Abstract
Background
Cardiac output (CO) is a major diagnostic and prognostic factor in pre-capillary pulmonary hypertension (PH). Reference methods for CO determination, like thermodilution (TD), require invasive procedures and allow only steady-state measurements. The Modelflow (MF) method is an appealing technique for this purpose as it allows non-invasive and beat-by-beat determination of CO.
Methods
We aimed to compare CO values obtained simultaneously from non-invasive pulse wave analysis by MF (COMF) and by TD (COTD) to determine its precision and accuracy in pre-capillary PH. The study was performed on 50 patients with pulmonary arterial hypertension (PAH) or chronic thrombo-embolic PH (CTEPH). CO was determined at rest in all patients (n = 50) and during nitric oxide vasoreactivity test, fluid challenge or exercise (n = 48).
Results
Baseline COMF and COTD were 6.18 ± 1.95 and 5.46 ± 1.95 L·min-1, respectively. Accuracy and precision were 0.72 and 1.04 L·min-1, respectively. Limits of agreement (LoA) ranged from -1.32 to 2.76 L·min-1. Percentage error (PE) was ±35.7%. Overall sensitivity and specificity of COMF for directional change were 95.2% and 82.4%, (n = 48) and 93.3% and 100% for directional changes during exercise (n = 16), respectively. After application of a correction factor (1.17 ± 0.25), neither proportional nor fixed bias was found for subsequent CO determination (n = 48). Accuracy was -0.03 L·min−1 and precision 0.61 L·min−1. LoA ranged from -1.23 to 1.17 L·min−1 and PE was ±19.8%.
Conclusions
After correction against a reference method, MF is precise and accurate enough to determine absolute values and beat-by-beat relative changes of CO in pre-capillary PH.
Introduction
Pre-capillary pulmonary hypertension (PH) is a haemodynamic condition that may lead to right heart failure, and that is defined by an increased resting pulmonary artery mean pressure (mPAP) due to elevated pulmonary vascular resistance (PVR) [1]. Consequently, cardiac output (CO), is a key diagnostic parameter and a major prognostic factor in diseases like pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) [1, 2], both characterized by such a condition.
Classical methods for CO measurement require right heart catheterisation (RHC) where the reference method is the direct Fick, although thermodilution (TD) or indirect Fick methods are widely preferred because of their relative simplicity in the clinical setting [1]. Hence, the availability of new, simple, reliable and non-invasive method for CO determination at rest and for CO changes in response to pharmacological interventions or during metabolic and volemic changes is desirable.
A promising technique for this purpose called Modelflow (MF) [3], relies on arterial pulse pressure wave analysis. Once corrected against a reference method, MF was shown to be a reliable and accurate procedure in healthy humans [4], requiring only the application of a finger plethysmographic cuff to the patient for the continuous monitoring of pulse pressure profiles, thus being simpler than any other method proposed so far.
To the best of our knowledge, the accuracy and precision of MF was never assessed in pre-capillary PH. This study aims to evaluate MF in PAH and CTEPH patients, by comparing CO values obtained by MF (COMF) with values simultaneously determined on the same patients by TD (COTD) during RHC procedures.
Materials and Methods
Study population
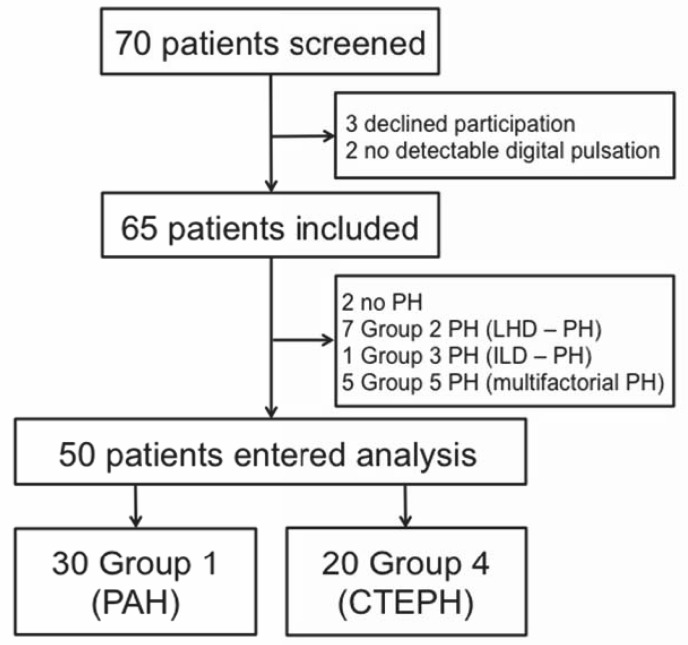
Seventy consecutive patients from the outpatient clinic and who underwent RHC for suspected or diagnosed pre-capillary PH within their routine workup were invited to participate (Fig 1). Patients with cardiac shunts were excluded, TD being potentially inaccurate in this condition. Patients with PH due to left heart disease (diagnostic Group 2 according to WHO classification, post-capillary PH) or PH with unclear or multifactorial mechanisms (diagnostic Group 5) were also excluded. In order to assess a homogeneous population, patients with PH due to lung disease were excluded because the physiopathology of pre-capillary PH is different in this group (diagnostic Group 3). 2 patients were also excluded from the study due to poor or no fingertip pulse pressure signal due to systemic sclerosis. Screening was not proposed to patients hospitalized in intensive care unit with acute right heart failure, haemodynamic shock or other life threatening condition. Analyses was finally performed on fifty patients with PAH (diagnostic Group 1, n = 30) or CTEPH (diagnostic Group 4, n = 20). This study was approved by a local ethics committee (comité de protection des personnes, Ile de France VIII, Hôpital Ambroise Paré, Boulogne-Billancourt, France) and was performed in the French reference centre for severe PH in Paris. All patients gave written informed consent.
Fig 1. Study profile.
PH: Pulmonary hypertension; LHD–PH: PH due to left heart disease; ILD–PH: PH due to interstitial lung disease; PAH: Pulmonary arterial hypertension; CTEPH: Chronic thromboembolic PH.
Right heart catheterisation
Haemodynamic evaluation was performed in a supine position. Electrocardiogram and arterial oxygen saturation (pulse oximetry) were monitored continuously. Mean systemic arterial pressure (MAP) was measured at the brachial artery (Dynamap 1800; Critikon, Tampa, FL, USA). RHC was performed using the modified Seldinger technique with an 8F sheath inserted in the jugular, basilic or cephalic vein. The Swan-Ganz catheter was a 7F, two-lumen, TD and pressure-measuring tipped catheter (Corodyn TD; Braun Medical, Bethlehem, PA, USA). The zero-level reference was determined at mid-thoracic line. Resting haemodynamic evaluation included measures of right atrial pressure (RAP), mPAP, pulmonary artery wedge pressure (PAWP), COTD. PVR was calculated as (mPAP–PAWP)/ COTD, and systemic vascular resistance as (MAP–RAP)/ COTD. Cardiac index was calculated as CO divided by body surface area. Fluid challenge was performed by infusion of 500 ml of isotonic saline solution in five minutes, and haemodynamic parameters reassessed afterward. Vasoreactivity test was performed with inhaled nitric oxide according to current recommendations [1] prior to haemodynamic reassessment.
Cardiac output by thermodilution
In each patient, the positioning of the Swan-Ganz probe was confirmed by fluoroscopic control. COTD was determined by injection of 10 ml of iced-cold sterile, isotonic glucosaline solution through the proximal catheter’s lumen. The time course of temperature changes was recorded by the thermistor at the distal end of the probe. Three consecutive bolus injections were performed for each condition. The mean value of the three measurements was calculated if the difference between highest and lowest values was ≤ 10%. Otherwise, two more measurements were performed, the highest and lowest values were deleted, and the mean value of the three remaining measurements was calculated.
Cardiac output by Modelflow
COMF was determined from continuous non-invasive recording of arterial pulse pressure profiles by a Portapres system (TNO-TPD, Amsterdam, The Netherlands). The photoplethysmographic cuff of Portapres was positioned on the index or middle finger contralateral to the vascular lines. The zero-level reference was placed according to the manufacturer’s instructions. From pulse pressure profiles, beat-by-beat heart rate (HR) and stroke volume (SV) were calculated with the MF model, using the Beatscope 1.1a software (TNO-TPD, Amsterdam, The Netherlands) developed for this purpose.
The MF method for the beat-by-beat assessment of CO makes it possible to reconstruct aortic blood flow by simulating a three-element non-linear and time varying model of aortic compliance [3]. Numerical integration of flow during systole then yields SV. COMF is finally computed as SV times the corresponding HR.
The COMF value in each condition was the mean value calculated over 100 consecutive beats, starting simultaneously to the first of each series of COTD procedure. Individual correction factors were calculated with the resting (basal) values as described elsewhere [4] and used to recalculate COMF (COMFcorr.) in other conditions.
Exercise haemodynamics
Exercise haemodynamic evaluation was performed supine on an electromagnetically-braked cycle ergometer (Cycline 100; Tecmachine, Andrezieux-Boutheon, France) secured to the catheterization table. Prior to exercise, 5 min of rest with feet installed on the bicycle (raised legs) were observed to ensure haemodynamic steady state after the expected increase of blood venous return to the heart (considered as “bicycle rest”). Then the patient pedalled at 60 rpm, the workload being increased stepwise by 20 W every 3 min from freeload cycling to a maximum of 60 W, depending on patient’s functional tolerance. CO was determined during the last minute of each exercise level. All measures were obtained at steady HR and pulmonary artery pressure.
Statistics
Data are given as mean ± standard deviation (SD), unless otherwise stated. Data comparisons were performed by t-test for paired observations. Differences were considered significant when p < 0.05. Coefficient of variation was calculated as mean divided by SD for baseline CO values. Linear relationships were analysed by linear regression, accounting for the error on both X- and Yaxis. The York algorithm [5] implemented under Matlab (version 7.13.0.564, MathWorks, Natick, MA, USA) was used, allowing calculation of confidence intervals for intercept (a ± 1.96*SD) and slope (b ± 1.96*SD), and estimation of proportional bias (a significantly different from 1) and fixed bias (b significantly different from 0) between the two methods. Bland–Altman analyses [6] were performed to determine the degree of agreement between COMF and COTD and between COMFcorr. and COTD. The mean bias (accuracy), precision, 95% limits of agreements (LoA) and percentage error (PE) were calculated. Sensitivity and specificity of COMF for directional COTD increase or decrease in response to any procedure were determined. A directional change was considered false-negative when an increase of COTD ≥ 10% was not accompanied by a COMF increase ≥ 10%. A directional change was considered false-positive when a COTD increase < 10% or any COTD decrease was accompanied by an increase of COMF ≥ 10%.
Results
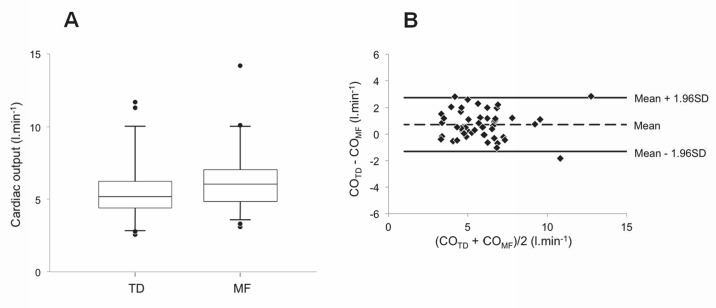
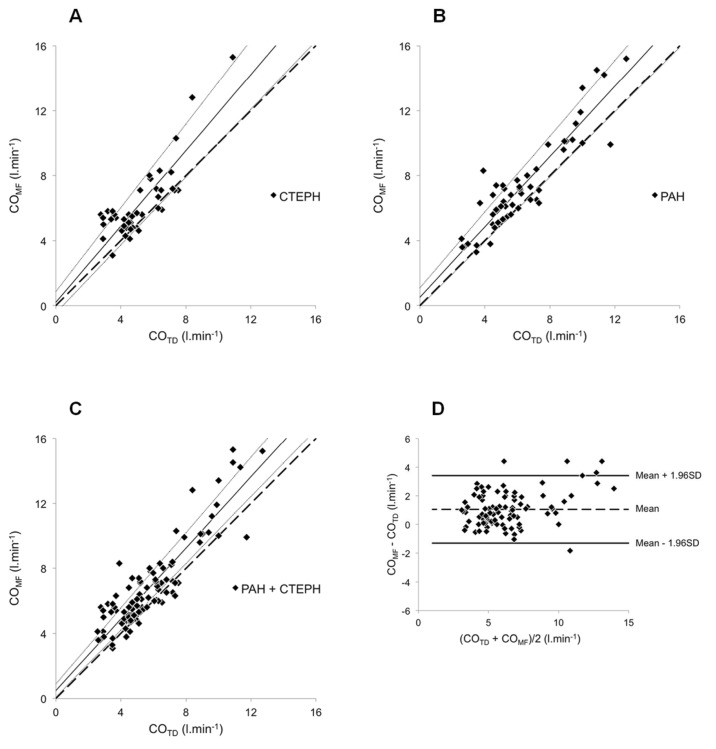
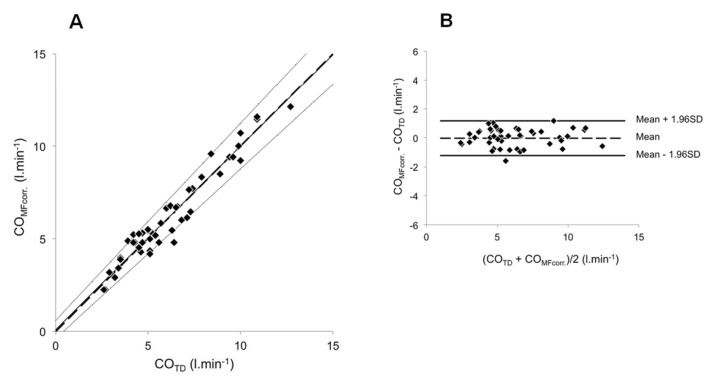
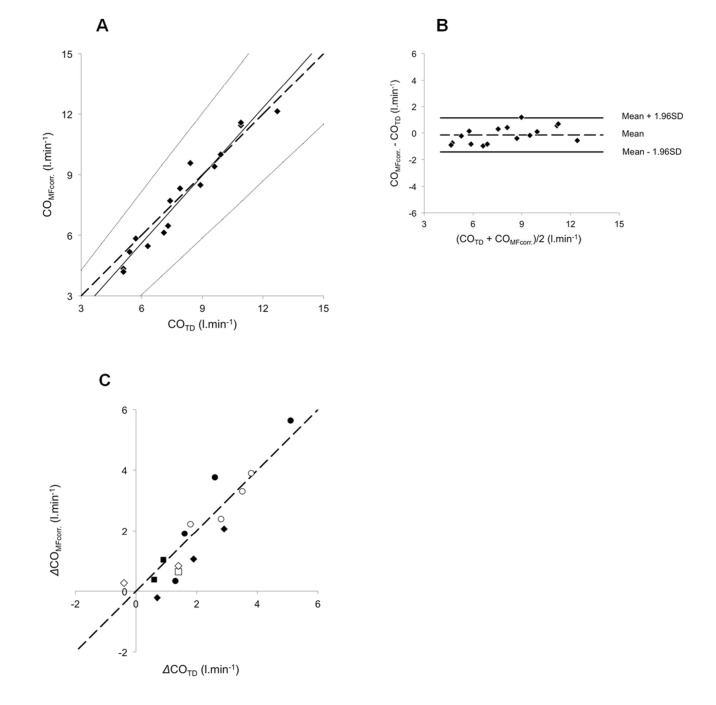
The clinical and haemodynamic data at baseline are shown in Tables 1, 2 and 3. The conditions and number of simultaneous COTD and COMF measurements are shown in Table 4. Baseline uncorrected COMF and COTD were 6.18 ± 1.95 and 5.46 ± 1.95 L·min-1, respectively, (p<0.05, Fig 2A). The coefficients of variation were 10.63% and 5.66% for COMF and COTD, respectively (p<0.01). The Bland–Altman plot for baseline CO values appears in Fig 2B. Accuracy and precision were 0.72 and 1.04 L·min-1, respectively. LoA ranged from -1.32 to 2.76 L·min-1. PE was ±35.7%. The relationships between COMF and the corresponding COTD are reported in Fig 3A, 3B and 3C. For CTEPH (n = 43), PAH (n = 55) and overall (n = 98), the intercept a was 0.207 ± 0.336, 0.516 ± 0.294 and 0.493 ± 0.209, respectively. Corresponding slope b was 1.167 ± 0.062, 1.082 ± 0.043 and 1.095 ± 0.034 L·min-1, respectively. A proportional bias was found for CTEPH (Fig 3A). Merging groups implied both proportional and fixed bias (Fig 3C). The Bland–Altman plot for merged groups is shown in Fig 3D. The accuracy was 1.05 L·min−1 (p <0.01). The precision was 1.20 L·min-1. LoA ranged from -1.30 to 3.40 L·min-1. PE was ±37.9%. The overall sensitivity and specificity of COMF for directional change in response to any of the experimental manoeuvres (nitric oxide vasoreactivity test, fluid challenge or exercise) were 95.2% and 82.4%, respectively (n = 48). Mean calibration factor for COMF was 1.17 ± 0.25. COMFcorr. in all conditions except baseline (which was used to determine the correction factor) are plotted against the corresponding COTD in Fig 4A. The regression line was characterized by a and b of 0.094 ± 0.254 L·min−1 and 0.990 ± 0.038, respectively. Neither proportional nor fixed bias was found. The Bland–Altman plot (Fig 4B) showed an accuracy of -0.03 l·min−1 (NS). Precision was 0.61 L·min−1. LoA ranged from -1.23 to 1.17 L·min-1. PE was ±19.8%. COMFcorr. values at exercise (n = 16) are plotted against their corresponding COTD in Fig 5A. The regression equation had intercept a and slope b of -1.093 ± 0.760 and 1.117 ± 0.091 L·min−1, respectively. Neither proportional nor fixed bias was found. The Bland–Altman plot (Fig 5B) revealed a -0.15 L·min−1 accuracy (NS). Precision was 0.65 L·min−1. LoA ranged from -1.42 to 1.12 L·min-1. PE was ±16.4%. The changes of COMFcorr (ΔCOMFcorr) from baseline for each subject are plotted against the corresponding ΔCOTD in Fig 5C. The sensitivity and specificity of COMF for directional change in response to exercise were 93.3%. and 100%, respectively (n = 16). In one case, a COMF decrease < 10% (-0.22 L·min-1) did not accompany a COTD increase ≥ 10% (+0.70 L·min-1) and was considered a false negative.
Table 1. Baseline characteristics of the study population.
| PAH (n = 30) | CTEPH (n = 20) | |||
|---|---|---|---|---|
| Not operated (n = 14) | Operated (n = 6) | |||
| Age | yrs | 48.7 ± 15.6 | 64.3 ± 11.0 | 56.2 ± 19.8 |
| Female | % | 60.0 | 57.1 | 66.7 |
| BMI | kg.m-2 | 25.0 ± 5.1 | 25.4 ± 4.9 | 31.5 ± 5.8 |
| BSA | m2 | 1.77 ± 0.19 | 1.76 ± 0.23 | 1.89 ± 0.18 |
| MAP | mmHg | 90.9 ± 14.2 | 104.3 ± 20.4 | 95.0 ± 19.5 |
| RAP | mmHg | 5.8 ± 3.1 | 7.4 ± 5.2 | 4.3 ± 3.9 |
| mPAP | mmHg | 48.6 ± 14.9 | 43.0 ± 13.0 | 26.3 ± 7.6 |
| PAWP | mmHg | 9.2 ± 3.7 | 6.2 ± 2.4 | 9.3 ± 3.3 |
| CO | L·min-1 | 6.0 ± 2.1 | 4.3 ± 1.3 | 5.5 ± 0.7 |
| CI | L·min-1·m-2 | 3.4 ± 1.0 | 2.4 ± 0.5 | 2.9 ± 0.2 |
| PVR | WU | 7.5 ± 4.4 | 9.5 ± 4.5 | 3.2 ± 1.3 |
| SVR | WU | 15.9 ± 6.1 | 24.7 ± 10.1 | 17.1 ± 5.8 |
Data are presented as mean ± SD unless otherwise stated. BMI: Body mass index; BSA: Body surface area; MAP: Mean systemic arterial blood pressure; RAP: Right atrial pressure; mPAP: Pulmonary artery mean pressure; PAWP: pulmonary artery wedge pressure; CO: cardiac output, determined by thermodilution; CI: Cardiac Index; PVR: Pulmonary vascular resistance; SVR: Systemic vascular resistance; WU: Wood units.
Table 2. Diagnosis and treatment of the PAH population (%).
| Subjects | 30 |
|---|---|
| WHO diagnostic subgroup | |
| Idiopathic | 17 (56.7) |
| Heritable | 2 (6.7) |
| Drugs and toxin induced | 1 (3.3) |
| Associated# | 9 (30.0) |
| Pulmonary veno-occlusive disease | 1 (3.3) |
| Treatment naive (newly diagnosed§) | 7 (23.3) |
| No specific drug therapy | 1 (3.3) |
| PAH-specific drug therapy | 22 (73.3) |
| ERA | 16 (72.7) |
| PDE5i | 12 (54.5) |
| Prostanoid | 7 (31.8) |
| CCB | 1 (4.5) |
| Monotherapy | 11 (50.0) |
| Double combination therapy | 7 (31.8) |
| Triple combination therapy | 4 (18.2) |
Table 3. Diagnosis and treatment of the CTEPH population (%).
| Subjects | 20 |
|---|---|
| Newly diagnosed § | 12 (60.0) |
| Inoperable** | 2 (10.0) |
| Operated ¶ | 6 (30.0) |
| PAH-specific drug therapy | 5 (25.0) |
| ERA | 5 (100) |
| PDE5i | 4 (80.0) |
| Monotherapy | 1 (20.0) |
| Double combination therapy | 4 (80.0) |
ERA: Endothelin receptors antagonists; PDE5i: phosphodiesterase-5 inhibitors; CCB: Calcium channel blockers. #Patients with PAH associated to connective tissue disease (n = 1), HIV infection (n = 2), Portal hypertension (n = 2) and Congenital heart disease after corrective cardiac surgery (n = 4).
§Patients diagnosed at the time of the present study.
**Due to distal lesions.
¶ Patients with persisting hemodynamic impairment at least 3 months after pulmonary endarterectomy (PEA).
Table 4. Conditions and number of simultaneous COTD and COMF measurements.
| n | Rest (baseline) | NO testing | Fluid Challenge | Bicycle rest | Bicycle exercise | Total |
|---|---|---|---|---|---|---|
| PAH | 30 | 7 | 4 | 5 | 9 | 55 |
| CTEPH | 20 | 9 | 1 | 6 | 7 | 43 |
| Total | 50 | 16 | 5 | 11 | 16 # | 98 |
Repartition of the different single simultaneous COTD and COMF measurements. A total of 98 CO measurements were performed in the 50 patients. Each COTD is the mean of 3 TD measurements (see Methods). Each COMF is the mean of 100 consecutive beat-by-beat values (see Methods). 26 patients (PAH n = 12, CTEPH n = 14) had a total of 48 measurements in conditions other than basal (PAH n = 25, CTEPH n = 23). #6 patients performed incremental exercise (PAH n = 4, CTEPH n = 2) with the workload being increased stepwise by 20 W every 3 min to a maximal workload of 60 W depending of patient functional tolerance for a total of 16 CO determined during steady exercise (PAH n = 9, CTEPH n = 7). NO: Nitric oxide vasoreactivity test; Fluid Challenge: CO determined after infusion of 500 ml of isotonic saline solution in five minutes; Bicycle rest: CO determined after 5 min of rest with feet positioned on the pedals with raised legs.
Fig 2. Comparison of baseline values of COMF and COTD.
Simultaneous determination of cardiac output by thermodilution (COTD) and Modelflow (COMF) in 50 patients with pre-capillary pulmonary hypertension. (A) The figure describes median (line), 25th to 75th percentile (box), 5th to 95th percentile (whiskers) and the dots represent outliers. The mean values for COTD and COMF were 5.46 ± 1.95 L·min-1 and 6.18 ± 1.95 L·min-1, respectively (p<0.05). (B) Difference between resting COMF and COTD values plotted against their mean. Broken line represents the mean (+ 0.72 L·min-1) and the solid lines the 95% limits of agreement (-1.32 to + 2.76 L·min-1).
Fig 3. Relationship between COMF and COTD.
COMF determined in 50 patients (98 values) under various conditions (rest, fluid challenge, NO testing and exercise). COMF values were plotted against the corresponding COTD values for CTEPH patients (A) PAH patients (B) and all 50 patients (C). (D) Difference between COMF and COTD values plotted against their mean. In (A), (B) and (C), the broken lines correspond to the lines of equality, solid lines are the mean regression lines and dotted lines delimit the confidence interval of the regression lines. In (D), broken line represents the mean (+ 1.05 L·min-1) and the solid lines the 95% limits of agreement (-1.30 to + 3.40 l.min-1).
Fig 4. Relationship between COMFcorr. and COTD.
COMFcorr. determined in 26 patients (48 values). (A) For each subject, COMFcorr. values were plotted against the corresponding COTD values. The broken line corresponds to the line of equality, solid line is the mean regression lines and dotted lines delimit the confidence interval of the regression lines. (B) Difference between COMFcorr. and COTD values plotted against their mean. Broken line represents the mean (-0.03 L·min-1) and the solid lines the 95% limits of agreement (-1.23 L·min-1 to +1.17 L·min-1).
Fig 5. Relationship between COMFcorr. and COTD during exercise.
COMFcorr determined in 6 patients during exercise procedure. (A) For each subject, COMFcorr values were plotted against the corresponding COTD values. The broken line corresponds to the line of equality, solid line is the mean regression lines and dotted lines delimit the confidence interval of the regression lines. (B) Difference between COMFcorr and COTD values plotted against their mean. Broken line represents the mean (-0.15 L·min-1) and the solid lines the 95% limits of agreement (-1.42 l.min-1 to +1.12 L·min-1). (C) For each subject and workload, the increase (Δ) in COMFcorr from rest was plotted against the same corresponding COTD increase (ΔCOTD). The six different targets correspond to the six different patients. The broken line corresponds to the line of equality.
Discussion
The results of the present study show that MF applied to non-invasive pulse pressure profiles, once corrected against a reference method, offers a precise and accurate determination of CO in patients with PAH and CTEPH.
Uncorrected COMF overestimation of COTD was confirmed by the ranges of regression lines in Fig 3, displaced above the equality line when plotting COMF against COTD in CTEPH and merging groups. When applied to the PAH group only (Fig 3B), however, linear regression showed neither fixed nor proportional bias. These results are consistent with previous data, comparing MF applied to peripheral pulse pressure profiles to different reference methods in various clinical settings and populations [4, 7, 8] and confirm the need of a correction when determination of absolute CO values, more than relative changes, is needed. Conversely, when compared to TD at rest, non calibrated MF applied to pulse waves obtained from intra-arterial signal in cardiac surgery patients was reliable and superior to other CO monitoring devices, with acceptable LoA and PE [9]. These results were confirmed by a recent meta-analysis [10]. Another study compared COMF from arterial pulse pressure profiles recorded with Portapres and from intra-arterial radial catheter in healthy subjects [11]: non-invasive CO was significantly and systematically higher than CO from intra-arterial signal. The systematic difference was attributed to differences in pulse wave between radial and finger arteries. This may also partially explain the overestimation of COMF as compared to COTD in our study. Here, PE of COMF slightly exceeded the ±30% limit admitted for allowing interchangeability of a new technology with TD, as suggested by a meta-analysis of CO validation studies published by Critchley and Critchley [12]. Still, the LoA and PE values of our study were smaller than those obtained in PH patients for TD against direct Fick [13], thoracic bioimpedance against TD [14] and transthoracic bioreactance against TD or indirect Fick [15]. It is noteworthy that these two latter alternative methods for non-invasive CO determination are also promising, as discussed in a recent review that focused its performances in the assessment of fluid responsiveness using passive leg raising test and cardiac output response to exercise stress testing [16]. In our study, COMF was not compared with direct or indirect Fick: any assumption of a better correlation between MF and these methods would be speculative. Previously, determination of CO changes by acetylene rebreathing showed good correlation with COTD changes in PAH patients [13]. That technique was considered acceptable in most cases, despite LoA and PE similar to the present ones. Other promising techniques, such as cardiac magnetic resonance [17, 18] or echocardiography [19, 20], raise real interest in CO determination but require the advanced expertise of a dedicated technician and a specific setting.
We didn’t aim at showing whether uncorrected MF could be interchangeable with TD. Previous studies that addressed this question showed that this is not so [21]. Moreover, contrary to other clinical conditions, RHC is mandatory for PAH or CTEPH diagnosis [1]. Thus, since these patients undergo anyway RHC, it would be easy to obtain a correction factor [4, 22] and calibrate MF with basal TD in order to use the former for subsequent CO determination during various conditions.
In this study, we also showed that MF detects significant directional changes of CO associated to vasoreactivity testing, fluid challenge or exercise (Figs 4 and 5). Also transthoracic bioreactance was claimed to reliably detect dynamic, directional CO changes following vasoreactivity testing with a sensitivity and specificity of 88.9% and 100%. Our results are similar to those reported in this publication [14]. In a consistent manner with previous reports [23], we assumed that a COTD variation would be significant when ≥10%, although an actual significant variation should overtake the magnitude of the least significant change. Thus, the minimum change that a device must measure to detect a real change is given by the formula: precision multiplied by √2 [24]. From our data, the least significant change corresponded to a 9.2% change. When applying this limit for calculation of sensitivity and specificity, no value was reclassified.
The third finding of our study is that, once corrected against a reference method, MF showed acceptable accuracy and precision in determining CO changes (Figs 4 and 5). In fact, COMFcorr revealed LoA and PE below the limits for inter-changeability with TD. This suggests that COMFcorr may constitute an alternative method to TD for serial CO measurements on the same patient during the same procedure. The range of individual correction factors corresponds to that of healthy subjects [4]. So, MF application may simplify and shorten any RHC protocol where several CO measurements are required [1, 25]. Moreover, it makes possible to explore CO changes with a beat-by-beat resolution, allowing opportunities to describe more precisely cardiovascular adjustments during transients, like exercise onset in PH patients. In fact, there is a growing interest in exploring exercise haemodynamics in pulmonary vascular diseases. For instance, the establishment of multipoint pressure-flow (mPAP–CO) relationships during exercise was recently shown to better describe pulmonary vascular resistive properties than PVR calculated only from resting values [26, 27]. Our group also recently showed that exercise haemodynamics may also give invaluable information for unmasking pulmonary vascular disease [28], assessing PAH severity and driving its therapeutic approach [29]. Likewise, haemodynamic response to exercise may be useful in patients with high risk of developing PAH such as relatives of patients with heritable PAH [30] or patients with diseases associated with high risk of developing PAH or resting mPAP values between 20 and 25 mmHg [31]. Cardiodynamic exploration with determination of PVR changes at exercise may also help unmasking PH due to left heart disease that could mimic PAH at rest and induce misdiagnosing [32]. Finally, the most recent guidelines for PAH treatment strongly recommend the inclusion of patients in supervised exercise rehabilitation programmes [2], where the possibility of easy-access non-invasive monitoring of CO changes is desirable.
This study has several limitations. TD is not the gold standard for CO determination: the comparison with the direct Fick method may provide different results. Yet, TD reflects everyday clinical reality and common practice in PH reference centres and we aimed at comparing MF to routine procedure. MF is unusable in patients with poor finger pulse-wave signal, as it was the case in the present study for two patients with PAH associated to connective tissue diseases (CTD). This may be an issue in centers were PAH associated to CTD is the most represented population. It is also unsure if MF and TD correlation would be different in patients with cardiac arrhythmias, which was not the case for the patients included in our study. Finally, it is still uncertain that the validity of the individual correction factor determined during RHC persists over time or in different clinical testing. To date, individual correction of MF needs to be determined against a reference method at every new exploration. That said, our data still confirm that MF is reliable for relative CO changes even if no correction factor is applied. It is also noteworthy that the model of aortic compliance used to compute COMF was developed from data obtained on healthy subjects [3]. So it may be that the characteristics of aortic compliance in PAH or CTEPH patients are different, inducing here a potential bias. In our study, this issue was counterbalanced by the individual correction factors. Yet it may well impair the utilization of a population correction factor, which moreover would have been unwise to use in our study, considering the size of the study population and the variables to be included in the analysis. We acknowledge that this may be seen as a limitation of our study. Nevertheless, the mean correction factor determined in the present work was similar to those obtained previously in healthy subjects against another reference method [4].
Conclusions
MF is a simple and reliable method for detecting CO changes in pre-capillary PH. Once corrected against a reference method, it is precise and accurate enough to evaluate absolute CO changes in response to exercise, fluid challenge or vasoreactivity testing. MF may simplify haemodynamic evaluation of pre-capillary PH in clinical practice and widen the range of follow-up and exploratory opportunities as it could potentially be used to study CO changes outside the RHC laboratory on a beat-by-beat basis.
Supporting Information
The table gives all the individual studied baseline parameters of the study population (n = 50) reported in the Fig 2, Tables 1–3.
(PDF)
This table gives all the individual conditions and number of simultaneous COTD and COMF measurements reported in the Fig 3 and Table 4 (n = 98).
(PDF)
This table gives the calculated confidence intervals of linear regression obtained with York algorithm for intercept (a ± 1.96*SD) and slope (b ± 1.96*SD), and confidence interval of COMF (Fig 3) or COMFcorr. (Figs 4 and 5) against any COTD.
(PDF)
This table gives all the conditions and number of simultaneous COTD and COMFcorr. measurements reported in the Fig 4 (n = 48).
(PDF)
Acknowledgments
The authors would like to thank Dr. Tiziano Binzoni for fruitful discussion about the data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by an educational grant from the European Respiratory Society (RESPIRE postdoctoral fellowship program—Co-funded by the European Commission Seventh Framework Program (FP7)—Marie Curie Actions; MC-1630-2010) to Frédéric Lador.
References
- 1. Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and Diagnosis of Pulmonary Hypertension. J Am Coll Cardiol. 2013; 62: D42–50. 10.1016/j.jacc.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 2. McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013; December 24; 62(25 Suppl):D73–81. 10.1016/j.jacc.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 3. Wesseling KH, Jansen JRC, Settles JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J. Appl. Physiol. 1993; 74, 2566–2573. [DOI] [PubMed] [Google Scholar]
- 4. Tam E, Azabji KM, Cautero M, Lador F, Antonutto G, di Prampero PE, et al. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci. 2004; 106: 371–376. [DOI] [PubMed] [Google Scholar]
- 5. York D, Evensen NM, López Martinez M, De Basabe Delgado J. Unified equations for the slope, intercept, and standard errors of the best straight line. American Journal of Physics. 2004; 72, 367. [Google Scholar]
- 6. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1: 307–310. [PubMed] [Google Scholar]
- 7. Houtman S, Oeseburg B, Hopman MT. Non-invasive cardiac output assessment during moderate exercise: pulse contour compared with CO2 rebreathing. Clin. Physiol. 1999; 19, 230–237. [DOI] [PubMed] [Google Scholar]
- 8. van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walløe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol. 2003; 90: 131–137. [DOI] [PubMed] [Google Scholar]
- 9. de Wilde RBP, Geerts BF, Cui J, van den Berg PCM, Jansen JRC. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia. 2009; 64, 762–769. 10.1111/j.1365-2044.2009.05934.x [DOI] [PubMed] [Google Scholar]
- 10. Schlöglhofer T, Gilly H, Schima H. Semi-invasive measurement of cardiac output based on pulse contour: a review and analysis. Can J Anaesth. 2014. May; 61(5):452–79. 10.1007/s12630-014-0135-8 [DOI] [PubMed] [Google Scholar]
- 11. Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, et al. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin. Sci. 2004; 106, 365–369. [DOI] [PubMed] [Google Scholar]
- 12. Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999; 15:85–91. [DOI] [PubMed] [Google Scholar]
- 13. Hoeper MM, Maier R, Tongers J, Niedermeyer J, Hohlfeld JM, Hamm M, et al. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med. 1999; 160: 535–541. [DOI] [PubMed] [Google Scholar]
- 14. Hedelin P, Agger E, Ekmehag B, Radegran G. Measurement of cardiac output with noninvasive Aesculon impedance versus thermodilution. Clin Physiol Funct Imaging. 2011; 31, 39–47. 10.1111/j.1475-097X.2010.00977.x [DOI] [PubMed] [Google Scholar]
- 15. Rich JD, Archer SL, Rich S. Noninvasive cardiac output measurements in patients with pulmonary hypertension. Eur Respir J. 2013; 42: 125–133. 10.1183/09031936.00102212 [DOI] [PubMed] [Google Scholar]
- 16. Jakovljevic DG, Trenell MI, MacGowan GA. Bioimpedance and bioreactance methods for monitoring cardiac output. Best Pract Res Clin Anaesthesiol. 2014. December; 28(4):381–94. 10.1016/j.bpa.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 17. Swift AJ, Rajaram S, Hurdman J, Hill C, Davies C, Sproson TW, et al. Noninvasive estimation of PA pressure, flow, and resistance with CMR imaging: derivation and prospective validation study from the ASPIRE registry. JACC Cardiovasc Imaging. 2013. October; 6(10):1036–47. 10.1016/j.jcmg.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 18. García-Alvarez A, Fernández-Friera L, Mirelis JG, Sawit S, Nair A, Kallman J, et al. Non-invasive estimation of pulmonary vascular resistance with cardiac magnetic resonance. Eur Heart J. 2011. October; 32(19):2438–45. 10.1093/eurheartj/ehr173 [DOI] [PubMed] [Google Scholar]
- 19. Lau EM, Vanderpool RR, Choudhary P, Simmons LR, Corte TJ, Argiento P, et al. Dobutamine Stress Echocardiography for the Assessment of Pressure-Flow Relationships of the Pulmonary Circulation. Chest. 2014. October;146 (4): 959–66. 10.1378/chest.13-2300 [DOI] [PubMed] [Google Scholar]
- 20. Argiento P, Chesler N, Mulè M, D'Alto M, Bossone E, Unger P et al. Exercise stress echocardiography for the study of the pulmonary circulation. Eur Respir J. 2010. June; 35(6): 1273–8. 10.1183/09031936.00076009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Remmen JJ, Aengevaeren WR, Verheugt FW, van ver Werf T, Luijten HE, Bos A, et al. Finapres arterial pulse wave analysis with Modelflow is not a reliable non-invasive method for assessment of cardiac output. Clin Sci (Lond). 2002. August; 103(2):143–9. [DOI] [PubMed] [Google Scholar]
- 22. Van Lieshout JJ, Karemaker JM. Tracking of cardiac output from arterial pulse wave. Clin Sci. 2003; 104, 239–240. [DOI] [PubMed] [Google Scholar]
- 23. Warburton DE, Haykowsky MJ, Quinney HA, Humen DP, Teo KK. Reliability and validity of measures of cardiac output during incremental to maximalaerobic exercise. Part I: conventional techniques. Sports Med. 1999; 27, 23–41. [DOI] [PubMed] [Google Scholar]
- 24. Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM. Bench-to-bedside review: The importance of the precision of the reference technique in method comparison studies–with specific reference to the measurement of cardiac output. Critical Care. 2009; 13:201 10.1186/cc7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006; 48: 2546–52. [DOI] [PubMed] [Google Scholar]
- 26. Provencher S, Hervé P, Sitbon O, Humbert M, Simonneau G, Chemla D. Changes in exercise haemodynamics during treatment in pulmonary arterial hypertension. Eur Respir J. 2008. August; 32(2): 393–8. 10.1183/09031936.00009008 [DOI] [PubMed] [Google Scholar]
- 27. Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005; 288: L419–L425. [DOI] [PubMed] [Google Scholar]
- 28. Herve P, Lau E, Sitbon O, Savale L, Montani D, Godinas L, et al. New Criteria for Diagnosis of Pulmonary Hypertension at Exercise. Eur Respir J. 2015. May 28 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29. Chaouat A, Sitbon O, Mercy M, Ponçot-Mongars R, Provencher S, Guillaumot A, et al. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014. September; 44(3):704–13. 10.1183/09031936.00153613 [DOI] [PubMed] [Google Scholar]
- 30. Grünig E, Weissmann S, Ehlken N, Fijalkowska A, Fischer C, Fourme T, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation. 2009. April 7; 119(13): 1747–57. 10.1161/CIRCULATIONAHA.108.800938 [DOI] [PubMed] [Google Scholar]
- 31. Saggar R, Khanna D, Furst DE, Shapiro S, Maranian P, Belperio JA, et al. Exercise-induced pulmonary hypertension associated with systemic sclerosis: four distinct entities. Arthritis Rheum. 2010. December; 62(12): 3741–50. 10.1002/art.27695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lador F, Herve P. A practical approach of pulmonary hypertension in the elderly. Semin Respir Crit Care Med. 2013. October; 34(5): 654–64. 10.1055/s-0033-1356549 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The table gives all the individual studied baseline parameters of the study population (n = 50) reported in the Fig 2, Tables 1–3.
(PDF)
This table gives all the individual conditions and number of simultaneous COTD and COMF measurements reported in the Fig 3 and Table 4 (n = 98).
(PDF)
This table gives the calculated confidence intervals of linear regression obtained with York algorithm for intercept (a ± 1.96*SD) and slope (b ± 1.96*SD), and confidence interval of COMF (Fig 3) or COMFcorr. (Figs 4 and 5) against any COTD.
(PDF)
This table gives all the conditions and number of simultaneous COTD and COMFcorr. measurements reported in the Fig 4 (n = 48).
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.