Abstract
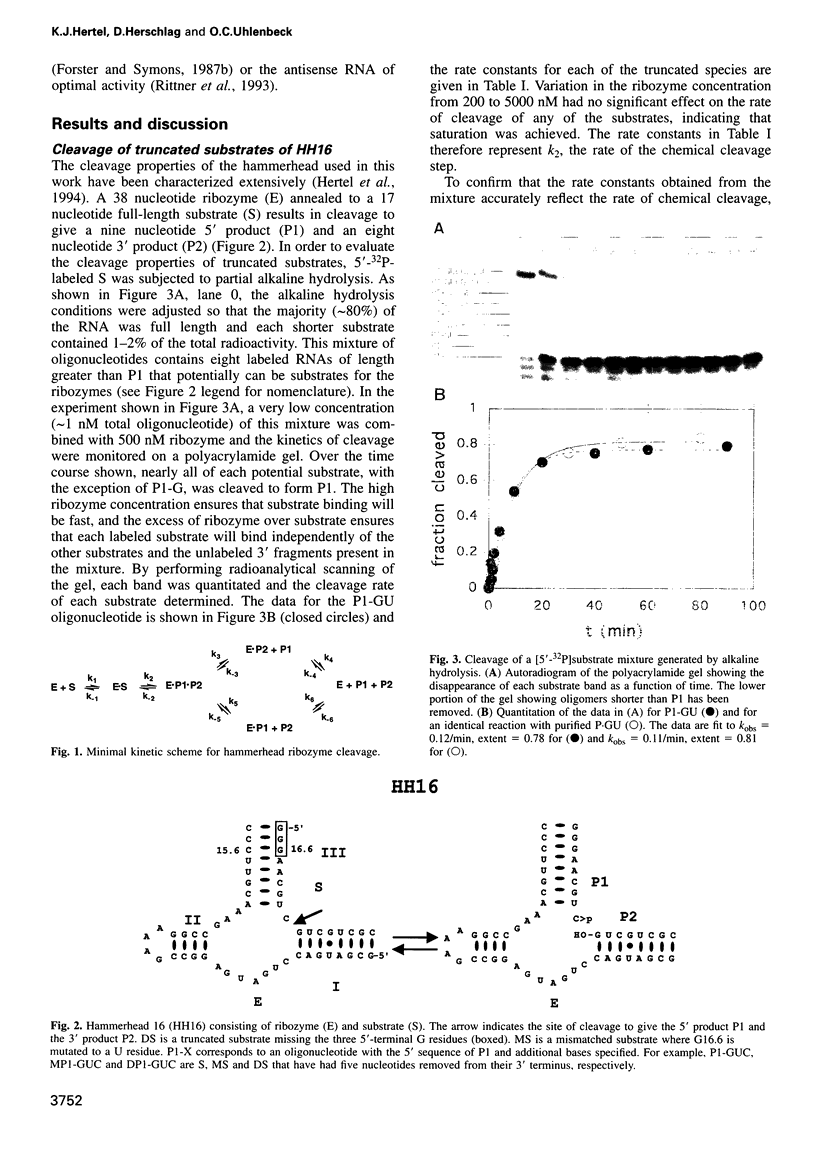
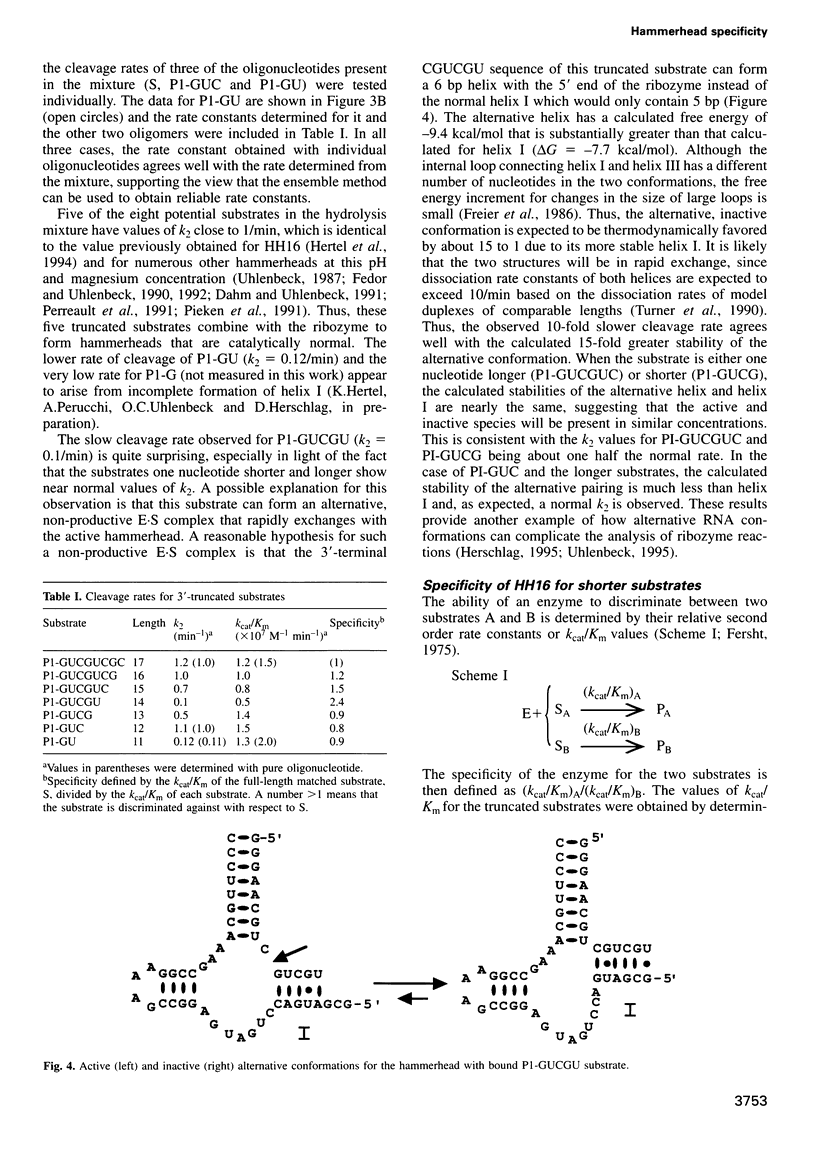
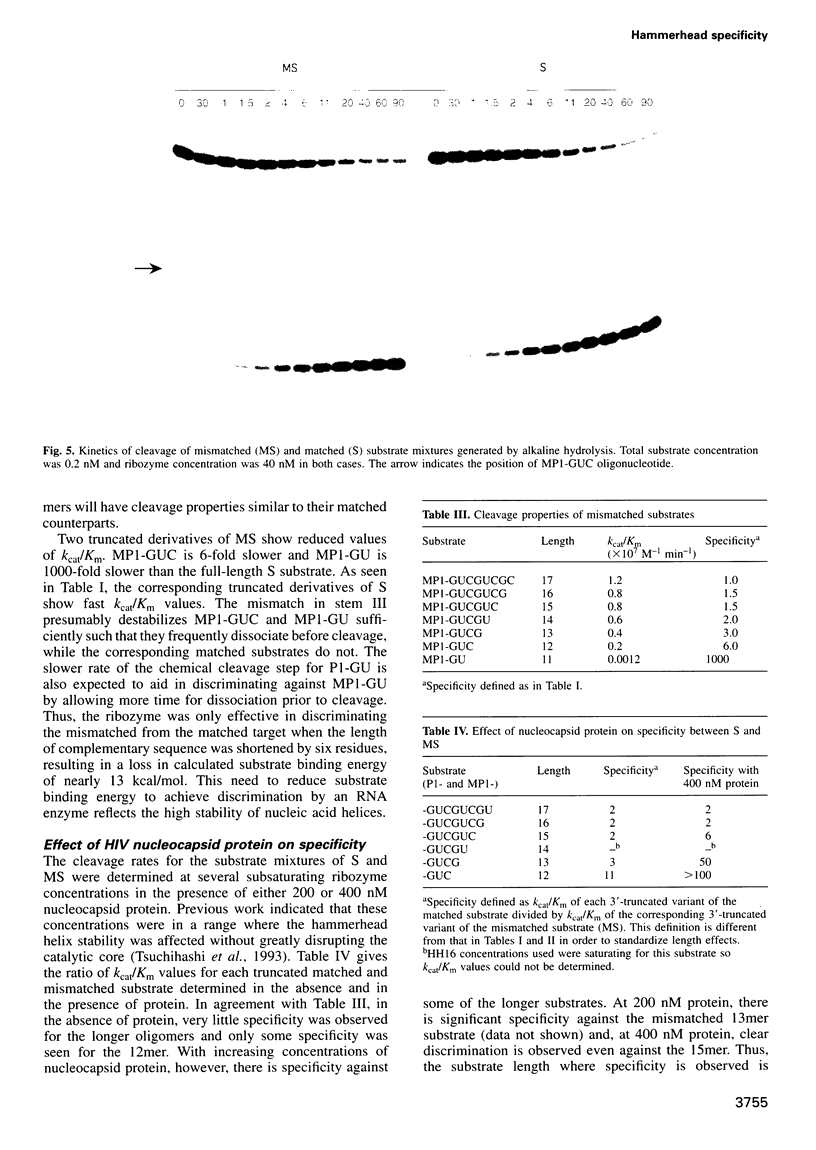
To be effective in gene inactivation, the hammerhead ribozyme must cleave a complementary RNA target without deleterious effects from cleaving non-target RNAs that contain mismatches and shorter stretches of complementarity. The specificity of hammerhead cleavage was evaluated using HH16, a well-characterized ribozyme designed to cleave a target of 17 residues. Under standard reaction conditions, HH16 is unable to discriminate between its full-length substrate and 3'-truncated substrates, even when six fewer base pairs are formed between HH16 and the substrate. This striking lack of specificity arises because all the substrates bind to the ribozyme with sufficient affinity so that cleavage occurs before their affinity differences are manifested. In contrast, HH16 does exhibit high specificity towards certain 3'-truncated versions of altered substrates that either also contain a single base mismatch or are shortened at the 5' end. In addition, the specificity of HH16 is improved in the presence of p7 nucleocapsid protein from human immunodeficiency virus (HIV)-1, which accelerates the association and dissociation of RNA helices. These results support the view that the hammerhead has an intrinsic ability to discriminate against incorrect bases, but emphasizes that the high specificity is only observed in a certain range of helix lengths.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand E. L., Rossi J. J. Facilitation of hammerhead ribozyme catalysis by the nucleocapsid protein of HIV-1 and the heterogeneous nuclear ribonucleoprotein A1. EMBO J. 1994 Jun 15;13(12):2904–2912. doi: 10.1002/j.1460-2075.1994.tb06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzayan J. M., Gerlach W. L., Bruening G. Satellite tobacco ringspot virus RNA: A subset of the RNA sequence is sufficient for autolytic processing. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8859–8862. doi: 10.1073/pnas.83.23.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F. H., Jennings P. A. Multiple domains in a ribozyme construct confer increased suppressive activity in monkey cells. Antisense Res Dev. 1994 Summer;4(2):87–94. doi: 10.1089/ard.1994.4.87. [DOI] [PubMed] [Google Scholar]
- Carey J., Cameron V., de Haseth P. L., Uhlenbeck O. C. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983 May 24;22(11):2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- Coetzee T., Herschlag D., Belfort M. Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev. 1994 Jul 1;8(13):1575–1588. doi: 10.1101/gad.8.13.1575. [DOI] [PubMed] [Google Scholar]
- Dahm S. C., Derrick W. B., Uhlenbeck O. C. Evidence for the role of solvated metal hydroxide in the hammerhead cleavage mechanism. Biochemistry. 1993 Dec 7;32(48):13040–13045. doi: 10.1021/bi00211a013. [DOI] [PubMed] [Google Scholar]
- Dahm S. C., Uhlenbeck O. C. Role of divalent metal ions in the hammerhead RNA cleavage reaction. Biochemistry. 1991 Oct 1;30(39):9464–9469. doi: 10.1021/bi00103a011. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Kinetics of intermolecular cleavage by hammerhead ribozymes. Biochemistry. 1992 Dec 8;31(48):12042–12054. doi: 10.1021/bi00163a012. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Substrate sequence effects on "hammerhead" RNA catalytic efficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1668–1672. doi: 10.1073/pnas.87.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D. Implications of ribozyme kinetics for targeting the cleavage of specific RNA molecules in vivo: more isn't always better. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D., Khosla M., Tsuchihashi Z., Karpel R. L. An RNA chaperone activity of non-specific RNA binding proteins in hammerhead ribozyme catalysis. EMBO J. 1994 Jun 15;13(12):2913–2924. doi: 10.1002/j.1460-2075.1994.tb06586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel K. J., Herschlag D., Uhlenbeck O. C. A kinetic and thermodynamic framework for the hammerhead ribozyme reaction. Biochemistry. 1994 Mar 22;33(11):3374–3385. doi: 10.1021/bi00177a031. [DOI] [PubMed] [Google Scholar]
- Mahieu M., Deschuyteneer R., Forget D., Vandenbussche P., Content J. Construction of a ribozyme directed against human interleukin-6 mRNA: evaluation of its catalytic activity in vitro and in vivo. Blood. 1994 Dec 1;84(11):3758–3765. [PubMed] [Google Scholar]
- Marschall P., Thomson J. B., Eckstein F. Inhibition of gene expression with ribozymes. Cell Mol Neurobiol. 1994 Oct;14(5):523–538. doi: 10.1007/BF02088835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Strack B., Dannull J., Sproat B. S., Surovoy A., Jung G., Moelling K. Amino acid requirements of the nucleocapsid protein of HIV-1 for increasing catalytic activity of a Ki-ras ribozyme in vitro. J Mol Biol. 1994 Sep 30;242(4):422–429. doi: 10.1006/jmbi.1994.1592. [DOI] [PubMed] [Google Scholar]
- Perreault J. P., Labuda D., Usman N., Yang J. H., Cedergren R. Relationship between 2'-hydroxyls and magnesium binding in the hammerhead RNA domain: a model for ribozyme catalysis. Biochemistry. 1991 Apr 23;30(16):4020–4025. doi: 10.1021/bi00230a029. [DOI] [PubMed] [Google Scholar]
- Pieken W. A., Olsen D. B., Benseler F., Aurup H., Eckstein F. Kinetic characterization of ribonuclease-resistant 2'-modified hammerhead ribozymes. Science. 1991 Jul 19;253(5017):314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- Rittner K., Burmester C., Sczakiel G. In vitro selection of fast-hybridizing and effective antisense RNAs directed against the human immunodeficiency virus type 1. Nucleic Acids Res. 1993 Mar 25;21(6):1381–1387. doi: 10.1093/nar/21.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. Q., Warrilow D., Wang L., Witherington C., Macpherson J., Symonds G. Ribozyme-mediated suppression of Moloney murine leukemia virus and human immunodeficiency virus type I replication in permissive cell lines. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9715–9719. doi: 10.1073/pnas.91.21.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. B., Hobom G., Luo D. Ribozyme mediated destruction of influenza A virus in vitro and in vivo. J Med Virol. 1994 Apr;42(4):385–395. doi: 10.1002/jmv.1890420411. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi Z., Brown P. O. DNA strand exchange and selective DNA annealing promoted by the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1994 Sep;68(9):5863–5870. doi: 10.1128/jvi.68.9.5863-5870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Werner M., Uhlenbeck O. C. The effect of base mismatches in the substrate recognition helices of hammerhead ribozymes on binding and catalysis. Nucleic Acids Res. 1995 Jun 25;23(12):2092–2096. doi: 10.1093/nar/23.12.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Bahner I. C., Larson G. P., Zaia J. A., Rossi J. J., Kohn E. B. Inhibition of HIV-1 in human T-lymphocytes by retrovirally transduced anti-tat and rev hammerhead ribozymes. Gene. 1994 Nov 4;149(1):33–39. doi: 10.1016/0378-1119(94)90409-x. [DOI] [PubMed] [Google Scholar]