithelial cells to undergo phenotypic activation or transition and to produce a large amount of ECM components. Therefore, inflammation after a sustained injury serves as a primer that sets up the fibrogenic stage and triggers tissue fibrogenesis.
Inflammation sets up the fibrogenic stage
The close correlation between inflammation and the extent of fibrosis has long been established and is the subject of several reviews published in the past 2 years.17–21 A consensus exists that inflammation as a whole has a crucial role in the initiation of renal fibrogenesis after injury, although it is becoming clear that the role of inflammation in the progression of fibrosis is more complicated than previously thought.19,20 Recruitment and activation of T lymphocytes could be an important early event that mediates the onset of renal fibrogenesis, as it typically precedes the influx of macrophages into the injured kidneys. Indeed, mice that lack mature B and T lymphocytes owing to a deficiency of V(D)J recombination-activating protein 1 (RAG1) are protected against fibrosis after obstructive injury.22 Similarly, depletion of CD4+ T cells in wild-type mice considerably reduces the extent of interstitial fibrosis, whereas reconstitution with purified CD4+ T cells in RAG1-knockout mice restores fibrogenesis after injury.22 These findings clearly establish a critical role for lymphocytes, specifically CD4+ T cells, in the onset of renal fibrosis.
The contribution of macrophages to renal fibrogenesis is well documented.18,20 When monocytes from circulating blood are recruited to the injured site in response to cytokine cues, they differentiate into two broad but distinct subsets of macrophages that are categorized as either classically activated (M1) or alternatively activated (M2).19,23 In general, M1 macrophages display a typical proinflammatory phenotype, produce a variety of chemokines, as well as ROS, and therefore have pathogenic functions that lead to tissue damage and fibrosis. Accordingly, depletion of macrophages is beneficial and ameliorates renal fibrosis after various injuries, while adoptive transfer of macrophages aggravates the fibrotic lesions, demonstrating their profibrotic roles in renal fibrogenesis.23–25 In addition to the number of macrophages, the activation status of infiltrating macrophages is another major determinant of injury. For instance, in mice with doxorubicin-induced nephropathy, intravenous infusion of macrophages preactivated by a Toll-like receptor 9 agonist, but not resting macrophages, substantially exaggerates disease progression.26
Dendritic cells, which derive from the same bone marrow myeloid progenitors as macrophages, are abundant in normal kidney interstitium.27–29 As they are antigen-presenting cells, dendritic cells have been shown to create antigenic peptides from albumin through a proteasome-dependent pathway in a remnant kidney model, which consequently activates syngeneic CD8+ T cells.30 In a mouse model of proteinuric diseases, renal dendritic cells capture and present filtered antigens to T cells, leading to the production of proinflammatory cytokines. Depletion of dendritic cells is able to attenuate disease progression.31,32 Altogether, these results indicate that activation of inflammatory lymphocytes, macrophages and dendritic cells is required for the initiation and progression of fibrotic kidney disease, although the role of mast cells in renal fibrogenesis remains controversial and uncertain.33–35 Consistent with this notion, renal fibrosis can be effectively ameliorated by inhibition of nuclear factor κB (NFκB) signaling and/or renal inflammation by a variety of means, such as administration of C–C chemokine receptor type 1 (CCR1) antagonist,36 CCR2 antagonist,37 vitamin D receptor activator,38 peroxisome proliferator-activated receptor γ agonist,39,40 anti-tumor necrosis factor blocking antibody,41 hepatocyte growth factor42,43 or an IL-1 receptor antagonist.44 Notably, the renal protection elicited by inhibition of angiotensin II is also, at least in part, attributable to its nonhemodynamic, anti-inflammatory effects.45
Mechanistic link of inflammation and fibrosis
The link between inflammation and fibrosis is also consistent with and supported by morphological evidence. As fibrotic foci in the injured kidneys are typically instigated and localized in the tissue surrounding renal blood vessels, an inflammatory microenvironment that is probably facilitated by endothelial dysfunction or activation.46 Albeit not tested, the concentration of local cytokines in the inflamed area is presumably very high, and as a result resident fibroblasts and neighboring tubular epithelial cells are readily susceptible to fibrogenic activation.47 The classic view on the connection between inflammation and fibrosis is that they are mediated in a paracrine fashion, whereby inflammatory cells secrete profibrotic cytokines that act on resident fibroblasts and tubular cells to promote fibrogenesis. This assumption is corroborated experimentally, as culture of tubular cells with activated leukocytes in vitro induces EMT and matrix production.48 In addition to the soluble mediators, findings also indicate that direct contact of monocytes and tubular cells might be required to induce tubular EMT by monocytes via an NFκB-dependent pathway.49
Evidence is emerging that an intrinsic connection at the molecular level exists between inflammatory signals and fibrosis within the same cells. For instance, activation of NFκB that is dependent on tumor necrosis factor stabilizes Snail1 by blocking its ubiquitin-mediated degradation.50 As Snail1 is a key transcription factor that promotes EMT, fibroblast migration and renal fibrosis,51–53 this finding provides a molecular link between inflammatory signaling and fibrosis. Activation of NFκB could directly contribute to fibroblast activation and renal fibrosis, as conditional expression of an inhibitor of κB dominant-negative transgene in interstitial fibroblasts attenuates renal fibro-genesis.54 Expression of inhibitor of differentiation 1 (Id1), a dominant-negative transcriptional antagonist, is rapidly and markedly induced in the degenerated kidney tubules after obstructive injury.55 Although Id1 represses the transcription of E-cadherin and tight junction protein ZO-1, leading to the dedifferentiation of tubular epithelial cells,55 it also augments NFκB signaling56,57 and potentiates the expression of CCL5 (also known as RANTES) in tubular cells. The notion that a single molecule such as Id1 promotes both epithelial dedifferentiation and chemokine expression in the same cells illustrates that inflammation and fibrosis are somehow intrinsically connected at the molecular level.
Nonresolving inflammation after chronic injury is a relentless driver of fibrogenesis because it creates a vicious cycle of inflammation, tissue damage and fibrosis. However, in normal wound healing, inflammation is an early beneficial response to injury, and it is spontaneously resolved once the repair and recovery is completed. What factor determines whether inflammation is a physiologic response or a driving force for tissue destruction remains elusive. Nevertheless, studies have begun to shed new light on this issue by identifying multiple mechanisms that normally ensure the resolution of inflammation.15,19 As is widely recognized,19,20,58 inflammatory cells exhibit heterogeneous phenotypes and have incredible functional plasticity, and they also have different roles in the development and recovery of kidney diseases. Unlike M1 macrophages, subsets of M2 macrophages can resolve inflammation and repair injury. In addition, macrophages can switch phenotypes in response to the ever-changing microenvironment and alter their effect from proinflammatory to anti-inflammatory. Along this line, FoxP3+ regulatory T cells (TREG cells) are known to inhibit the inflammatory response and reduce kidney injury,59 and macrophages modified ex vivo by IL-10 or transforming growth factor β (TGF-β) attenuate renal inflammation and structural injury, and preserve kidney function in a mouse model of nephropathy induced by doxorubicin.60 These new data demonstrating the plasticity of inflammatory cell function call for conceptual and strategic innovations in order to develop effective anti-inflammation therapies for the treatment of patients with CKD. Sorting out ‘good’ from ‘bad’ inflammation over the disease models and stages is a daunting task and warrants more and careful investigations.
Activation
The upsurge of profibrotic cytokine pressure in the inflamed microenvironment after kidney injury inevitably leads to the activation of matrix-producing cells, which is arguably a central event in renal fibrogenesis. Although many cell types in the tubulointerstitium of the kidneys, such as fibroblasts, tubular epithelial cells, vascular smooth muscle cells and a subset of macrophages, are capable of producing ECM, fibroblasts are commonly regarded as the principal matrix-producing cells that generate a large amount of interstitial matrix components, including fibronectin and type I and type III collagens. Activated fibroblasts in diseased kidneys often express α smooth muscle actin (αSMA), and are also referred to as myofibroblasts. In this context, one of the fundamental issues in the field is to delineate the origin, activation and regulation of these matrix-producing myofibroblasts.61–63
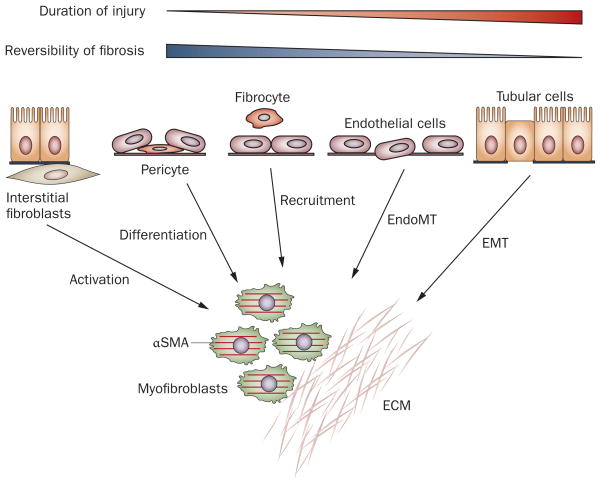
At least five different sources, with diverse mechanisms, have been proposed as contributors to the myofibroblast pool in diseased kidneys (Figure 2). These include activation of interstitial fibroblasts, differentiation of pericytes, phenotypic conversion of tubular epithelial cells and endothelial cells and recruitment of circulating fibrocytes.64 The relative contribution, and even the very existence, of each particular myofibroblast-generating pathway to renal fibrosis is a matter of intense debate and is highly controversial. This controversy is largely attributable to the inherent difficulty in identifying and tracking fibroblasts owing to the lack of specific markers for this cell type. Two other major problems in determining the contributions of the different pathways are that fibroblasts exhibit enormous phenotypic heterogeneity, probably reflecting their diverse origins, and they change their phenotypes depending on the activation status, localization and stage of renal fibrogenesis.
Figure 2.
Multiple origins of myofibroblasts have been proposed in renal fibrosis. Myofibroblasts can be derived from at least five different sources through various mechanisms: phenotypic activation from interstitial fibroblasts; differentiation from vascular pericytes; recruitment from circulating fibrocytes; capillary EndoMT; and tubular EMT. The relative contribution of each source to the myofibroblast pool in renal fibrosis is controversial. Conceivably, local activation of resident fibroblasts remains the major route for the generation of myofibroblasts in diseased kidneys, at least in the early stage. By contrast, EMT could be a late event, and contribute to the irreversible progression of fibrosis. Abbreviations: αSMA, α smooth muscle actin; ECM, extracellular matrix; EMT, epithelial–mesenchymal transition; EndoMT, endothelial–mesenchymal transition.
Activation of fibroblasts and pericytes
Historically, matrix-producing myofibroblasts were assumed to derive from resident fibroblasts by phenotypic activation after renal injury.65 Although this concept has lately been challenged, it remains largely authenticated.62,66 The majority of myofibroblasts could conceivably originate from local activation and proliferation of interstitial fibroblasts in the injured kidneys.
In normal adult kidneys, fibroblasts are situated in the interstitial space between the capillaries and the epithelia and form a network throughout the renal parenchyma, thereby stabilizing tissue architecture.27 Morphologically, these cells are stellate shaped and exhibit abundant rough endoplasmic reticulum, collagen-secreting granules and actin filaments. They possess multiple cell processes, which connect them to the tubular and capillary basement membranes.27 In the resting, quiescent state, interstitial fibroblasts express CD73 (also known as ecto-5′-nucleotidase) in their plasma membrane, and produce erythropoietin.27,67 They also express platelet- derived growth factor receptor β (PDGFRβ),8,68,69 and fibroblast-specific protein 1 (FSP1; also known as S100A4), a small protein that binds calcium and is associated with the cytoskeleton.7,70,71 Fibroblasts maintain interstitial matrix homeostasis in physiologic conditions by producing a basal level of ECM components. Upon activation by profibrotic cytokines and mechanical stress in vivo, fibroblasts acquire a myofibroblast phenotype by expressing αSMA and producing a large amount of ECM components. Myofibroblasts also retain FSP1 and PDGFRβ and express vimentin (an intermediate filament protein) de novo. In many respects, myofibroblasts resemble both activated fibroblasts and smooth muscle cells because of their excessive production of matrix components and their expression of αSMA and contractility, respectively.
Several markers have been used to characterize fibroblasts and myofibroblasts in the kidneys (Table 1). Unfortunately, none of these markers is specific. In addition, they are rarely expressed by all fibroblasts and myofibroblasts or are hardly ever present all the time. Even αSMA, a classic marker of myofibroblast activation in organ fibrosis of all types,72,73 is not without problems. The expression of αSMA is not exclusive to myofibroblasts, as it is also present in vascular smooth muscle cells.62,66 In addition, not all activated fibroblasts express αSMA all the time. Although the majority of cells that produce collagen I are shown to also express αSMA in a collagen I α1-green fluorescent protein reporter mouse model,74 production of αSMA and matrix components are not always functionally coupled in vivo.75 Despite these problems, extensive studies indicate that the abundance of αSMA is closely correlated with the severity of renal fibrosis and predicts the decline of kidney function.65
Table 1.
Markers for renal interstitial fibroblasts and myofibroblasts
| Marker | Fibroblasts | Myofibroblasts | Function | Other cell types |
|---|---|---|---|---|
| αSMA | Negative | Very positive | Stress fiber, cell contractility | Vascular smooth muscle cells |
| FSP1 | Positive | Positive | Actin-binding protein, cell motility | CD45+, leukocytes |
| Vimentin | Negative | Very positive | Intermediate filament protein, cytoskeleton | Glomerular podocytes |
| Nestin | Negative | Positive | Intermediate filament protein, cytoskeleton | Glomerular podocytes |
| CD73 | Very positive | Positive | Ecto-5′-nucleotidase, conversion of 5′-AMP to adenosine | Proximal tubular cells, mesangial cells, T cells |
| PDGFRβ | Positive | Positive | Cell proliferation, matrix production | Vascular smooth muscle cells, pericytes |
Abbreviations: αSMA, α smooth muscle actin; CD, cluster of differentiation; FSP1, fibroblast-specific protein 1; PDGFRβ, platelet-derived growth factor receptor β.
Vimentin is another marker for fibroblast activation, as it is expressed de novo specifically in the activated but not quiescent fibroblasts of adult kidneys.27 However, vimentin is also expressed in glomerular podocytes.76 Similarly, nestin (another intermediate filament protein) could be a useful marker of fibroblast activation,8 but is also found in glomerular podocytes.76 Other fibroblast markers have their own drawbacks. For instance, although FSP1 was initially characterized as specific for fibroblasts and myofibroblasts, studies suggest that it also co-localizes with leukocytes.27,74 With regard to CD73, it is expressed not only in interstitial fibroblasts, but also in several other cell types, including proximal tubular cells, mesangial cells and T cells.27 PDGFRβ is found in fibroblasts, myofibroblasts, vascular smooth muscle cells and pericytes.8,69
Activated fibroblasts are often characterized by two key features: proliferation and myofibroblastic activation. The latter is illustrated by αSMA expression and matrix production. Both fibroblasts and myofibroblasts have the capacity to proliferate in response to cytokine cues, which leads to expansion of the fibroblast population and interstitial space in diseased kidneys. Several fibrogenic growth factors, including PDGF, TGF-β, basic fibroblast growth factor (FGF2) and connective tissue growth factor (CTGF), are well-known mitogens for fibroblasts.65,68,77–80 In addition to these classic cytokines, studies indicate that tissue-type plasminogen activator is another critical factor that promotes fibroblast survival, proliferation and myofibroblastic activation.81–84 The action of tissue-type plasminogen activator is independent of its protease activity, and is mediated by its ability to recruit β1 integrin.84,85 Interestingly, two downstream effectors of integrin signaling—focal adhesion kinase and integrin-linked kinase (ILK)—control fibroblast proliferation and matrix production, respectively.82 Therefore, it seems that cell proliferation and matrix production, two events that characterize fibroblast activation, could be uncoupled at the molecular level.
Studies suggest that vascular pericytes are a major source of myofibroblasts in fibrotic kidneys.63,74,86,87 Pericytes are a subset of the stromal cells that partially cover capillary walls, thereby stabilizing the endothelium. Following kidney injury, pericytes are detached from the endothelium, undergo migration and proliferation, and differentiate into myofibroblasts (Figure 2).74,87 The story of pericytes is quite interesting, because pericyte detachment and differentiation into myofibroblasts under pathological conditions not only results in destabilization of the microvasculature, but also contributes to myofibroblast activation, which leads to interstitial fibrosis. However, because the markers for pericytes, such as PDGFRβ, are not specific and are present in many cell types including fibroblasts, and because fibroblasts are intricately connected to the capillaries via cell processes in renal interstitium, whether the pericytes identified and interstitial fibroblasts are the same entity remains to be elucidated.27
Epithelial–mesenchymal transition
Another source of matrix-producing cells could be tubular epithelium that undergoes EMT, a cell phenotypic conversion process that occurs during embryonic development, tumor metastasis and organ fibrosis.47,53,88–90 Similarly, fibroblasts and/or myofibroblasts might derive from capillary endothelium by endothelial–mesenchymal transition (EndoMT).91,92 EndoMT is considered to be a unique form of EMT, as endothelial cells are a specialized type of epithelia. The contribution of EMT to renal fibrosis is controversial and is the focus of several reviews and debates.86,93–99
Broad agreement exists that tubular epithelial cells in vitro can undergo EMT, characterized by loss of epithelial features and acquisition of mesenchymal markers, under the bombardment of various profibrotic cytokines, particularly TGF-β1.87,100 However, whether this transition merely represents an in vitro artifact or does occur in vivo is at the center of the argument. Using a genetic-lineage tracking, fate-mapping technique, an early study demonstrated that over one-third of FSP1+ interstitial fibroblasts were derived from tubular epithelia in a mouse model of obstructive nephropathy.101 Likewise, two independent studies also show that endothelial cells make a substantial contribution to the generation of fibroblasts and/or myofibroblasts via EndoMT in a variety of fibrotic kidney diseases.91,92 However, these results have been challenged by a number of similar cell-fate-mapping studies in which no epithelial or endothelial origin of fibroblasts is evident.87,102 Thus far, the reason behind these discrepancies is unsettled.
A large number of studies have demonstrated a potential role of EMT in kidney fibrosis by examining the phenotypic conversion of tubular cells in animal models as well as in renal biopsy samples from patients with CKD.103–107 Tubular expression of fibroblast markers such as αSMA, vimentin and FSP1 in fibrotic kidneys is well documented.8,103,105,106,108 Key mediators of EMT-regulatory signaling such as TGF-β1/Smad, ILK, Wnt/β-catenin and Snail1 are preferentially activated in renal tubular epithelia after injury.109,110 Furthermore, morphological evidence indicates that epithelial cells do indeed traverse the tubular basement membrane (TBM) into the interstitium after injury.111
EMT is a dynamic process in which epithelial cells and fibroblasts represent two extremes of a continual spectrum of intermediate cell phenotypes. The frequency at which epithelial cells complete the entire EMT course and ultimately become fibroblasts is probably limited, and depends heavily on the disease models, stages and persistence of raised cytokine pressure in the inflamed milieu.112 In most circumstances, tubular cells undergo a partial EMT, in which epithelial cells only change one or two phenotypic markers, although the transcriptional program of EMT is activated. Such a partial EMT, however, is closely associated with poor outcomes and predicts the progression toward interstitial fibrosis in humans.107 Not surprisingly, blockade of EMT by a variety of agents, such as hepatocyte growth factor,105 bone morphogenetic protein 7,106 ILK inhibitor,110 Wnt antagonists,109,113 paricalcitol,114 or induction of endogenous heat shock protein 72,115 ameliorates renal fibrosis and preserves kidney function.
Recruitment of circulating fibrocytes
Fibrocytes are a subset of bone marrow-derived, circulating monocytes with fibroblast-like features in the peripheral blood.116 They are spindle-shaped cells that express hematopoietic cell marker CD45 and are capable of producing type I collagen.117,118 Fibrocytes also express certain chemokine receptors, such as CCR7. In response to kidney injury, fibrocytes mobilize, infiltrate renal parenchyma and participate in fibrogenesis. The differentiation of fibrocytes is modulated by other inflammatory cells, such as CD4+ T cells, through secreted cytokines.119 Profibrotic cytokines IL-4 and IL-13 promote fibrocyte differentiation, whereas antifibrotic cytokines IFN-γ and IL-12 inhibit this process, suggesting that an inflamed milieu that contains a complex mixture of cytokines is a major determinant of fibrocyte differentiation.120 Interestingly, ciclosporin, but not sirolimus, promotes the development of fibrocytes, which could partly explain the nephrotoxicity of ciclosporin (a calcineurin inhibitor) in the clinical setting.119 In mouse models of renal fibrosis, pharmacological inhibition of angiotensin II type 1 receptor reduces the number of fibrocytes in both the kidney and the bone marrow and ameliorates renal fibrosis.121 This finding suggests that activation of the renin–angiotensin system could contribute to the pathogenesis of renal fibrosis by regulating the differentiation and expansion of fibrocytes.121
The relative importance of fibrocytes in renal fibro-genesis is another area full of controversy. As specific markers for these cells are lacking, clear discrimination of fibrocytes from monocytes, macrophages, fibroblasts and myofibroblasts is a great challenge. In addition, subpopulations of fibrocytes seem to exist.118 Thus far, results from experimental studies on the involvement of fibrocytes, and bone marrow-derived cells in general, in renal fibrosis are inconsistent.74,101,119,122 Although some studies indicate that a considerable percentage of all collagen-producing fibroblasts in a mouse model of obstructive nephropathy originate from fibrocytes or bone marrow-derived cells,101,119 other studies in the same model show that bone marrow-derived cells hardly contribute to the pool of collagen-producing cells at all.23,74,122 Clarification of this issue needs further investigation.
The different origins of fibroblasts probably contribute to their phenotypic heterogeneity. The relative contribution of each lineage to the myofibroblast pool might depend on the disease model and specific stage of the disease. Conceivably, myofibroblast activation from fibroblasts, pericytes or fibrocytes is an early event,27 whereas EMT often takes place at a late stage after a sustained injury (Figure 2).112 The pathological effects of the early activation of fibroblasts and EMT on renal fibrosis might be different. Although activation of fibroblasts is important for the onset of renal fibrosis, EMT could be a major determinant of fibrosis progression and irreversibility (Figure 2).
Execution
Regardless of their different origins, activated fibroblasts from all sources produce a large amount of ECM components, leading to excessive accumulation and deposition of interstitial matrix and formation of collagenous fibers that predominantly contain type I and type III collagens and fibronectin. How these fibrotic matrix proteins are synthesized, secreted, properly assembled in the interstitial space and finally modified to resist proteolysis is a central question to understanding the execution phase of renal fibrogenesis.
Assembly of fibrogenic molecular machinery
The expression and synthesis of ECM proteins by the matrix-producing cells is primarily controlled at the level of gene transcription in response to various extracellular fibrogenic cues. Key fibrogenic factors include TGF-β1, PDGF, FGF2, CTGF and angiotensin II, whereas hepatocyte growth factor and bone morphogenetic protein 7 inhibit the production of matrix components primarily by antagonizing TGF-β1 action.68,123–127 Through their respective receptors and specific downstream intra-cellular signal cascades, these fibrogenic cytokines activate a host of transcription factors that act on the cognate elements in the promoter regions of the collagen and fibronectin genes to activate their transcription. Such signal transduction cascades and expression of matrix genes are also regulated by a variety of microRNAs.128–132 These studies have provided important insights into understanding the regulation of matrix genes at the molecular level. However, whether the data obtained from these in vitro studies truly reflect the regulation of matrix genes in fibrotic kidneys in vivo remains uncertain. As fibroblasts in the fibrotic milieu are typically bombarded with a cocktail of cytokines, how they respond to these stimuli in a coordinated fashion is an unsolved conundrum.
Activated fibroblasts contain stress fibers and display abundant transmembrane connections (also known as fibronexus)133 between extracellular fibronectin-containing matrix and intracellular actin microfilaments, suggesting that an increased interaction of ECM and integrins occurs.8 Integrins are a family of heterodimeric transmembrane receptors that consist of α and β subunits and bind to the ECM and to the cytoskeleton, thereby integrating the ‘outside-in’ and ‘inside-out’ signals between cells and their extracellular environment.134,135 Integrins transmit their signals by activating the downstream effector kinase, focal adhesion kinase and ILK, as they possess no enzymatic or actin-binding activity. Extensive studies indicate that ILK, a scaffolding or adaptor protein and a serine/threonine protein kinase, is especially suited to serve as a molecular platform that integrates various fibrogenic signals.136
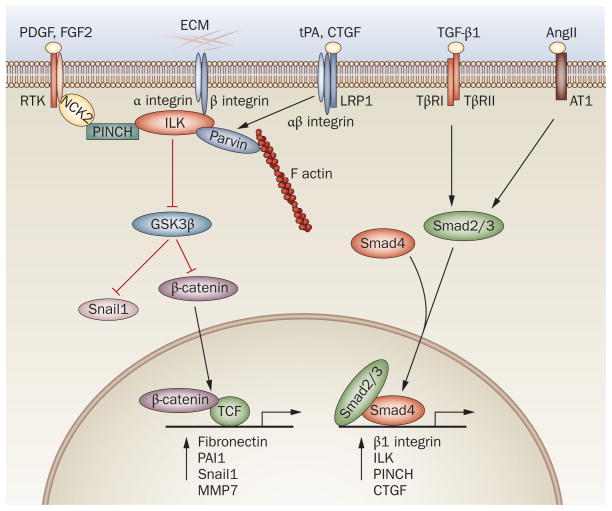
Structurally, ILK contains four ankyrin repeats, a pleckstrin homology domain, a kinase catalytic domain and an integrin-binding domain. Whether ILK is a true kinase remains to be resolved,137,138 but a consensus exists that overexpression of ILK leads to glycogen synthase kinase 3β (GSK3β) phosphorylation and inactivation, which results in stabilization of Snail1 and β-catenin.11,110 β-catenin directly controls the transcription of fibronectin and plasminogen activator inhibitor 1,139,140 whereas Snail1 regulates fibroblast motility and tubular EMT.90 Perhaps more importantly, ILK, as a scaffolding protein, assembles a multicomponent protein complex that contains ILK, PINCH and parvin.136,141 These features enable ILK to bridge the integrins, actin cytoskeleton and other growth factor signal cascades, as PINCH connects to receptor tyrosine kinase signaling via cytoplasmic protein NCK2 and parvin binds to F actin (Figure 3). Interestingly, either disruption of this complex formation or inhibition of ILK activity blunts fibronectin expression, deposition and extracellular assembly, which suggests that the integrity and activity of this complex are obligatory for matrix production.110,142,143
Figure 3.
A multicomponent, integrin-associated protein complex constitutes the molecular machinery that integrates various fibrogenic signals and orchestrates matrix production and assembly. Integrins and ILK and their associated proteins constitute the core components of this machinery. TGF-β1 regulates the expression of major components of this complex such as β1 integrin, ILK and PINCH via Smad signaling. AngII activates Smad3 signaling through pathways that are either dependent on or independent of TGF-β1, which leads to upregulation of the components of this complex. PDGF and FGF2 activate their respective receptor tyrosine kinases and influence this complex via cytoplasmic protein NCK2. CTGF and tPA bind to LRP1 and recruit β1 integrin, and then activate this machinery. This complex also activates β-catenin and Snail1 through inhibition of GSK3β, and thereby directly controls the expression of various fibrosis-related genes. The red lines indicate inhibition, the black lines indicate promotion. Abbreviations: AngII, angiotensin II; AT1, angiotensin II type I receptor; CTGF, connective tissue growth factor; ECM, extracellular matrix; FGF2, basic fibroblast growth factor; GSK3β, glycogen synthase kinase 3β; ILK, integrin-linked kinase; LRP1, LDL receptor-related protein 1; MMP7, matrix metalloproteinase 7; NCK2, non-catalytic region of tyrosine kinase adaptor protein 2; PAI1, plasminogen activator inhibitor 1; PDGF, platelet-derived growth factor; PINCH, particularly interesting new cysteine-histidine rich protein; RTK, receptor tyrosine kinase; TCF, T cell factor; TGF-β1, transforming growth factor β1; TβR1, type I TGF-β receptor; TβRII, type II TGF-β receptor; tPA, tissue-type plasminogen activator.
Increasingly, it is becoming clear that integrins, ILK and their associated proteins constitute a fibrogenic molecular machinery that orchestrates the production and assembly of the ECM. Analogous to the concept of the inflammasome,14 we propose this multicomponent, integrin-associated protein complex as a ‘matrisome’, which is a molecular platform activated as a result of injury that integrates various fibrogenic signal inputs and triggers the production and assembly of matrix components (Figure 3). This view is supported by copious studies demonstrating that integrin signaling is essential for the production and assembly of matrix proteins.134,144 More importantly, it seems that almost all the key fibrogenic cues somehow promote the production of matrix components by regulating this molecular machinery, either directly or indirectly. For example, TGF-β1 is known to induce the expression of β1 integrin, ILK and PINCH1, the major components of this complex, and promote their assembly.134,142,144,145 ILK is also upregulated by many fibrogenic factors, including CTGF and high levels of glucose.11,145–147 Angiotensin II is known to induce the expression of TGF-β1, and activates Smad signaling in both TGF-β-dependent and TGF-β-independent manners.148–150 Intriguingly, angiotensin II also induces the expression of β1 integrin and ILK,147 and thus its profibrotic effects might be attributable to facilitating formation of the integrin–ILK complex. PDGF and FGF2 signaling is potentially connected to this machinery through PINCH1.141 In fibroblasts, tissue-type plasminogen activator promotes collagen I expression by triggering the LDL receptor-related protein 1 (LRP1)-mediated recruitment of β1 integrin and ILK activation.84 Of interest, CTGF can bind to both integrins and LRP1, leading to activation of ILK and production of matrix components. In essence, this molecular machinery serves as a platform that integrates diverse ‘outside-in’ fibrogenic signals and controls matrix production in a coordinated fashion (Figure 3).
Matrix deposition and modification
Assembly and deposition of fibrillar collagens in vivo are also an integrin-dependent event. In the early stage of renal fibrosis, deposition of fibronectin precedes the production of fibrillar collagens. Fibronectin activates integrins, co-localizes with procollagen secretion and forms loose scaffolding for fibrillar collagens. This process is followed by the production of a variety of new matrix proteins, such as secreted protein acidic and rich in cysteine (SPARC) and type IV collagen, as well as vitro-nectin, thrombospondin, decorin and heparan sulfate proteoglycan.151 In addition to fibronectin, SPARC seems to be particularly important in regulating the assembly of fibrillar collagen.152 SPARC is a so-called matricellular protein that binds collagen, and its expression in adult tissues is frequently associated with excessive deposition of collagen.152 SPARC-null mice fail to generate a robust fibrotic response to a variety of stimuli.152 Interestingly, SPARC is able to bind to β1 integrin and activates ILK signaling,153 which suggests a potential connection between the integrin–ILK complex and matrix assembly.
In the early stage of renal fibrosis, the collagen matrix is susceptible to proteolysis, and therefore the fibrosis is potentially reversible, which leads to wound healing. However, as fibrosis progresses, matrix in the fibrotic kidneys is considerably modified compared with normal tissues. Biochemical modifications of the matrix proteins by cross-linking are induced by enzymes such as tissue transglutaminase and lysyl oxidase, rendering them stiff and resistant to proteolysis.151 In biopsy samples from patients with CKD, the expression of tissue transglutaminase is upregulated and correlates closely with the severity of renal fibrosis.8 Consistently, both genetic ablation and pharmacological inhibition of tissue transglutaminase in mice substantially reduce renal fibrosis after injury.154,155
Progression
The concept that renal fibrosis is solely the result of excessive accumulation of matrix components is overly simplistic and inaccurate, and might be erroneous. Whether fibrosis itself actually causes kidney failure remains an open question; after all, it is the normal early response to wound healing. Many cellular and molecular events beyond the production of matrix components promote the progression of fibrotic injury and are actually responsible for the progressive loss of kidney function. In many ways, the events that occur in the progression phase determine the reversibility and ultimate outcome of renal fibrogenesis.
Tubular injury and atrophy
Tubular epithelial cells comprise the bulk of renal parenchyma and are the primary target of a variety of metabolic, immunologic, ischemic and toxic insults. Depending on the severity and duration of injury, tubular cells exhibit a wide range of responses, such as proliferation, autophagy, growth arrest, EMT and apoptosis. In patients with CKD, the histopathological presentation of tubular damage is often characterized as tubular atrophy, presumably as a consequence of apoptosis and EMT.
The potential involvement of tubular epithelia in renal fibrosis is illustrated by the preferential activation of major inflammatory and fibrogenic signaling in renal tubules after injury. In response to cell stress or injuries, NFκB signaling is activated in renal tubular epithelium, which triggers the production and release of inflammatory cytokines, and initiates peritubular inflammation. Tubular epithelial cells are susceptible to TGF-β1, produced by both the injured tubule and the infiltrating cells, and instigate a fibrogenic program that includes EMT. In tubular epithelial cells, sustained injury also activates ILK,145 β-catenin,109,156 Notch1157,158 and hypoxia-inducible factor 1 (HIF1),159,160 all of which are key intracellular signaling mediators implicated in renal fibrogenesis. In essence, tubular activation of major fibrogenic signaling, such as NFκB, Smad and β-catenin is characteristic of various fibrotic kidney diseases.38,131,156
Damage to tubular epithelia often instigates protective and regenerative responses such as autophagy and cell proliferation, at least initially. Autophagy, a process of cell ‘self-eating’, is regarded as a mechanism of cell survival and protection that mediates adaptation to calorie restriction or hypoxia in renal tubules in vivo.102,161,162 However, excessive autophagy is proposed as the underlying mechanism that leads to total decomposition of tubular cells, as demonstrated in tetracycline-controlled TGF-β overexpression transgenic mice.163 Although cell proliferation is increased in the renal tubules of fibrotic kidneys, the net mass of epithelial cells is decreased, presumably owing to an increased rate of apoptosis and EMT, leading to tubular atrophy. Furthermore, defects in progression of the cell cycle after injury, such as arrest at G2/M phase, cause tubular cells to switch to a pro-fibrotic phenotype with an increased expression of TGF-β, thereby promoting fibrosis.164
Tubular injury and atrophy undoubtedly impair tubular function and diminish the effectiveness of its endogenous protective mechanisms. For instance, levels of bone morphogenetic protein 7, an antifibrotic factor produced by tubular cells in normal kidneys, are progressively reduced in patients with CKD.165 Proximal tubules are also the site of active vitamin D synthesis through the action of 1 α hydroxylase. Therefore, tubular atrophy could cause vitamin D deficiency, while defective vitamin D receptor signaling promotes EMT, tubular atrophy and renal fibrosis, establishing a vicious cycle.156,166–168 Chronic injury not only damages tubular cells but impairs the integrity of the underlying TBM as well. Although a thickened TBM is a major pathological feature of advanced CKD, the integrity of the TBM is impaired and type IV collagen, a major component of TBM, is transiently downregulated in renal fibrogenesis.111,169 Consistently, matrix metallo-proteinase 2 (MMP2) and MMP9, the proteases that degrade type IV collagen and laminin, are induced in the injured kidneys,111 and transgenic expression of MMP2 or knock out of MMP9 promotes or ameliorates renal fibrosis, respectively.169,170 Therefore, MMPs could have a dual function in the pathogenesis of renal fibrosis that is specific to the stage of fibrosis.171 Although MMPs might protect the kidney by reducing matrix accumulation via promoting degradation of the ECM in the advanced stage, their activation in the early stage is generally pathogenic by impairing the integrity of the TBM and facilitating EMT.111,169
Microvascular rarefaction
Renal fibrosis is typically associated with peritubular microvascular rarefaction, particularly in the advanced stage. Multiple mechanisms could account for the loss of peritubular capillaries in the fibrotic kidneys. As discussed above, the process of EndoMT not only generates the matrix-producing fibroblasts and/or myofibroblasts, but also directly leads to the loss of endothelial cells. Accordingly, blockade of EndoMT by a specific inhibitor of Smad3 (SIS3) can preserve endothelium, reduce renal fibrosis and retard the progression of diabetic nephropathy.172 In addition, activation of myofibroblasts from pericytes under pathological conditions causes pericyte deficiency and destabilizes the endothelium, eventually resulting in microvascular rarefaction.63 When PDGFRβ signaling in pericytes is blocked by circulating soluble receptor ectodomains, capillary rarefaction and renal fibrosis are attenuated in an animal model of progressive CKD.173 Furthermore, pericyte–myofibroblast differentiation triggers a switch in secretion of vascular endothelial growth factor (VEGF) isomers (from VEGF164 to VEGF120 and VEGF188), leading to endothelial loss and microvascular rarefaction in renal fibrogenesis.173
Peritubular vasculature is susceptible to damage induced by apoptosis of endothelial cells in advanced CKD. Ischemia and oxidant stress are the known apoptotic stimuli for endothelial cells, which could be a potential mechanism that leads to rarefaction of peritubular capillaries.174,175 Likewise, the integrity of peritubular capillaries could be impaired by endothelial dysfunction. In endothelial cells, activation of the receptor for advanced glycation end products, a common finding in advanced renal fibrosis, induces superoxide generation, activation of NADPH oxidase and p38 mitogen-activated protein kinase and nuclear translocation of NFκB.176 In mice that lack functional Crim1, a protein involved in endothelial maintenance and integrity, the peritubular capillaries are dysfunctional, permeable and encircled by collagen deposition, which results in age-related fibrosis.177
Chronic hypoxia
Chronic hypoxia in renal interstitium is characteristic of advanced CKD, and is a common pathology in patients with this condition.178–180 Several mechanisms lead to chronic hypoxia in diseased kidneys, including microvascular rarefaction, decreased oxygen diffusion as a result of fibrosis and increased metabolic demands of tubular cells. Hypoxia can lead to tubular EMT or apoptosis, activate resident fibroblasts and impair peritubular capillaries, thereby creating a cycle of chronic hypoxia and progressive kidney failure. HIF1 is a key regulator of cell responses to hypoxia.181 Although HIF1 controls a battery of genes that mediate adaptive responses against hypoxia, inappropriate activation of HIF1 can be deleterious as it evokes EMT and induces the expression of fibrogenic genes.159,160
Chronic hypoxia often coexists with increased oxidative stress and generation of ROS, which incites damage to biologically important macromolecules, including proteins, lipids and carbohydrates, and causes them to undergo structural modifications, leading to generation of advanced glycation end products, advanced oxidation protein products and advanced lipoperoxidation end products.182,183 These modified macromolecules are not merely a reliable surrogate marker for estimating the degree of oxidant-mediated damage in CKD, they also affect various kidney cells as pathogenic mediators and trigger renal inflammation and fibrogenic responses, thereby promoting the progression of kidney injury and renal fibrosis.184–187 In addition, the hypoxic and fibrotic microenvironment might lead to perturbed cellular signaling and induce epigenetic modifications, which could contribute to increased fibroblast proliferation, activation and fibrogenesis.188
Conclusions
Renal fibrogenesis is an enormously complex, dynamic process that involves almost all the cell types in the kidneys, as well as the infiltrated cells. Over the past decade, our understanding of renal tubulointerstitial fibrosis, particularly as to how inflammation, fibroblast activation, tubular and microvascular injury contribute to fibrogenesis, has considerably evolved and improved.7,8 We now have a better appreciation than previously for the diverse origins and phenotypic heterogeneity of the matrix-producing fibroblasts. Furthermore, the newly characterized novel actions of several well-known factors in fibrosis, such as proteases and macrophages, have challenged the established dogma in the field and represent a paradigm shift in our thinking about their roles in renal fibrogenesis and injury repair.19,189
Many gaps exist in our understanding of renal fibrogenesis, and the challenges ahead are daunting. The relative contribution of each fibroblast-generating pathway to renal fibrogenesis is difficult to delineate, and is perhaps dependent on the disease context and stage. Another important area that deserves attention is how the matrix-producing cells integrate the innumerable fibrogenic signals in the inflamed microenvironment and produce matrix in a coordinated fashion. New findings on macrophage heterogeneity and plasticity also necessitate conceptual and strategic innovations in the development of future therapeutics. As a variety of strategies that target key pathogenic mediators and pathways to halt renal fibrosis are effective in animal models, some of these remedies should eventually become clinically relevant for patients with CKD in the future.
Key points.
Despite having various initial causes, renal fibrogenesis is a converging and highly dynamic process, which consists of four overlapping phases: priming, activation, execution and progression
After a sustained injury, nonresolving inflammation sets up a fibrogenic stage (priming) and triggers the activation and expansion of matrix-producing fibroblasts from multiple sources through a range of mechanisms
Upon activation, the matrix-producing cells build a multicomponent, integrin-associated, ternary protein complex, which integrates various fibrogenic signals and orchestrates the production of extracellular matrix and its assembly
Many cellular and molecular events, such as tubular atrophy, vascular rarefaction and hypoxia, promote the progressive loss of kidney function and determine the outcome of renal fibrosis
Review criteria.
The PubMed database was searched for English-language articles published up to June 2011, using the following search terms: “renal fibrosis”, “kidney fibrosis”, “myofibroblast”, “epithelial–mesenchymal transition”, “integrins” and “renal inflammation”. This Review is primarily focused on recent literature published after January 2006, although older papers that are very relevant to this topic were also selected.
Acknowledgments
I apologize to all colleagues whose important findings could not be cited due to space limitations. Our works described in this Review were supported by the National Institutes of Health grants DK064005 and DK071040.
Footnotes
Competing interests
The author declares no competing interests.
References
- 1.Coresh J, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System. Annual Data Report 2009. 2010 [online], http://www.usrds.org/adr.htm.
- 3.Sharma SK, et al. Burden of CKD, proteinuria, and cardiovascular risk among Chinese, Mongolian, and Nepalese participants in the International Society of Nephrology screening programs. Am J Kidney Dis. 2010;56:915–927. doi: 10.1053/j.ajkd.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008;51:373–384. doi: 10.1053/j.ajkd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 8.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi Y, et al. Epithelial-mesenchymal transition as an explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–664. doi: 10.1053/j.ajkd.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Kang YS, et al. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010;78:363–373. doi: 10.1038/ki.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 13.Chung AC, Lan HY. Chemokines in renal injury. J Am Soc Nephrol. 2011;22:802–809. doi: 10.1681/ASN.2010050510. [DOI] [PubMed] [Google Scholar]
- 14.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Vielhauer V, Kulkarni O, Reichel CA, Anders HJ. Targeting the recruitment of monocytes and macrophages in renal disease. Semin Nephrol. 2010;30:318–333. doi: 10.1016/j.semnephrol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Vernon MA, Mylonas KJ, Hughes J. Macrophages and renal fibrosis. Semin Nephrol. 2010;30:302–317. doi: 10.1016/j.semnephrol.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Harris DC. Macrophages in renal disease. J Am Soc Nephrol. 2011;22:21–27. doi: 10.1681/ASN.2010030269. [DOI] [PubMed] [Google Scholar]
- 21.Grande MT, Pérez-Barriocanal F, López-Novoa JM. Role of inflammation in tubulointerstitial damage associated to obstructive nephropathy. J Inflamm. 2010;7:19. doi: 10.1186/1476-9255-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapmeier TT, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78:351–362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 23.Lin SL, Castaño AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 24.Henderson NC, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2008;23:842–852. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, et al. By homing to the kidney, activated macrophages potently exacerbate renal injury. Am J Pathol. 2008;172:1491–1499. doi: 10.2353/ajpath.2008.070825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochem Cell Biol. 2008;130:247–262. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John R, Nelson PJ. Dendritic cells in the kidney. J Am Soc Nephrol. 2007;18:2628–2635. doi: 10.1681/ASN.2007030273. [DOI] [PubMed] [Google Scholar]
- 29.Teteris SA, Engel DR, Kurts C. Homeostatic and pathogenic role of renal dendritic cells. Kidney Int. 2011;80:139–145. doi: 10.1038/ki.2011.129. [DOI] [PubMed] [Google Scholar]
- 30.Macconi D, et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol. 2009;20:123–130. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heymann F, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119:1286–1297. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochheiser K, et al. Kidney dendritic cells become pathogenic during crescentic glomerulonephritis with proteinuria. J Am Soc Nephrol. 2011;22:306–316. doi: 10.1681/ASN.2010050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timoshanko JR, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:150–159. doi: 10.1681/ASN.2005080799. [DOI] [PubMed] [Google Scholar]
- 34.Holdsworth SR, Summers SA. Role of mast cells in progressive renal diseases. J Am Soc Nephrol. 2008;19:2254–2261. doi: 10.1681/ASN.2008010015. [DOI] [PubMed] [Google Scholar]
- 35.Kanamaru Y, et al. Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. J Immunol. 2006;176:5607–5615. doi: 10.4049/jimmunol.176.9.5607. [DOI] [PubMed] [Google Scholar]
- 36.Anders HJ, et al. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayyed SG, et al. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011;80:68–78. doi: 10.1038/ki.2011.102. [DOI] [PubMed] [Google Scholar]
- 38.Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-κB signaling. J Am Soc Nephrol. 2008;19:1741–1752. doi: 10.1681/ASN.2007060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen X, Li Y, Liu Y. Opposite action of peroxisome proliferator-activated receptor-γ in regulating renal inflammation: functional switch by its ligand. J Biol Chem. 2010;285:29981–29988. doi: 10.1074/jbc.M110.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawai T, et al. PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab Invest. 2009;89:47–58. doi: 10.1038/labinvest.2008.104. [DOI] [PubMed] [Google Scholar]
- 41.Khan SB, et al. Antibody blockade of TNF-α reduces inflammation and scarring in experimental crescentic glomerulonephritis. Kidney Int. 2005;67:1812–1820. doi: 10.1111/j.1523-1755.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 42.Giannopoulou M, et al. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-κB signaling. Am J Pathol. 2008;173:30–41. doi: 10.2353/ajpath.2008.070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong R, Rifai A, Dworkin LD. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J Am Soc Nephrol. 2006;17:2464–2473. doi: 10.1681/ASN.2006020185. [DOI] [PubMed] [Google Scholar]
- 44.Jones LK, et al. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant. 2009;24:3024–3032. doi: 10.1093/ndt/gfp214. [DOI] [PubMed] [Google Scholar]
- 45.Yu C, Gong R, Rifai A, Tolbert EM, Dworkin LD. Long-term, high-dosage candesartan suppresses inflammation and injury in chronic kidney disease: nonhemodynamic renal protection. J Am Soc Nephrol. 2007;18:750–759. doi: 10.1681/ASN.2006070770. [DOI] [PubMed] [Google Scholar]
- 46.Pate M, Damarla V, Chi DS, Negi S, Krishnaswamy G. Endothelial cell biology: role in the inflammatory response. Adv Clin Chem. 2010;52:109–130. [PubMed] [Google Scholar]
- 47.López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nightingale J, et al. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J Am Soc Nephrol. 2004;15:21–32. doi: 10.1097/01.asn.0000102479.92582.43. [DOI] [PubMed] [Google Scholar]
- 49.Li Q, et al. Monocytes induce proximal tubular epithelial mesenchymal transition through NF-κB dependent upregulation of ICAM-1. J Cell Biochem. 2011;112:1585–1592. doi: 10.1002/jcb.23074. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, et al. Stabilization of snail by NF-κB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boutet A, et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe RG, et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J Cell Biol. 2009;184:399–408. doi: 10.1083/jcb.200810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Inoue T, et al. Fibroblast expression of an IκB dominant-negative transgene attenuates renal fibrosis. J Am Soc Nephrol. 2010;21:2047–2052. doi: 10.1681/ASN.2010010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Yang J, Luo JH, Dedhar S, Liu Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J Am Soc Nephrol. 2007;18:449–460. doi: 10.1681/ASN.2006030236. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y, Liou HC, Sun XH. Id1 potentiates NF-κB activation upon T cell receptor signaling. J Biol Chem. 2006;281:34989–34996. doi: 10.1074/jbc.M608078200. [DOI] [PubMed] [Google Scholar]
- 57.Lin J, et al. Inhibitor of differentiation 1 contributes to head and neck squamous cell carcinoma survival via the NF-κB/survivin and phosphoinositide 3-kinase/Akt signaling pathways. Clin Cancer Res. 2010;16:77–87. doi: 10.1158/1078-0432.CCR-08-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duffield JS. Macrophages in kidney repair and regeneration. J Am Soc Nephrol. 2011;22:199–201. doi: 10.1681/ASN.2010121301. [DOI] [PubMed] [Google Scholar]
- 59.Gandolfo MT, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 60.Cao Q, et al. IL-10/TGF-β-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grande MT, López-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol. 2009;5:319–328. doi: 10.1038/nrneph.2009.74. [DOI] [PubMed] [Google Scholar]
- 63.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 64.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol. 2009;296:F1239–F1244. doi: 10.1152/ajprenal.90521.2008. [DOI] [PubMed] [Google Scholar]
- 66.Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17:2992–2998. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- 67.Paliege A, et al. Hypoxia-inducible factor-2α-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int. 2010;77:312–318. doi: 10.1038/ki.2009.460. [DOI] [PubMed] [Google Scholar]
- 68.Boor P, Floege J. Chronic kidney disease growth factors in renal fibrosis. Clin Exp Pharmacol Physiol. 2011;38:391–400. doi: 10.1111/j.1440-1681.2011.05487.x. [DOI] [PubMed] [Google Scholar]
- 69.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 70.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grigorian M, Ambartsumian N, Lukanidin E. Metastasis-inducing S100A4 protein: implication in non-malignant human pathologies. Curr Mol Med. 2008;8:492–496. doi: 10.2174/156652408785747942. [DOI] [PubMed] [Google Scholar]
- 72.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinz B, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeji M, et al. Smooth muscle α-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J Biol Chem. 2006;281:40193–40200. doi: 10.1074/jbc.M602182200. [DOI] [PubMed] [Google Scholar]
- 76.Zou J, et al. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006;448:485–492. doi: 10.1007/s00428-005-0134-9. [DOI] [PubMed] [Google Scholar]
- 77.Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor-(CTGF, CCN2)—a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp Nephrol. 2010;114:e83–e92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- 78.Böttinger EP. TGF-β in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 79.Strutz F, et al. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 80.Ostendorf T, Eitner F, Floege J. The PDGF family in renal fibrosis. Pediatr Nephrol. doi: 10.1007/s00467-011-1892-z. http://dx.doi.org/10.1007/s00467-011-1892-z. [DOI] [PubMed]
- 81.Hu K, et al. tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J Am Soc Nephrol. 2008;19:503–514. doi: 10.1681/ASN.2007030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hao S, Shen H, Hou Y, Mars WM, Liu Y. tPA is a potent mitogen for renal interstitial fibroblasts: role of β1 integrin/focal adhesion kinase. Am J Pathol. 2010;177:1164–1175. doi: 10.2353/ajpath.2010.091269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin L, et al. tPA activates LDL receptor-related protein 1-mediated mitogenic signaling involving the p90RSK and GSK3β pathway. Am J Pathol. 2010;177:1687–1696. doi: 10.2353/ajpath.2010.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest. 2007;117:3821–3832. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu K, et al. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 86.Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 2011;79:494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 87.Humphreys BD, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sleeman JP, Thiery JP. SnapShot: the epithelial-mesenchymal transition. Cell. 2011;145:162.e1. doi: 10.1016/j.cell.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J, Bertram JF. Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology. 2010;15:507–512. doi: 10.1111/j.1440-1797.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- 97.Burns WC, Thomas MC. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev Mol Med. 2010;12:e17. doi: 10.1017/S1462399410001481. [DOI] [PubMed] [Google Scholar]
- 98.Grgic I, Duffield JS, Humphreys BD. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol. doi: 10.1007/s00467-011-1772-6. http://dx.doi.org/10.1007/s00467-011-1772-6. [DOI] [PMC free article] [PubMed]
- 99.Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 2011;80:41–50. doi: 10.1038/ki.2011.77. [DOI] [PubMed] [Google Scholar]
- 100.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iwano M, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li L, Zepeda-Orozco D, Black R, Lin F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol. 2010;176:1767–1778. doi: 10.2353/ajpath.2010.090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Togawa H, et al. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300:F511–F520. doi: 10.1152/ajprenal.00038.2010. [DOI] [PubMed] [Google Scholar]
- 104.Boonla C, et al. Fibrosis and evidence for epithelial-mesenchymal transition in the kidneys of patients with staghorn calculi. BJU Int. 2011;108:1336–1345. doi: 10.1111/j.1464-410X.2010.10074.x. [DOI] [PubMed] [Google Scholar]
- 105.Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13:96–107. doi: 10.1681/ASN.V13196. [DOI] [PubMed] [Google Scholar]
- 106.Zeisberg M, et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 107.Hertig A, et al. Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol. 2008;19:1584–1591. doi: 10.1681/ASN.2007101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galichon P, Hertig A. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: are we ready for the bedside? Fibrogenesis Tissue Repair. 2011;4:11. doi: 10.1186/1755-1536-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He W, et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, et al. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:1907–1918. doi: 10.1681/ASN.2008090930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang J, et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110:1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 113.Surendran K, Schiavi S, Hruska KA. Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 114.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 115.Zhou Y, et al. HSP72 inhibits Smad3 activation and nuclear translocation in renal epithelial-to-mesenchymal transition. J Am Soc Nephrol. 2010;21:598–609. doi: 10.1681/ASN.2009050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010;38:548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wada T, et al. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin Exp Nephrol. 2011;15:8–13. doi: 10.1007/s10157-010-0372-2. [DOI] [PubMed] [Google Scholar]
- 118.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niedermeier M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci USA. 2009;106:17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sakai N, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens. 2008;26:780–790. doi: 10.1097/HJH.0b013e3282f3e9e6. [DOI] [PubMed] [Google Scholar]
- 122.Roufosse C, et al. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J Am Soc Nephrol. 2006;17:775–782. doi: 10.1681/ASN.2005080795. [DOI] [PubMed] [Google Scholar]
- 123.Yang J, Dai C, Liu Y. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol. 2003;163:621–632. doi: 10.1016/S0002-9440(10)63689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schnaper HW, et al. TGF-β signal transduction in chronic kidney disease. Front Biosci. 2009;14:2448–2465. doi: 10.2741/3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-β1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402–1412. doi: 10.1097/01.asn.0000130568.53923.fd. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287:F7–F16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- 127.Luo DD, Phillips A, Fraser D. Bone morphogenetic protein-7 inhibits proximal tubular epithelial cell Smad3 signaling via increased SnoN expression. Am J Pathol. 2010;176:1139–1147. doi: 10.2353/ajpath.2010.090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang B, et al. miR-200a prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes. 2011;60:280–287. doi: 10.2337/db10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kato M, et al. A microRNA circuit mediates transforming growth factor-β1 autoregulation in renal glomerular mesangial cells. Kidney Int. 2011;80:358–368. doi: 10.1038/ki.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 131.Chung AC, Huang XR, Meng X, Lan H. Y miR-192 mediates TGF-β/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21:1317–1325. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Q, et al. TGF-β-induced MiR-491-5p expression promotes Par-3 degradation in rat proximal tubular epithelial cells. J Biol Chem. 2010;285:40019–40027. doi: 10.1074/jbc.M110.141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eyden B. Fibronexus junctions associated with in vivo human endothelium. Ultrastruct Pathol. 2009;33:28–32. doi: 10.1080/01913120802625822. [DOI] [PubMed] [Google Scholar]
- 134.Margadant C, Sonnenberg A. Integrin-TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Legate KR, Fässler R. Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 136.Legate KR, Montañez E, Kudlacek O, Fässler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 137.Maydan M, et al. Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3β) phosphorylation. PLoS ONE. 2010;5:e12356. doi: 10.1371/journal.pone.0012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fukuda K, Gupta S, Chen K, Wu C, Qin J. The pseudoactive site of ILK is essential for its binding to α-parvin and localization to focal adhesions. Mol Cell. 2009;36:819–830. doi: 10.1016/j.molcel.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He W, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J Biol Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.ten Berge D, et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15:460–466. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 142.Li Y, Dai C, Wu C, Liu Y. PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J Am Soc Nephrol. 2007;18:2534–2543. doi: 10.1681/ASN.2007030315. [DOI] [PubMed] [Google Scholar]
- 143.Guo L, Wu C. Regulation of fibronectin matrix deposition and cell proliferation by the PINCH–ILK–CH–ILKBP complex. FASEB J. 2002;16:1298–1300. doi: 10.1096/fj.02-0089fje. [DOI] [PubMed] [Google Scholar]
- 144.Yeh YC, et al. Transforming growth factor-β1 induces Smad3-dependent β1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177:1743–1754. doi: 10.2353/ajpath.2010.091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu XC, Liu BC, Zhang XL, Li MX, Zhang JD. Role of ERK1/2 and PI3-K in the regulation of CTGF-induced ILK expression in HK-2 cells. Clin Chim Acta. 2007;382:89–94. doi: 10.1016/j.cca.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 147.Han SY, et al. High glucose and angiotensin II increase β1 integrin and integrin-linked kinase synthesis in cultured mouse podocytes. Cell Tissue Res. 2006;323:321–332. doi: 10.1007/s00441-005-0065-4. [DOI] [PubMed] [Google Scholar]
- 148.Yang F, et al. Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J Pathol. 2010;221:390–401. doi: 10.1002/path.2721. [DOI] [PubMed] [Google Scholar]
- 149.Carvajal G, et al. Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int. 2008;74:585–595. doi: 10.1038/ki.2008.213. [DOI] [PubMed] [Google Scholar]
- 150.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-β-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 151.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–365. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 152.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin β1 and activation of integrin-linked kinase. J Biol Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shweke N, et al. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-β activation and cell infiltration. Am J Pathol. 2008;173:631–642. doi: 10.2353/ajpath.2008.080025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang L, et al. Transglutaminase inhibition ameliorates experimental diabetic nephropathy. Kidney Int. 2009;76:383–394. doi: 10.1038/ki.2009.230. [DOI] [PubMed] [Google Scholar]
- 156.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharma S, Sirin Y, Susztak K. The story of Notch and chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20:56–61. doi: 10.1097/MNH.0b013e3283414c88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bielesz B, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Higgins DF, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun S, et al. Hypoxia-inducible factor-1α induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75:1278–1287. doi: 10.1038/ki.2009.62. [DOI] [PubMed] [Google Scholar]
- 161.Kume S, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koesters R, et al. Tubular overexpression of transforming growth factor-β1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang S, et al. Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol. 2006;17:2504–2512. doi: 10.1681/ASN.2006030278. [DOI] [PubMed] [Google Scholar]
- 166.Tan X, He W, Liu Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int. 2009;76:1248–1257. doi: 10.1038/ki.2009.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Zeeuw D, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 168.Mirkovic K, van den Born J, Navis G, de Borst MH. Vitamin D in chronic kidney disease: new potential for intervention. Curr Drug Targets. 2011;12:42–53. doi: 10.2174/138945011793591572. [DOI] [PubMed] [Google Scholar]
- 169.Wang X, et al. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2010;299:F973–F982. doi: 10.1152/ajprenal.00216.2010. [DOI] [PubMed] [Google Scholar]
- 170.Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J. 2006;20:1898–1900. doi: 10.1096/fj.06-5898fje. [DOI] [PubMed] [Google Scholar]
- 171.Zeisberg M, et al. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li J, et al. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:2612–2624. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin SL, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Venkatachalam MA, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F923–F931. doi: 10.1152/ajprenal.00205.2009. [DOI] [PubMed] [Google Scholar]
- 176.Guo ZJ, et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10:1699–1712. doi: 10.1089/ars.2007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wilkinson L, et al. Loss of renal microvascular integrity in postnatal Crim1 hypomorphic transgenic mice. Kidney Int. 2009;76:1161–1171. doi: 10.1038/ki.2009.345. [DOI] [PubMed] [Google Scholar]
- 178.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 179.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 180.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 181.Gunaratnam L, Bonventre JV. HIF in kidney disease and development. J Am Soc Nephrol. 2009;20:1877–1887. doi: 10.1681/ASN.2008070804. [DOI] [PubMed] [Google Scholar]
- 182.D’Agati V, Schmidt AM. RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol. 2010;6:352–360. doi: 10.1038/nrneph.2010.54. [DOI] [PubMed] [Google Scholar]
- 183.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou LL, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76:1148–1160. doi: 10.1038/ki.2009.322. [DOI] [PubMed] [Google Scholar]
- 185.Shi XY, et al. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology. 2008;149:1829–1839. doi: 10.1210/en.2007-1544. [DOI] [PubMed] [Google Scholar]
- 186.Daroux M, et al. Advanced glycation end-products: implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 2010;36:1–10. doi: 10.1016/j.diabet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 187.Shanmugam N, et al. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57:879–888. doi: 10.2337/db07-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bechtel W, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu K, Mars WM, Liu Y. Novel actions of tissue-type plasminogen activator in chronic kidney disease. Front Biosci. 2008;13:5174–5186. doi: 10.2741/3073. [DOI] [PMC free article] [PubMed] [Google Scholar]