Abstract
Heat Shock Transcription Factor 1 (HSF1) is a major transcriptional regulator of the heat shock response in eukaryotic cells. HSF1 is also evoked in response to a variety of cellular stressors including elevated temperatures, oxidative stress, and other proteotoxic stressors. Previously, we demonstrated that HSF1 is activated in naive T cells at fever range temperatures (39.5°C) and is critical for in vitro T cell proliferation at fever temperatures. In this study, we demonstrated thatmurine HSF1 became activated to the DNA-binding form and trans-activated a large number of genes in lymphoid cells strictly as a consequence of receptor activation in the absence of apparent cellular stress. Microarray analysis comparing HSF1+/+ and HSF1−/− gene expression in T cells activated at 37°C revealed a diverse set of 323 genes significantly regulated by HSF1 in non-stressed T cells. In vivo proliferation studies revealed a significant impairment of HSF1−/− T cell expansion under conditions mimicking a robust immune response (staphylococcal enterotoxin B induced T cell activation). This proliferation defect due to loss of HSF1 is observed even under non-febrile temperatures. HSF1−/− T cells activated at fever temperatures show a dramatic reduction in cyclin E and cyclin A proteins during the cell cycle, although the transcription of these genes was not affected. Finally, B cell, and hematopoietic stem cell proliferation from HSF1−/− mice, but not HSF1+/+ mice were also attenuated under stressful conditions, indicating that HSF1 is critical for the cell cycle progression of lymphoid cells activated under stressful conditions.
Introduction
Heat shock transcription factor 1 (HSF1) is a major transcriptional regulator of the eukaryotic cellular heat shock response and is evoked by a variety of stress stimuli including elevated temperatures (1–3), radiation(4), oxidative stress(5), toxic chemicals(6,7), infectious agents (8,9), and other proteotoxic stressors. Upon sensing stress, HSF1 is rapidly converted from an inactive monomeric form to atrimeric DNA-binding form in the nucleus, which then interacts with DNA sequences carrying inverted repeat nGAAn sequences named heat shock elements (HSE), and regulates target gene expression. The most widely studied genes regulated by HSF1 encode the heat shock proteins (HSPs). HSPs serve a variety of critical functions within the cell, acting as chaperones, assisting in correct protein folding, and helping to target damaged or unfolded proteins to the proteasome for degradation.
While initially studied in the context of the heat shock response, HSF1 is now considered to be part of a larger network of protein homeostasis or proteostasis(10–13). The proteostasis network is ancient and evolutionarily conserved and consists of various cellular pathways dedicated to maintaining protein homeostasis in both “normal” and stress conditions. These include degradative pathways such as the ubiquitin proteasome system and the ER associated degradation systems, post translational modification including phosphorylation, acylation, and oxidation, and protein synthesis/folding/unfolding including ribosomes, HSF1, and the unfolded protein response(10, 12). Thus, HSF1 can be considered as one of the important sensors of proteostasis, with the capability of regulating a series of genes necessary to maintain proper proteostasis. It is clear that the needs of proteostasis will differ between cell types and between different environmental conditions. So far, the role of proteostasis in the immune system is poorly understood.
In yeast, the single HSF gene is required for viability, probably because of the requirement of HSF to maintain basal HSP activity(14, 15). In drosophila, HSF mutants are lethal, but conditional knock outs show that it is mainly required for early embryogenesis and is dispensable for viability of the adult(16). In mammals, there are several forms of HSF, with HSF1 serving the major stress responsive function. HSF1 knock-out mice exhibit embryonic lethality, but on a mixed genetic background, viable knock-out mice are obtained (17). These mice, while capable of surviving to old age in laboratory conditions, are generally smaller than HSF1+/+ mice, and show an increased susceptibility to stress including heat and oxidative stress. Interestingly, these mice also show increased lethality to endotoxin (17), and infection with listeria (18). In the latter case, we have shown that the increased lethality is at least in part due to an overproduction of TNF α, in agreement with other studies showing HSF1 to be a negative regulator of TNF α(19, 20). HSF1 also plays important roles in lung protection (7), neurogenesis(21), proliferation(22), apoptosis(23), cell cycle(24, 25), and carcinogenesis(26).
While HSF1 affects a variety of cellular processes, a particularly striking common thread is the role of HSF1 in cellular proliferation. HSF1 knock-down or knock-out has profound effects on cell cycle progression in both yeast and mammalian cells. In most cases, the requirement for HSF1 becomes more acute as the stress level increases. In drosophila and mice, the requirement for HSF1 is most acute in early embryogenesis(24, 27). Female HSF1−/− mice are sterile due to a requirement for maternal HSF1 for cell division of the early pre-implantation fertilized oocyte(24). The critical importance for HSF1 in cell division is underscored by the finding that HSF1−/− mice are highly resistant to carcinogenesis (26, 28). Virtually all tumors and cell lines show constitutively active HSF1 and it appears that continual activation of HSF1 is necessary for most tumors to progress to high levels of mitotic activity.
With respect to the immune system, HSF1−/− mice are defective in cross presentation of antigen, probably due to the fact that HSF1 is required for the inducible form of HSP70, known to be important in cross presentation(29–31). HSF1−/− mice have normal lymphoid architecture and cellularity, indicating that HSF1 has no critical role in lymphoid development. However, we and others have shown clear defects in induced immune responses in HSF1−/− mice. For example, induced antibody responses are compromised in HSF1−/− mice (32) andin vitro proliferation of T cells from HSF1−/− mice are defective at elevated temperatures in the fever range in response to T cell receptor triggering(22). These studies suggest that HSF1 serves an important role in proteostasis within the immune system.
Here we demonstrate that HSF1 becomes activated in T cells shortly after T cell receptor ligation, even at normal temperatures. This in turn activates a genetic program, regulating transcription of over 200 genes, including a signature of proteostasis-related genes. We further demonstrate that HSF1 is necessary for optimal T cell proliferation at normal temperatures, in the presence of robust stimuli in vivo and in vitro. These results suggest that HSF1 is a critical proteostasis regulator in lymphoid cells, one function of which is to maintain optimal proliferation in the face of proteotoxic stress.
Materials and Methods
Mice
HSF1−/− mice on the 129Sv. BALB/c mixed background were colonized from a breeding pair provided by Ivor Benjamin (University of Utah, Salt Lake City, UT) and maintained by breeding heterozygotes as described previously (22). HSF1+/+ mice and HSF1−/− mice were derived from the same breeding colony and identified by PCR. DO11.10 and BALB/c mice were obtained from Jackson laboratories (Bar Harbor, Maine). All mice were maintained under specific pathogen- free conditions at the Division of Laboratory Animal Resources, University of Kentucky Medical Center (Lexington, KY). All animal experiments have been approved by the University of Kentucky Institutional Animal Care and Use committee.
Lymphocyte cultures
T cells were purified from spleens and/or peripheral lymph nodes using either anti-CD3 magnetic beads or Pan T cells isolation kit (Miltenyi Biotech) and the autoMACS® (Miltenyi Biotech, Cambridge, MA), according to the manufacturers protocol. B cells were purified by depletion of T cells with a cocktail of anti-T cell Ab and complement as described previously(33). Unless specified otherwise, cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 1mM glutamine 1mM sodium pyruvate, 1X MEM non-essential amino acids, 50 μM β-mercaptoethanol, 100 u/ml penicillin and 100u/ml streptomycin (cRPMI medium). Hematopoietic bone marrow cells were isolated by removing bone marrows from femurs of HSF1−/− or HSF1+/+ mice. The single cell suspensions were then washed with 1X HBSS and re-suspended in cRPMI medium.
In vitro Proliferation assays
For proliferation assays, 2 × 105 spleen cells were cultured alone or with staphylococcal enterotoxin B (SEB) (20μg/ml) (Sigma Aldrich, St. Louis, MO) or anti-CD3 (1μg/ml) and anti-CD28 (1μg/ml) in 96 well plates for 72 hours at indicated temperatures. 3H-thymidine (1 μCi/well) was added to each culture during the last 18h and proliferation was determined by scintillation counting. Alternatively, spleen cells or purified T cells obtained through anti-CD3 magnetic bead purification were labeled with CFSE as previously described(34) and cultured in 24-well plates at 1 × 106 cells per well in medium alone or with SEB or anti-CD3/CD28 under the conditions described. Following activation, the cells were harvested, stained and analyzed using FACScan® flow cytometer. For co-culture studies, flow through cells (untouched negative fraction) obtained during the T cell purification step were used at indicated ratios as APCs. For trans-well studies, 1 × 106 spleen cells were added to individual wells of 24 well plates (“bottom well”) in 1ml of cRPMI media with or without SEB or anti-CD3/CD28. For the “top well” An equal number of spleen cells was then added to a 10mm tissue culture insert containing a 0.4μM membrane bottom (NalgeNunc International, Rochester, NY) placed inside an individual well of the 24 well plate. Following activation, the cells in bottom wells were harvested and analyzed by flow cytometry after staining the cells with PE conjugated anti-Vβ8 and APC conjugated anti-CD3ε antibodies. For B cell proliferation studies, purified B cells at 2×105 cells/well were cultured in 96 well plates in cRPMI medium with or without LPS or goat (Fab′)2 anti-IgM(10μg/ml) + rat anti-mouse CD40 (2.5 μg/ml)(BD Bioscience) for 72h at indicated temperatures. 3H thymidine was added to the cultures during the last 18h and proliferation was measured using a scintillation counter. For hematopoietic stem cell proliferation studies, CFSE labeled bone marrow cells were cultured with GM-CSF in cRPMI medium for 72h at indicated temperatures. For the six day time point, bone marrow cells were cultured in GM-CSF for 72h, labeled with CFSE, and cultured for an additional 72h in GM-CSF. The CFSE profile of the total cell population was determined by flow cytometry.
In vivo T cell proliferation Assay
Mice were injected intravenously with 150 mg SEB. At 44h after SEB injection, each mouse received 1mg BrdU. All mice were sacrificed at 48h to isolate peripheral lymph nodes and spleens. Single cell suspensions of spleen and lymph nodes were surface labeled with APC-conjugated anti-CD3, and PE-conjugated anti-Vβ8 antibodies (cocktail of anti Vβ8.1, 8.2 and 8.3) (eBioscience, San Diego, CA). Following this step, cells were fixed, permeabilized and stained with anti-BrdU antibody using BrdU Staining Kit (BD Pharmingen). The Vβ8 T cell expansion was then analyzed using aFACScan® flow cytometer by gating on CD3+ cells and analyzing Vβ8+, BrdU+ cells within this gate.
Native Gel Shift Assay
Native protein extracts were prepared by lysing cells as described previously (35, 36). Briefly, cell pellets were rapidly frozen in liquid nitrogen and then suspended in lysis buffer containing 20mM HEPES (pH; 7.9), 25% glycerol, 0.42 M NaCl, 1.5mM MgCl2, 0.2mM EDTA, 0.5 mM PMSF, and 0.5mM DTT. The lysates were then centrifuged at 15,000 rpm for 15 min at 4°C and the supernatants were stored at −80°C until further analysis. For the EMSA, 5μg of extract was mixed with 0.1ng of 32P labeled HSE oligonucleotide probe in a 25μl reaction mixture containing 0.5μg of poly (dI-dC), 2μg BSA, 10mM Tris (pH 7.8),50mM NaCl, 1mM EDTA, 0.5mM DTT, and 5% glycerol. The reaction mixture was incubated for 30 min at 25°C, and a dye solution (50% glycerol, and 0.2% bromophenol blue) was added and directly loaded onto a 4% polyacrylamide gel in 0.5X Tris-borate-EDTA buffer. The gels were run for 2.5h at 130V, dried, and subjected to auto radiography. [32P]HSE oligonucleotides were prepared by 5′ end labeling one strand with T4 Kinase as described previously (35).
Western blot analysis
Cells were washed once with 1X PBS, lysed in RIPA buffer (150mM NaCl, 1%NP-40, 0.5% Sodium deoxycholate, 0.1% SDS, and 50mM Tris, pH8.0) containing protease inhibitor cocktail (Thermo Scientific, Waltham, MA) and centrifuged at 13,000 rpm for 15 min. Protein concentrations in the supernatants were determined using the Bradford method (Thermo Scientific, Waltham, MA). The supernatants were then boiled for 5min in 1X SDS loading buffer and loaded directly on to an 8% SDS-polyacrylamide gel and subjected to electrophoresis at 120V for 90 min. The proteins were then transferred to a PVDF membrane. The membranes were blocked with 5% non-fat milk (Sigma-Aldrich, Saint Louis, MO) and probed at 4°C overnight with protein specific primary antibodies:rabbit polyclonal anti-cyclin D2 (sc-452, Santa Cruz Biotechnology, Santa Cruz, CA), cyclin E1 (sc-481), cyclin A2 (sc-751), p27, and monoclonal mouse anti-β Actin as a loading control. Blots were then washed and developed with HRP conjugated goat-anti rabbit secondary antibody or goat-anti-mouse secondary antibody and the signal was detected using ECL detection system (Thermo Scientific, Waltham, MA).
Microarray analysis
Purified T cells from pooled spleen and lymph nodes of HSF1−/− or HSF1+/+mice were activated with plate bound anti-CD3 for 5h at either 37°C or 40°C. Following activation, cells were harvested, washed with 1X PBS and centrifuged. RNA from the cell pellets was isolated using an RNA easy Kit (QIAGEN, Valencia, CA) with a DNA clean up step included in the protocol. RNA quality and quantity was measured using spectrometry and a Model 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). For each treatment, equal amounts of RNA isolated from mice (n=3) were pooled and stored at −80°C until further analysis. The total RNA (2.5μg) was then used to transcribe to biotinylated cRNA by the Microarray Core Facility at University of Kentucky and was hybridized to the whole mouse genome microarray chip (Affymetrix mouse genome 430 2.0) using two chips for each RNA sample. Hybridization of cRNA on to the chips was carried out for 16 h and was then labeled using streptavidin anti-biotin and streptavidin-pyhcoerythrin. The chips were then scanned using Affymetrix GCS3000 7G Scanners and the data were recorded for further analysis.
Microarray Data Analysis
The Affymetrix mouse genome 430 2.0 gene chip has 45,101 probe sets out of which 27,518 probe sets were selected for further analysis after removing 17,583 probe sets that were always absent. A two-way ANOVA with repeated measurement test was used to identify the probe sets with significance in expression. Probe set hybridization levels with a P-value of ≤ 0.01 between HSF1−/− and HSF1+/+ at 37°C (37_KO vs. 37_WT), and HSF1−/− and HSF+/+ at 40°C (40_KO vs. 40_WT) and a fold change of at least 0.5 on log2 scale between the treatments were selected for further analysis. A complete listing of the gene expression data has been deposited with the Gene Expression Omnibus (GEO) data repository (accession number GSE41005)
RT-PCR analysis
Total RNA (250ng) isolated from the cells were reverse transcribed using QuantiTect reverse transcription kit (QIAGEN, Valencia, CA) containing genomic DNA elimination buffer. Reverse transcribed product (2μl) was then used for the PCR reaction. The PCR reaction was carried out with gene specific primers for 35 cycles with the following PCR conditions: 1x denaturing step, 95°C for 5min; 35 X amplification step, 95°C for 30 sec, 55°C for 1min, and 72°C for 30sec; 1x final elongation step, 72°C for 5 min. The amplified PCR products were electrophoresed in 0.5 % agarose gel and the band intensities were quantified using Quanta One® Imaging and analysis Software (Bio-rad, Hercules, CA). The PCR products were detected using primers as follows; cyclin A2 5′-CCT CTC CTC CAT GTC TGT GTT AAG-3′ and 5′-GTG CTC CAT TCT CAG AAC CTG CTT-3′ and GAPDH 5′-TGA AGG TCG GTG TGA ACG GAT TTG-3′ and 5′-ACG ACA TAC TCA GCA CCA GCA TCA-3′ were as described previously(37). Cyclin E1 primers 5′-TGC AGA TCG CAG AGC TTC TA-3′ and 5′-CTT TCT TTG CTT GGG CTT TG-3′ were designed using the primer-BLAST primer designing tool.
Results
HSF1 transactivates a proteostasis network in T cells
In previous studies, we have demonstrated that HSF1 lacked DNA binding activity and was inactive in resting spleen cells or purified resting T cells at 37°C, but was activated to the DNA binding form upon incubation at 39.5°C for one hour(22, 36). We also showed that this activation was accompanied by the induction of HSP70i protein (the product of the nearly identical hspa1a and hspa1b genes), indicating that the acquisition of HSF1 DNA binding activity was accompanied by transcriptional activity. The lack of HSP70i protein in HSF1−/− cells supports the notion that HSP70i is strictly dependent on HSF1 (17, 22). We also noted that HSP70i protein was induced upon T cell activation at 37°C and more strongly at 39.5°C, in an HSF1 dependent manner (22). These results indirectly suggest that HSF1 is capable of transactivating target genes following T cell activation in the absence of thermal stress.
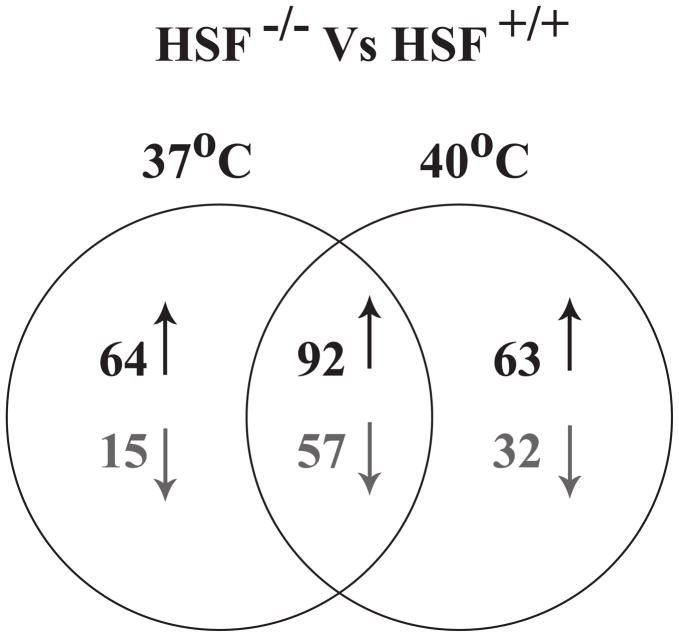
To determine the gene program regulated by HSF1 in freshly activated T cells, we performed a microarray analysis. We used a whole mouse genome microarray to identify the genes differentially regulated in anti-CD3 activated T cells from HSF−/− and HSF1+/+ mice after 5h of activation at normal (37°C) or febrile (40°C) temperatures. We chose 5h of T cell activation in order to allow for HSF1 to transactivate gene expression of target genes, but to limit the influence of secondary transactivation by genes not directly regulated by HSF1. Out of 27,518 probes that were always present across the treatment groups, 323probe sets showed a differential regulation between HSF1−/− and HSF+/+ at either physiological (37°C) or febrile temperatures (40°C) based on the selection criteria of a minimum of log2 0.5 fold change and p-value less than 0.01. A complete listing of the gene expression data has been deposited with the NCBI’s Gene Expression Omnibus data repository (accessible through GEO series accession GSE41005; URL; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE41005). Surprisingly, the majority of these genes (228) were regulated by HSF1 at 37°C. In addition, 156 of those genes showed higher expression in HSF1−/− cells suggesting negative regulation by HSF1. There were subsets of genes regulated by HSF1 only at 37°C, or only at 40°C, indicating that temperature has a critical modulating effect on genes regulated by HSF1 (Fig. 1). For those genes regulated by HSF1 at both 37 and 40°C, the effect was generally greater at 40°C, consistent with the higher levels of HSF1 activation at 40°C(36).
FIGURE 1.
Venn diagram representing the probe sets that showed differential regulation between HSF1−/− and HSF1+/+ mice. Probe sets that had p-value ≤ 0.01 and fold change of log2 0.5 in at least one of the two conditions (37°C or 40°C) were chosen for representation. P-values were calculated using two-way ANOVA of mean intensities of probe sets among the treatments. Up arrows indicate genes expressed at higher levels in HSF1−/− T cells compared to HSF1+/+ T cells, and therefore negatively regulated by HSF1. Down arrows indicate genes expressed at lower levels in HSF1−/− T cells compared to HSF1+/+ T cells, and therefore positively regulated by HSF1.
The genes most significantly regulated by HSF1 are grouped by function (supplemental Table SI). Many of these genes are involved in the stress response, apoptosis, cell cycle, inflammation, signal transduction, transcription and translation, and metabolism. Chaperones, including heat shock proteins, are the most well-known targets of activated HSF1. As expected, expression of several of the heat shock proteins was significantly lower in activated HSF1−/− T cells at febrile temperatures indicating positive regulation. Interestingly, all of these HSPs were also positively regulated by HSF1 at 37°C. Of the 323 genes regulated by HSF1, the HSPa1a and HSPa1b genes, encoding the inducible HSP70 proteins, showed greatest reduction in expression in the HSF1−/− T cells compared to HSF+/+, indicating strong positive regulation. Many of the genes regulated by HSF1 in T cells fit the proteostasis signature common to a wide range of organisms (Table I) (12). These include genes involved in the heat shock response, ubiquitin pathway, and the unfolded protein response. Thus, activation of T cells through T cell receptor ligation activates a proteostasis network through the action of HSF1.
Table I.
Proteostasis signature genes regulated by HSF1 in T cells
| Gene Title | Gene Symbol | Log2 (KO37/WT37) | P-Value | Log2 (KO40/WT40) | P-Value |
|---|---|---|---|---|---|
| AHA1, activator of heat shock 90kDa protein ATPase homolog 1 | Ahsa1 | −0.74 | 0.002 | −0.85 | 0.001 |
| Autophagy-related 7 (yeast) (Atg7) | Apg7l | 0.50 | 0.009 | 0.55 | 0.008 |
| Beta-2 microglobulin (B2m), mRNA | B2m | 0.69 | 0.002 | 0.90 | 0.001 |
| Bcl2-associated athanogene 3 | Bag3 | −1.01 | 0.005 | −1.41 | 0.002 |
| Cullin 1 | Cul1 | 1.40 | 0.001 | 0.34 | 0.125 |
| DnaJ (Hsp40) homolog, subfamily A, member 1 | Dnaja1 | −0.99 | 0.008 | −1.08 | 0.006 |
| DnaJ (Hsp40) homolog, subfamily A, member 4 | Dnaja4 | −1.26 | 0.005 | −0.90 | 0.013 |
| DnaJ (Hsp40) homolog, subfamily B, member 1 | Dnajb1 | −0.91 | 0.000 | −1.04 | 0.000 |
| FK506 binding protein 4 | Fkbp4 | −0.61 | 0.008 | −0.74 | 0.003 |
| Histone deacetylase 5 | Hdac5 | 0.76 | 0.001 | 0.25 | 0.153 |
| Hect domain and RLD 4 (Herc4), mRNA | Herc4 | 0.64 | 0.007 | 0.19 | 0.180 |
| Heat shock factor 1 | Hsf1 | −0.70 | 0.011 | −0.99 | 0.003 |
| Heat shock factor 2 | Hsf2 | 0.58 | 0.014 | 1.22 | 0.001 |
| Heat shock protein 110 | Hsp110 | −1.80 | 0.001 | −1.64 | 0.001 |
| Heat shock protein 1A | Hspa1a | −3.55 | 0.003 | −4.51 | 0.001 |
| Heat shock protein 1B | Hspa1b | −4.00 | 0.001 | −4.79 | 0.000 |
| Heat shock protein 8 | Hspa8 | −0.54 | 0.025 | −0.68 | 0.009 |
| Heat shock protein 1, alpha | Hspca | −0.51 | 0.011 | −0.61 | 0.005 |
| Heat shock protein 1, beta | Hspcb | −0.38 | 0.001 | −0.50 | 0.000 |
| Heat shock protein 1 (chaperonin 10) | Hspe1 | −0.77 | 0.009 | −0.94 | 0.004 |
| Lectin, mannose-binding 2-like | Lman2l | −0.89 | 0.005 | −0.88 | 0.002 |
| Stress-induced phosphoprotein 1 | Stip1 | −0.75 | 0.005 | −0.79 | 0.003 |
Activation of lymphocytes resulted in the activation of HSF1 to the DNA-binding form in the absence of apparent cellular stress
HSF1 is constitutively expressed in an inactive form within the nucleus of virtually all cell types. Upon sensing stress, HSF1 is rapidly converted to the DNA-binding form and, if properly phosphorylated, regulates gene expression. This conversion can be readily detected by EMSA using labeled HSE oligonucleotides(38). We have previously demonstrated that resting T cells had no detectable HSF1 DNA binding activity at 37°C, but were very sensitive to HSF1 activation, only requiring a temperature increase of two and half degrees to 39.5°C(36). In our microarray analysis, the T cells were activated with anti-CD3 for 5 hours, and in that case, we observed that the majority HSF1 dependent genes were regulated at 37°C. These results suggest that T cell activation alone may result in the conversion of HSF1 to the DNA binding form.
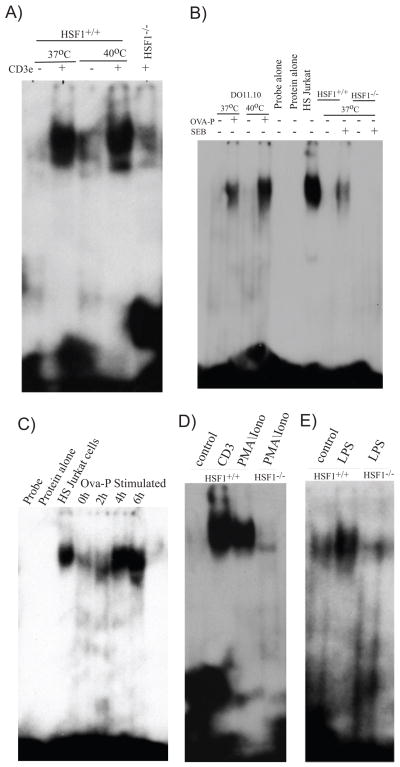
To address this question, T cells were activated by a variety of stimuli and whole cell native protein extracts from these cells were subjected to EMSA analysis using a32P labeled HSE oligonucleotide probe. There was no visible retarded band in resting T cells, but a prominent retarded band was present after 20h of anti-CD3 activation at 37°C or 40°C (Fig. 2A). The prominent retarded band was largely absent in anti-CD3, 37°C activated T cells from HSF1−/− mice indicating that the retarded species was specific for HSF1. Some weak residual binding was observed in activated HSF1−/− T cells which is likely due to weak binding of the other constitutive HSF species to the HSE probe (39, 40). To determine whether antigen-specific T cell activation would also activate the DNA binding activity of HSF1, spleen cells from DO11.10 TCR transgenic mice were activated with OVA323–339 for 20h. As we saw in anti-CD3 stimulated T cells, a prominent retarded band corresponding to HSF1 was present in OVA activated spleen cells at both 37°C and 40°C (Fig. 2B). SEB stimulation of HSF1+/+ spleen cells also resulted in a retarded band that was absent in HSF1−/− spleen cells. Heat-shocked Jurkat cells, widely used as a control for HSF1 activation produced a retarded band migrating to the same position. Thus, T cell activation through the T cell receptor results in prominent HSF1 activation to the transcriptionally active DNA binding form in the apparent absence of external stress. Nevertheless, in both cases, more HSF1 binding was present at 40°C indicating a synergy between cellular activation signals and mild temperature stress for the activation of HSF1.
FIGURE 2.
HSF1 is activated to the DNA-binding form in activated T cells under non-stressful conditions. (A) Purified T cells from HSF1+/+ mice were activated with plate-bound anti-CD3 for 20h at either 37°C or 40°C. HSF1−/− cells were activated at 37°C. (B) Spleen cells from DO11.10 TCR transgenic mice were cultured with or without OVA323–339 for 20h at either 37°C or 40°C. Spleen cells from HSF1−/− or HSF1+/+ mice were cultured with or without SEB for 20h at 37°C. (C) Spleen cells from DO11.10 mice were cultured in the presence of OVA323–339 (Ova-P Stimulated) for the indicated times at 37°C. (D) Purified T cells from HSF1+/+ or HSF1−/− mice were activated with either plate bound anti-CD3 or PMA and ionomycin at 37°C for 5h. (E) Purified B cells from HSF1+/+ or HSF1−/− mice were stimulated at 37°C for 20h with media (control) or LPS. Following activation, nuclear extracts were obtained from the cells and were subjected to EMSA using a 32P-labeled HSE oligonucleotide probe. Probe: Labeled HSE with no protein in the binding reaction. HS Jurkat cells: Jurkat cells heat shocked at 45°C for 30 min. Data are representative of two (C, D, E) or three (A, B) independent experiments with cells obtained from two at least 2 mice from each strain.
The previous experiments showed that HSF1 was activated after 20h of T cell activation. To determine how rapidly HSF1 was activated following T cell activation, DO11.10 T cells were activated with OVA323–339 for 2, 4, or 6 hours and HSF1 DNA binding evaluated. Within 4h of T cell activation, prominent HSF1 DNA binding activity was present and was not further increased at 6h (Fig. 2C). Thus, we conclude that activation of HSF1 DNA binding occurs rapidly, between 2 and 4 hours, following T cell activation. To determine whether early TCR signaling events were required for HSF1 activation, we activated purified T cells with PMA and ionomycin. This mitogen provides full T cell activation signals by directly activating protein kinase C and opening calcium channels, thus bypassing proximal TCR signaling events. Again, prominent HSF1 DNA binding activity was induced within 5h indicating that cellular activation events downstream of PKC are involved in activation of HSF1(Fig. 2D).
Because HSF1 was activated rapidly in T cells through general mitogenic signals, and given the known importance of HSF1 in cellular proliferation, we reasoned that HSF1 would be activated in other lymphoid cell types receiving proliferative signals. To test this, we activated purified B cells from HSF1+/+or HSF1−/− mice with LPS for 20h at 37°C and assessed HSF1 DNA binding activity. As expected, we observed a single prominent retarded band corresponding to HSF1 in HSF1+/+ but not HSF1−/− B cells (Fig. 2E). Thus, HSF1 activation is associated with proliferative stimuli in both T and B lymphocytes in the absence of thermal stress.
HSF1 is required for optimal proliferation of T cells in vivo at non-febrile temperatures
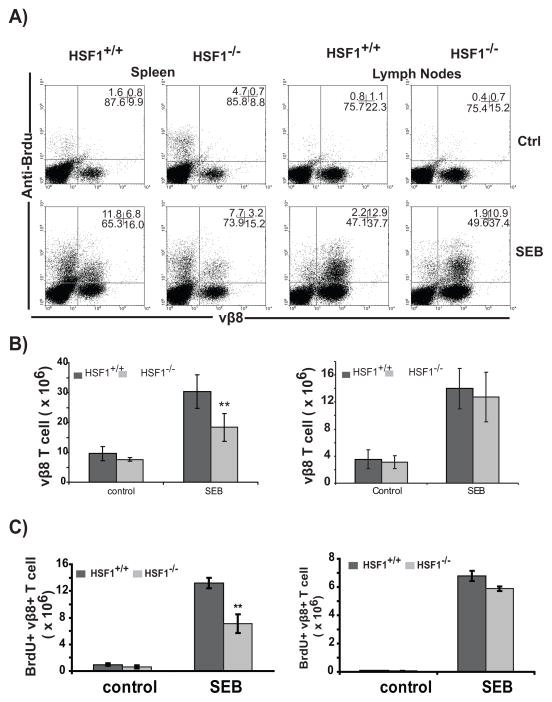
In our previous studies, we demonstrated that proliferation of purified T cells from HSF1−/− mice was severely affected upon activation with plate bound anti-CD3 in vitro under a stress paradigm that did not affect wild-type T cells (40°C and low [CO2]). Nevertheless, comparable proliferation was observed in both HSF1+/+, and HSF1−/− T cells at 37°C(22). An experiment reproducing these results is shown in Figure S1A. Therefore, it was important to understand how loss of HSF1 affected T cell proliferation under more physiologic conditions in vivo. An ideal model system would be to create HSF1−/−, TCR transgenic mice. However, we and others have found that viable homozygous HSF1−/− pups cannot be obtained after backcrossing to either BALB/c or C57BL/6 mice, and the line must be maintained on a mixed BALB/c and 129 background (17), precluding the use of adoptive transfer studies. To evaluate in vivo T cell proliferation in this mixed background strain, we used a SEB mediated T cell activation model. SEB is a super-antigen that cross-links MHCII molecules on the antigen presenting cells to Vβ chains of the T cell receptor and results in T cell activation and proliferation of all T cells expressing appropriate Vβ chains in an antigen independent manner(41). SEB was injected intravenously into HSF1−/− or HSF1+/+mice followed by an intraperitoneal injection of BrdU to label dividing cells. We monitored the division of Vβ8 bearing T cells, which represents the major Vβ population of T cells responding to SEB in these mice, by flow cytometry after staining with anti-Vβ8 and anti-BrdU antibodies. Administration of SEB to the HSF1+/+mice induced a significant expansion of Vβ8+ T cells in vivo during the 48h period (Fig. 3A). In addition, these mice showed significant enlargement of spleen and lymph nodes (visual observation and data not shown) compared to control mice after SEB injection. In contrast, HSF1−/− mice had similar lymph node enlargement, but the spleens showed much less enlargement and remained significantly smaller compared to HSF1+/+ spleens, which was reflected in total cell counts (Fig. S1B). Analysis of the proliferating Vβ8+ T cells in the HSF1−/− mice confirmed the visual observation showing that SEB induced expansion was significantly impaired in the spleen, but not lymph nodes of HSF1−/− mice. We observed a marked reduction in the percentages (Fig. 3A) and total number of Vβ8+, and Vβ8+BrdU+ cells in spleen but not lymph node(Fig. 3B, 3C). Thus, HSF1−/− T cells show an impaired proliferation in response to SEB in spleen, but not lymph nodes.
FIGURE 3.
HSF1−/− T cells show reduced proliferation in vivo in response to SEB stimulation at non-febrile temperatures. (A) HSF1−/− or HSF+/+ mice were injected i.v. with (bottom row), or without (top row) 150μg SEB. After 44 hours, mice were injected with BrdU and spleen or LN cells isolated 4h later. Cells were stained with anti-CD3, anti-Vβ8 and anti-BrdU and analyzed by flow cytometry. Shown are representative anti-Vβ8 vs. anti-BrdU dot-plots of gated CD3+ cells. Numbers in each dot-plot represent the percentage of CD3+ cells in each respective quadrant. (B) Total Vβ8+ T cells were calculated from the experiment in (A) using total spleen or LN numbers and average Vβ8+ T cell percentages from 6–8 mice in each treatment. (C) Total BrdU+, Vβ8+ T cells were calculated from the experiment in (A) using total spleen or LN numbers and average BrdU+, Vβ8+ T cell percentages from 6–8 mice in each treatment. P-values were calculated from student’s T-test with one-tailed distribution and two-sample unequal variance. ** represent p-value ≤ 0.01. Standard error is calculated as ± 1 standard deviation. This experiment was repeated three times with similar results.
In our previous in vitro studies and Figure S1A, HSF1−/− cells stimulated with anti-CD3 or anti-CD3/CD28 showed impaired proliferation only at 39°C (22). Thus, we hypothesized that the impaired T cell proliferation observed in vivo in response to SEB was a consequence of an increase in body temperature due to the effects of SEB. Using implanted temperature transponders, we directly tested whether SEB administration would significantly raise mouse body temperature. To our surprise, we did not see any significant change in core body temperature of either wild-type or HSF1−/− mice injected with SEB over a 48h period (Fig. S2). In contrast, we readily detected fever range temperatures in mice infected with listeria(18). Thus, the impaired proliferation of Vβ8 T cells in HSF1−/− mice occurred under non-febrile temperatures.
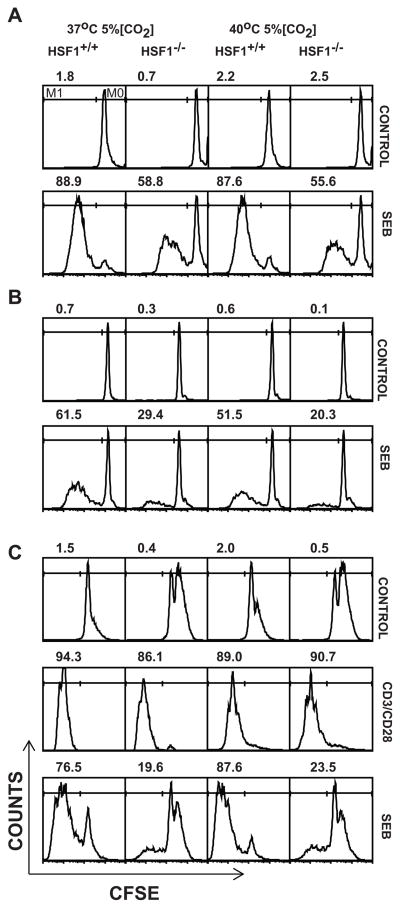
These data suggested that, unlike in vitro anti-CD3 stimulation, in vivo SEB stimulation imparted a sufficient level of stress to impair the proliferation of HSF1−/− T cells at normal temperatures within the spleen microenvironment, but not lymph nodes. To determine whether HSF1−/− T cells were impaired in vitro in response to SEB, we activated spleen and lymph node cells separately with SEB at indicated concentrations and measured the Vβ8+ T cell proliferation using CFSE dilution and thymidine incorporation assays. Both spleen and LN cells were impaired in proliferation when stimulated with SEB at 37°C and to a greater extent at 40°C (Fig. 4A, 4B, and S1C). Thus, SEB stimulation of T cells in vitro revealed a defect in proliferation of HSF1−/− cells that anti-CD3 stimulation did not. This suggested the possibility that SEB stimulation resulted in a heightened level of stress compared to anti-CD3 stimulation sufficient to impair proliferation of the HSF1−/− T cells. To test this, spleen cells from HSF1+/+ or HSF1−/− mice were labeled with CFSE, split in half, and stimulated with either anti-CD3 or SEB. The proliferation of the Vβ8+ T cells in the culture was then evaluated by flow cytometric analysis of CFSE dilution in the live Vβ8+ T cell population. Consistent with our previous report and the data shown in Fig S1A, the HSF1−/− cells showed no impairment in proliferation when stimulated with anti-CD3/anti-CD28. In contrast, only the HSF1+/+ cells showed robust proliferation in response to SEB, revealing a striking qualitative difference between SEB stimulation and anti-CD3 stimulation of T cells in vitro (Fig. 4C). Thus, proliferation of HSF1−/− T cells in response to SEB is impaired both in vitro and in vivo. However, in vivo, this impairment was only observed in the spleen, but not lymph node microenvironment.
FIGURE 4.
HSF1−/− T cells show compromised proliferation in vitro in response to SEB simulation at both physiologic and febrile temperatures. CFSE labeled spleen cells (A) or Lymph node cells (B) from HSF1−/− or HSF1+/+ mice were cultured in media alone or with 20μg/ml SEB in 24 well plates for 3 days at 37°C or 40°C and 5% CO2. (C) CFSE labeled spleen cells from HSF1−/− or HSF1+/+ mice were activated at indicated temperatures, either alone (Control), or with anti-CD3 and anti-CD28 Abs or SEB (20μg/ml). Following activation, cultures were harvested, washed, labeled with anti-Vβ8 and anti-CD3 and CFSE profiles on live gated Vβ8+CD3+ T cells were analyzed using FACScan® flow cytometer. Numbers above each histogram represent the percent of total gated live cells (average of three replicates) which have undergone cell division (M1 gate, left line). Histograms are representative of two (C) or three (A, B) independent experiments with 3 replicates in each treatment. In each experiment cells were obtained from 2 mice in each strain.
The defective proliferation of HSF1−/− T cells in response to SEB stimulus is T cell intrinsic
When anti-CD3 was used as a stimulus, we were able to use purified T cells and demonstrate that the impaired proliferation of HSF1−/− T cells under stressful conditions is T cell intrinsic (22). In contrast, when SEB was used as a stimulus, cultures necessarily included antigen presenting cells. Thus, it is possible that the impaired proliferation was due to lack of HSF1 in the APC and not to a T cell defect.
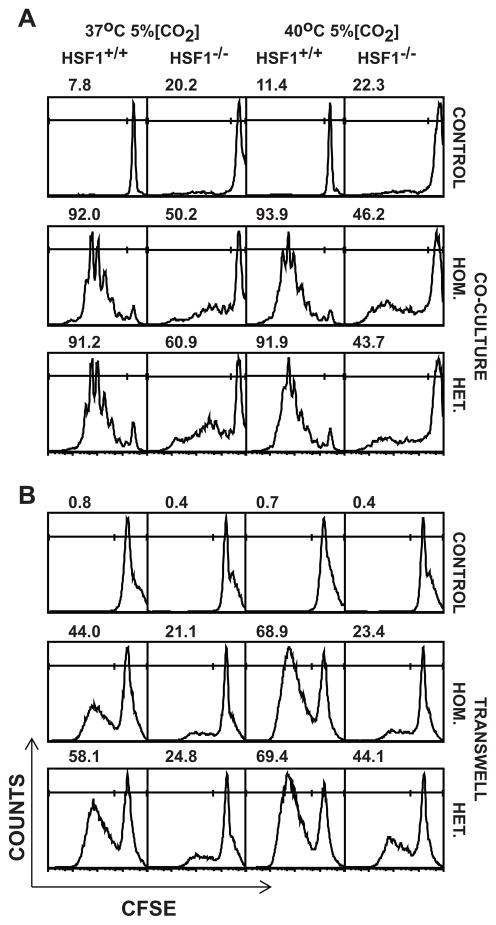
To determine whether APCs play a role contributing to the defective proliferation of HSF1−/− T cells in response to SEB, we labeled purified T cells with CFSE and stimulated them with SEB in a co-culture system containing APCs from HSF1+/+ or HSF−/− mice added at equal ratios to the T cell cultures. Cells were harvested 3 days after activation and CFSE dilution profiles for CD4+, Vβ8+ T cells were analyzed by flow cytometry. As shown previously, HSF1+/+ T cells proliferated vigorously to SEB at both 37°C and 40°C, and this proliferation was the same whether the APC were from HSF1+/+ or HSF1−/− mice (Fig. 5A). Also as expected, HSF1−/− T cells were impaired in their response to SEB, and this impairment was apparent whether the APC were from HSF1+/+ or HSF1−/− mice. There was a modest enhancement of proliferation in HSF1−/− T cells in the presence of HSF1+/+ APC (heterogeneous co-culture) compared to HSF1−/− APC (homogeneous co-culture). However, this difference was small compared to the proliferation of HSF1+/+ T cells, and was not apparent at 40°C. Thus, the impaired proliferation in response to SEB is due to a lack of HSF1 in the T cell and not due to changes in APC function.
FIGURE 5.
Defective proliferation of HSF1−/− T cells in response to SEB stimulation is not due to a defect in antigen presenting cell interactions nor influenced by stimulatory or inhibitory factors released from APC-T cell interactions. (A) Purified T cells from HSF1−/− or HSF1+/+ mice were CFSE labeled and mixed with unlabeled APCs (flow through fraction obtained during T cell purification) from HSF1−/− or HSF1+/+ spleens at 1:1 ratio and activated with 20 μg/ml SEB in 24 well plates for 3 days at the indicated temperatures. Cells were then labeled with anti-Vβ8, and anti-CD3 and the CFSE fluorescence of the Vβ8+, CD3+ T cells were analyzed. Histograms represent CFSE profiles of control, un-stimulated (top row), or SEB-Stimulated T cells in homogenous co-cultures (HSF1+/+ T cells, and HSF1+/+APCs or HSF1−/− T cells, and HSF1−/−APCs) (center row) or heterogeneous co-culture (HSF1+/+ T cells and HSF1−/−APCs or HSF1−/− T cells, and HSF1+/+APCs (bottom row). (B) Spleen cells from HSF1−/− or HSF+/+ mice were CFSE labeled and activated with SEB in 24 well trans-well plates for 3 days at indicated temperatures. Histograms represent CFSE profiles of control, un-stimulated (top row) or SEB stimulated homogenous transwell-cultures (HSF1−/− or HSF1+/+ spleen cells in both top and bottom wells) (center row) or heterogeneous transwell-cultures (HSF1+/+ spleen cells in the bottom wells and HSF1−/− cells in the top wells and vice versa) (bottom row). After 3 days, cells were cells were harvested from the bottom well of each transwell, washed and labeled with PE-conjugated anti-Vβ8, and APC-conjugated anti-CD3 and the CFSE fluorescence of the Vβ8+ CD3+ T cells were analyzed. Numbers in each histogram represent the percent of total gated live cells (average of three replicates) which have undergone cell division (M1 gate, left line). Histograms are representative of data from 3 repeated experiments with 3 replicates in each treatment and cells obtained from two mice/strain were pooled in each experiment.
Because T cell proliferation depends, in part, on autocrine growth factors like IL-2 and IL-15, and can be negatively impacted by inhibitory factors like IL-10, we investigated, whether soluble factors released as a result of APC-T cell interactions during SEB stimulation affected the proliferation of HSF1−/− T cells. To test this, we used a trans-well system where spleen cells from HSF1+/+and HSF1−/− mice were cultured in the same well but separated by a permeable membrane. CFSE labeled spleen cells from HSF1+/+ or HSF1−/− mice were added to bottom wells of a 24 well plate and unlabeled spleen cells from HSF1+/+ or HSF1−/− mice respectively were added to the trans-membrane inserts on the top to make homogenous or heterogeneous trans well cultures. The cultures were then stimulated with SEB for 72h at 37 or 40°C. Following the activation, cells in the bottom well were harvested and CFSE profiles of CD4+ Vβ8+ gated T cells were analyzed by flow cytometry. HSF1−/− T cells proliferated poorly compared to HSF1+/+ T cells, and this proliferation was not rescued by soluble factors from HSF1+/+ cells (Fig. 5B). Similarly, the normal proliferation of HSF1+/+ T cells was not inhibited by soluble factors from HSF1−/− cells. Thus, the proliferation defect in HSF1−/− T cells is not simply due to a lack of autocrine growth factors like IL-2, nor to an over-production of an inhibitory factor like IL-10. Collectively, the above studies indicate that the impaired proliferation of HSF1−/− T cells in response to SEB is T cell intrinsic, resulting in heightened sensitivity to SEB induced stress.
HSF1−/− T cells show dysregulated cyclin expression when activated with anti-CD3 under stressful conditions
We have demonstrated that HSF1−/− T cells have a defect in cell cycle progression under two different experimental conditions, both of which create an apparent stress on cells. In the case of anti-CD3 stimulation, the block in proliferation was apparent at elevated temperatures and low [CO2]. In the case of SEB stimulation, the block in proliferation occurred at 37°C. Thus, it is likely that the underlying mechanism is similar: in the absence of HSF1, T cell proliferation is hypersensitive to proteotoxic stress. Collectively, our data herein, and from previous studies(22), point to an intrinsic block in T cell cycle progression. To further understand the mechanism of this block in cell cycle, we stimulated purified T cells with anti-CD3 under benign (37°C) or stressful (40°C and 2% [CO2])conditions and visualized the appearance of key cell cycle related proteins by western blot. We chose these conditions because anti-CD3 stimulation allows the use of purified T cells and stimulates the majority of T cells in the culture. Furthermore, the stress paradigm used (40°C, 2% [CO2]) induces a profound block in HSF1−/− T cell proliferation, but does not impair HSF1+/+ T cell proliferation.
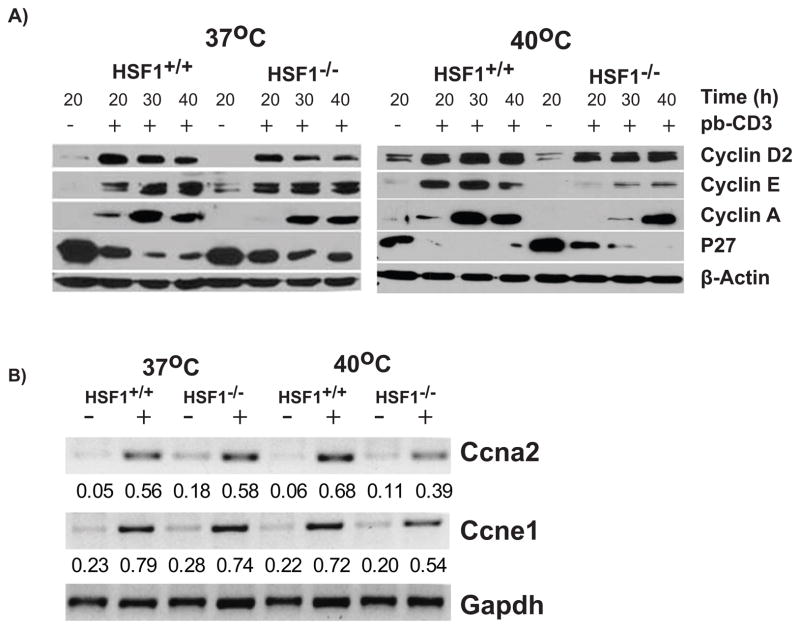
T cell proliferation is initiated by membrane proximal signaling events which ultimately trigger a MAPK cascade, NFAT, and NFκB which, in turn, activate transcription of a variety of genes required for cellular activation and proliferation. Entry into the cell cycle is initiated by expression of cyclin D2. Immunoblot analysis revealed no marked changes in the expression of cyclin D2 in the lysates from HSF1+/+or HSF1−/− T cells activated with anti-CD3 at either stressful or non-stressful conditions (Fig. 6A). This confirmed our previous conclusion that early activation events were not affected in HSF1−/− cells(22). In contrast, while the expression of cyclin E1 and cyclin A2 were comparable between lysates from HSF1−/− and HSF1+/+ T cells atnon-stressful conditions, the expression of these cyclins were significantly reduced in HSF1−/− T cells activated under stressful conditions (Fig. 6A). In addition, we also observed increased levels of p27 protein in lysates from HSF1−/− T cells activated at stressful conditions, while no p27 was observed in these conditions in the lysates from HSF1+/+ T cells.
FIGURE 6.
Activated HSF1−/− T cells show dysregulation of cell cycle regulatory proteins cyclin E, cyclin A, and P27 when activated under stressful conditions. (A) Purified T cells from HSF1−/− or HSF1+/+ mice were cultured in media alone or with plate-bound anti-CD3 for the indicated time and temperatures. Following activation, the cell lysates were subjected to western blot analysis. Shown are western blots of cell extracts probed with cyclin D2, cyclin E1, cyclin A2, p27, or β-Actin antibodies. (B) Purified T cells from HSF1−/− or HSF1+/+ mice were cultured in media alone or with plate-bound anti- CD3 for 24h at indicated temperatures. Following activation, total RNA from these cells were isolated, reverse transcribed, and analyzed using semi-quantitative PCR for the expression of Ccna2(cyclinA2), Ccne2(cyclinE1) mRNA, and GAPDH as a loading control. The numbers below each lane represent the normalized expression level relative to GADPH expression. Data are representative of 3 independent experiments with cells from 2 mice/group in each experiment.
To determine if the lack of cyclin E and A in HSF1−/− cells was reflected at the transcriptional level, we carried out semi-quantitative PCR analysis of cyclins in activated T cells. The mRNA expression analysis revealed a 43% reduction in the levels of cyclin A2 mRNA and a 25% reduction in the levels of cyclin E1 mRNA in HSF1−/− T cells compared to HSF1+/+ T cells activated under stressful conditions (Fig. 6B). This reduction in mRNA levels appears to be much less than the almost complete elimination of protein seen in Figure 6A. These results suggest that the reduction of cyclins E and A in stressed HSF1−/− cells may be due to a combined reduction in gene transcription as well as a decrease in protein stability.
Loss of HSF1 also affected the proliferation of activated B cells and bone marrow hematopoietic cells activated under stressful conditions
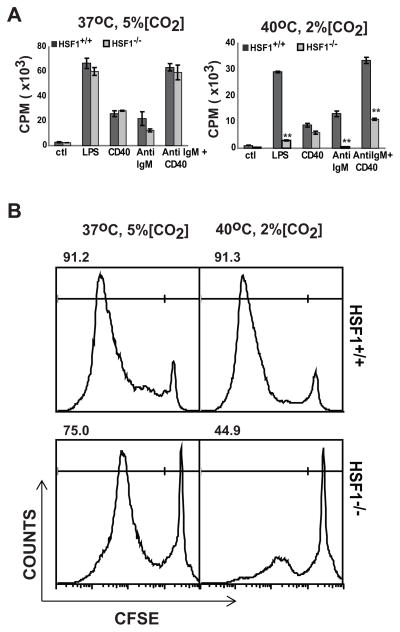
Our results suggest that the proliferation block in HSF1−/− T cells activated under stressful conditions is due to reduced expression of important cell cycle proteins. Since this pathway is common to all proliferating cells, we postulated that other cell types would show the same blockage in proliferation under similar stress. Hence, we evaluated proliferation of B cells and differentiating bone marrow monocytic cells derived from HSF1+/+ or HSF1−/− mice. Purified resting B cells from HSF1+/+ or HSF1−/− mice were activated with either LPS, or anti-IgM and anti-CD40, and monitored for proliferation after incubation at either non-stressful or stressful conditions (Fig. 7A). As we observed for T cells, there was significant impairment in B cell proliferation in the HSF1−/− B cells compared to HSF1+/+ B cells when activated with either LPS or anti-IgM and anti-CD40 under stressful conditions. These results were also consistent with our previous gel shift analysis (Fig. 2)which showed activation of HSF1 DNA-binding activity in B cells upon stimulation with LPS. We also cultured CFSE labeled bone marrow cells isolated from HSF1−/− or HSF1+/+ mice in presence of GM-CSF and monitored the proliferation under non-stressful or stressful conditions. Again, we observed that bone marrow cells from HSF1−/− mice incubated under stressful conditions showed a significant block in cell divisions at day three and day six of culture, as evidenced by significantly higher percent of cells that did not divide in HSF1−/− mice compared to HSF1+/+ mice. These studies show that HSF1 is critical for proliferation not only in T cells, but other hematopoietic cells as well when activated or cultured under stressful conditions.
FIGURE 7.
Proliferation of purified B cells and hematopoietic stem cells from HSF1−/− mice is compromised upon activation under stressful conditions. (A) Purified B cells from HSF1−/− (grey bars) or HSF1+/+ (black bars) mice were cultured in media alone or with LPS, anti-CD40, anti-IgM, or anti-IgM + anti-CD40 for 72h at the indicated conditions. The proliferation of B cells was determined by incorporation of 3H thymidine during the last 18 h of culture. Double asterisks indicate a p –value ≤ 0.01 estimated using student’s T-test performed with one-tailed distribution and samples with equal variance. (B) Bone marrow cells from HSF1−/− or HSF1+/+ mice were CFSE labeled and cultured in media containing GM-CSF at the indicated conditions and cell divisions were monitored by flow cytometry using CFSE histograms on day 3. Numbers above each histogram represent the percent of total gated live cells (average of three replicates) which have undergone cell division (M1 gate, left line) for either HSF1+/+ or HSF1−/− mice. Scatter plots revealed similar viability in all cultures. Data are representative of two independent experiments with cells pooled from 2 mice in ach group.
Discussion
HSF1 has long been considered strictly a stress responsive factor, providing for a rapid response to cellular stressors like elevated temperature or reactive oxygen species (ROS). However, it is now clear that HSF1 plays a much broader role in regulating a proteostasis network, critical for protein homeostasis in diverse cell types in the face of stress or changes in metabolic activity (42–44). The major findings of this study are that HSF1 is rapidly activated in lymphocytes as a normal consequence of proliferative signals and, in turn, activates a transcriptional program that includes proteostasis signature genes. We also show that lymphocyte proliferation in the absence of HSF1 is hypersensitive to stress, resulting in a block in the G1 to S phase of the cell cycle. This impaired proliferation is cell intrinsic, and associates with a reduction of key cell cycle proteins cyclins A and E. Thus, HSF1 plays a crucial role in the maintenance of optimal proliferation in lymphocytes and HSF1 becomes increasingly important as stress levels increase. We suggest that this cellular protection involves the activation of a gene program affecting a variety of proteostasis related genes as well as other genes whose function is yet to be understood in this context. This requirement for HSF1 is shown in both lymphocytes and proliferating bone marrow cells, suggesting that HSF1 plays a role in a wide range of cell types involved in rapid proliferation.
Since the observation in 1962 that elevated temperature induces transcriptionally active puffs in Drosophila polytene chromosomes, investigators have attempted to understand the mechanism cells use to sense stress (45). HSF1 has emerged as the key stress sensing molecule for the heat shock response from yeast to mammalian cells (43, 44). However, how HSF1 senses diverse stressors including, heat, ROS, heavy metals and small molecule mediators, remains unclear. Several non-mutually exclusive models have been put forward to explain the stress sensing mechanism of HSF1. One common thread among the stressors that activate HSF1 is that they usually cause the accumulation of misfolded or nonnative proteins (46). Indeed, injection of denatured proteins into Xenopus oocytes can trigger HSF1 dependent HSP gene expression (47). The observation that HSP90 binds to monomeric HSF1, keeping it in a monomeric state, has led to a model whereby proteotoxic insults titrate HSP90 away from HSF1, allowing rapid trimerization(48, 49). The identification of a ribonucleoprotein complex that controls the heat shock response of HSF1 has led to a model in which RNA serves as the temperature sensor (50). Purified recombinant HSF1 also has an intrinsic ability to trimerize when exposed to heat, low pH, increased calcium concentrations, and H2O2, suggesting an innate capacity of HSF1 itself to sense stress (51–55). Finally, elegant studies in C elegans show that the organismal heat shock response is regulated by thermosensory neurons, suggesting that there may be neuronal control of the stress response (56). Thus, it is likely that multiple mechanisms are involved in the stress sensing capability of HSF1, and this likely differs in different cell types. Furthermore, changes in metabolic activity are now known to activate HSF1 and need to be included in our concept of stress (57). Our data provide a new paradigm for the study of the mechanism of HSF1 activation. This is possible because lymphocytes can be isolated as resting, non-proliferating cells, largely in the G0 stage of the cell cycle. At this stage, activated HSF1 and inducible HSP70 are almost undetectable (22, 36). Within four hours of activation with a mitogenic signal, HSF1 DNA binding and transcriptional activity is induced. Thus, in this model of HSF1 activation, there is no obvious stressor. However, if one considers the enormous cellular changes that must occur when a small resting lymphocyte is activated, then perhaps it is not surprising that HSF1 senses these changes as stress and becomes activated. Possibilities include changes in intracellular calcium, increases in mitochondrial activity, including the electron transport chain and ROS, increased gene transcription and protein synthesis.
What are the consequences of HSF1 activation in T cells? In this study, we used whole genome mouse microarrays to investigate the role of HSF1 in the regulation of gene expression in activated T cells at normal and febrile temperatures. We identified a diverse set of 323 genes differentially regulated between activated T cells from HSF1−/− and HSF1+/+ mice, either at normal or febrile temperatures. About 143 genes in the group showed significant differences between HSF1+/+ and HSF1−/− cells both at normal and febrile temperatures, clearly indicating that these genes are influenced by HSF1 mediated gene regulation. The identification of so many genes regulated by HSF1 at 37°C in activated T cells is noteworthy because it identifies a gene program regulated by HSF1 independent of heat shock. Several of these genes are either already known to be directly or indirectly regulated by HSF1, or are known to be differentially regulated from previous microarray studies characterizing HSF1 mediated gene regulation under various conditions (23, 58–60). The inducible form of HSP70 is the most extensively studied HSF1 regulated gene, probably because it shows the greatest fold induction upon HSF1 activation and appears to be completely dependent on HSF1 for its expression (22, 61). Consistent with this, the hsp70a and hsp70b genes (the two inducible forms of HSP70) showed the largest fold-difference in expression between HSF1−/− and HSF1+/+ mice in our microarray analysis. This is in agreement with several reports showing that inducible HSP70 is almost exclusively regulated by HSF1 (22, 61). This result also validates the accuracy of the microarray analysis in identifying HSF1 regulated genes in T cells. Other heat shock proteins and co-chaperones regulated by HSF1 in our microarray are known to be regulated by HSF1 in other cells (58–60). These include genes encoding HSP110, HSP90 alpha, HSP90 beta, HSP40, and HSP10. Our microarray analysis identified an HSP70 co-chaperone, Bag3 as being positively regulated by HSF1 in T cells. Bag3 is known to be regulated by HSF1 in response to heat shock and plays a role in protecting cells from apoptosis through its interaction with HSP70 (23, 62–65). Also in agreement with previous studies (59), our microarray studies showed positive regulation of genes encoding anti-apoptotic proteins like clusterin, and caspase 6 and negative regulation of pro-apoptotic genes, including BCL2l11, Card6, Card11, and Traf1, by HSF1 in activated T cells. Several reports show that HSF1 plays a role in inflammation and the immune response through transcriptional regulation of genes involved in the immune response (6, 17, 32). Consistent with these data, expression of genes involved in the NFκB pathway, including IKK2, NFκB2, and NFκBζ, was higher in HSF1−/− T cells, indicating negative regulation by HSF1 of such pro-inflammatory genes. Likewise, the proinflammatory cytokine IL16 showed negative regulation by HSF1, while the Th2 cytokines IL-4 and IL-13 displayed positive regulation. In addition, we found a large number of genes regulated by HSF1 that are involved in signal transduction, metabolism, transcription and translation (Table I). Finally, many of the genes regulated by HSF1 in T cells fit a “proteostasis signature” of genes regulated in organism as diverse as S. cerevisiae, C.elegans, drosophila, and mammals (42).
We previously demonstrated that T cells from HSF1−/− mice are able to proliferate normally under benign in vitro conditions of anti-CD3 stimulation in vitro at 37°C. However, when the temperature is increased to 40°C, proliferation of HSF1−/− T cells is markedly reduced. The [CO2] in the incubator also has a modifying effect on the proliferation block at 40°C, with lower concentrations in the 2% range resulting in severe inhibition of T cell proliferation, and elevated [CO2] showing a protective effect. This CO2 effect is at least partly related to changes in intracellular ROS levels(22). These modified conditions have no deleterious effect on proliferation of T cells from HSF1+/+ mice (22). Because HSF1−/− mice must be maintained on a mixed genetic background to prevent embryonic lethality, we were unable to perform adoptive transfer experiments to assess T cell proliferation in vivo. Instead, we monitored Vβ8 T cell proliferation in response to SEB in individual mice. HSF1−/− mice consistently showed reduced Vβ8 T cell proliferation in the spleen. Interestingly, there was no impairment of proliferation in lymph nodes. Because these mice were not febrile during the time frame of the experiment, it suggests that the stress resulting in the impaired proliferation of HSF1−/− cells is derived from events other than hyperthermia. This was confirmed in in vitro experiments showing decreased proliferation of HSF1−/− T cells in response to SEB. Both spleen and LN T cells from HSF1−/− mice showed impaired proliferation in response to SEB in vitro, indicating that the normal proliferation of LN cells in vivo must be related to differences between the LN and spleen microenvironment. A particularly revealing experiment compared the expansion of the same population of Vβ8 T cells from HSF1−/− mice stimulated by anti-CD3 with those stimulated with SEB. The fact that only SEB stimulation resulted in impaired proliferation indicates a fundamental qualitative difference between anti-CD3 and SEB stimulation, the latter producing a sufficient level of stress on the T cell as to require HSF1 for normal proliferation.
We have previously shown that the HSF1 requirement for anti-CD3 stimulated proliferation under stress is apparent in purified T cells, indicating that the effect is T cell intrinsic, but manifested only in the face of extrinsic stress. Because SEB stimulated proliferation requires cross-linking MHC class II on APC with the Vβ region of the TCR, APC were necessarily included in these cultures. However, cross mixing experiments between T cells and APC from either HSF1−/− or HSF1+/+ mice reveals that the impaired proliferation in response to SEB is independent of the genotype of the APC and therefore also T cell intrinsic. Furthermore, in transwell experiments, our data show that the impaired proliferation is not simply due to the lack of a required cytokine like IL-2, or the over production of an inhibitory cytokine like IL-10. Collectively, these data point to stressful conditions present during SEB stimulation which, in the absence of HSF1, result in impaired T cell proliferation.
Although further studies are required to determine qualitative differences in the T cell immune responses after activation with SEB and plate bound anti-CD3, or anti-CD3/CD28, there are several obvious differences between anti-CD3 T cell activation and SEB mediated T cell activation that can explain the apparent increased stress level. Activation of T cells with plate bound anti-CD3 cross links CD3 receptors and is capable of inducing T cell activation in the absence of APC. In contrast, activation of T cells with SEB involves cross linking of MHC II on APCs and TCR on T cells and also involves additional secondary signaling molecules, including CD4/CD8, and CD28(66, 67). Thus, in addition to T cell signaling, SEB induced T cell-APC cross linking activates signaling pathways in MHC II expressing cells(68) including B cells, DCs (69, 70), and macrophages (71, 72). In addition, in vivo administration of SEB induces maturation and migration of DCs in spleen (69, 70). Recently, SEB has been shown to engage CD28 in addition to MHCII and the TCR, inducing high levels of IL-2, IFNγ and TNFα (67). Because of this, SEB induces a more complete T cell response, more closely mimicking an antigen specific response, complete with a wide range of proinflammatory cytokines (67, 73–75). Proinflammatory cytokines like TNFα, IL-1, and IFNγ are known to induce nitric oxide causing oxidative stress in a variety of cell types (76, 77). Thus, it may be that these cytokines are the source of stress resulting in impaired proliferation of SEB stimulated HSF1−/− T cells. Future experiments adding purified cytokines to anti-CD3 stimulated HSF1−/− cells will address this question.
We have previously demonstrated that the impairment of T cell proliferation in HSF1−/− T cells under stress is due to an intrinsic block in the cell cycle, localized to the G1-S phase transition (22). In this study we extend these findings by showing that stressed HSF1−/− T cells entered the cell cycle normally, as revealed by normal expression of cyclin D2 protein. However there was a dramatic reduction of cyclins E and A within HSF1−/− T cells only under conditions of stress. Thus, it is likely that this disappearance of cyclin E and A is the primary reason for the block in cell cycle progression observed in stressed HSF1−/− T cells. Transcriptional assays revealed some reduction of cyclin E and cyclinA mRNA in HSF1−/− T cells activated under stressful conditions, suggesting that the reduced cyclin E and A protein is due to a combination of transcriptional and post-transcriptional mechanisms.
The cell cycle has emerged as a process that is particularly sensitive to proteotoxic stress. The cell cycle progresses through the precise sequential orchestration of cyclins and kinases, many of which are regulated at the level of protein stability. HSF1 is known to be important in cell cycle progression in a number of systems, including yeast, drosophila, and mammalian cells. Yeast cells carrying an HSF null mutation show significant defects in cell cycle progression through G2/M phase mainly due to the loss of HSP90 mediated cdc2 protein stabilization (78). Likewise, the HSP70 family member, HSc73 (Hspa8; heat shock cognate protein), directly interacts with p27Kip protein suggesting a possible role of cognate HSPs in targeting these proteins to ubiquitin mediated protein degradation (79). p27 is an important cell cycle inhibitory protein controlling G1/S phase transition. In agreement with these studies, our studies also showed inefficient clearance of P27Kip protein in activated HSF1−/− T cells under stressful conditions. Furthermore, our microarray data revealed significant down regulation of HSc73 gene expression in HSF1−/− T cells. Thus, it is possible that dysregulated p27Kip degradation observed in HSF1−/− T cell could partially affect the kinase activity of CDk4/cyclin D and CDk2/cyclin E complexes thereby eventually affecting the transition through G1 phase. While the mechanism of HSF1 protection of cyclin E and A levels in the face of stress is unclear at present, our results indicate that these proteins are acutely sensitive to proteotoxic stress and that the HSF1 regulated proteostasis network is critical for maintaining normal levels of these cyclins during the cell cycle.
The defective proliferation in HSF−/− cells provides a window on stressful situations that ordinarily are not apparent using HSF sufficient cells. Thus, this phenotype revealed a level of stress associated with SEB stimulation that was not apparent in anti-CD3 stimulation. SEB stimulation is more reflective of a “complete” immune response involving APC-T cell interaction and stimulates a potent pro-inflammatory cytokine response. This level of stress is likely to be present in the microenvironment of many immune or inflammatory responses in vivo. Stressors could include cytokines, reactive oxygen species, nitric oxide, as well as fever. While our work did not identify the precise mediators of stress, the use of HSF1−/− cells provides an approach to further characterize the varied stressors with the potential to affect the immune response. Identification of these stressors involved in immune responses is particularly critical for understanding the age-related decline in immune responses. HSF1 activity and concomitant loss of proteostasis is known to decline with age (42–44). Based on our results, this would leave the immune system particularly vulnerable to the “normal” stressors present in the immune response, and result in an impairment of clonal expansion in both T and B cells in response to pathogen challenge. Identification of these stressors could lead to approaches designed to augment the immune response in the elderly.
Supplementary Material
Acknowledgments
We thank Jennifer Strange and Greg Bauman for their expert technical assistance in Flow Cytometry. We thank Kuey-Chu Chen for her help in performing the microarray experiments and Arnold Stromberg for his assistance on the statistical analysis of the microarray data. We are grateful to Ivor Benjamin for providing the HSF1−/− mice.
This work was supported by National Institutes of Health R01 grants EY14060 (to J.G.W.) and GM61053 and GM64606 (to K.D.S.). Flow cytometry and microarray analysis was carried out at the University of Kentucky Flow Cytometry and Microarray Core Facilities, respectively, which are supported in part by the Office of the Vice President for Research, the Markey Cancer Center and a grant from the NIH Shared Instrument Program (S10 RR026827-01A1).
Abbreviations used in this article
- HSE
heat shock element
- HSF1
heat shock factor 1
- HSP
heat shock protein
- ROS
reactive oxygen species
- SEB
staphylococcal enterotoxin B
References
- 1.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 2.Travers KJ, Patil CK, Weissman JS. Functional genomic approaches to understanding molecular chaperones and stress responses. Adv Protein Chem. 2001;59:345–390. doi: 10.1016/s0065-3233(01)59011-7. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol. 1995;268:R28–32. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, V, Tron A, Li G, Trotter MJ. Heat shock transcription factor-1 regulates heat shock protein-72 expression in human keratinocytes exposed to ultraviolet B light. J Invest Dermatol. 1998;111:194–198. doi: 10.1046/j.1523-1747.1998.00266.x. [DOI] [PubMed] [Google Scholar]
- 5.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 6.Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-alpha regulation. J Leukoc Biol. 2008;84:1335–1345. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth D, Christians E, Li X, Benjamin IJ, Gustin P. Use of Hsf1(−/−) mice reveals an essential role for HSF1 to protect lung against cadmium-induced injury. Toxicol Appl Pharmacol. 2003;192:12–20. doi: 10.1016/s0041-008x(03)00256-4. [DOI] [PubMed] [Google Scholar]
- 8.Kowalczyk A, Guzik K, Slezak K, Dziedzic J, Rokita H. Heat shock protein and heat shock factor 1 expression and localization in vaccinia virus infected human monocyte derived macrophages. J Inflamm (Lond) 2005;2:12. doi: 10.1186/1476-9255-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele AD, Hutter G, Jackson WS, Heppner FL, Borkowski AW, King OD, Raymond GJ, Aguzzi A, Lindquist S. Heat shock factor 1 regulates lifespan as distinct from disease onset in prion disease. Proc Natl Acad Sci U S A. 2008;105:13626–13631. doi: 10.1073/pnas.0806319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth DM, Balch WE. Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol. 2011;23:126–134. doi: 10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaneo M, Dominici R, Cardano M, Diaferia G, Rovida E, Biunno I. Molecular chaperones as therapeutic targets to counteract proteostasis defects. J Cell Physiol. 2011 doi: 10.1002/jcp.22856. [DOI] [PubMed] [Google Scholar]
- 12.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorger PK, Pelham HR. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- 15.Gallo GJ, Prentice H, Kingston RE. Heat shock factor is required for growth at normal temperatures in the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1993;13:749–761. doi: 10.1128/mcb.13.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jedlicka P, Mortin MA, Wu C. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 1997;16:2452–2462. doi: 10.1093/emboj/16.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murapa P, Ward MR, Gandhapudi SK, Woodward JG, D’Orazio SE. Heat shock factor 1 protects mice from rapid death during Listeria monocytogenes infection by regulating expression of tumor necrosis factor alpha during fever. Infect Immun. 2011;79:177–184. doi: 10.1128/IAI.00742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh IS, He JR, Calderwood S, Hasday JD. A high affinity HSF-1 binding site in the 5′-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J Biol Chem. 2002;277:4981–4988. doi: 10.1074/jbc.M108154200. [DOI] [PubMed] [Google Scholar]
- 20.Singh IS, Viscardi RM, Kalvakolanu I, Calderwood S, Hasday JD. Inhibition of tumor necrosis factor-alpha transcription in macrophages exposed to febrile range temperature. A possible role for heat shock factor-1 as a negative transcriptional regulator. J Biol Chem. 2000;275:9841–9848. doi: 10.1074/jbc.275.13.9841. [DOI] [PubMed] [Google Scholar]
- 21.Takaki E, Fujimoto M, Sugahara K, Nakahari T, Yonemura S, Tanaka Y, Hayashida N, Inouye S, Takemoto T, Yamashita H, Nakai A. Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice. J Biol Chem. 2006;281:4931–4937. doi: 10.1074/jbc.M506911200. [DOI] [PubMed] [Google Scholar]
- 22.Murapa P, Gandhapudi S, Skaggs HS, Sarge KD, Woodward JG. Physiological Fever Temperature Induces a Protective Stress Response in T Lymphocytes Mediated by Heat Shock Factor-1 (HSF1) J Immunol. 2007;179:8305–8312. doi: 10.4049/jimmunol.179.12.8305. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs AT, Marnett LJ. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J Biol Chem. 2009;284:9176–9183. doi: 10.1074/jbc.M808656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metchat A, Akerfelt M, Bierkamp C, Delsinne V, Sistonen L, Alexandre H, Christians ES. Mammalian heat shock factor 1 is essential for oocyte meiosis and directly regulates Hsp90alpha expression. J Biol Chem. 2009;284:9521–9528. doi: 10.1074/jbc.M808819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YJ, Lee HJ, Lee JS, Jeoung D, Kang CM, Bae S, Lee SJ, Kwon SH, Kang D, Lee YS. A novel function for HSF1-induced mitotic exit failure and genomic instability through direct interaction between HSF1 and Cdc20. Oncogene. 2008;27:2999–3009. doi: 10.1038/sj.onc.1210966. [DOI] [PubMed] [Google Scholar]
- 26.Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26:5086–5097. doi: 10.1038/sj.onc.1210317. [DOI] [PubMed] [Google Scholar]
- 27.Christians E, Davis AA, Thomas SD, Benjamin IJ. Maternal effect of Hsf1 on reproductive success. Nature. 2000;407:693–694. doi: 10.1038/35037669. [DOI] [PubMed] [Google Scholar]
- 28.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/s0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 30.Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Brauchle C, Mayer MP, Buchner J, Issels RD, Noessner E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem. 2007;282:31688–31702. doi: 10.1074/jbc.M704129200. [DOI] [PubMed] [Google Scholar]
- 31.Zheng H, Li Z. Cutting edge: cross-presentation of cell-associated antigens to MHC class I molecule is regulated by a major transcription factor for heat shock proteins. J Immunol. 2004;173:5929–5933. doi: 10.4049/jimmunol.173.10.5929. [DOI] [PubMed] [Google Scholar]
- 32.Inouye S, Izu H, Takaki E, Suzuki H, Shirai M, Yokota Y, Ichikawa H, Fujimoto M, Nakai A. Impaired IgG production in mice deficient for heat shock transcription factor 1. J Biol Chem. 2004;279:38701–38709. doi: 10.1074/jbc.M405986200. [DOI] [PubMed] [Google Scholar]
- 33.Mullins MW, Pittner BT, Snow EC. CD40-mediated induction of p21 accumulation in resting and cycling B cells. Mol Immunol. 1998;35:567–580. doi: 10.1016/s0161-5890(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 34.Egan RM, Yorkey C, Black R, Loh WK, Stevens JL, Storozynsky E, Lord EM, Frelinger JG, Woodward JG. In vivo behavior of peptide-specific T cells during mucosal tolerance induction: antigen introduced through the mucosa of the conjunctiva elicits prolonged antigen-specific T cell priming followed by anergy. J Immunol. 2000;164:4543–4550. doi: 10.4049/jimmunol.164.9.4543. [DOI] [PubMed] [Google Scholar]
- 35.Mosser DD, Theodorakis NG, Morimoto RI. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988;8:4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gothard LQ, Ruffner ME, Woodward JG, Park-Sarge OK, Sarge KD. Lowered temperature set point for activation of the cellular stress response in T-lymphocytes. J Biol Chem. 2003;278:9322–9326. doi: 10.1074/jbc.M209412200. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho LD, Teixeira LK, Carrossini N, Caldeira AT, Ansel KM, Rao A, Viola JP. The NFAT1 transcription factor is a repressor of cyclin A2 gene expression. Cell Cycle. 2007;6:1789–1795. doi: 10.4161/cc.6.14.4473. [DOI] [PubMed] [Google Scholar]
- 38.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroeger PE, Sarge KD, Morimoto RI. Mouse heat shock transcription factors 1 and 2 prefer a trimeric binding site but interact differently with the HSP70 heat shock element. Mol Cell Biol. 1993;13:3370–3383. doi: 10.1128/mcb.13.6.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto N, Takemori Y, Sakurai M, Sugiyama K, Sakurai H. Differential recognition of heat shock elements by members of the heat shock transcription factor family. FEBS J. 2009;276:1962–1974. doi: 10.1111/j.1742-4658.2009.06923.x. [DOI] [PubMed] [Google Scholar]
- 41.Hong SC, Waterbury G, Janeway CA., Jr Different superantigens interact with distinct sites in the Vbeta domain of a single T cell receptor. J Exp Med. 1996;183:1437–1446. doi: 10.1084/jem.183.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 43.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 44.Anckar J, Sistonen L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol. 2007;594:78–88. doi: 10.1007/978-0-387-39975-1_8. [DOI] [PubMed] [Google Scholar]
- 45.Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–98. doi: 10.1379/1466-1268(1996)001<0097:dothsr>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 47.Ananthan J, Goldberg AL, Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986;232:522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 48.Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morimoto RI. Dynamic remodeling of transcription complexes by molecular chaperones. Cell. 2002;110:281–284. doi: 10.1016/s0092-8674(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 50.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–560. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 51.Mosser DD, Kotzbauer PT, Sarge KD, Morimoto RI. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci U S A. 1990;87:3748–3752. doi: 10.1073/pnas.87.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodson ML, Sarge KD. Heat-inducible DNA binding of purified heat shock transcription factor 1. J Biol Chem. 1995;270:2447–2450. doi: 10.1074/jbc.270.6.2447. [DOI] [PubMed] [Google Scholar]
- 53.Farkas T, Kutskova YA, Zimarino V. Intramolecular repression of mouse heat shock factor 1. Mol Cell Biol. 1998;18:906–918. doi: 10.1128/mcb.18.2.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol Cell. 1998;2:101–108. doi: 10.1016/s1097-2765(00)80118-5. [DOI] [PubMed] [Google Scholar]
- 55.Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–814. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harbor symposia on quantitative biology. 2011;76:91–99. doi: 10.1101/sqb.2012.76.010637. [DOI] [PubMed] [Google Scholar]
- 58.Trinklein ND, Murray JI, Hartman SJ, Botstein D, Myers RM. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell. 2004;15:1254–1261. doi: 10.1091/mbc.E03-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Page TJ, Sikder D, Yang L, Pluta L, Wolfinger RD, Kodadek T, Thomas RS. Genome-wide analysis of human HSF1 signaling reveals a transcriptional program linked to cellular adaptation and survival. Mol Biosyst. 2006;2:627–639. doi: 10.1039/b606129j. [DOI] [PubMed] [Google Scholar]
- 60.Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 62.Gentilella A, Khalili K. BAG3 expression in glioblastoma cells promotes accumulation of ubiquitinated clients in an Hsp70-dependent manner. J Biol Chem. 2011;286:9205–9215. doi: 10.1074/jbc.M110.175836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gentilella A, Khalili K. Autoregulation of co-chaperone BAG3 gene transcription. J Cell Biochem. 2009;108:1117–1124. doi: 10.1002/jcb.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franceschelli S, Rosati A, Lerose R, De Nicola S, Turco MC, Pascale M. Bag3 gene expression is regulated by heat shock factor 1. J Cell Physiol. 2008;215:575–577. doi: 10.1002/jcp.21397. [DOI] [PubMed] [Google Scholar]
- 65.Du ZX, Zhang HY, Meng X, Gao YY, Zou RL, Liu BQ, Guan Y, Wang HQ. Proteasome inhibitor MG132 induces BAG3 expression through activation of heat shock factor 1. J Cell Physiol. 2009;218:631–637. doi: 10.1002/jcp.21634. [DOI] [PubMed] [Google Scholar]
- 66.Lamphear JG, Stevens KR, Rich RR. Intercellular adhesion molecule-1 and leukocyte function-associated antigen-3 provide costimulation for superantigen-induced T lymphocyte proliferation in the absence of a specific presenting molecule. J Immunol. 1998;160:615–623. [PubMed] [Google Scholar]
- 67.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, Supper E, Shpilka T, Minis A, Kaempfer R. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS biology. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mourad W, al-Daccak R, Chatila T, Geha RS. Staphylococcal superantigens as inducers of signal transduction in MHC class II-positive cells. Semin Immunol. 1993;5:47–55. doi: 10.1006/smim.1993.1007. [DOI] [PubMed] [Google Scholar]
- 69.Yoon S, Bae KL, Shin JY, Yoo HJ, Lee HW, Baek SY, Kim BS, Kim JB, Lee HD. Analysis of the in vivo dendritic cell response to the bacterial superantigen staphylococcal enterotoxin B in the mouse spleen. Histol Histopathol. 2001;16:1149–1159. doi: 10.14670/HH-16.1149. [DOI] [PubMed] [Google Scholar]
- 70.Muraille E, De Trez C, Pajak B, Brait M, Urbain J, Leo O. T cell-dependent maturation of dendritic cells in response to bacterial superantigens. J Immunol. 2002;168:4352–4360. doi: 10.4049/jimmunol.168.9.4352. [DOI] [PubMed] [Google Scholar]
- 71.Piotrowicz P, Kwiatkowski K. Foreign body of the bronchus in adults. Pneumonol Alergol Pol. 1993;61:189–190. [PubMed] [Google Scholar]
- 72.Hendricks A, Leibold W, Kaever V, Schuberth HJ. Prostaglandin E2 is variably induced by bacterial superantigens in bovine mononuclear cells and has a regulatory role for the T cell proliferative response. Immunobiology. 2000;201:493–505. doi: 10.1016/S0171-2985(00)80069-8. [DOI] [PubMed] [Google Scholar]
- 73.Rajagopalan G, Sen MM, Singh M, Murali NS, Nath KA, Iijima K, Kita H, Leontovich AA, Gopinathan U, Patel R, David CS. Intranasal exposure to staphylococcal enterotoxin B elicits an acute systemic inflammatory response. Shock. 2006;25:647–656. doi: 10.1097/01.shk.0000209565.92445.7d. [DOI] [PubMed] [Google Scholar]
- 74.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer PH, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fraser JD. Clarifying the mechanism of superantigen toxicity. PLoS biology. 2011;9:e1001145. doi: 10.1371/journal.pbio.1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mir M, Asensio VJ, Tolosa L, Gou-Fabregas M, Soler RM, Llado J, Olmos G. Tumor necrosis factor alpha and interferon gamma cooperatively induce oxidative stress and motoneuron death in rat spinal cord embryonic explants. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 77.Mir M, Tolosa L, Asensio VJ, Llado J, Olmos G. Complementary roles of tumor necrosis factor alpha and interferon gamma in inducible microglial nitric oxide generation. J Neuroimmunol. 2008;204:101–109. doi: 10.1016/j.jneuroim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Nakai A, Ishikawa T. Cell cycle transition under stress conditions controlled by vertebrate heat shock factors. EMBO J. 2001;20:2885–2895. doi: 10.1093/emboj/20.11.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakamura S, Tatuno I, Noguchi Y, Kitagawa M, Kohn LD, Saito Y, Hirai A. 73-kDa heat shock cognate protein interacts directly with P27Kip1, a cyclin-dependent kinase inhibitor, during G1/S transition. Biochem Biophys Res Commun. 1999;257:340–343. doi: 10.1006/bbrc.1999.0442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.