Abstract
Protection of beta cells from autoimmune destruction potentially cures type 1 diabetes mellitus (T1D). During antigen presentation, interactions between cytotoxic T-lymphocyte antigen-4 (CTLA4) and B7 molecules, or programmed death 1 (PD1) and its ligand PDL1, negatively regulate immune responses in a non-redundant manner. Here, we employed beta cell-targeted adeno-associated virus serotype 8 (AAV8)-based vectors to over-express an artificial PDL1-CTLA4Ig polyprotein or IL10. Beta cell-targeted expression of PDL1-CTLA4Ig or IL10 preserved beta cell mass and protected NOD mice from T1D development. When NOD mice were treated with vectors at early onset of hyperglycemia, PDL1-CTLA4Ig or IL10 alone failed to normalize the early onset of hyperglycemia. When drug-induced diabetic mice received MHC-matched allo-islets, with or without pretreatment of the PDL1-CTLA4Ig-expressing vector, PDL1-CTLA4Ig-expressing islets were protected from rejection for at least 120 days. Similarly, transplantation of PDL1-CTLA4Ig-expressing MHC-matched islets into mice with established T1D resulted in protection of allo-islets from acute rejection, although islet grafts were eventually rejected. Thus, the present study demonstrates the potent immuno-suppressive effects of beta cell-targeted PDL1-CTLA4Ig overexpression against T1D development and allo-islet rejection. The gene-based simultaneous inhibition of PD1 and CTLA4 pathways provides a unique strategy for immunosuppression-free tissue/organ transplantation, especially in the setting of no established autoimmunity.
Keywords: AAV vector, type 1 diabetes, islet transplant, β-cell regeneration, immune suppression
INTRODUCTION
Type 1 Diabetes Mellitus (T1D) is caused by autoimmune destruction of the insulin producing β-cells. The susceptibility to develop T1D has been linked to the HLA genotypes, DR and DQ, and to a lesser degree to other genes, including CTLA-4, PTPN22 and insulin-VNTR1. β-cell-targeted autoimmune responses involve an expansion of auto-reactive CD4+ and CD8+ T cells, autoantibody-producing B cells and activation of the innate immune system2, 3. Continuing β-cell destruction results in progressive β-cell mass decline, ultimately leading to absolute insulin deficiency requiring life-long insulin therapy4. Additionally, fluctuating blood glucose levels often result in diabetes-associated complications, such as retinopathy, neuropathy and cardiorenal diseases4.
Immunotherapy arresting the β-cell mass decline at early onset provides an opportunity for immune intervention to replenish β-cell mass. Indeed, earlier studies have demonstrated high doses of non-specific immune suppression, by anti-CD3 antibody, preserved C-peptide production in new onset T1D5, 6. Unfortunately, recent phase III immunotherapy trials with CD3 antibody have failed to meet therapeutic end points7, 8.
Transplantation of pancreatic islets has emerged as another promising therapy for T1D, offering a potential cure. For instance, the Edmonton protocol, first described by Shapiro and colleagues, has achieved long-term islet survival; 20% of islet recipients remained insulin-therapy-free at five years after transplantation9, 10. Over 50% of subjects showed favorable glycemic control, demonstrating the advantage of tight glucose regulation over the regular insulin replacement therapies11, 12. Recent studies have also shown improvements in primary efficacy, safety outcomes, and insulin independence 3 years post-transplant11, 12. Despite such significant advances13, challenges have precluded the widespread use of islet transplantation. One major limitation is the requirement of life-long immunosuppression. In the glucocorticoid-free Edmonton protocol, daclizumab is transiently used immediately after transplantation, while sirolimus (rapamycin) and tacrolimus (FK506) are given for life. However, these agents cause side effects, such as anemia, hyperlipidemia and renal toxicity14, 15. Additionally, commonly used immunosuppressants, calcineurin inhibitors (FK506 and cyclosporine) and rapamycin, have been linked to impaired islet function and insulin action16, 17, which may play a role in the difficulty of achieving long-term islet survival in transplant recipients18.
During the T-cell receptor complex activation by an antigen presented on MHC molecules, costimulation via interaction between B7 molecules on antigen presenting cells and the coreceptor CD28 on T cells is required for optimal T-cell activation19. Cytotoxic T-lymphocyte antigen-4 (CTLA4) on T cells binds to B7 molecules on the antigen-presenting cells (APCs) and attenuates the immune response. Similarly, interaction between the programmed cell death 1 (PD1) receptor on T cells and its ligand PDL1 on the APCs leads to negative regulation of immune responses20. Notably, CTLA4 and PD1 inhibitory pathways are non-redundant21, and PD1 and CTLA4 combination blockade has demonstrated synergistic effects on immune activation in peripheral immune tolerance22. Importantly, in a T1D immunotherapy trial, inhibition of the CTLA4 pathway by abatacept (CTLA4-Ig) delayed, but not blocked, the decline of β-cell function in recent-onset T1D23.
In this study, we employ a non-pathogenic, highly β-cell-tropic adeno-associated virus serotype 8 (AAV8)-based vectors for β-cell-targeted overexpression of PDL1, CTLA4Ig, and IL10 transgenes. We assessed the effects of β-cell-targeted immunomodulation on T1D development and islet rejection in spontaneous autoimmune diabetes and matched islet transplantation models.
RESULTS and DISCUSSION
Generation of a β-cell-targeted AAV vector expressing an artificial PDL1-CTLA4Ig polyprotein
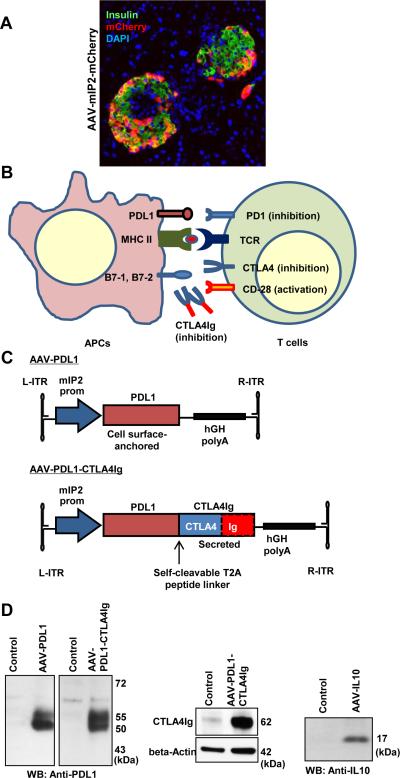
Previously, we and others have demonstrated efficient β-cell-targeted gene delivery by AAV8 and AAV9 vector with rat insulin promoter sequences24, 25. We and others also found that AAV8 vector with a mouse insulin 2 gene (mIP2) promoter show robust, and β-cell-specific, transgene expression26. We therefore generated AAV8 vector expressing mCherry gene, under the control of mIP2 promoter. As shown in Fig. 1A, intraperitoneal injection of the AAV8-mIP2-mCherry vector resulted in β-cell-targeted mCherry expression.
Figure 1. Generation of a β-cell-targeted AAV vector expressing an artificial PDL1-CTLA4Ig.
A. Pancreas sections were observed for mCherry expression. β-cell-specific mCherry expression (red) was observed in insulin-positive β-cells (green), but not in acinar cells. Nuclei were counterstained by DAPI (blue). B. Schematic representation of immuno modulatory molecules, including CTLA4Ig protein. C. Schematic representation of AAV vectors. D. Western blotting analysis verified expression of PDL1, CTLA4Ig and IL10 by specific antibodies.
During antigen presentation, interactions between CTLA4 and B7 molecules or PD1 and PDL1 negatively regulate immune responses in a non-redundant manner (Fig. 1B). We designed and synthesized an artificial, codon-optimized, PDL1-CTLA4Ig gene encoding both full-length PDL1 and CTLA4Ig proteins, conjugated with the T2A self-cleaving peptide linker. We cloned the PDL1-CTLA4Ig sequence, or the PDL1 ORF alone into pAAV-mIP2 vector (Fig. 1C). Western blotting analysis with a specific anti-PDL1 antibody verified PDL1 expression, including unmodified 50 kDa and glycosylated 55 kDa proteins, from the Ins1 β-cell line, upon transfection with the two constructs (Fig. 1D). CTLA4Ig expression was also verified by anti-CTLA4 antibody. We also generated an AAV vector expressing IL10 (AAV-IL10), which expressed the approximately 17 kDa IL10 protein upon transfection into Ins1 cells.
β-cell-targeted PDL1-CTLA4Ig or IL10 expression blocked development of autoimmune diabetes in NOD mice
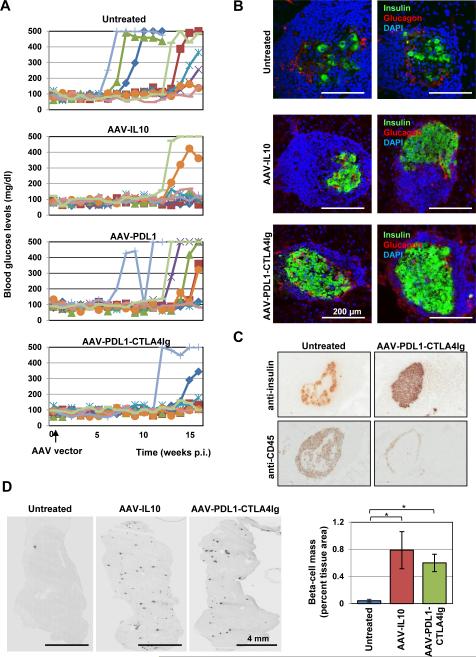
We injected 8 weeks-old NOD mice with the β-cell-targeted AAV8 vectors expressing PDL1 alone, PDL1-CTLA4Ig, or IL10. Fasting blood glucose levels were monitored for 16 weeks post injection (P.I.). Mice with no treatment showed high incidences of hyperglycemia (7 out of 9 mice) (Fig. 2A). Hyperglycemia onset was sudden and progressed rapidly, leading to marked weight loss, increased urine output, hunched back, and rough coating. AAV8-PDL1 vector-treated mice showed delayed hyperglycemic development (Fig. 2A), 7 out of 9 animals developed hyperglycemia by study termination. In contrast, the groups which received AAV8-PDL1-CTLA4Ig or AAV8-IL10 vectors showed significant protection from the development of autoimmune-mediated diabetes; in both groups, only 2 out of 9 mice showed hyperglycemia (Fig. 2A). At study termination, we sacrificed the mice and examined for pathological changes. Diabetic mice commonly showed a pale pancreas with minimal fat tissue. No notable changes were found in normoglycemic mice treated by AAV8-PDL1 or AAV8-PDL1-CTLA4Ig. However, all AAV8-IL10-treated mice showed splenomegaly, as described previously in IL10-treated mice27. Immunohistochemistry (IHC) was then performed to assess the levels of autoimmune destruction of β-cells in control and treated mice. Pancreatic tissue sections at 16 weeks p.i. were stained for insulin and glucagon by specific antibodies. Untreated control mice showed notable destructive infiltration involving most of the islet area and excessive loss of β-cells (Fig. 2B and 2C). Pancreatic IL10 over-expression showed similar peri-islet infiltration but with preserved β-cell mass (Fig. 2B, 2C and 2D). AAV8-PDL1-CTLA4Ig treated animals showed no or minimal peri-islet immune infiltration with well-preserved β-cell mass (Fig. 2B, 2C and 2D). These data demonstrated that β-cell-targeted PDL1-CTLA4Ig over-expression can protect NOD mice from autoimmune diabetes through preserved β-cell mass.
Figure 2. β-cell-targeted PDL1-CTLA4Ig or IL10 expression blocked development of autoimmune diabetes in NOD mice.
A. Female NOD mice were treated with indicated AAV vectors and fasting blood glucose levels were monitored for 16 weeks. Fisher's exact test analysis indicated AAV-IL10 and AAV-PDL1-CTLA4Ig treatments reduced the incidence of spontaneous diabetes (p<0.05). B. Representative pancreatic islets of AAV8 vector-treated or control mice stained with anti-insulin (green) and anti-glucagon (red) antibodies. Nuclei were counterstained with DAPI. C. Islets with infiltrating cells in untreated and AAV-PDL1-CTLA4Ig-treated mice were stained for insulin and CD45 by specific antibodies. D. Insulin-positive beta-cells in whole pancreatic sections were visualized by anti-insulin antibody (representative images from control, AAV-IL10 and AAV-PDL1-CTLA4Ig-treated mice), and percent beta cell areas were compared between the treatments (n=3 each). *p<0.05, Student's t-test.
β-cell-targeted PDL1-CTLA4Ig and IL10 expression reduced blood glucose levels in early onset diabetic NOD mice
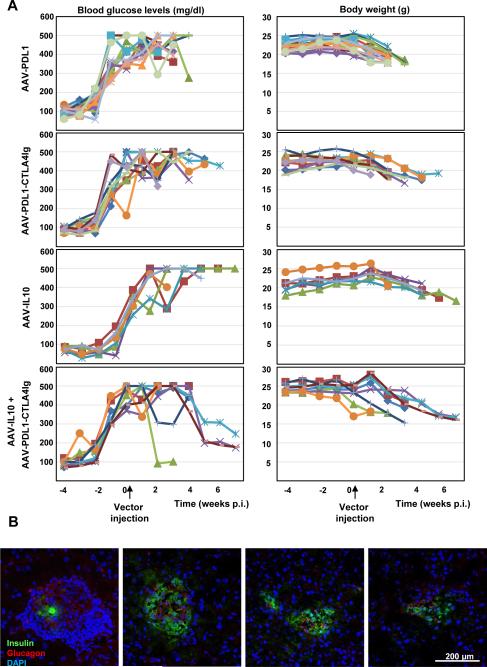
Next, we assessed whether β-cell-targeted PDL1, PDL1-CTLA4Ig, or IL10 expression could restore normoglycemia at early onset of autoimmune diabetes in NOD mice. Early onset diabetic NOD mice, with over 200 mg/dl blood glucose levels for two consecutive weeks, received intraperitoneal injection of AAV8 vectors at 2 × 1013 gc/kg. All control, AAV8-IL10-, AAV8-PDL1-, or AAV8-PDL1-CTLA4Ig-treated mice developed full-blown diabetes (Fig. 3A, left panels, not shown for an untreated group), and were euthanized when they lost more than 20% of their initial body weight (Fig. 3A, right panels). Intriguingly, 4 of 6 mice treated with both AAV8-IL10 and AAV8-PDL1-CTLA4Ig vectors exhibited notable reductions in blood glucose levels (Fig. 3A, bottom left panel; p<0.05 vs. other groups). Those mice appeared healthier than untreated mice, with smooth coats, no hunching and normal/increased appetites. Nevertheless, they lost more than 20% of their initial body weight over the course of the study (Fig. 3A, bottom right panel), necessitating euthanasia. Subsequent IHC analysis of pancreatic tissues of mice with reduced blood glucose levels revealed typical immune cell-infiltrated islets with minimal insulin-positive cells (Fig. 3B, left panel), as well as small immunoinfiltration-free islets (Fig. 3B), rich in α-cells and substantial β-cell mass (Fig. 3B).
Figure 3. β-cell-targeted PDL1-CTLA4Ig and IL10 expression reduced blood glucose levels in early onset diabetic NOD mice.
A. Fasting blood glucose levels in female NOD mice were monitored to detect early onset hyperglycemia. Mice were then treated with indicated AAV vectors and were further monitored for the blood glucose levels and body weights. B. Representative pancreatic islets of the mice showing reduced blood glucose levels were shown. Islets were stained with anti-insulin (green) and anti-glucagon (red) antibodies. Nuclei were counterstained with DAPI.
The effects of β-cell-targeted PDL1-CTLA4Ig expression on rejection of allo-islets grafts in MHC-matched recipient mice in the absence of autoimmunity against β-cells
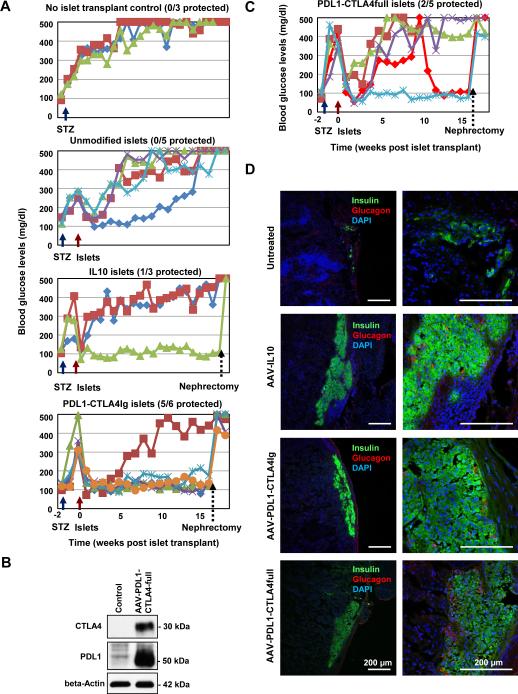
The failure in normalizing blood glucose levels by PDL1-CTLA4Ig in the early onset diabetes in NOD mice can be either due to a lack of sufficient immunosuppression or insufficient remaining β-cells to achieve glucose level homeostasis. To assess the effects of PDL1-CTLA4Ig-mediated β-cell protection, with sufficient numbers of healthy islets, we used the MHC-matched islet transplant models. First, to assess the effects of PDL1-CTLA4Ig in the absence of autoimmunity, we used the allo-islet transplant model with drug-induced diabetes. Specifically, we induced diabetes in DBA2 mice by low dose, multiple intraperitoneal injections of streptozotocin (STZ). We also treated Balb/c mice with the AAV vectors expressing PDL1, PDL1-CTLA4Ig or IL10. Two weeks post AAV vector administration, we harvested pancreatic islets from Balb/c mice, which were then transplanted into the kidney capsules of the diabetic DBA2 mice. Fasting blood glucose levels were monitored for 16 weeks. As predicted, animals without grafts continued to show a progressive course of hyperglycemia due to STZ-mediated β-cell damage (Fig. 4A, top panel). In contrast, untreated Balb/c islet cell grafts were able to ameliorate the hyperglycemic phenotype of DBA2 mice (Fig. 4A). However, this effect was transient, and DBA2 mice started to show an increase in their blood glucose levels 10 to 14 days post transplantation. At 16 weeks post transplantation, all mice transplanted with unmodified allo-islets became hyperglycemic (mean fasting blood glucose level >500 mg/dl, n=5). PDL1-CTLA4Ig expressing islet cell grafts were able to completely reverse the diabetes in STZ treated DBA mice and protected this phenotype for 16 weeks post transplantation (PT) in 5 out of 6 mice (p<0.05). Of note, unilateral nephrectomies of the kidney capsule grafts at 16 weeks post transplantation, rendered normoglycemic graft recipient mice hyperglycemic immediately post nephrectomy (Fig. 4A, bottom panel), verifying that normalized glucose levels were due to the islet grafts, rather than spontaneous recovery of endogenous pancreatic β-cells. Although not statistically significant, one of three diabetic DBA mice, treated with IL10 expressing allo-islets, also showed reversal of hyperglycemia for 16 weeks (Fig. 4A).
Figure 4. The effects of β-cell-targeted PDL1-CTLA4Ig expression on rejection of allo-islets grafts in MHC-matched recipient mice in the absence of autoimmunity against β-cells.
A. STZ-treated DBA2 mice, transplanted with genetically modified Balb/c mouse islets, were monitored for fasting blood glucose levels. At 16 weeks after islet transplantation, unilateral nephrectomy was performed to remove the islet grafts to see the recurrence of hyperglycemia. B. Western blotting analysis was performed to verify the expression of PDL1 and CTLA4 using beta cell line (Min6 cells) transfected with pAAV-PDL1-CTLA4-full plasmid. C. Same as A, except for the islets were modified to express the PDL1-CTLA4full protein. D. Representative images of recovered islet grafts in the renal capsules were shown. Islets were stained with anti-insulin (green) and anti-glucagon (red) antibodies.
β-cell-targeted CTLA4Ig expression can lead to secretion of CTLA4Ig, with the potential to induce systemic immune suppression. We postulated that beta-cell-targeted expression of the cell surface-anchored, full-length CTLA4 could block the trafficking of B7 molecules in the ER of the islet cells, leading to their down regulation and protection of beta-cells. We therefore tested the utility of the full-length CTLA4, instead of CTLA4Ig, along with PDL1 (designated as AAV8-PDL1-CTLA4full). Expression of CTLA4 and PDL1 was verified by Western blot (Fig. 4B). Pre-treatment of Balb/c allo-islets with the AAV vector expressing the PDL1-CTLA4full polyprotein showed complete reversal of hyperglycemia in 1 out of 5 diabetic DBA2 mice (Fig. 4C, no significance). Intriguingly, one recipient mouse, which initially showed failed normalization of blood glucose levels, became normoglycemic 10 weeks post transplantation. Again, unilateral nephrectomies of the two normoglycemic mice 16 weeks post transplantation abrogated the therapy.
We then performed IHC analysis of the islet cell grafts at 16 weeks post transplantation. Control, untreated Balb/c islets were almost completely destroyed by inflammatory cells in DBA2 mice, with detection of a paucity of insulin-positive cells (Fig. 4D, top panels). In contrast, the DBA2 mice with reversed diabetes upon transplantation of PDL1-CTLA4Ig- or PDL1-CLTA4full-treated islets, showed significant preservation of β-cells and no infiltrative inflammatory cells (Fig. 4D). IL10-treated grafts showed a minimal degree of destructive inflammatory infiltration with significant preservation of insulin positive β-cells (Fig, 4D).
β-cell-targeted PDL1-CTLA4Ig expression transiently protects islet cell allografts from hyperacute rejection in the setting of autoimmune diabetes
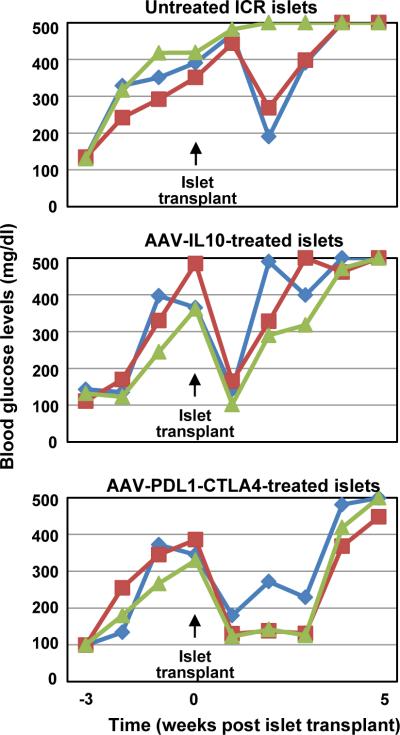
We repeated the previous experiment using ICR/HaJ mice as donors for islet cell grafts and autoimmune diabetic NOD mice as recipients. ICR/HaJ strain carries the same H2g7 MHC haplotype as NOD mice. ICR/HaJ mice do not develop insulitis or diabetes, thus allowing MHC-matched islet transplantation for NOD mice. When fasting blood glucose levels were monitored; control, untreated islet cell grafts exhibited transient reductions in blood glucose levels in 2 out of 3 diabetic mice (Fig. 5, top panel). Similarly, IL10-treated islets normalized hyperglycemia, but only for one week. On the other hand, PDL1-CTLA4Ig expressing islet cell grafts successfully reversed diabetes in NOD mice for 3 weeks (n=3).
Figure 5. β-cell-targeted PDL1-CTLA4Ig expression transiently protects islet cell allografts from hyperacute rejection in the setting of autoimmune diabetes.
Autoimmune diabetic NOD mice received genetically modified ICR/HaJ mouse islets, and were monitored for fasting blood glucose levels.
Pre-T1D is characterized by ongoing islet β-cell destruction in which sufficient β-cell mass and functionality remain to preserve glucose homeostasis. Silencing the diabetogenic attack at this stage could prevent full-blown diabetes. Recently, co-stimulation modulation with CTLA4Ig (abatacept) has demonstrated a slowing decline of β-cell function in recent-onset T1D. Notably, the decrease in β-cell function with abatacept parallels the placebo after 6 months, despite continued administration of abatacept over 2 years23. Here, we showed that combination blockade of two non-redundant immune inhibitory pathways, CTLA4 and PD1, in pre-diabetic NOD mice prevented T1D development through preserved β-cell mass. As reported previously28, β-cell-targeted IL10 over-expression also blocked T1D progression; however, treated mice showed notable splenomegaly, which was also reported in a previous study using IL10 gene therapy27. Similarly, in an allo-islet transplantation model, pretreatment of Balb/c islets to express PDL1-CTLA4Ig, and a lesser degree with IL10 or PDL1-CTLA4full expression, is sufficient to protect acute rejection of allo-islet grafts in STZ-induced diabetic DBA2 mice, leading to long-term allo-graft survival in the absence of systemic immunosuppressive regimen. Our results therefore demonstrate the potency of immunosuppressive effects by simultaneous blockade of CTLA4 and PD1 pathways, a plausible therapeutic intervention for pre-T1D.
In sharp contrast to the successful β-cell protection in the settings with no pre-existing autoimmunity to β-cells, β-cell-targeted over-expression of PDL1-CTLA4Ig or IL10 genes in the early onset diabetes failed to reverse hyperglycemia in NOD mice. Moreover, although PDL1-CTLA4Ig expression facilitated extended allo-islet grafts survival in autoimmune diabetic mice, the protective effect was transient and the mice relapsed in hyperglycemia 4 weeks after islet transplantation. Those observations indicate that the simultaneous suppression of the PD1 and CTLA4 axes can block the development of autoimmunity, or adaptive immunity in the case of islet transplantation, to β-cells, but is ultimately insufficient for reversing fully activated β-cell-targeted immunity. It is possible that additional therapies, such as low dose anti-CD3 treatment and transient exogenous insulin therapy, may be required at this stage of disease. These auxiliary therapies would support the immunosuppressive effects of PDL1-CTLA4Ig while reducing the stress of β-cells due to prolonged hyperglycemia. Nevertheless, our data highlights the challenges in controlling established autoimmunity through β-cell-targeted immunomodulatory gene therapy. In this context, it is notable that treatment with both PDL1-CTLA4Ig- and IL10-expressing AAV vectors led to reduced hyperglycemia in diabetic NOD mice. Unexpectedly, however, this combination therapy failed to control weight loss even though treated mice showed overall improvement, including a healthy appetite, smooth coat, and better preserved β-cell mass. Further studies are necessary to understand the underlying mechanism for weight loss. Since NOD mice also have the highly unique, autoimmunity-susceptible genetic background29, it is plausible that β-cell-targeted immunotherapy was not sufficient to control other spontaneous autoimmune diseases in NOD mice, such as Sjorgren's syndrome.
Initially, we speculated that the failure in normalizing blood glucose levels by the PDL1-CTLA4Ig gene therapy approach in the early onset diabetes might be due to insufficient remaining β-cells to achieve glucose level homeostasis. However, we observed comparable results with the genetically modified islet transplantation approach. Both AAV vectors expressing IL10 or PDL1-CTLA4Ig showed clear therapeutic effects in the setting of no pre-existing auto-immunity to beta-cells but not after the onset of autoimmune diabetes. Thus, immune modulatory therapies with PDL1-CTLA4Ig or IL10 are not sufficient to block established auto-immune diabetes, either in the gene therapy or in the gene and cell therapy settings. In addition, apart from splenomegaly in mice treated with IL10-expressing AAV vectors, we found no notable differences between AAV-PDL1-CTLA4Ig and AAV-IL10 groups. It is possible that marked intra-group variations in the timing of autoimmune diabetes onsets and severity of immune infiltration among individual NOD mice have masked the differences between the two treatments. Further studies with increased numbers of mice and analyses of beta-cell-infiltrating/recruited immune cells may provide better mechanistic insights into the therapeutic effects between IL10- and PDL1-CTLA4Ig-mediated therapies.
In conclusion, our data demonstrate the potent immunosuppressive effects of β-cell-targeted PDL1-CTLA4Ig overexpression against T1D development and allo-islet rejection. The gene-based simultaneous inhibition of PD1 and CTLA4 pathways would provide a unique strategy for immunosuppression-free tissue/organ transplantation, especially in a setting without established autoimmunity.
MATERIALS and METHODS
Mice
All studies were approved by Mayo Clinic Institutional Animal Care and Use Committee. Six-weeks old BALB/CJ, DBA/2J, ICR/HaJ and NOD/ShiLtJ mice were purchased from Jackson Laboratories. Mice were maintained under a 12-hours light-dark cycle and were provided with irradiated Rodent Laboratory Chow (Purina 5053). Fasting (4-6 hours) blood glucose levels were monitored weekly or bi-weekly by FreeStyle Lite Blood Glucose Monitor (Abbott Laboratories, Illinois). Mice received AAV8 vectors at a final dose of approximately 2 × 1013 genome copies (gc)/kg.
Cells
HEK293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were kept at 37°C with 5% CO2.
Plasmids
The codon-optimized, PDL1-CTLA4Ig or PDL1-CTLA4 fusion constructs were synthesized by GenScript (Piscataway, NJ). The PDL1 and CTLA4Ig (or CTLA4) open-reading frames are linked by the T2A self-cleaving peptide linker. The 1.13 kbp mouse insulin 2 promoter (MIP2) region was PCR amplified from mouse genomic DNA with primers Forward Mlu1, GCCACACGCGTCCCTCCTCTTGCATTTCAAAT and Reverse BamH1, TCCACAGGATCCTGTTGAAACAATAACCTGGAA. pAAV-MIP2-Luciferase vector was generated through cloning the MIP2 promoter sequence into the Mlu1-BamHI sites of pAAV-CMV-Luc vector, while pAAV-MIP2-eGFP vector was generated by replacing the luciferase gene in the pAAV-MIP2-Luciferase with the emerald GFP gene (Aurora). pAAV-MIP2-PDL1, pAAV-MIP2-PDL1-CTLA4Ig and pAAV-MIP2-PDL1-CTLA4 vectors were generated by replacing the emerald GFP transgene with PDL1, PDL1-CTLA4Ig and PDL1-CTLA4 by utilizing the unique restriction sites BamH1 and Xho1. pAAV-mRIP vector was previously described24. IL10 cDNA was cloned into this vector plasmid, resulting in pAAV-mRIP-IL10.
AAV8 vectors
The AAV8 vector stocks were produced in human 293T cells using the helper-free three-plasmid transfection method and titrated as described previously 24, with modification of the use of AAV8 capsid-expressing plasmid pRC-2/8 (kindly provided by Dr. James Wilson through National Gene Vector Biorepository).
Detection of protein expression
To verify the expression of the AAV8 delivered transgene(s), 293T cell lysates, transfected with either pAAV-mRIP-IL10 or pAAV-MIP2-PDL1-CTLA4Ig, were used for immunoblotting with anti-IL10 (Abcam, JES5-2A5) and anti-PDL1 (goat anti-mouse B7-H1, R&D Systems) antibodies.
Immunohistochemistry staining
Seven μm cryo-sections of OCT (Sakura)-embedded pancreatic tissues and left kidneys with grafts were immediately fixed in 4% paraformaldehyde, permeabilized with PBS containing 0.3% Triton-X for 10 min, blocked in PBS containing 5% FBS. Sections were incubated with Guinea Pig anti-insulin and mouse anti-glucagon antibodies (Dako), followed by confocal microscopy with either Zeiss LSM 510 or Zeiss LSM 780.
Streptozotocin (STZ) administrations
STZ was prepared in Hanks Balanced Salt Solution (Sigma). DBA2 mice received approximately 50 mg/kg STZ through intraperitoneal injection on five consecutive daily doses.
Islets isolation and culture
Two weeks after AAV8 vectors administration, mice were sacrificed and pancreatic islets were isolated through perfusion and digestion of the pancreas with collagenase V (Sigma) as described30. Untreated islets were also prepared from untreated age-matched mice.
Islets culture and transplantation
Isolated islets were cultured for 4-5 days to enhance healing in glucose adjusted (25 mM D-glucose) RPMI medium supplemented with 10% calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin, in non-sticking Petri dishes at 28°C with 5% CO2. High quality islets were manually picked and counted. Each animal received approximately 350 IE (Islet Equivalent) units. Islets were grafted in a pocket under the left kidney capsule as described by Zmuda and colleagues30. Fasting blood glucose levels were monitored weekly or bi-weekly. 120 days post-transplantation (PT), nephrectomies were performed to harvest the grafts. Blood glucose levels were monitored for 2 weeks after removal of grafts.
Sample size and statistical analysis
For beta-cell mass analysis, groups were compared by unpaired Student's t-test, and data were presented as means ± SEM. Significance was accepted for p<0.05. For the incidence of spontaneous auto-immune diabetes or reversal of hyperglycemia, data sets were analyzed by Fisher's exact test, and significance was accepted for p<0.05.
Acknowledgments
FUNDING: This work was supported by Mayo Foundation, Eisenberg Stem Cell Trust, Minnesota Partnership Grant (YI), Mayo Center for Regenerative Medicine (YI and TS), and National Institutes of Health (HL098502 to Y. Ikeda).
Footnotes
Authors have no competing interests to disclose.
REFERENCES
- 1.Daneman D. Type 1 diabetes. Lancet. 2006;367(9513):847–58. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatzigeorgiou A, Harokopos V, Mylona-Karagianni C, Tsouvalas E, Aidinis V, Kamper EF. The pattern of inflammatory/anti-inflammatory cytokines and chemokines in type 1 diabetic patients over time. Ann Med. 2010;42(6):426–38. doi: 10.3109/07853890.2010.495951. [DOI] [PubMed] [Google Scholar]
- 4.Holditch SJ, Terzic A, Ikeda Y. Concise review: pluripotent stem cell-based regenerative applications for failing beta-cell function. Stem Cells Transl Med. 2014;3(5):653–61. doi: 10.5966/sctm.2013-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. The New England journal of medicine. 2002;346(22):1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 6.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C- peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54(6):1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Jr., et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes. 2013;62(1):9–17. doi: 10.2337/db12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. The New England journal of medicine. 2006;355(13):1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 10.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 11.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes care. 2012;35(7):1436–45. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(11):2463–70. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. The New England journal of medicine. 2004;350(7):694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 14.Hirshberg B, Rother KI, Digon BJ, 3rd, Lee J, Gaglia JL, Hines K, et al. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes care. 2003;26(12):3288–95. doi: 10.2337/diacare.26.12.3288. [DOI] [PubMed] [Google Scholar]
- 15.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. The New England journal of medicine. 2003;349(10):931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 16.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. The Journal of clinical investigation. 2007;117(9):2553–61. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rostambeigi N, Lanza IR, Dzeja PP, Deeds MC, Irving BA, Reddi HV, et al. Unique cellular and mitochondrial defects mediate FK506-induced islet beta-cell dysfunction. Transplantation. 2011;91(6):615–23. doi: 10.1097/TP.0b013e3182094a33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potter KJ, Westwell-Roper CY, Klimek-Abercrombie AM, Warnock GL, Verchere CB. Death and dysfunction of transplanted beta-cells: lessons learned from type 2 diabetes? Diabetes. 2014;63(1):12–9. doi: 10.2337/db12-0364. [DOI] [PubMed] [Google Scholar]
- 19.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. The Journal of experimental medicine. 1991;173(3):721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10(11):1185–92. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes care. 2014;37(4):1069–75. doi: 10.2337/dc13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonne JM, Sakuma T, Deeds MC, Munoz-Gomez M, Barry MA, Kudva YC, et al. Global gene expression profiling of pancreatic islets in mice during streptozotocin-induced beta-cell damage and pancreatic Glp-1 gene therapy. Dis Model Mech. 2013;6(5):1236–45. doi: 10.1242/dmm.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(13):E1211–20. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonne JM, Sakuma T, Munoz-Gomez M, El Khatib M, Barry MA, Kudva YC, et al. Beta cell regeneration after single-round immunological destruction in a mouse model. Diabetologia. 2014 doi: 10.1007/s00125-014-3416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller C, Braag SA, Martino AT, Tang Q, Campbell-Thompson M, Flotte TR. The pros and cons of immunomodulatory IL-10 gene therapy with recombinant AAV in a Cftr−/−-dependent allergy mouse model. Gene therapy. 2009;16(2):172–83. doi: 10.1038/gt.2008.156. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Chen M, Wu R, Fialkow LB, Bromberg JS, McDuffie M, et al. Suppression of autoimmune diabetes by viral IL-10 gene transfer. Journal of immunology. 2002;168(12):6479–85. doi: 10.4049/jimmunol.168.12.6479. [DOI] [PubMed] [Google Scholar]
- 29.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 30.Zmuda EJ, Powell CA, Hai T. A method for murine islet isolation and subcapsular kidney transplantation. J Vis Exp. 2011;(50) doi: 10.3791/2096. [DOI] [PMC free article] [PubMed] [Google Scholar]