Summary
Background
Several risk factors for inhibitors have recently been described for hemophilia A. It has been assumed that similar risk factors are also relevant for hemophilia B, but there is limited data to confirm this notion.
Objectives
To determine the prevalence of and risk factors associated with inhibitors in hemophilia B
Methods
The database of the Universal Data Collection (UDC) project of the Centers for Disease Control for the years 1998 – 2011 was queried to determine the prevalence of inhibitors in hemophilia B subjects. In addition, disease severity, race/ethnicity, age, factor exposure, and prophylaxis usage were evaluated to determine their impact on inhibitor prevalence.
Results
Of the 3800 male subjects with hemophilia B enrolled in the UDC database, 75 (2%) were determined to have an inhibitor at some point during the study period. Severe disease (OR 13.1, 95% CI 6.2-27.7), black race (OR 2.2, 95% CI 1.2-4.1), and age less than 11 (OR 2.5, 95% CI 1.5-4.0) were found to be significantly associated with having an inhibitor. There was insufficient data to determine if type of factor used and prophylaxis were associated with inhibitors.
Conclusions
Inhibitors in hemophilia B are much less prevalent than hemophilia A, especially in patients with mild disease. Similar factors associated with inhibitors in hemophilia A also seem to be present for hemophilia B. The information collected by this large surveillance project did not permit evaluation of potential risk factors related to treatment approaches and exposures, and additional studies will be required.
Keywords: Ethnicity, Factor IX, Hemophilia B, Inhibitors, Race, UDC
Introduction
The development of an inhibitor is one of the most devastating complications of hemophilia. In recent years, several risk factors for inhibitor formation in patients with hemophilia have been proposed. These include severity of disease, type of mutation, race, intensity of coagulation factor use at first exposure, type of coagulation product used, prophylaxis, surgery, and other immune related genetic polymorphisms [1]. Data supporting the importance of these risk factors for inhibitor development have derived primarily from the study of hemophilia A. It is assumed that similar risk factors for inhibitor development are present in patients with hemophilia B. However, this assumption may not be valid, especially considering that the clinical behavior of factor IX inhibitors differs from factor VIII inhibitors in important ways. The most significant of these are that factor IX inhibitors may be associated with allergic and hypersensitivity reactions; attempts to eliminate factor IX inhibitors with immune tolerance induction (ITI) regimens can lead to the development of nephrotic syndrome; and standard ITI succeeds in a minority of attempts [2-4]. Risk factors for inhibitor development in patients with hemophilia B have never been evaluated in an independent, systematic way. Likewise, the prevalence of inhibitors in patients with hemophilia B has generally been estimated using data from small, single institution studies, or from clinical trials of new factor IX products [5-7]. A large survey of North American Hemophilia Treatment Centers (HTC) found a prevalence of inhibitors in hemophilia B patients of 1.5%. However, nearly half the HTCs failed to respond to the survey, and the results of this survey may have been subject to bias [8].
To address these issues, we performed a descriptive analysis of a large database of bleeding disorders patients enrolled in the Universal Data Collection (UDC) study sponsored by the Centers for Disease Control (CDC) in Atlanta, U.S.A. The focus of this review was to determine the prevalence of and risk factors associated with inhibitors in hemophilia B subjects enrolled in the UDC database.
Materials and Methods
The UDC was established by the United States CDC as a national public health surveillance system to monitor treatment and outcomes of people with bleeding disorders.[9] Patients with hemophilia A and B, Von Willebrand Disease, and rare coagulation factor deficiencies who receive treatment at one of the 130 federally funded Hemophilia Treatment Center (HTC) in the United States are eligible to participate in the UDC. The 130 federally funded HTCs comprise the Hemophilia Treatment Center Network (HTCN), and investigators from each site contributed data to this study.
Data were collected by HTC staff from 1998 - 2011 using standardized data collection forms. At study enrollment data were collected regarding age, sex, race/ethnicity, bleeding disorder diagnosis, severity of factor deficiency, age and site of first bleed, family history of a bleeding disorder, history of intracranial hemorrhage, and genotype if available (not required for enrollment). For children less than 2 years of age at study enrollment, details regarding the birth history were also collected. For all age groups, data regarding allergic or hypersensitivity reactions, a prior history of an inhibitor, prior factor usage, treatment type (episodic/prophylactic infusions, or immune tolerance induction) prior to enrollment, and intensity of exposure at first usage were not collected. Race/Ethnicity was based on self-report and categorized as White (non-Hispanic), White (Hispanic), Black (non-Hispanic), Black (Hispanic), Asian/Pacific Islander, Native American, and other.
At subsequent UDC visits data regarding factor product(s) received, frequency of bleeds, treatment type (episodic, prophylaxis, ITI), joint range of motion, HIV and hepatitis C risk management and status, and highest inhibitor titer measured in Bethesda Units (BU) ml−1 since the last visit were collected.
The Institutional Review Boards of each participating HTC and the CDC approved the UDC and all participants or parents of minor children gave informed consent for data collection and transmission.
The database was queried to identify all hemophilia B patients entered into the database from May 1998 until September 2011. Of this subset, potential hemophilia B inhibitor cases were identified by the presence of any inhibitor titer ≥ 0.5 BU ml−1, or by the report of treatment to establish immune tolerance, or by the use of bypassing agents. At each site with potential hemophilia B inhibitor subjects, the participating institution (PI) was given the highest recorded inhibitor titer in the UDC database for potential inhibitor subjects from their center and asked via email to 1) confirm the accuracy of the submitted UDC data; and 2) to verify whether or not the patient had an inhibitor. Institutions that did not respond to the initial emails were sent up to two additional requests to confirm the data.
If the reported titer was incorrect, the PI was asked to provide the correct titer value. If the PI verified that the patient had an inhibitor, the PI was asked to classify the inhibitor into one of the following categories: 1) high titer inhibitor if the inhibitor titer was ≥5 BU ml−1 on at least two occasions; 2) low titer if the inhibitor titer was between the upper limit of normal for the PI’s lab and 5 BU ml−1 on at least two occasions, lasted > 6 months, and was not associated with an anamnestic response; and 3) transient if the inhibitor titer was greater than the upper limit of normal for the PI’s lab no matter what the peak titer was, lasted ≤ 6 months, and has never recurred. If the PI verified that the patient never had an inhibitor, the patient was categorized as inhibitor negative for the study. Whenever there were discrepancies between the PI confirmed data and the UDC data, the PI confirmed data were used for the analysis.
Inhibitor status for the subjects whose PI did not respond to our inquiries was based solely on the submitted UDC data and was categorized as follows: 1) high titer if the inhibitor titer was ≥ 5 BU ml−1 at any UDC visit; 2) low titer if the highest recorded inhibitor titer in the UDC database was greater than 1 BU ml−1 and <5 BU ml−1; and 3) if the titer was between 0.5 and 1 BU ml−1 then inhibitor status was positive for a low titer inhibitor if the subject ever received bypassing agents or immune tolerance induction (ITI) at any UDC visit; otherwise, the status was categorized as negative for an inhibitor.
From the UDC dataset, information on disease severity, age, race/ethnicity, prophylaxis and type of coagulation concentrates used for each subject was obtained.
Hemophilia severity was determined according to their factor IX activity: severe if less than 1%, moderate if between 1% and less than or equal to 5.0 %, and mild if greater than 5.0% and less than 40%.[10]
Patients were considered on prophylaxis if they received treatment products to prevent bleeding or to prevent re-bleeding. Subjects receiving either intermittent or continuous prophylaxis were both considered on prophylaxis for the purposes of this study. Patients were considered to have received episodic care if they received treatment products only in response to bleeding complications.
ITI was defined by the UDC as successful if the patient could be effectively treated for a bleeding episode with a factor dosage appropriate to his/her hemophilia severity and its type, otherwise it was unsuccessful. This definition of ITI success was established by the UDC at the inception of the UDC study and was determined for this study from the standardized annual UDC collection form.
Statistical analysis: Differences in the proportion of patients with inhibitors in each category of demographic and clinical characteristics were evaluated for statistical significance using chi-square tests. Independent associations between these characteristics and inhibitor prevalence were assessed using the prevalence odds ratio resulting from a logistic regression model. All analysis used the SAS software (SAS Institute, Cary, NC) and associations were considered statistically significant when p-values were ≤ 0.05.
Results
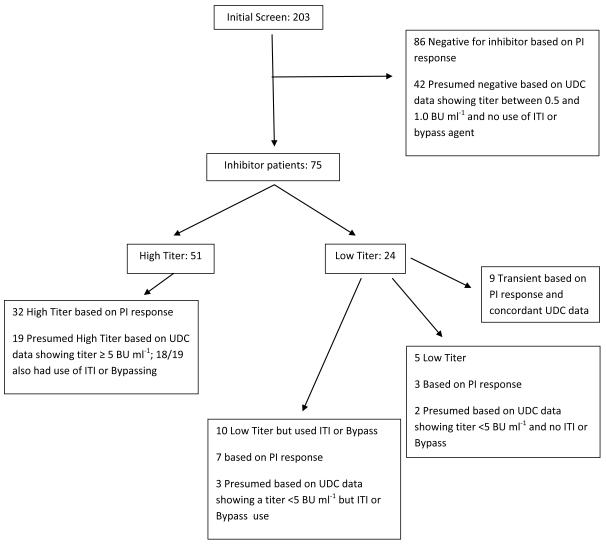
At the time of our database query in September, 2011, 3785 male patients with hemophilia B had been enrolled in the UDC database. Of these, 985 (26%) had mild, 1418 (38%) had moderate, and 1367 (36%) had severe hemophilia B. In 15 subjects (0.4%) these data were missing. There were 203 potential subjects with a reported inhibitor titer ≥0.5 BU ml−1 or who were reported to have received immune tolerance therapy or bypass agents at one of more UDC visits. Further data were available from the PI for 137 subjects with regard to the accuracy of the submitted UDC data and verification of inhibitor status. For the remaining 66 subjects, inhibitor status was based solely on the data available in the UDC database. Figure 1 provides the details of how both UDC data and PI-supplied data were used to classify the inhibitor status of the 203 subjects identified as potential inhibitor cases from the initial screening.
Figure 1.
Identification of Hemophilia B Inhibitor Patients in the UDC Database
Reveals how inhibitor subjects were identified. The initial screen of the database revealed 203 potential subjects with an inhibitor based on a titer of 0.5 Bu ml−1 or greater. Of these 128 were shown not to have an inhibitor based on PI response to inquiries of their submitted data or UDC data alone for those subjects whose PI did not respond to inquiries. This left 75 subjects with an inhibitor. ITI= immune tolerance induction therapy; Bypass= use of a bypassing agent such as recombinant factor VIIa or an activate prothrombin complex concentrate
When the PI responded to the question regarding the accuracy of the inhibitor titer data in the UDC data base, 49 (39%) indicated it was correct, and 78 (61%) indicated that it was not. In 10 subjects, the PI did not respond to the question regarding the accuracy of the inhibitor titer data, but did respond to other questions during confirmatory correspondence so that it could be determined that 9 of these subjects had high titer inhibitors, and one did not have an inhibitor. Almost 90% of the time that the PI indicated the inhibitor titer data were not correct, the titer in the UDC database was between 0.5 and ≤ 1 BU ml−1, and the PI did not consider this subject to have an inhibitor. This was because the titer was below the upper limit of normal for the local lab but still ≥ 0.5 BU ml−1, or the PI incorrectly rounded any positive titer up to 1 BU ml−1. Of the 87 subjects with inhibitor titers between 0.5 and ≤ 1 BU ml−1 and no ITI or bypass agent use where the PI responded to our email, 99% were categorized as never having an inhibitor based on PI response to our query.
Of the 3785 hemophilia B subjects enrolled into the UDC surveillance project, 75 (2%) had an inhibitor at some point during the study period. The majority of subjects (59) had evidence of an inhibitor (either elevated inhibitor titer, or receipt of ITI or bypass agent) at time of enrollment into the UDC. Among those with an inhibitor, 24 (0.6%) had low titer inhibitors, while the remaining 51(1.3%) had high titer inhibitors. Of those with a low titer inhibitor, 10 (42%) received treatment with ITI or a bypassing agent during the study period.
Table 1 shows the prevalence of inhibitors across categories of the studied risk factors. Age, race/ethnicity, and disease severity were all found to have a statistically significant impact on inhibitor prevalence. Children ≤ 10 years of age were more likely than those who were older to have a prevalent inhibitor. White non-Hispanic subjects were less likely than subjects of any other race or ethnicity to have a prevalent inhibitor. As expected, subjects with severe disease were far more likely to have an inhibitor than those with less severe disease. Associations between prevalent inhibitors and age and race were not as strong among those with low titer inhibitors compared to those whose inhibitor was high titer.
Table 1.
Characteristics of 3,785 Males with Hemophilia B and Relations with Prevalent Inhibitors
| Characteristic | Total N (%)* |
Inhibito N (%) |
p-value | Low Titer N (%) |
p-value | High Titer N (%) |
p-value |
|---|---|---|---|---|---|---|---|
| (n=75) | (n=24) | (n=51) | |||||
| Age | |||||||
| <2 years | 89 (2.4) | 3 (3.4) | <0.001 | 2 (2.2) | 0.055 | 1 (1.1) | < 0.01 |
| 2-5 years | 310 (8.2) | 12 (3.9) | 3 (1.0) | 9 (2.9) | |||
| 6-10 years | 455 (12.0) | 18 (4.0) | 6 (1.3) | 12 (2.6) | |||
| 11-20 years | 1039 (27.4) | 17 (1.6) | 7 (0.7) | 10 (1.0) | |||
| 21-44 years | 1173 (31.0) | 18 (1.5) | 3 (0.3) | 15 (1.3) | |||
| 45+ years | 719 (19.0) | 7 (1.0) | 3 (0.4) | 4 (0.6) | |||
| Race/Ethnicity | |||||||
| White | 2821 (74.5) | 37 (1.3) | < 0.001 | 15 (0.5) | NS | 22 (0.8) | < 0.001 |
| Black | 391 (10.3) | 18 (4.6) | 4 (1.0) | 14 (3.6) | |||
| Hispanic | 357 (9.4) | 12 (3.4) | 3 (0.8) | 9 (2.5) | |||
| Other | 207 (5.5) | 8 (3.9) | 2 (1.0) | 6 (2.9) | |||
| Severity | |||||||
| Mild | 985 (26.0) | 1 (0.1) | < 0.001 | 1 (0.1) | < 0.001 | 0 | < 0.001 |
| Moderate | 1418 (37.5) | 7 (0.5) | 5 (0.4) | 2 (0.1) | |||
| Severe | 1367 (36.1) | 67 (4.9) | 18 (1.3) | 49 (3.6) |
Percents do not all total to 100% due to missing data
The results of a multivariate analysis are shown in table 2. After adjusting for the effects of all the studied factors, subjects under the age of 11 years had 2.5 (95% CI 1.5 – 4.0) times the odds of having an inhibitor than subjects who were older. In contrast to the bivariate results, only black subjects had higher odds of prevalent inhibitors than white subjects. Finally, subjects with severe hemophilia had 13.2 (95% CI 6.2 - 27.7) times the odds of having an inhibitor than those with mild or moderate hemophilia B. These associations also held true when subjects with high titer inhibitors only were included in the analysis (table 3).
Table 2.
Independent associations between patient characteristics and prevalent inhibitors among 3785 hemophilia B patients
| Characteristic | Odds Ratio (95% CI) |
p-value |
|---|---|---|
| Age (vs. 11+ years) | ||
| <11 years | 2.5 (1.5 - 4.0) | < 0.001 |
| Race/Ethnicity (vs. White) | ||
| Black | 2.2 (1.2 - 4.1) | < 0.05 |
| Hispanic | 1.4 (0.7 - 2.7) | NS |
| Other | 1.7 (0.8 - 3.8) | NS |
| Severity (vs. Mild/Moderate) | ||
| Severe | 13.1 (6.2 – 27.7) | < 0.001 |
CI = Confidence Interval
Table 3.
Independent associations between patient characteristics and prevalent high titer inhibitors among 3761* hemophilia B patients
| Characteristic | Odds Ratio (95% CI) |
p-value |
|---|---|---|
| Age (vs. 11+ years) | ||
| <11 years | 2.3 (1.3 - 4.1) | 0.004 |
| Race/Ethnicity (vs. White) | ||
| Black | 2.8 (1.4 – 5.5) | ≤0.05 |
| Hispanic | 1.7 (0.7 - 3.7) | NS |
| Other | 2.1 (0.8 – 5.2) | NS |
| Severity (vs. Mild/Moderate) | ||
| Severe | 36.8 (8.9 – 152.8) | < 0.001 |
CI = Confidence Interval
24 patients with low titer inhibitors were excluded from this analysis
Of the 3785 Hemophilia B patients enrolled in the UDC database, there were a total of 114 (3%) reported deaths. There was only one (1.3%) reported death among the 75 inhibitor subjects.
Among the 75 patients with an inhibitor, 20 (27%) were reported to have received ITI at any UDC visit during the study period. Of these, 8 were reported as failures, 6 were successes, and in 6 ITI was ongoing as of the last UDC visit.
Discussion
Our review of this large surveillance database showed that 2% of hemophilia B subjects had inhibitors. Because we could not confirm in all cases the presence or absence of an inhibitor in subjects prior to enrollment in the UDC, we could not determine the actual proportion of inhibitors in this population, but it is unlikely to be much higher than 2% [8]. The majority of inhibitors were high titer, with a ratio of almost 2:1 high titer versus low titer. This is similar to the ratio found by Katz in his survey of North American Hemophilia Treatment Centers [8]. It is interesting to note that almost half of the low titer inhibitor subjects received ITI or bypassing agents, suggesting a significant clinical relevance to some low titer inhibitor subjects. To our knowledge, this approach has not been reported previously. It is possible that low titer subjects were treated with bypassing agents because of or out of concern for anaphylactic reactions. Unfortunately, the UDC database did not collect information regarding allergic reactions to factor IX products. In addition, the surveillance data collected in the UDC does not allow the capture of information regarding whether changes to dosing frequency or amount were occasioned by low titer inhibitors. Because this would be associated with increased clinical or financial burden, there may have been a further clinical relevance to low titer inhibitors.
The reasons for a lower prevalence of inhibitors in hemophilia B compared to hemophilia A are unclear. As with hemophilia A we found that underlying disease severity was significantly associated with inhibitor prevalence, with an odds ratio of an inhibitor in severe hemophilia B of 13.1 (95% CI 6.2 – 27.7) as compared to subjects with mild or moderate hemophilia B.
Interestingly, the profile of mutations found in severe hemophilia B differs from that found in hemophilia A. Higher proportions of severe hemophilia B patients are a result of missense mutations and are cross-reacting material positive (CRM+) [2, 4, 11]. Null mutations in hemophilia patients confer a higher risk of inhibitor development. This is true for both hemophilia A and hemophilia B [2, 12]. The differences in the ratio of CRM+ to CRM – patients in hemophilia A and B may in part explain the lower prevalence of inhibitors in hemophilia B. There may also be differences in immunogenicity between the factor VIII and factor IX molecule [4]. Our data showing a much lower proportion of prevalent inhibitors in mild hemophilia B subjects compared to mild hemophilia A patients supports this notion.
Race/Ethnicity was also related to the presence of an inhibitor. Bivariate analyses suggested that non-white race/ethnicity had a higher prevalence of inhibitor formation. However, on multivariate analysis, only black race remained associated. This is in contrast to hemophilia A, in which this same UDC database revealed that both black race and Hispanic ethnicity were related to higher rates of inhibitor formation [13]. However, given the small sample size, weak associations cannot be excluded.
Age was the third factor in this study to show an association with inhibitor status, with age less than 11 showing an odds ratio of 2.5 (95% CI 1.5 - 4.0) for prevalent inhibitor. At first glance, this is not a surprising result. Since the majority of inhibitors occur within the first 50 exposure days, one might expect a higher ratio of inhibitors in younger subjects. However, even if the incidence of new inhibitor formation is lower in older subjects compared to younger subjects, it is not clear why the population prevalence of inhibitors reported at annual UDC visits might decrease with advancing age. With hemophilia A, one would expect a fall off in inhibitor prevalence with age as 75% of hemophilia A subjects with inhibitors are successfully immune tolerized. The success rate for immune tolerance induction in hemophilia B subjects is thought to be much lower [2, 3]. We could not determine the success rate for ITI in the UDC population because almost one-third of the subjects had ongoing ITI therapy at the time of study closure. Alternatively, subjects who develop an inhibitor and who subsequently are not exposed to factor IX (for instance, subjects whose bleeding episodes are managed exclusively with bypassing agents) may experience a diminution in the titer of the factor IX inhibitor to undetectable levels with time. Although such an individual would see a rise in inhibitor titer should he be exposed to factor IX in the future, he would not have a prevalent inhibitor that would be recorded at the time of the UDC visit. Spontaneous loss of high-responder factor VIII inhibitor responsiveness has been described in HIV infected individuals with hemophilia A [10, 14]. To our knowledge spontaneous loss of high responder factor IX inhibitors has not been described in immunodeficient or immunocompetent individuals. Another potential explanation is mortality rate. If the mortality rate for inhibitor patients was higher than non-inhibitor subjects, then one would expect a drop off in prevalent inhibitors with age. In this study, the mortality rate of inhibitor subjects was not higher than that of non-inhibitor subjects.
Finally, the type(s) of factor to which subjects were exposed could explain the difference in prevalent inhibitors between the older population and younger population. Recombinant factor IX was introduced to the U.S market over 15 years ago, which may have led to a higher exposure to recombinant factor IX at initial exposure in younger subjects as compared to older. Several studies of inhibitors arising in hemophilia A suggest a higher risk of inhibitor formation in subjects exposed to recombinant factor VIII versus plasma derived factor VIII, though definitive studies are lacking [1, 15, 16] We attempted to determine if the type of coagulation factor concentrate used was associated with inhibitor formation in this study, but could not because we could not adequately determine the type of factor IX used prior to inhibitor formation. A recent study looked at anaphylactic reactions and inhibitor formation in hemophilia B subjects exposed to recombinant factor IX alone, and both recombinant and plasma derived factor IX, and found no difference in the rate of inhibitor and anaphylactic reactions between the two groups [17]. However, the sample sizes in these groups were small – 7 patients total.
This study has several strengths compared to previous studies of inhibitors in hemophilia B. The sample size in this study was the largest population ever studied for hemophilia B and inhibitors. In addition, the population in this study was racially and ethnically diverse, which reduced the likelihood of a founder effect. To limit the possibility of ascertainment bias, we contacted care providers to obtain additional information for potential inhibitor cases identified by screening the UDC data on reported titers and use of bypassing agents and ITI. We were able to obtain information for two-thirds of such cases to validate the presence of an inhibitor. Most of the patients with reported titers below 1 BU ml−1 did not have an inhibitor. Therefore, for the remaining one-third of potential cases for whom we had no confirmatory data, we adjusted our algorithm by raising the lower inhibitor titer limit from ≥ 0.5 to > 1.0 BU ml−1 to decrease the chance of incorrectly classifying patients with these lower titer values as having an inhibitor. We believe these efforts improved the validity of our findings.
There were several limitations that should be considered when interpreting the results of this study. First, as part of the data validation effort we identified a number of apparent discrepancies in the presence or absence of an inhibitor as defined by the study on the initial query of the UDC database, and that reported in response to our queries of the PI. Fortunately, the majority of these discrepancies were the result of our initial assumption that reported titers in the UDC database ≥ 0.5 BU ml−1 represented an inhibitor. In fact, values reported in the range of 0.5-1.0 BU ml−1 were substantially influenced by variations in the limits of detection of low titer inhibitors in different laboratories, and, in some cases due to rounding an actual inhibitor titer of < 0.5 BU ml−1 up to 1 rather than down to 0 on the UDC data form. Our validation efforts did not extend into the confirmation of the accuracy of submitted data on subjects identified on initial query as never having an inhibitor titer ≥ 0.5 BU ml−1. This was due to the size of the sample in this study, and the difficulty in getting PI to respond to our email inquiries. To the extent that subjects who actually had an inhibitor but never had an inhibitor titer ≥ 0.5 BU ml−1 in the UDC database during their participation in the UDC, our study would underestimate the prevalence of inhibitors in this population.
Another limitation was the use of a database designed for disease surveillance to determine prevalence and risk. The lack of information on subjects prior to entry in to the UDC hampered efforts to determine the true prevalence of inhibitors, and the potential role of previous factor exposure in inhibitor risk. The small number (16) of subjects who developed inhibitors after enrollment in the UDC was too small to determine if type of factor used or use of prophylaxis prior to inhibitor development was associated with prevalent inhibitors.
We believe this study provides valuable information on a rare complication of a rare disease, and serves as a starting point for future studies aimed at determining risk factors for inhibitors in hemophilia B patients.
Acknowledgements
The authors would like to acknowledge the assistance of the Universal Data Collection Working Group and the Centers for Disease Control for their help in designing and completing this study, and for the use of the UDC data. The collection and analysis of the data were supported by the Grant/Cooperative Agreement “Prevention of Bleeding Disorder Complications through Regional Hemophilia Treatment Centers.” We also thank the patients, staff members and physicians of the Hemophilia Treatment Centers who contributed to the UDC database, and especially those who responded to our queries regarding the data.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosures
The authors have no relevant conflicts of interest to report.
Contributor Information
John Puetz, Saint Louis University Department of Pediatrics Division of Hematology/Oncology 1465 S. Grand St. Louis MO 63104.
J. Michael Soucie, National Center for Birth Defects and Developmental Disabilities Division of Blood Disorders 1600 Clifton Road, MS E64 Atlanta, GA 30333.
Christine L. Kempton, Department of Pediatrics and Department of Hematology/Oncology Emory University 2015 Uppergate Drive Atlanta, Ga 30322.
Paul E. Monahan, University of North Carolina at Chapel Hill CB #7236, 1185 1st Floor Physicians Office Building 170 Manning Drive Chapel Hill, NC 27599-7236.
Hemophilia Treatment Center Network (HTCN) Investigators, National Center for Birth Defects and Developmental Disabilities Division of Blood Disorders 1600 Clifton Road, MS E64 Atlanta, GA 30333.
Reference
- 1.ter Avest PC, Fischer K, Mancuso ME, Santagostino E, Yuste VJ, van den Berg HM, van der Bom JG. Risk stratification for inhibitor development at first treatment for severe hemophilia A: a tool for clinical practice. J Thromb Haemost. 2008;6(12):2048–2054. doi: 10.1111/j.1538-7836.2008.03187.x. [DOI] [PubMed] [Google Scholar]
- 2.Chitlur M, Warrier I, Rajpurkar M, Lusher JM. Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997-2006) Haemophilia. 2009;15(5):1027–1031. doi: 10.1111/j.1365-2516.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 3.DiMichele DM, Kroner BL. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87(1):52–57. [PubMed] [Google Scholar]
- 4.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138(3):305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 5.Briet E, Reisner HM, Roberts HR. Inhibitors in Christmas disease. Prog Clin Biol Res. 1984;150:123–139. [PubMed] [Google Scholar]
- 6.Roth DA, Kessler CM, Pasi KJ, Rup B, Courter SG, Tubridy KL. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98(13):3600–3606. doi: 10.1182/blood.v98.13.3600. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro AD, Di Paola J, Cohen A, Pasi KJ, Heisel MA, Blanchette VS, Abshire TC, Hoots WK, Lusher JM, Negrier C, et al. The safety and efficacy of recombinant human blood coagulation factor IX in previously untreated patients with severe or moderately severe hemophilia B. Blood. 2005;105(2):518–525. doi: 10.1182/blood-2004-06-2283. [DOI] [PubMed] [Google Scholar]
- 8.Katz J. Prevalence of factor IX inhibitors among patients with haemophilia B: results of a large scale North American survey. Haemophilia. 1996;2:28–31. doi: 10.1111/j.1365-2516.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 9.Soucie JM, McAlister S, McClellan A, Oakley M, Su Y. The universal data collection surveillance system for rare bleeding disorders. Am J Prev Med. 2010;38(4 Suppl):S475–481. doi: 10.1016/j.amepre.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 10.White GC, 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85(3):560. [PubMed] [Google Scholar]
- 11.Belvini D, Salviato R, Radossi P, Pierobon F, Mori P, Castaldo G, Tagariello G. Molecular genotyping of the Italian cohort of patients with hemophilia B. Haematologica. 2005;90(5):635–642. [PubMed] [Google Scholar]
- 12.Astermark J. Why do inhibitors develop? Principles of and factors influencing the risk for inhibitor development in haemophilia. Haemophilia. 2006;12(Suppl 3):52–60. doi: 10.1111/j.1365-2516.2006.01261.x. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter SL, Michael Soucie J, Sterner S, Presley R. Increased prevalence of inhibitors in Hispanic patients with severe haemophilia A enrolled in the Universal Data Collection database. Haemophilia. 2012;18:e260–265. doi: 10.1111/j.1365-2516.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 14.Bray GL, Kroner BL, Arkin S, Aledort LW, Hilgartner MW, Eyster ME, Ragni MV, Goedert JJ. Loss of high-responder inhibitors in patients with severe hemophilia A and human immunodeficiency virus type 1 infection: a report from the Multi-Center Hemophilia Cohort Study. Am J Hematol. 1993;42(4):375–379. doi: 10.1002/ajh.2830420408. [DOI] [PubMed] [Google Scholar]
- 15.Goudemand J, Rothschild C, Demiguel V, Vinciguerrat C, Lambert T, Chambost H, Borel-Derlon A, Claeyssens S, Laurian Y, Calvez T. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood. 2006;107(1):46–51. doi: 10.1182/blood-2005-04-1371. [DOI] [PubMed] [Google Scholar]
- 16.Mannucci PM, Gringeri A, Peyvandi F, Santagostino E. Factor VIII products and inhibitor development: the SIPPET study (survey of inhibitors in plasma-product exposed toddlers) Haemophilia. 2007;13(Suppl 5):65–68. doi: 10.1111/j.1365-2516.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- 17.Recht M, Pollmann H, Tagliaferri A, Musso R, Janco R, Neuman WR. A retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia B. Haemophilia. 2011;17(3):494–499. doi: 10.1111/j.1365-2516.2011.02436.x. [DOI] [PubMed] [Google Scholar]