Abstract
Background
Alcohol is frequently co-abused with smoking. In humans, nicotine use can increase alcohol craving and consumption. The objectives of the current study were to assess the acute effects of nicotine on alcohol seeking and relapse at two different time points.
Method
Adult female alcohol-preferring (P) rats were trained in 2-lever operant chambers to self-administer 15% EtOH (v/v) and water on a concurrent fixed-ratio 5 – fixed-ratio 1 (FR5-FR1) schedule of reinforcement in daily 1-hr sessions. Following 10 weeks of daily 1-hr sessions, rats underwent 7 extinction sessions, followed by 2 weeks in their home cages. Rats were then returned to the operant chambers without EtOH or water being present for 4 sessions (Pavlovian Spontaneous Recovery [PSR]). Rats were then given a week in their home cage before being returned to the operant chambers with access to EtOH and water (relapse). Nicotine (0, 0.1, 0.3, or 1.0 mg/kg) was injected s.c. immediately or 4-hr prior to PSR or relapse testing.
Results
Injections of nicotine immediately prior to testing reduced (5–10 responses PSR; 50–60 responses relapse), whereas injections of nicotine 4-hr prior to testing increased (up to 150 responses for PSR; up to 400 responses for relapse with 1.0 mg/kg dose) responses on the EtOH lever during PSR and relapse tests.
Discussion
The results of this study demonstrate that acute effects of nicotine on EtOH-seeking and relapse behaviors may be time-dependent, with the immediate effects being a result of nicotine possibly acting as a substitute for EtOH whereas, with a delay of 4-hr, priming effects of nicotine alterations in nicotinic receptors, and/or the effects of nicotine’s metabolites (i.e., cotinine, nornicotine) may enhance the expression of EtOH-seeking and relapse behaviors.
Keywords: Ethanol-seeking, Ethanol-relapse, Pavlovian Spontaneous Recovery, nicotine, alcohol preferring rat
INTRODUCTION
Nicotine is commonly co-abused with alcohol. Epidemiological and clinical studies estimated that 50–80% of alcohol-dependent individuals are regular smokers (Hurt et al., 1994; Pomerleau et al., 1997; Romberger and Grant, 2004). Alcohol dependent individuals also have higher rates of nicotine dependence (Hughes, 1996; Room, 2004) and twin studies have shown that there may be common genetic mechanisms between alcohol dependence and nicotine dependence (Nurnberger et al., 2004; True et al., 1999; Volk et al., 2007). Alcohol intake is also significantly higher in smokers compared to only alcohol users (York and Hirsch, 1995).
Human and animals studies have demonstrated that the effects of nicotine on alcohol intake are complex. For example, two recent double-blind clinical studies demonstrated that transdermal nicotine or nicotine delivered by tobacco smoke can increase alcohol consumption in men (Acheson et al., 2006 and Barrett et al., 2006). In contrast, transdermal nicotine has been shown to decrease alcohol intake in women (Acheson et al., 2006) and delay alcohol drinking in heavy drinkers (McKee et al., 2008). Pre-clinical findings have provided further evidence that nicotine can increase ethanol (EtOH)-intake (Blomqvist et al., 1996; Clark et al., 2001; Ericson et al., 2000; Lallemand et al., 2007; Le et al., 2003; Olausson et al., 2001; Potthoff et al., 1983; Smith et al., 1999), as wells as decrease it (Dyr et al., 1999; Nadal et al., 1998; Sharpe and Samson, 2002) in rodents. It has been suggested that the different effects observed after nicotine administration may be time-dependent due to the length of nicotine exposure (i. e., acute vs. repeated) or to the length of EtOH access (30-min vs. 60-min) (see Le, 2002).
Previous studies primarily focused on examining the effects of nicotine on EtOH intake. However, the concurrent use of alcohol with nicotine is also thought to increase the risk of alcohol-seeking and relapse further than alcohol use alone (Taylor et al., 2000). Given that relapse is a major problem in the treatment of alcohol addiction, some attention has been focused on how nicotine may affect EtOH-seeking and relapse drinking (Le et al., 2003; Lopez-Moreno et al., 2004).
Le et al. (2003) demonstrated that nicotine can reinstate EtOH-seeking behavior in Long-Evans rats under operant conditions, whereas Lopez-Moreno et al. (2004) showed that nicotine can dose-dependently increase operant EtOH intake during alcohol deprivation in Wistar rats. These studies provide some evidence that nicotine use may be involved in increasing EtOH-seeking and relapse drinking in non-selective rats. However, the effects of nicotine on EtOH-seeking and relapse drinking in selectively bred high alcohol consuming rats have not been studied.
The selectively bred alcohol-preferring (P) line of rat has been well characterized both behaviorally and neurobiologically (McBride and Li, 1998; Murphy et al., 2002) and satisfies criteria proposed as essential for an animal model of alcoholism (Cicero, 1979; Lester and Freed, 1973). Alcohol-naïve P rats readily self-administer more nicotine as well as express greater nicotine-seeking behavior than the alcohol non-preferring (NP) rats (Le et al., 2006). Nicotine also has greater reinforcing effects in P than NP rats (Le at al., 2006), which supports the hypothesis that alcohol and nicotine addiction share some common genetic risk factors.
Research assessing EtOH-seeking behavior through the expression of Pavlovian Spontaneous Recovery (PSR) paradigm has been conducted in P rats (Rodd-Henricks et al., 2002a, b). PSR is a unique phenomenon in that it is time dependent, and the behavior appears to be dependent on the re-exposure of the organism to cues in the behavioral environment previously associated with the reinforcer. The expression of a PSR is directly correlated to reward saliency (Robbins, 1990), contextual cues associated with first-learned signals, and the amount of first- and second-learned associations (Brooks, 2000). The PSR phenomenon has been asserted to be the result of an intrinsic shift away from the recent extinction (second-) learning to the initial reinforced learning responses, which reflects a motivation to obtain the previously administered reward (Bouton 2002, 2004; Rescorla 2001). Therefore, the PSR model may represent a unique paradigm to study EtOH-seeking behaviors. P rats readily express a PSR for EtOH (Rodd-Henricks et al. 2002a, b; Rodd et al. 2006) and this expression can be enhanced by exposure to EtOH odor cues or EtOH priming (Rodd-Henricks et al. 2002a, b). In addition, P rats express pronounced relapse drinking under 24-hr free choice and operant conditions (McKinzie et al 1998; Rodd-Henricks et al., 2000; Rodd et al., 2003; Toalston et al., 2008).
The objective of the current study was to test the hypothesis that nicotine will increase EtOH-seeking behavior and EtOH-relapse of the alcohol preferring P rats in a time-dependent manner.
MATERIALS AND METHODS
Animals
Adult female P rats from the 55th – 56th generations weighing 250–325g at the start of the experiment were used. Previous research indicated that EtOH intake of female P rats was not affected by the estrus cycle (McKinzie et al., 1998). Rats were maintained on a 12-hr reversed light-dark cycle (lights off at 0900-hr). Food and water were available ad libitum throughout the experiment, except during operant testing. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996).
aOperant Apparatus
EtOH self-administration procedures were conducted in standard two-lever experimental chambers (Coulbourn Instruments, Whitehall, PA, USA) contained within ventilated, sound-attenuated enclosures. Two operant levers, located on the same wall, were 15 cm above a grid floor and 13 cm apart. A trough was directly beneath each lever, from which a dipper cup could raise to present fluid. Upon a reinforced response on the respective lever, a small light cue was illuminated in the drinking trough and 4 seconds of dipper cup (0.1 ml) access was presented. A personal computer controlled all operant chamber functions while recording lever responses and dipper presentations.
Operant Training
Without prior training, naïve P rats were placed into the operant chamber. Operant sessions were 60-min in duration and occurred daily (including weekends) for 10 weeks (Rodd et al., 2006). The EtOH concentration used for operant administration was 15% (vol/vol). During the initial 4 weeks of daily operant access, both solutions (water and EtOH) were reinforced on an FR-1 schedule. At the end of this time, the response requirement for EtOH was increased to an FR-3 schedule for 3 weeks, and then to FR-5 schedule for 3 weeks. After the P rats had established stable levels of responding on the FR5 schedule for EtOH and FR1 for water, they underwent 7 days of extinction training (60-min/day), with neither water nor EtOH available (Rodd et al,. 2006). With the exception of no fluid being presented, the delivery system operated exactly as the preceding EtOH self-administration sessions.
Pavlovian Spontaneous Recovery (PSR) testing
After extinction training, all rats were maintained in the home cages for 14 days. Following the abstinence period, rats received additional operant sessions under the extinction protocol conditions. Lever contingencies and dipper functioning were maintained, but EtOH and water were absent. Rats were given 4 consecutive PSR test sessions (Rodd-Henricks et al., 2002a, b; Rodd et al., 2006). There are 4 consecutive PSR session because previous studies have shown that exposure to EtOH odor cues or EtOH priming (Rodd-Henricks et al., 2002a, b) and some drugs (Dhaher et al., 2009) may enhance PSR responding for more than one session.
Relapse
Following the PSR phase of the experiment, all rats were maintained in the home cages for 7 days. Rats were then transferred to the operant chambers with both 15% EtOH and water available for the 60-min sessions. The EtOH lever was maintained on a FR5 schedule and the water lever on a FR1 schedule (Rodd et al., 2006).
Nicotine Effects on EtOH-Seeking and Relapse Drinking
Nicotine HCl was purchased from Sigma (St. Louis, USA). Nicotine HCl was dissolved in saline. Following extinction training, adult female P rats (n = 55) rats were randomly assigned to groups that received injections of nicotine (0, 0.1, 0.3, or 1.0 mg/kg, free base) immediately or 4-hr prior to the first PSR test session only (n = 6–10/dose/time point). Pilot testing revealed that nicotine injected 30-min prior to testing suppressed operant behaviors similar to that observed in the immediate condition; therefore, this time point was removed from the study. To reduce the number of rats used in the experiment, the saline group consisted of 9 rats distributed at each time point (n = 4–5/time point).
These same rats were also used to test the effects of nicotine during relapse responding, using a counterbalanced design (i.e., rats that were administered 1.0 mg/kg nicotine immediately prior to PSR test sessions were randomly assigned to separate groups that received one of the 4 doses of nicotine). For relapse testing, rats received 0, 0.1, 0.3, or 1.0 mg/kg nicotine (n = 7–9/group/time point) immediately or 4-hr prior to the 1st relapse session only. Similarly, to reduce the number of rats used in the experiment, the saline group consisted of 9 rats distributed at each time point (n = 4–5/time point).
Statistical Analyses
Overall operant responding (60-min) data were analyzed with a mixed factorial ANOVA with a between subject factors of dose and time point and a repeated measure of ‘session’. For the PSR experiments, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH lever for the last 3 extinction sessions. For the relapse studies, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH lever for the 3 sessions immediately prior to deprivation. Operant responding data were also analyzed in 10-min blocks, which required the additional repeated measure of time. Post-hoc Tukey’s b tests were performed to determine individual differences.
RESULTS
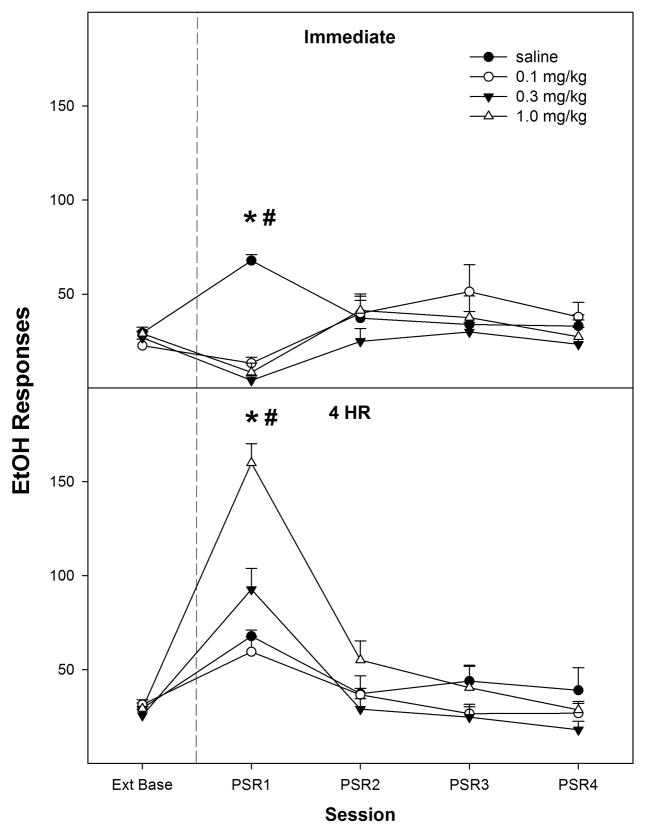
Nicotine administered immediately prior to PSR testing reduced operant responding on the EtOH lever (top panel, Fig. 1), whereas nicotine administered 4 hr prior to PSR testing increased responding on the EtOH lever (bottom panel, Fig. 1). There was a significant effect of ‘session’ (F4, 44 = 35.8; p < 0.001), ‘dose’ (F3, 47 = 4.5; p = 0.007), and ‘session’ by ‘dose’ by ‘time of injection’ interaction (F12, 138 = 4.5; p < 0.001). Decomposing the interaction term by holding ‘time of injection’ constant revealed, for rats injected immediately prior to PSR testing, there was a significant ‘session’ by ‘dose’ interaction (F12, 63 = 2.7; p < 0.001). Individual One-way ANOVAs performed on each session indicated that only during the 1st PSR test session was there a significant ‘dose’ effect (F3, 22 = 34.5; p < 0.001). Post-hoc comparisons indicated that all 3 nicotine groups responded less than the saline treated rats during the 1st PSR test session. Comparison with extinction baseline levels of responding indicated that the saline treated rats increased responding on the lever previously associated with the delivery of EtOH during the 1st PSR session, whereas rats treated with each dose of nicotine reduced responding (paired t-tests, p values < 0.001).
Fig. 1.
Mean (± S.E.M.) responses per session on the lever previously associated with the delivery of EtOH in P rats given saline (n = 4–5/time point) or 0.1, 0.3, or 1.0 mg/kg nicotine (n = 6–10/dose/time point) subcutaneously immediately or 4-hr prior to the first PSR session. Upper Panel: asterisk (*) indicates that rats administered saline responded significantly (p < 0.05) more on the EtOH lever during the first PSR session compared to extinction baseline levels, whereas rats administered 0.1, 0.3, or 1.0 mg/kg nicotine immediately prior to the first PSR session responded significantly less than extinction baseline. Pound (#) indicates that all doses of nicotine reduced EtOH responding during the first PSR session compared to the saline group (p<0.05). Lower Panel: asterisk (*) indicates that rats administered saline, 0.1, 0.3, or 1.0 mg/kg nicotine 4-hr prior to the first session responded significantly (p < 0.05) more on the EtOH lever during the first PSR session compared to extinction baseline levels. Pound symbol (#) indicates that 1.0 mg/kg nicotine increased EtOH responding during the first PSR session compared to all other groups (p < 0.05).
For rats injected 4-hr prior to PSR testing, a significant ‘session’ by ‘dose’ interaction (F12, 72 = 2.9; p < 0.001) was revealed (Fig. 1). The ‘dose’ groups differed only during the 1st PSR test session (F3,25 = 25.6; p < 0.001), and post-hoc comparisons indicated that rats administered 1.0 mg/kg nicotine 4-hr prior to testing increased EtOH-lever responses compared to all other groups. Comparison with extinction baseline levels of responding indicated that all groups increased responding on the lever previously associated with the delivery of EtOH during the 1st PSR session (paired t-tests, p values < 0.008).
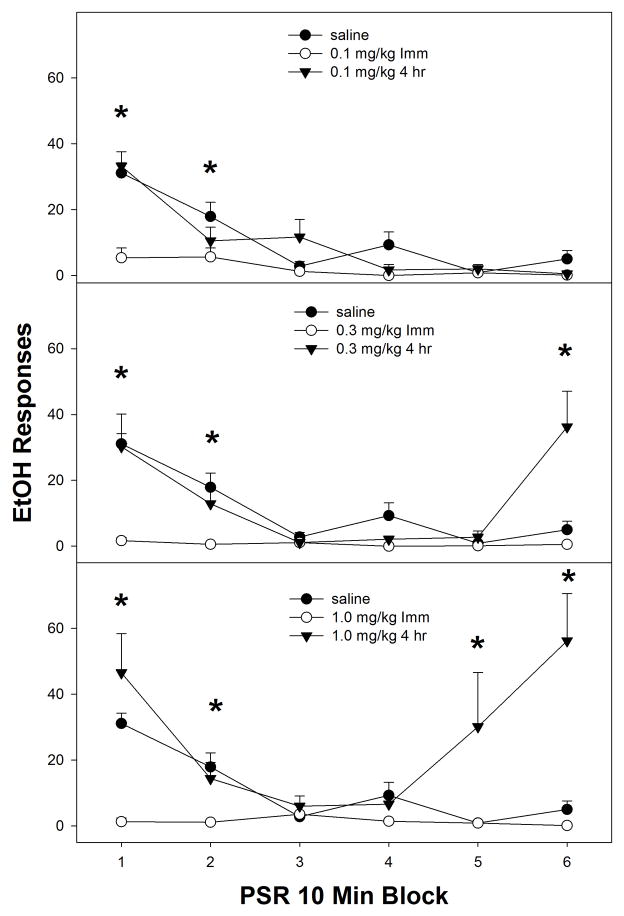
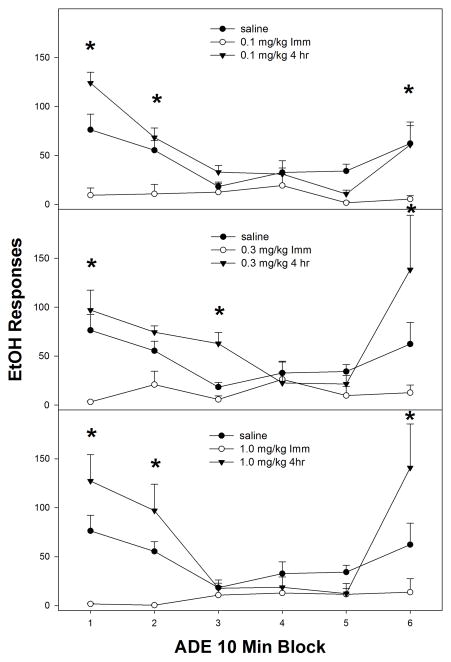
Examining the time course effects of nicotine (in 10-minute blocks) within the first PSR session (Fig. 2), indicated a significant ‘time of injection’ x ‘dose’ x ‘session time’ block interaction (F15, 135 = 2.8; p < 0.001). Reducing the interaction term by holding ‘dose’ constant, indicated a significant ‘time of injection’ x ‘session time’ interaction (p values < 0.004) in rats administered 0.1, 0.3 or 1.0 mg/kg nicotine. Analysis performed on the saline group revealed no effect of ‘time of injection’ x ‘session time’ interaction (p values < 0.68) in rats administered 0.1, 0.3 or 1.0 mg/kg nicotine. Rats treated with nicotine immediately prior to testing (open circles, all panels Fig. 2) displayed a reduction in responding compared to saline during 0–20-min of PSR testing and this reduced responding continued throughout the 60-min session. The rats injected 4-hr prior to the 1st PSR session showed that saline, 0.1, 0.3, and 1.0 mg/kg all showed significantly more responding than the group given nicotine immediately prior to the session during the beginning of the 60-min sessions. Further, the rats administered 0.3 or 1.0 mg/kg nicotine 4-hr prior to PSR testing also displayed an increase in responding during the end of the 60-min test session compared to saline treated rats (middle and bottom panel, Fig. 2). The time pattern of high responding at the beginning and at end of the session for the 0.3 and 1.0 mg/kg groups resulted in the overall enhanced PSR responding for both groups compared to saline and 0.1 mg/kg.
Fig. 2.
Mean (± S.E.M.) responses per 10-min blocks on the lever previously associated with the delivery of EtOH in P rats given saline (n = 4–5/time point), or 0.1, 0.3, or 1.0 mg/kg nicotine (n = 6–10/dose/time point) immediately or 4-hr prior to only the first PSR session. Upper Panel: asterisk (*) indicates that rats administered 0.1 mg/kg nicotine immediately prior to the test session responded significantly (p < 0.05) less on the EtOH lever during 0–20-min of PSR testing compared to saline. Middle Panel: asterisk (*) indicates that rats administered 0.3 mg/kg nicotine immediately prior to first the PSR session responded significantly (p < 0.05) less on the EtOH lever during 0–20-min of PSR testing compared to saline and rats administered 0.3 mg/kg nicotine 4-hr prior testing. Rats administered 0.3 mg/kg nicotine 4-hr prior to PSR testing displayed an increase in EtOH lever responses during the 50–60 period. Lower Panel: asterisk (*) indicates that rats administered 1.0 mg/kg nicotine immediately prior to test session responded significantly (p < 0.05) less on the EtOH lever during 0–20-min of PSR testing compared to saline and rats administered 1.0 mg/kg nicotine 4-hr prior testing. Rats administered 1.0 mg/kg nicotine 4-hr prior to PSR testing displayed an increase in EtOH responses during the 40–60 period.
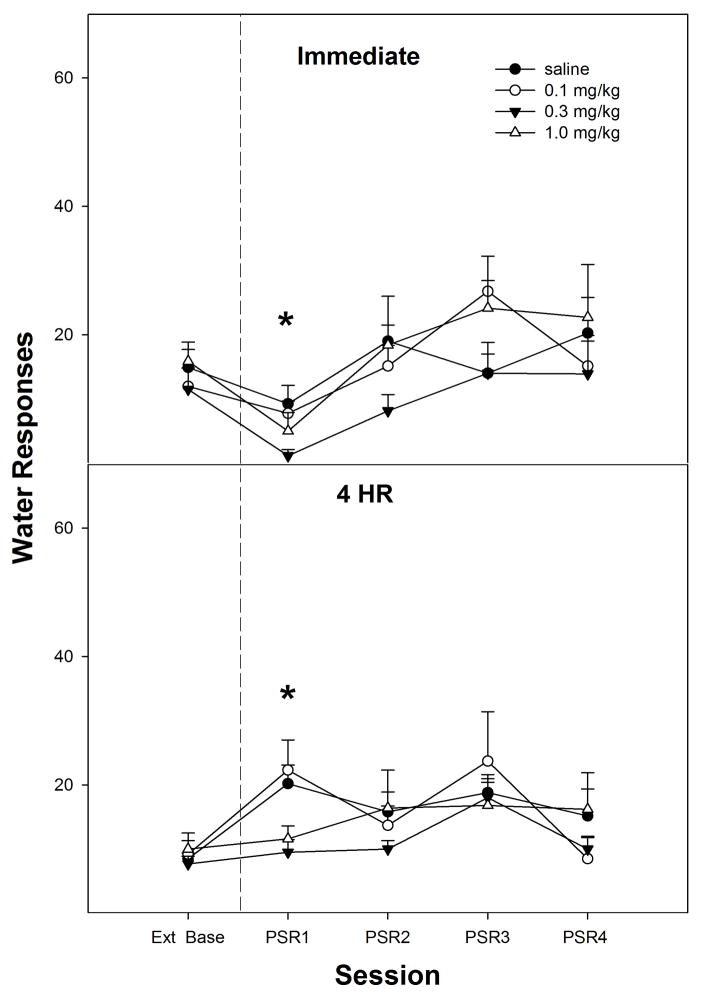
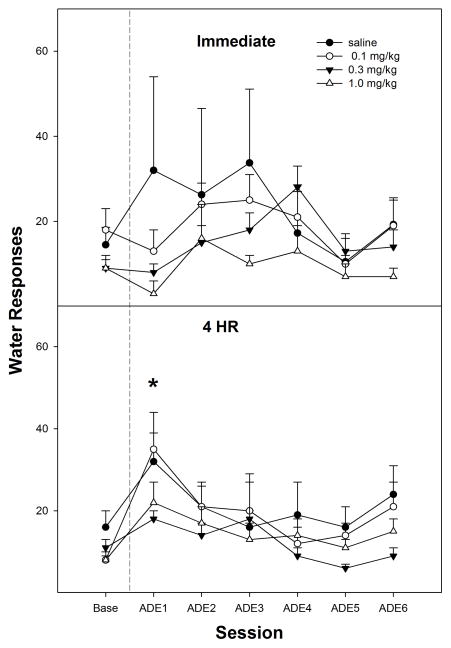
Responding on the lever previously associated with water was significantly lower (paired t-tests, p =0.033) in the 0.3 mg/kg nicotine group compared to extinction baseline values (top panel, Fig. 3). There were no a significant differences in water responding for the 1st PSR session for the saline, 0.1 mg/kg or 1.0 mg/kg nicotine groups compared to extinction baseline values (paired t-tests, p ≥ 0.060). In addition, there were no significant group differences (p values > 0.05) on water lever responses between saline, 0.1 mg/kg, 0.3 mg/kg, and 1.0 mg/kg nicotine during the 1st PSR session (top panel, Fig. 3).
Fig. 3.
Mean (± S.E.M.) responses per session on the lever previously associated with the delivery of water in P rats given saline (n = 4–5/time point), or 0.1, 0.3, or 1.0 mg/kg nicotine (n = 6–10/dose/time point) subcutaneously immediately or 4-hr prior to the first PSR session. Upper Panel: asterisk (*) indicates that rats administered 0.3 mg/kg nicotine responded significantly (p < 0.05) less on the water lever during the first PSR session compared to extinction baseline levels. There were no significant water responding differences when animals were administered saline or 0.1 or 1.0 mg/kg of nicotine immediately prior to the first PSR session. Lower Panel: asterisk (*) indicates that rats administered saline or 0.1 mg/kg nicotine 4-hr prior to the first session responded significantly (p < 0.05) more on the water lever during the first PSR session compared to extinction baseline levels. There were no significant water responding differences when animals were administered 0.3 or 1.0 mg/kg nicotine 4-hr prior to the first PSR session.
Responding on the lever previously associated with water was significantly higher in the saline (paired t-tests, p =0.015) and 0.1 mg/kg nicotine (paired t-tests, p =0.021) groups compared to extinction baseline values (bottom panel, Fig. 3) for the 4-hr delay. There were no significant differences in responses on the water lever for the 0.3 mg/kg or 1.0 mg/kg nicotine groups compared to extinction baseline values during the 1st PSR session (paired t-tests, p ≥ 0.456). When the 4-hr delay groups were compared to each other the analysis revealed that responding on the water lever for saline and 0.1 mg/kg nicotine groups were significantly higher (p values < 0.05) than 0.3 mg/kg and 1.0 mg/kg nicotine during the initial PSR session (bottom panel, Fig. 3).
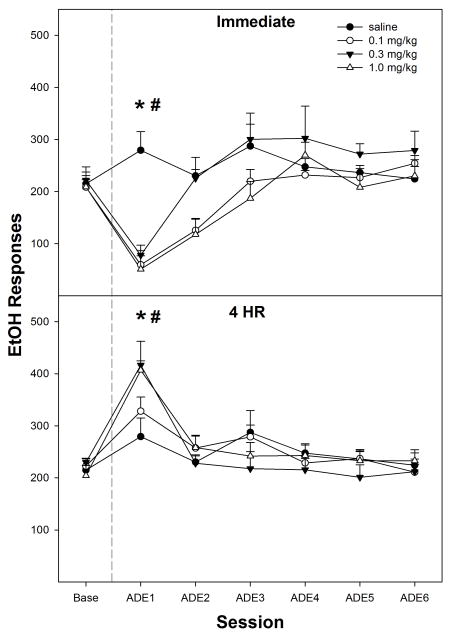
A similar time-dependent pattern to that found during PSR testing was also observed when nicotine was administered immediately or 4-hr prior to EtOH relapse testing (top panel, Fig. 4). Decomposing the interaction term by holding ‘time of injection’ constant indicated that in rats injected immediately prior to EtOH reinstatement (top panel, Fig. 4), there was a significant ‘session’ x ‘dose’ interaction (F18,57 = 5.3; p = 0.002). Individual ANOVAs indicated there was a significant ‘dose’ effect during the 1st relapse session (F3, 22 = 7.6; p < 0.001). Post-hoc comparisons indicated that responding by the saline group was significantly higher than all nicotine groups and baseline values. There was no significant effect of group during the 2nd relapse session (p = 0.063) or subsequent sessions. In addition, responses on the EtOH lever for all nicotine groups were lower than baseline values during the 1st session; during the 2nd session only the 0.1 and 1.0 mg/kg nicotine doses reduced responses below baseline.
Fig. 4.
Mean (± S.E.M.) responses per session on the EtOH lever by P rats given saline (n = 4–5/time point), or 0.1, 0.3, or 1.0 mg/kg nicotine (n = 7–9/group/time point) immediately or 4-hr prior to only the first relapse session. Upper Panel: asterisk (*) indicates that rats administered 0.1, 0.3, or 1.0 mg/kg nicotine immediately prior to the first relapse session responded significantly (p < 0.05) less than baseline, and rats given saline had higher responding than baseline. Pound symbol (#) indicates that all doses of nicotine reduced EtOH responding during the first relapse session compared to the saline group (p<0.05). Lower Panel: asterisk (*) indicates that rats administered saline or 0.1, 0.3, or 1.0 mg/kg nicotine 4-hr prior to the first relapse session responded significantly (p < 0.05) more on the EtOH lever compared to baseline levels. Pound symbol (#) indicates that 0.3 and 1.0 mg/kg nicotine increased EtOH responding significantly more than the saline group (p < 0.05).
For rats injected with nicotine 4-hr prior to EtOH relapse testing (bottom panel, Fig. 4), there was a significant ‘session’ effect (F6, 20 = 10.8; p < 0.001). An ANOVA performed on the 1st relapse session (p = 0.033) indicated that rats given 0.3 or 1.0 mg/kg nicotine 4-hr prior to the relapse session responded more on the EtOH lever than the saline group (Fig. 4). Comparison with baseline indicated that all rats in the 4-hr group responded more during the 1st relapse session than during baseline (p values ≤ 0.03).
The average baseline for EtOH intake prior to relapse testing for both immediate and 4-hr groups was approximately 1.5 g/kg in a 60-min sessions. The immediate and 4-hr delay saline groups during EtOH relapse increased EtOH intakes to around 2 g/kg. The immediate effects of nicotine reduced EtOH intake to 0.4–0.6 g/kg. The 4-hr delay effects of nicotine increased EtOH intakes as high 3 g/kg.
Examining the time course effects (in 10-min bins) of administration of nicotine on EtOH relapse during the 1st relapse session (Fig. 5) indicated that the EtOH lever responding temporal profile was influenced by both nicotine dose and ‘time of injection’. An analysis of the EtOH relapse responding separated into 10-min blocks indicated a significant ‘time of injection’ x ‘session time’ interaction (F5, 43 = 10.4; p < 0.001). In general, nicotine administered immediately prior to the 1st relapse session reduced EtOH self-administration throughout the 60-min test session compared to saline values. In general, administration of nicotine 4-hr prior to the relapse session increased responding during the beginning and the end of the 1st relapse session. For rats administered 0.1 mg/kg nicotine 4-hr prior to relapse (top panel, Fig. 5), responding was greater than saline during the 1st 10-min block. For rats administered 0.3 mg/kg nicotine 4-hr prior to relapse (middle panel, Fig-5), responding was greater than saline during the 3rd and 6th 10-min blocks. For rats administered 1.0 mg/kg nicotine 4-hr prior to the 1st relapse session (bottom panel, Fig. 5), responding was greater than saline during the 1st, 2nd, and 6th 10-min blocks.
Fig. 5.
Mean (± S.E.M.) responses per 10-min blocks on the EtOH lever by P rats given saline (n = 4–5/time point), or 0.1, 0.3, or 1.0 mg/kg nicotine (n = 7–9/group/time point) immediately or 4-hr prior to the first relapse session. Upper Panel: asterisk (*) indicates that rats administered saline or 0.1 mg/kg 4-hr prior to test session responded significantly (p < 0.05) more on the EtOH lever during initial 0–20-min and final 10-min of relapse testing compared to rats injected with 0.1 mg/kg nicotine immediately prior to the test session. Middle Panel: asterisk (*) indicates that rats administered 0.3 mg/kg nicotine immediately responded significantly (p < 0.05) less on the EtOH lever during 0–20-min of relapse testing compared to saline, and rats administered 0.3 mg/kg nicotine 4-hr prior to relapse testing displayed an increase in EtOH responding during the 3rd and 6th 10-min block compared to saline. Lower Panel: asterisk (*) indicates that rats administered 1.0 mg/kg nicotine immediately responded significantly (p < 0.05) less on the EtOH lever during 0–20-min of relapse testing compared to saline, and rats administered 1.0 mg/kg nicotine 4-hr prior to relapse testing displayed an increase in EtOH lever responses during 1st, 2nd, and 6th 10-min block compared to saline.
There were no significant differences in water relapse responding when the rats were administered saline or nicotine immediately prior to the 1st relapse session compared to baseline values (paired t-tests, p values ≥ 0.085), nor were there water responding differences when the groups were compared to each other (p values = 0.112, top panel, Fig. 6). However, rats administered saline or 0.1 mg/kg of nicotine 4-hr prior to the 1st relapse session responded significantly (paired t-tests, p = 0.015) more on the water lever compared to baseline values (bottom panel, Fig. 6). There were no significant differences in water responding when rats were administered 0.3 or 1.0 mg/kg nicotine 4-hr prior to the 1st relapse session (paired t-tests, p values > 0.05). In addition, there were no significant group differences on water responding between rats administered saline or 0.1, 0.3 or 1.0 mg/kg nicotine 4-hr prior to the 1st relapse session (bottom panel, Fig. 6).
Fig. 6.
Mean (± S.E.M.) responses per session on the water lever by P rats given saline (n = 4–5/time point), or 0.1, 0.3, or 1.0 mg/kg nicotine (n = 7–9/group/time point) immediately or 4-hr prior to only the first relapse session. Upper Panel: There were no significant differences in water responding when the rats were administered saline or 0.1, 0.3, or 1.0 mg/kg nicotine immediately prior to the first relapse. Lower Panel: asterisk (*) indicates that rats administered saline or 0.1 mg/kg of nicotine 4-hr prior to the first relapse session responded significantly (p < 0.05) more on the water lever compared to baseline levels. There were no significant differences water responding when rats were administered 0.3 or 1.0 mg/kg nicotine 4-hr prior to the first relapse test.
DISCUSSION
The major findings of this study demonstrated that the enhanced expression of EtOH-seeking and EtOH-relapse by acute doses of nicotine is time-dependent. In the present study, the PSR and relapse paradigms were used to access EtOH-seeking and EtOH- relapse behaviors, respectively.
The doses of nicotine are comparable to the nicotine doses used in previous experiments (Le et al., 2003; Lopez-Moreno et al., 2004). The 4-hr delay between nicotine administration and EtOH access showed that nicotine dose–dependently increased both PSR and EtOH-relapse (lower panels, Figs. 1 and 4), whereas the saline and 0.1 mg/kg of nicotine groups showed increased responses on the water lever for both PSR and water relapse (lower panels, Figs. 3 and 6). The mid- (0.3 mg/kg) and high- (1.0 mg/kg) dose of nicotine did not have any effect on water lever responding for PSR or relapse (lower panels, Figs. 3 and 6). These results reveal that the 4-hr delay of nicotine did not lead to general enhancement of locomotor activity because water lever responses for the mid- and high-dose nicotine groups did not differ from baseline water lever responses. In addition, the saline group’s water lever responses were very similar to responses observed for the low-dose nicotine groups. The enhancement of EtOH-seeking and EtOH-relapse like self-administration that we observed is also not likely due to nicotine withdrawal because it was single administration of nicotine and the animals did not exhibit any of the somatic symptoms of nicotine withdrawal (i.e., abdominal constrictions, facial fasciculation, increased eye blinks, and ptosis).
Our results are in line with Le et al. (2003) findings that demonstrated repeated nicotine administration can increase EtOH-seeking behavior, as well as the findings of Lopez-Moreno et al. (2004) that showed repeated nicotine administrations can enhance EtOH-relapse-like drinking for up to two weeks. The difference between our study and the previous studies is that a single administration of nicotine and 4-hr delay prior to the operant sessions increased EtOH-seeking and relapse responding in P rats. Interestingly, Katner et al. (1997) showed that EtOH intake in P rats starts to recovery 4-hr after acute nicotine administration. Taken together, these studies suggest that nicotine’s acute priming effects may lead to enhancement of EtOH-seeking and relapse long after the dissipation of nicotine.
The desensitization or inactivation of nicotinic acetylcholine receptors (nAChRs) may be involved in the delayed enhancement of PSR and EtOH relapse responding by nicotine. Nicotine can rapidly desensitize nAChR subtypes (i.e., α4β2) for prolonged periods of time (Mansvelder et al., 2002; Pidoplichko et al., 1997, 2004). It has been reported that a single exposure of nicotine can reduce nAChRs function for 24-hr and that these receptors remain desensitized even after repeated exposure to nicotine (Vann et al., 2006). There is also evidence that EtOH can enhance desensitization of nAChRs caused by nicotine (Nagata et al., 1996), as well as the behavioral desensitization induced by pretreatment with nicotine (de Fiebre and Collins, 1989). Therefore, it is possible that the enhancement of EtOH-seeking and EtOH-relapse observed in the current study may be due to the desensitization of nAChRs caused by nicotine.
An alternative reason for the enhancement of EtOH-seeking and EtOH-relapse drinking for the 4-hr delay group may be due to active nicotine metabolites. Nicotine has several metabolites that have a longer half-life than nicotine itself. Cotinine is major metabolite of nicotine and its half-life ranges from 5 to ~ 7 hours in rat plasma (Kyerematen et al., 1988; Miller et al., 1977) and 5.6 hours in the brain (Ghosheh et al., 1999). Studies have shown that cotinine can stimulate nicotinic mechanisms to release noradrenaline (Vainio et al., 1998) and DA (Dwonski et al., 1999b), inhibit the binding of cholinergic receptors (Anderson and Arneric, 1994; Riah et al., 1999; Sloan et al., 1984), and desensitize nicotinic receptors (Dwoskin et al., 1999b). However, the cotinine concentrations necessary to increase extracellular DA have to be relatively high (Dwoskin et al., 1999b). Interestingly, not all of cotinine actions are mediated through cholinergic receptors. Riah et al. (2000) results provided the first molecular evidence that cotinine may have its own distinctive cotinine receptors. Cotinine has also been shown to reduce neuronal 5-HT turnover (Fuxe et al., 1979). Nicotine’s second largest metabolite nornicotine has a half-life of 2.8 hours in the brain (Ghosheh et al., 1999) to 3.3 hours in plasma (Kyerematen and Vesell, 1991). Nornicotine can also cause nicotinic receptor desensitization (Dwoskin et al., 1999a, 2001); it has reinforcing actions similar to nicotine (Bardo et al., 1999), and can act as a substitute for nicotine (Reichel et al., 2010). Although there is no current research on how nicotine’s metabolites may affect EtOH-seeking behavior or relapse like drinking, it is possible that one of nicotine’s metabolites may enhance EtOH-seeking behavior and EtOH- relapse like drinking after the 4-hr delay.
The present results showed that all doses of nicotine injected immediately prior to testing reduced both PSR and EtOH-relapse responding of P rats (top panels, Figs. 1 and 4), whereas only 0.3 mg/kg of nicotine injected immediately prior to PSR testing reduced responses on the lever previously associated with water (top panel, Fig. 3). The low (0.1 mg/kg) and the high (1.0 mg/kg) immediate doses of nicotine did not have any effect on water lever responding in the PSR test. None of the immediate doses of nicotine had any effect on water relapse responding when compared to baseline or among groups (top panel, Fig. 6). Interestingly, all doses of nicotine, given immediately before the operant session, reduced responding on the EtOH lever to approximately the same level in the PSR test and during relapse (upper panel, Figs. 1 and 4). This reduction is not likely due to a general suppressant effect on general locomotor activity since a dose of 0.8 mg/kg has been reported to enhance locomotor activity of P rats (Gordon et al., 1993b), and there was no definitive reduction in responding on the water lever during the PSR test or during relapse by P rats treated with nicotine (top panel, Figs. 3 and 6). The lack of a dose-dependent effect on EtOH lever responding during the PSR test and relapse by nicotine, when given immediately prior to the session, may be a result of the P rat being more sensitive to the stimulating and EtOH discriminative effects of nicotine (Gordon et al., 1993a, b). Therefore, it is possible that the EtOH PSR and relapse responding by the P rats may be sensitive to the immediate effects of nicotine, such that the 0.1 mg/kg dose is producing a maximal effect, which may reflect the greater EtOH discriminative stimulus and locomotor stimulating effects of nicotine for the P compared to the NP rat (Gordon et al., 1993a, b).
The current findings for the non-delayed groups of nicotine are in line with previous studies that showed when nicotine was administered immediately-to-30-min prior to EtOH access, there was a reduction in EtOH intake (see Le, 2002). Administration of nicotine into the lateral ventricles 15-min before limited EtOH access dose-dependently decreased EtOH intake within the first 30-min without altering saccharin intake of P rats (Katner et al., 1997). Local application of nicotine into the nucleus accumbens 10-min prior to EtOH access decreased EtOH operant self-administration in Long-Evans rats without altering sucrose self-administrations (Nadal et al., 1998). Peripheral administration of nicotine immediately prior to EtOH-access has also been shown to decrease EtOH consumption (Dyr et al., 1999). On the other hand, Gauvin et al. (1993) found a biphasic effect of acute nicotine on EtOH consumption in Sprague-Dawley rats. Low-dose nicotine (0.1 mg/kg), administered 15-min before EtOH access, increased EtOH intake, whereas higher doses (0.18 and 0.32 mg/kg), which are similar concentrations used in more recent studies, reduced EtOH intake. For P rats, a biphasic effect of nicotine on EtOH-seeking or EtOH relapse like self-administration was not observed (top panels, Figs. 1 and 4). The low-dose produced similar reductions as the mid and high doses of nicotine, suggesting that P rats may be more sensitive to the effects of nicotine than non-selective rats (top panels, Figs. 1 and 4).
One possible explanation for the reduction of EtOH-seeking and EtOH-relapse responding by P rats is that the initial effects of nicotine may be to substitute for EtOH. Interestingly, drug discrimination studies have shown that EtOH discriminative stimulus generalizes to nicotine in P rats compared to the NP rats (Gordon et al., 1993a; McMillan et al., 1999) and that P rats are more likely to substitute nicotine for EtOH (McMillan et al., 1999). Le at al. (2006) findings showed that EtOH-naïve P rats will self-administer more nicotine and express greater nicotine-seeking behavior than NP rats. Le et al. (2006) findings in P rats provided support for the hypothesis that nicotine and alcohol addiction may share a common genetic vulnerability and this shared common genetic vulnerability may also help explain why the initial effects of nicotine reduced EtOH-seeking and EtOH-relapse self-administration drinking behavior in P rats.
The mechanisms underlying nicotine’s effect on EtOH-seeking or relapse behavior are not understood. However, activation of the mesolimbic dopamine (DA) system is thought to be involved in mediating compulsive drug seeking and drug taking behaviors. EtOH can enhance DA neurotransmission by increasing the firing rate of DA neurons (Brodie et al., 1990; Gessa et al., 1985) and somatodendritic DA release in the VTA (Campbell et al., 1996; Kohl et al., 1998), subsequently leading to an increase in extracellular levels of DA in the nucleus accumbens (Imperato and Di Chiara, 1986; Kohl et al., 1998; Yoshida et al., 1993). Similar findings have shown that nicotine can also enhance DA neurotransmission by increasing the firing rate of DA neurons (Grenhoff et al., 1986) and somatodendritic DA release in the VTA (Rahman et al., 2003, 2004), subsequently leading to an increase in extracellular levels of DA in the nucleus accumbens (Ferrari et al., 2002; Imperato et al., 1986b; Nisell et al., 1994; Tizabi et al., 2002).
Nicotine has a half-life that ranges from 55 to 64-min in plasma (Miller et al., 1977) and 30 to 60-min in brain (Ghosheh et al., 1999; Rossi et al., 2005; Sastry et al., 1995). Nicotine brain concentration levels can reach their peak in less than 10-min (Rossi et al., 2005). A single administration of nicotine can stimulate DA neurotransmission for over an hour after nicotine administration (Di Chiara and Imperato, 1988; Imperato et al., 1986b; Pidoplichko et al., 2004; Schilstrom et al., 1998). This prolonged DA neurotransmission is due to α7 nAChRs on the presynaptic glutamate terminals, which do not desensitize to nicotine, but continue to enhance glutamatergic excitation in the presence of nicotine (Pidoplichko et al., 2004).
Some studies have shown that blocking nAChR can decrease EtOH-self administrations in animals (Blomqvist et al., 1996; Farook et al., 2009; Hendrickson et al., 2009; Kuzmin et al., 2009; Le et al., 2000), alcohol drinking in humans (Chi and deWit, 2003), and block EtOH induced extracellular DA release (Blomqvist et al., 1993, 1997), thus providing evidence that some of EtOH reinforcing effects may be mediated via nAChRs. It is noteworthy to mention that the majority of pre-clinical studies, which observed nicotine increasing EtOH intake, administered nicotine repeatedly, or at a constant rate (Blomqvist et al., 1996; Clark et al., 2001; Ericson et al., 2000; Olausson et al., 2001; Pothoff et al., 1983; Smith et al. 1999), whereas those that examine acute effects of nicotine on EtOH intake observed a reduction (Dyr et al., 1999; Gauvin et al., 1993; Katner et al., 1997; Nadal et al., 1998). Thus, it is possible that the initial DA stimulating effects of nicotine, when it is administered immediately prior to the tests, may be a viable substitute for EtOH under seeking and relapse conditions. However, taken into account that nicotine doses have significantly decreased after 4-hr suggests that enhancement of EtOH-seeking and EtOH-relapse responding are occurring long after the stimulatory effects of nicotine on DA release. Therefore, in the current study it seems unlikely that the enhancement of EtOH-seeking and EtOH-relapse responding by nicotine is due to increased DA release.
Another factor to take into consideration is that neuroadaptations can occur after chronic drinking and that repeated alcohol deprivations may produce further increases in the reinforcing effects and the sensitivity of EtOH within the mesolimbic DA system (Rodd et al., 2005a, b). Furthermore, it is thought that drugs of abuse can prime responding by activating the mesolimbic DA system, which has become sensitized with repeated drug use in a long lasting manner (Robinson and Berridge, 1993). Alcohol can also enhance the function of several subtypes of nAChRs (i.e., α4β2, α4β4, α2β2, and α2β4) (see Davis and de Fiebre, 2006; Cardoso et al. 1999; Narahashi et al., 1999, 2001), have little to no effect on other subtypes (i.e., α3β2 and α3β4) (see Davis and de Fiebre, 2006; Cardoso et al., 1999), or inhibit other nAChR subtypes (i.e., α7) (Cardoso et al. 1999; see Davis and de Fiebre, 2006; de Fiebre and de Fiebre 2005; Narahashi et al., 1999, 2001; Yu et al. 1996). In the current study, PSR was examine after 21 days of EtOH deprivation and EtOH-relapse after 32 days of EtOH deprivation, nicotine’s effects may be acting on a sensitized mesolimbic DA system with various nAChR alterations. Hence, further alterations of this system after chronic drinking and abstinence may also contribute to the effects of nicotine that were observed in these animals during PSR and EtOH relapse.
In order to obtain a better understanding of nicotine’s time-dependent effects on EtOH-seeking and relapse behaviors, EtOH responding in 10-min bins was examined (Figs. 2 and 5). Previous research demonstrated that only the 0.8 mg/kg dose of nicotine suppressed EtOH responding of non-selective Wistar rats in the first 20-min; however, EtOH responding started to increase after this time point, resulting in enhanced EtOH intake (Le et al., 2000). Le (2002) suggested that the suppression of EtOH intake observed in studies that administered nicotine acutely may be due to the length of EtOH access, suggesting that shorter durations (30-min) to EtOH access may mask nicotine’s effect on EtOH intake, because nicotine initially reduces EtOH intake in the first 20-min and increases EtOH intake towards the end of the 60-min session (Le et al., 2000; see Le, 2002). The current10-min bin data revealed that all nicotine doses suppressed the initial response of EtOH-seeking and relapse responding within the first 20-min in the non-delayed groups compared to the saline group (Fig. 2). However, unlike the Le et al. (2000) study, the suppression of these behaviors in P rats continued throughout the entire 60-min session. Taken together, these results provide evidence that P rats are more sensitive to the effects of nicotine than non-selective rats because the effects of nicotine did not rebound after the initial 20-min suppression. In addition, further examination of the time course for the 4-hr delayed group showed that the increase of EtOH-seeking and relapse generally occurred in the first 30-min and/or last 10-min of the 60-min sessions (Fig. 5). For example, the mid- (middle panels, Figs. 2 and 5) and high- (lower panels, Figs. 2 and 5) doses of nicotine for the 4-hr delayed group tended to increase EtOH-seeking and relapse at the beginning and towards the end of the 60-min sessions, whereas the saline group only increased EtOH-seeking at the beginning followed by reduction in responses from 30-min to the end of the session. This led to the overall enhancement of PSR and EtOH-relapse for 0.3 mg/kg and 1.0 mg/kg nicotine 4-hr delayed groups compared to saline and shows that priming effects of the higher doses of nicotine actively motivated the animals to obtain EtOH at beginning and end of the 60-min sessions even when EtOH was unavailable (PSR). The 0.1 mg/kg nicotine dose was similar to saline group and the enhancement of EtOH-seeking was only observed at the beginning of session regardless of time, whereas the enhancement of relapse drinking was observed at the beginning and towards the end for both saline and the 0.1 mg/kg nicotine groups. Taken together, these results suggest that time and dose of nicotine administration are important factors in the enhanced expression of EtOH-seeking and relapse in P rats.
In conclusion, these findings suggest that the effects of nicotine on EtOH-seeking and EtOH-relapse behaviors in P rats may depend on the temporal assessment of the behaviors post drug administration. Further alterations of neuronal systems after chronic drinking and abstinence may also contribute to nicotine’s effect on the enhancement of EtOH-seeking and EtOH-relapse responding observed in these animals.
Acknowledgments
Sources of support: Grants AA07611, AA10721, AA07462, and AA019366.
The skillful technical assistance of Tylene Pommer and Victoria McQueen is gratefully acknowledge.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology (Berl) 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Arneric SP. Nicotinic receptor binding of [3H] cytisine, [3H]nicotine and [3H]methylcarbamylcholine in rat brain. Eur J Pharmacol. 1994;253:261–267. doi: 10.1016/0014-2999(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Brooks DC. Recent and remote extinction cues reduce spontaneous recovery. Q J Exper Psycho. 2000;153:25–58. doi: 10.1080/027249900392986. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, Ambiguity, and unlearning: Sources of relapse after behavioral extinction. Bio Psych. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Engel JA, Soderpalm B. Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Cicero TJ. A critique of animal analogues of alcoholism. In: Majchrowicz E, Noble EP, editors. Biochemistry and Pharmacology of Ethanol. Vol. 2. New York: Plenum Press; 1979. pp. 533–560. [Google Scholar]
- Clark A, Lindgren S, Brooks SP, Watson WP, Little HJ. Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology. 2001;41:108–117. doi: 10.1016/s0028-3908(01)00037-5. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- de Fiebre CM, Collins AC. Behavioral desensitization to nicotine is enhanced differentially by ethanol in long-sleep and short-sleep mice. Alcohol. 1989;6:45–51. doi: 10.1016/0741-8329(89)90072-4. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, de Fiebre CM. alpha7 Nicotinic acetylcholine receptor knockout selectively enhances ethanol-, but not beta-amyloid-induced neurotoxicity. Neurosci Lett. 2005;373:42–47. doi: 10.1016/j.neulet.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but not Alcohol-Seeking, in Alcohol-Preferring (P) Rats. J Addict Med. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyr W, Koros E, Bienkowski P, Kostowski W. Involvement of nicotinic acetylcholine receptors in the regulation of alcohol drinking in Wistar rats. Alcohol Alcohol. 1999;34:43–47. doi: 10.1093/alcalc/34.1.43. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng LH, Crooks PA. Nornicotine, a nicotine metabolite and tobacco alkaloid: desensitization of nicotinic receptor-stimulated dopamine release from rat striatum. Eur J Pharmacol. 2001;428:69–79. doi: 10.1016/s0014-2999(01)01283-3. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology (Berl) 1999a;145:442–451. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, Crooks PA. (S)-(−)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp Ther. 1999b;288:905–911. [PubMed] [Google Scholar]
- Ericson M, Engel JA, Soderpalm B. Peripheral involvement in nicotine-induced enhancement of ethanol intake. Alcohol. 2000;21:37–47. doi: 10.1016/s0741-8329(99)00099-3. [DOI] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Effects of mecamylamine on alcohol consumption and preference in male C57BL/6J mice. Pharmacology. 2009;83:379–384. doi: 10.1159/000219488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Le NN, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Everitt BJ, Hokfelt T. On the action of nicotine and cotinine on central 5-hydroxytryptamine neurons. Pharmacol Biochem Behav. 1979;10:671–677. doi: 10.1016/0091-3057(79)90319-8. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Moore KR, Holloway FA. Do rat strain differences in ethanol consumption reflect differences in ethanol sensitivity or the preparedness to learn? Alcohol. 1993;10:37–43. doi: 10.1016/0741-8329(93)90051-o. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2’-(14)C]nicotine. Drug Metab Dispos. 1999;27:1448–1455. [PubMed] [Google Scholar]
- Gordon TL, Meehan SM, Schechter MD. P and NP rats respond differently to the discriminative stimulus effects of nicotine. Pharmacol Biochem Behav. 1993a;45:305–308. doi: 10.1016/0091-3057(93)90243-m. [DOI] [PubMed] [Google Scholar]
- Gordon TL, Meehan SM, Schechter MD. Differential effects of nicotine but not cathinone on motor activity of P and NP rats. Pharmacol Biochem Behav. 1993b;44:657–659. doi: 10.1016/0091-3057(93)90182-s. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, ston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. The future of smoking cessation therapy in the United States. Addiction. 1996;91:1797–1802. doi: 10.1046/j.1360-0443.1996.911217974.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr, Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin Exp Res. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Katner SN, McBride WJ, Lumeng L, Li TK, Murphy JM. Effects of cholinergic agents on locomotor activity of P and NP rats. Alcohol Clin Exp Res. 1996;20:1004–1010. doi: 10.1111/j.1530-0277.1996.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Katner SN, McBride WJ, Lumeng L, Li TK, Murphy JM. Involvement of CNS cholinergic systems in alcohol drinking of P rats. Addict Biol. 1997;2:215–223. doi: 10.1080/13556219772769. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986a;239:219–228. [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986b;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology (Berl) 1998;139:79–85. doi: 10.1007/s002130050692. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology (Berl) 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Vesell ES. Metabolism of nicotine. Drug Metab Rev. 1991;23:3–41. doi: 10.3109/03602539109029754. [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Taylor LH, deBethizy JD, Vesell ES. Pharmacokinetics of nicotine and 12 metabolites in the rat. Application of a new radiometric high performance liquid chromatography assay. Drug Metab Dispos. 1988;16:125–129. [PubMed] [Google Scholar]
- Lallemand F, Ward RJ, De WP. Nicotine increases ethanol preference but decreases locomotor activity during the initial stages of chronic ethanol withdrawal. Alcohol Alcohol. 2007;42:207–218. doi: 10.1093/alcalc/agm023. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le AD. Effects of nicotine on alcohol consumption. Alcohol Clin Exp Res. 2002;26:1915–1916. doi: 10.1097/01.ALC.0000040963.46878.5D. [DOI] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester D, Freed EX. Criteria for an animal model of alcoholism. Pharmacol Biochem Behav. 1973;1:103–107. doi: 10.1016/0091-3057(73)90062-2. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Trigo-Diaz JM, Rodriguez de FF, Gonzalez CG, Gomez de HR, Crespo GI, Navarro M. Nicotine in alcohol deprivation increases alcohol operant self-administration during reinstatement. Neuropharmacology. 2004;47:1036–1044. doi: 10.1016/j.neuropharm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl) 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li T-K. The alcohol deprivation effect in the alcohol-preferring P rat under free drinking and operant access conditions. Alcohol Clin Exp Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- McMillan DE, Li M, Shide DJ. Differences between alcohol-preferring and alcohol-nonpreferring rats in ethanol generalization. Pharmacol Biochem Behav. 1999;64:415–419. doi: 10.1016/s0091-3057(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Miller RP, Rotenberg KS, Adir J. Effect of dose on the pharmacokinetics of intravenous nicotine in the rat. Drug Metab Dispos. 1977;5:436–443. [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Nadal R, Chappell AM, Samson HH. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- Nagata K, Aistrup GL, Huang CS, Marszalec W, Song JH, Yeh JZ, Narahashi T. Potent modulation of neuronal nicotinic acetylcholine receptor-channel by ethanol. Neurosci Lett. 1996;217:189–193. [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int. 1999;35:131–141. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Soderpalm B, Ericson M, Olausson P, Engel JA, Zhang X, Nordberg A, Marszalec W, Aistrup GL, Schmidt LG, Kalouti U, Smolka AM, Hedlund L. Mechanisms of alcohol-nicotine interactions: alcoholics versus smokers. Alcohol Clin Exp Res. 2001;25:152S–156S. doi: 10.1097/00000374-200105051-00026. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Aubin HJ, Pomerleau OF. Self-reported alcohol use patterns in a sample of male and female heavy smokers. J Addict Dis. 1997;16:19–24. doi: 10.1300/J069v16n03_02. [DOI] [PubMed] [Google Scholar]
- Potthoff AD, Ellison G, Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav. 1983;18:489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Local perfusion of nicotine differentially modulates somatodendritic dopamine release in the rat ventral tegmental area after nicotine preexposure. Neurochem Res. 2004;29:1687–1693. doi: 10.1023/b:nere.0000035803.64724.17. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: in vivo microdialysis study. Neurosci Lett. 2003;348:61–64. doi: 10.1016/s0304-3940(03)00723-7. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA. Extinction with varenicline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology. 2010;58:1237–1245. doi: 10.1016/j.neuropharm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Experimental Extinction. In: Mowrer RR, Klein SB, editors. Handbook of Contemporary Learning Theories. NJ: Mahwah: Erlbaum; 2001. pp. 119–154. [Google Scholar]
- Riah O, Dousset JC, Bofill-Cardona E, Courriere P. Isolation and microsequencing of a novel cotinine receptor. Cell Mol Neurobiol. 2000;20:653–664. doi: 10.1023/a:1007094623775. [DOI] [PubMed] [Google Scholar]
- Riah O, Dousset JC, Courriere P, Stigliani JL, Baziard-Mouysset G, Belahsen Y. Evidence that nicotine acetylcholine receptors are not the main targets of cotinine toxicity. Toxicol Lett. 1999;109:21–29. doi: 10.1016/s0378-4274(99)00070-3. [DOI] [PubMed] [Google Scholar]
- Robbins SJ. Mechanisms underlying spontaneous recovery in authoshaping. J Exper Psychol Anim Behav Processes. 1990;16:235–249. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005a;29:358–66. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005b;315:648–57. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149:217–24. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Room R. Smoking and drinking as complementary behaviours. Biomed Pharmacother. 2004;58:111–115. doi: 10.1016/j.biopha.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rossi S, Singer S, Shearman E, Sershen H, Lajtha A. Regional heterogeneity of nicotine effects on neurotransmitters in rat brains in vivo at low doses. Neurochem Res. 2005;30:91–103. doi: 10.1007/s11064-004-9690-7. [DOI] [PubMed] [Google Scholar]
- Sastry BV, Chance MB, Singh G, Horn JL, Janson VE. Distribution and retention of nicotine and its metabolite, cotinine, in the rat as a function of time. Pharmacology. 1995;50:128–136. doi: 10.1159/000139274. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Repeated nicotine injections decrease operant ethanol self-administration. Alcohol. 2002;28:1–7. doi: 10.1016/s0741-8329(02)00238-0. [DOI] [PubMed] [Google Scholar]
- Sloan JW, Todd GD, Martin WR. Nature of nicotine binding to rat brain P2 fraction. Pharmacol Biochem Behav. 1984;20:899–909. doi: 10.1016/0091-3057(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl) 1999;142:408–412. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- Taylor BJ, Graham JW, Cumsille P, Hansen WB. Modeling prevention program effects on growth in substance use: Analysis of five years of data from the Adolescent Alcohol Prevention Trial. Prevention Science. 2000;1:183–197. doi: 10.1023/a:1026547128209. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Toalston JE, Oster SM, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA. Effects of alcohol and saccharin deprivations on concurrent ethanol and saccharin operant self-administration by alcohol-preferring (P) rats. Alcohol. 2008;42:277–284. doi: 10.1016/j.alcohol.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Vainio PJ, Viluksela M, Tuominen RK. Nicotine-like effects of cotinine on protein kinase C activity and noradrenaline release in bovine adrenal chromaffin cells. J Auton Pharmacol. 1998;18:245–250. doi: 10.1046/j.1365-2680.1998.18490.x. [DOI] [PubMed] [Google Scholar]
- Vann RE, James JR, Rosecrans JA, Robinson SE. Nicotinic receptor inactivation after acute and repeated in vivo nicotine exposures in rats. Brain Res. 2006;1086:98–103. doi: 10.1016/j.brainres.2006.02.075. [DOI] [PubMed] [Google Scholar]
- Volk HE, Scherrer JF, Bucholz KK, Todorov A, Heath AC, Jacob T, True WR. Evidence for specificity of transmission of alcohol and nicotine dependence in an offspring of twins design. Drug Alcohol Depend. 2007;87:225–232. doi: 10.1016/j.drugalcdep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- York JL, Hirsch JA. Drinking patterns and health status in smoking and nonsmoking alcoholics. Alcohol Clin Exp Res. 1995;19:666–673. doi: 10.1111/j.1530-0277.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Tanaka T, Mizoguchi K, Emoto H, Ishii H, Tanaka M. Facilitatory modulation of mesolimbic dopamine neuronal activity by a mu-opioid agonist and nicotine as examined with in vivo microdialysis. Brain Res. 1993;624:277–280. doi: 10.1016/0006-8993(93)90087-4. [DOI] [PubMed] [Google Scholar]
- Yu ZJ, Morgan DG, Wecker L. Distribution of three nicotinic receptor alpha 4 mRNA transcripts in rat brain: selective regulation by nicotine administration. J Neurochem. 1996;66:1326–1329. doi: 10.1046/j.1471-4159.1996.66031326.x. [DOI] [PubMed] [Google Scholar]