Abstract
APP695 is a transmembrane precursor of Abeta amyloid. In familial Alzheimer's disease (FAD), three mutations V642I/F/G were discovered in APP695, which has been suggested by multiple studies to be a cell surface signaling receptor. We previously reported that normal APP695 encodes a potential GO-linked receptor with ligand-regulated function and that expression of the three FAD mutants (FAD-APPs), not normal APP, induces cellular outputs by GO-dependent mechanisms. This suggests that FAD-APPs are constitutively active GO-linked receptors. Here, we provide direct evidence for this notion. Reconstitution of either recombinant FAD-APP with GO vesicles induced activation of GO, which was inhibitable by pertussis toxin, sensitive to Mg2+ and proportional in quantity to the reconstituted amounts of FAD-APP. Consistent with the dominant inheritance of this type of FAD, this function was dominant over normal APP, because little activation was observed in APP695-GO vesicles. Experiments with antibody competition and sequence deletion indicated that His657-Lys676 of FAD-APP, which has been specified as the ligand-dependent GO-coupling domain of normal APP, was responsible for this constitutive activation, confirming that the three FAD-APPs are mutationally activated APP695. This study identifies the intrinsic signaling function of APP to be a novel target of hereditary Alzheimer's disease mutations, providing an in vitro system for the screening of potential FAD inhibitors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allinquant B., Hantraye P., Mailleux P., Moya K., Bouillot C., Prochiantz A. Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J Cell Biol. 1995 Mar;128(5):919–927. doi: 10.1083/jcb.128.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond R. A., Leff P., Johnson T. D., Milano C. A., Rockman H. A., McMinn T. R., Apparsundaram S., Hyek M. F., Kenakin T. P., Allen L. F. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the beta 2-adrenoceptor. Nature. 1995 Mar 16;374(6519):272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- Breen K. C., Bruce M., Anderton B. H. Beta amyloid precursor protein mediates neuronal cell-cell and cell-surface adhesion. J Neurosci Res. 1991 Jan;28(1):90–100. doi: 10.1002/jnr.490280109. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991 Oct 31;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Doherty P., Ashton S. V., Moore S. E., Walsh F. S. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991 Oct 4;67(1):21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Caceres A., Kosik K. S. Intraneuronal compartments of the amyloid precursor protein. J Neurosci. 1993 Jul;13(7):3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore F., Zambrano N., Minopoli G., Donini V., Duilio A., Russo T. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer's amyloid precursor protein. J Biol Chem. 1995 Dec 29;270(52):30853–30856. doi: 10.1074/jbc.270.52.30853. [DOI] [PubMed] [Google Scholar]
- Frank D. A., Greenberg M. E. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994 Oct 7;79(1):5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. A pertussis toxin-sensitive G protein in hippocampal long-term potentiation. Science. 1989 May 26;244(4907):980–983. doi: 10.1126/science.2543072. [DOI] [PubMed] [Google Scholar]
- Gong X., Dubois D. H., Miller D. J., Shur B. D. Activation of a G protein complex by aggregation of beta-1,4-galactosyltransferase on the surface of sperm. Science. 1995 Sep 22;269(5231):1718–1721. doi: 10.1126/science.7569899. [DOI] [PubMed] [Google Scholar]
- Ikezu T., Okamoto T., Giambarella U., Yokota T., Nishimoto I. In vivo coupling of insulin-like growth factor II/mannose 6-phosphate receptor to heteromeric G proteins. Distinct roles of cytoplasmic domains and signal sequestration by the receptor. J Biol Chem. 1995 Dec 8;270(49):29224–29228. doi: 10.1074/jbc.270.49.29224. [DOI] [PubMed] [Google Scholar]
- Ikezu T., Okamoto T., Komatsuzaki K., Matsui T., Martyn J. A., Nishimoto I. Negative transactivation of cAMP response element by familial Alzheimer's mutants of APP. EMBO J. 1996 May 15;15(10):2468–2475. [PMC free article] [PubMed] [Google Scholar]
- Ito I., Okada D., Sugiyama H. Pertussis toxin suppresses long-term potentiation of hippocampal mossy fiber synapses. Neurosci Lett. 1988 Jul 19;90(1-2):181–185. doi: 10.1016/0304-3940(88)90808-7. [DOI] [PubMed] [Google Scholar]
- Jarriault S., Brou C., Logeat F., Schroeter E. H., Kopan R., Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995 Sep 28;377(6547):355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Jin L. W., Ninomiya H., Roch J. M., Schubert D., Masliah E., Otero D. A., Saitoh T. Peptides containing the RERMS sequence of amyloid beta/A4 protein precursor bind cell surface and promote neurite extension. J Neurosci. 1994 Sep;14(9):5461–5470. doi: 10.1523/JNEUROSCI.14-09-05461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Karlinsky H., Vaula G., Haines J. L., Ridgley J., Bergeron C., Mortilla M., Tupler R. G., Percy M. E., Robitaille Y., Noldy N. E. Molecular and prospective phenotypic characterization of a pedigree with familial Alzheimer's disease and a missense mutation in codon 717 of the beta-amyloid precursor protein gene. Neurology. 1992 Aug;42(8):1445–1453. doi: 10.1212/wnl.42.8.1445. [DOI] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986 Jun 25;261(18):8182–8191. [PubMed] [Google Scholar]
- Kjelsberg M. A., Cotecchia S., Ostrowski J., Caron M. G., Lefkowitz R. J. Constitutive activation of the alpha 1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region which constrains receptor activation. J Biol Chem. 1992 Jan 25;267(3):1430–1433. [PubMed] [Google Scholar]
- Knops J., Johnson-Wood K., Schenk D. B., Sinha S., Lieberburg I., McConlogue L. Isolation of baculovirus-derived secreted and full-length beta-amyloid precursor protein. J Biol Chem. 1991 Apr 15;266(11):7285–7290. [PubMed] [Google Scholar]
- Lang J., Nishimoto I., Okamoto T., Regazzi R., Kiraly C., Weller U., Wollheim C. B. Direct control of exocytosis by receptor-mediated activation of the heterotrimeric GTPases Gi and G(o) or by the expression of their active G alpha subunits. EMBO J. 1995 Aug 1;14(15):3635–3644. doi: 10.1002/j.1460-2075.1995.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc A. C., Kovacs D. M., Chen H. Y., Villaré F., Tykocinski M., Autilio-Gambetti L., Gambetti P. Role of amyloid precursor protein (APP): study with antisense transfection of human neuroblastoma cells. J Neurosci Res. 1992 Apr;31(4):635–645. doi: 10.1002/jnr.490310407. [DOI] [PubMed] [Google Scholar]
- Levitan D., Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995 Sep 28;377(6547):351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Mendel J. E., Korswagen H. C., Liu K. S., Hajdu-Cronin Y. M., Simon M. I., Plasterk R. H., Sternberg P. W. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science. 1995 Mar 17;267(5204):1652–1655. doi: 10.1126/science.7886455. [DOI] [PubMed] [Google Scholar]
- Milward E. A., Papadopoulos R., Fuller S. J., Moir R. D., Small D., Beyreuther K., Masters C. L. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992 Jul;9(1):129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Murayama Y., Okamoto T., Ogata E., Asano T., Iiri T., Katada T., Ui M., Grubb J. H., Sly W. S., Nishimoto I. Distinctive regulation of the functional linkage between the human cation-independent mannose 6-phosphate receptor and GTP-binding proteins by insulin-like growth factor II and mannose 6-phosphate. J Biol Chem. 1990 Oct 15;265(29):17456–17462. [PubMed] [Google Scholar]
- Murrell J., Farlow M., Ghetti B., Benson M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Naruse S., Igarashi S., Kobayashi H., Aoki K., Inuzuka T., Kaneko K., Shimizu T., Iihara K., Kojima T., Miyatake T. Mis-sense mutation Val----Ile in exon 17 of amyloid precursor protein gene in Japanese familial Alzheimer's disease. Lancet. 1991 Apr 20;337(8747):978–979. doi: 10.1016/0140-6736(91)91612-x. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Finch E. A., Dawes L. R. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988 Oct;1(8):669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
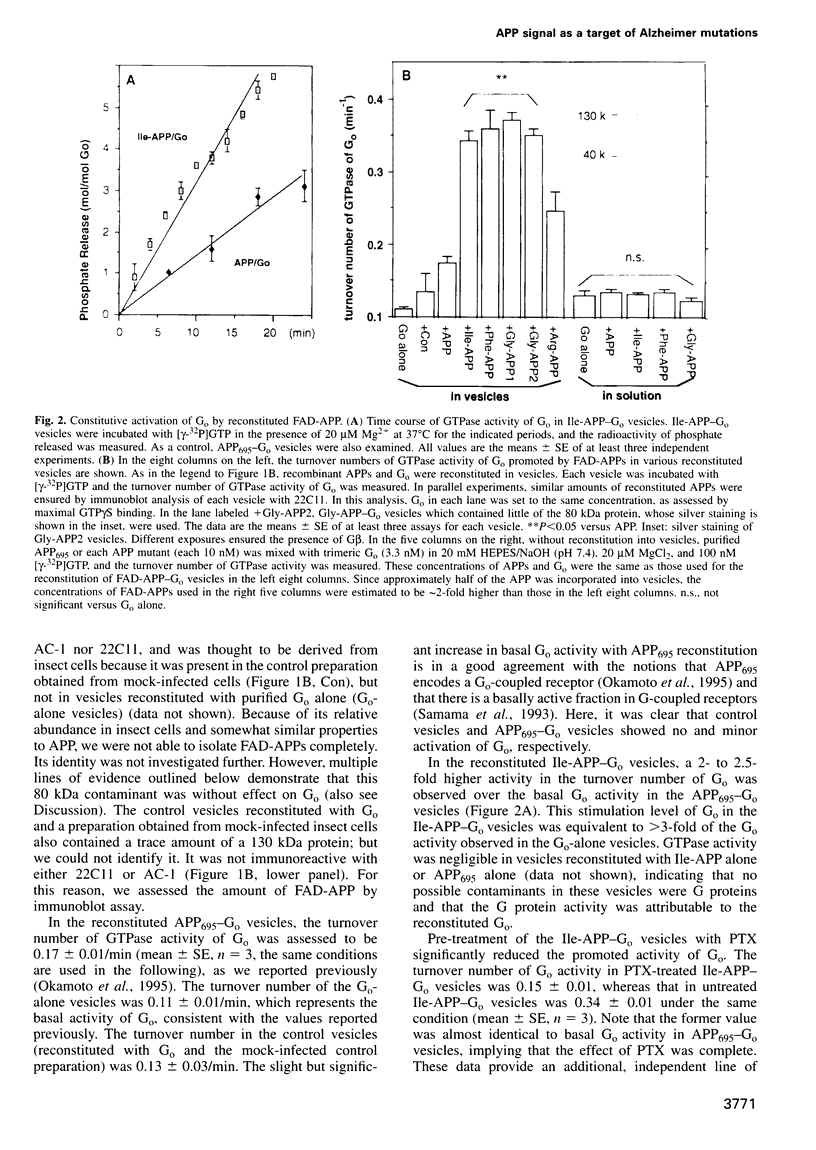
- Nishimoto I., Okamoto T., Matsuura Y., Takahashi S., Okamoto T., Murayama Y., Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G(o) Nature. 1993 Mar 4;362(6415):75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Katada T., Murayama Y., Ui M., Ogata E., Nishimoto I. A simple structure encodes G protein-activating function of the IGF-II/mannose 6-phosphate receptor. Cell. 1990 Aug 24;62(4):709–717. doi: 10.1016/0092-8674(90)90116-v. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Murayama Y., Strittmatter S. M., Katada T., Asano S., Ogata E., Nishimoto I. An intrinsic guanine nucleotide exchange inhibitor in Gi2 alpha. Significance of G-protein self-suppression which antagonizes receptor signal. J Biol Chem. 1994 May 13;269(19):13756–13759. [PubMed] [Google Scholar]
- Okamoto T., Nishimoto I. Detection of G protein-activator regions in M4 subtype muscarinic, cholinergic, and alpha 2-adrenergic receptors based upon characteristics in primary structure. J Biol Chem. 1992 Apr 25;267(12):8342–8346. [PubMed] [Google Scholar]
- Okamoto T., Takeda S., Murayama Y., Ogata E., Nishimoto I. Ligand-dependent G protein coupling function of amyloid transmembrane precursor. J Biol Chem. 1995 Mar 3;270(9):4205–4208. doi: 10.1074/jbc.270.9.4205. [DOI] [PubMed] [Google Scholar]
- Parma J., Duprez L., Van Sande J., Cochaux P., Gervy C., Mockel J., Dumont J., Vassart G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993 Oct 14;365(6447):649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995 Aug 31;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Samama P., Cotecchia S., Costa T., Lefkowitz R. J. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993 Mar 5;268(7):4625–4636. [PubMed] [Google Scholar]
- Schubert W., Prior R., Weidemann A., Dircksen H., Multhaup G., Masters C. L., Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991 Nov 1;563(1-2):184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- Schuch U., Lohse M. J., Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1989 Jul;3(1):13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Sebok K., Woodside D., al-Aoukaty A., Ho A. D., Gluck S., Maghazachi A. A. IL-8 induces the locomotion of human IL-2-activated natural killer cells. Involvement of a guanine nucleotide binding (Go) protein. J Immunol. 1993 Feb 15;150(4):1524–1534. [PubMed] [Google Scholar]
- Shenker A., Laue L., Kosugi S., Merendino J. J., Jr, Minegishi T., Cutler G. B., Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993 Oct 14;365(6447):652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995 Jun 29;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
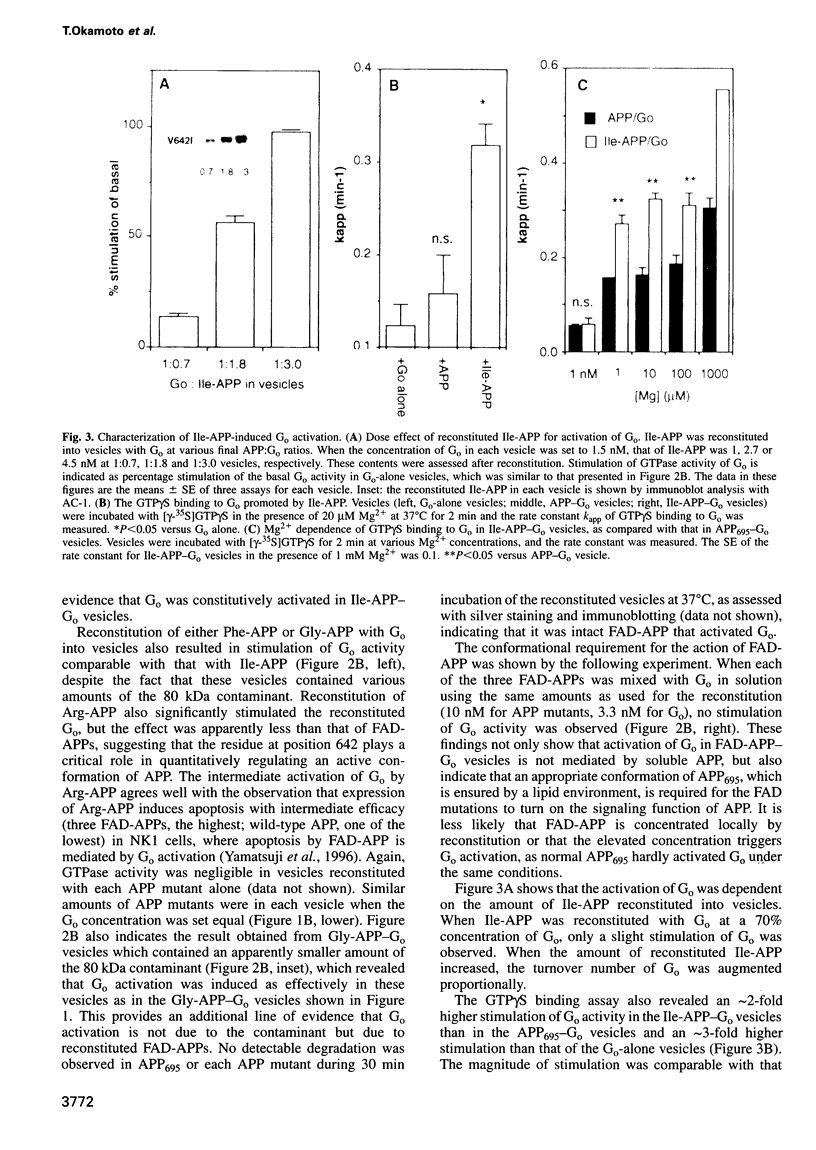
- Small D. H., Nurcombe V., Reed G., Clarris H., Moir R., Beyreuther K., Masters C. L. A heparin-binding domain in the amyloid protein precursor of Alzheimer's disease is involved in the regulation of neurite outgrowth. J Neurosci. 1994 Apr;14(4):2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kamps M. P., Cao H. Oncogenic activation of p185neu stimulates tyrosine phosphorylation in vivo. Mol Cell Biol. 1988 Sep;8(9):3969–3973. doi: 10.1128/mcb.8.9.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Cannon S. C., Ross E. M., Higashijima T., Fishman M. C. GAP-43 augments G protein-coupled receptor transduction in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5327–5331. doi: 10.1073/pnas.90.11.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Fishman M. C., Zhu X. P. Activated mutants of the alpha subunit of G(o) promote an increased number of neurites per cell. J Neurosci. 1994 Apr;14(4):2327–2338. doi: 10.1523/JNEUROSCI.14-04-02327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Sudo Y., Linder M. E., Fishman M. C. An intracellular guanine nucleotide release protein for G0. GAP-43 stimulates isolated alpha subunits by a novel mechanism. J Biol Chem. 1991 Nov 25;266(33):22465–22471. [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Ségalat L., Elkes D. A., Kaplan J. M. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science. 1995 Mar 17;267(5204):1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Yamatsuji T., Okamoto T., Takeda S., Murayama Y., Tanaka N., Nishimoto I. Expression of V642 APP mutant causes cellular apoptosis as Alzheimer trait-linked phenotype. EMBO J. 1996 Feb 1;15(3):498–509. [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T., Hayashi Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature. 1992 Sep 3;359(6390):64–67. doi: 10.1038/359064a0. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Miki T., Katsuya T., Ogihara T., Sakaki Y. The 717Val----Ile substitution in amyloid precursor protein is associated with familial Alzheimer's disease regardless of ethnic groups. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1141–1146. doi: 10.1016/0006-291x(91)91011-z. [DOI] [PubMed] [Google Scholar]
- Zheng H., Jiang M., Trumbauer M. E., Sirinathsinghji D. J., Hopkins R., Smith D. W., Heavens R. P., Dawson G. R., Boyce S., Conner M. W. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995 May 19;81(4):525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]