Abstract
Many low-birth-weight infants experience failure to thrive. The amino acid leucine stimulates protein synthesis in skeletal muscle of the neonate, but less is known about the effects of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB). To determine the effects of HMB on protein synthesis and the regulation of translation initiation and degradation pathways, overnight-fasted neonatal pigs were infused with HMB at 0, 20, 100, or 400 μmol·kg body wt−1·h−1 for 1 h (HMB 0, HMB 20, HMB 100, or HMB 400). Plasma HMB concentrations increased with infusion and were 10, 98, 316, and 1,400 nmol/ml in the HMB 0, HMB 20, HMB 100, and HMB 400 pigs. Protein synthesis rates in the longissimus dorsi (LD), gastrocnemius, soleus, and diaphragm muscles, lung, and spleen were greater in HMB 20 than in HMB 0, and in the LD were greater in HMB 100 than in HMB 0. HMB 400 had no effect on protein synthesis. Eukaryotic initiation factor (eIF)4E·eIF4G complex formation and ribosomal protein S6 kinase-1 and 4E-binding protein-1 phosphorylation increased in LD, gastrocnemius, and soleus muscles with HMB 20 and HMB 100 and in diaphragm with HMB 20. Phosphorylation of eIF2α and elongation factor 2 and expression of system A transporter (SNAT2), system L transporter (LAT1), muscle RING finger 1 protein (MuRF1), muscle atrophy F-box (atrogin-1), and microtubule-associated protein light chain 3 (LC3-II) were unchanged. Results suggest that supplemental HMB enhances protein synthesis in skeletal muscle of neonates by stimulating translation initiation.
Keywords: amino acid, protein metabolism, protein degradation, mtor, infant
during the neonatal period, an infant's growth and protein turnover are higher than during any other period of life (5). The high growth rate of neonatal skeletal muscle is mainly due to the high rate of protein synthesis (5, 6). Many low-birth-weight (LBW) infants experience failure to thrive and remain small throughout their lives (10, 40). Providing a diet high in protein can increase weight and length of LBW infants at discharge (24, 29, 47). However, providing an ideal diet high in protein is often unachievable, secondary to feeding intolerance, concerns regarding fluid balance, and possible adverse events associated with the escalation of feeds (29).
Previous work, utilizing the neonatal pig model as a surrogate for the human infant, has shown that protein synthesis is acutely increased in neonates in response to supplementation of the branched-chain amino acid (BCAA) leucine (15). Leucine serves not only as a substrate for protein synthesis but also as a nutrient signaling molecule that stimulates protein synthesis through activation of the intracellular signal transduction pathway that regulates mRNA translation (1, 14, 15). Leucine positively regulates the mammalian target of rapamycin (mTOR) signaling pathway, promoting eukaryotic initiation factor (eIF)4E-binding protein-1 (4E-BP1) phosphorylation, which increases the formation of the active eIF4E·eIF4G complex that mediates the binding of the mRNA to the 43S ribosomal complex. mTOR also phosphorylates ribosomal protein S6 kinase-1 (S6K1), an activator of S6, and this protein has been reported to promote mRNA translation of proteins involved in the regulation of translation (38).
Protein homeostasis is the byproduct of a complex interplay of catabolic and anabolic pathways. Skeletal muscle serves as a reservoir of amino acids during periods of fasting and when dietary intake is unable to meet metabolic demands (11). The ubiquitin-proteasome and autophagy-lysosome pathways have been identified as being particularly important proteolytic pathways in skeletal muscle (11, 26, 41, 49). Polyubiquitination by ubiquitin ligases is the rate-limiting step of the ubiquitin-proteasome system, the two most important muscle-specific ubiquitin ligases being muscle atrophy F-box (atrogin-1) and muscle RING finger 1 protein (MuRF1). Microtubule-associated protein light chain 3 (LC3-II), a ubiquitin-like molecule, has been identified as a marker of the autophagosome (41), although increasing amounts of LC3-II may not be a direct measure of protein degradation (11).
The leucine metabolite β-hydroxy-β-methylbutyrate (HMB) has been tested for its potential effectiveness as a supplement to promote lean gain and strength in adults (19, 30). Studies suggest that HMB can mitigate protein degradation in the adult model (23, 25); however, no studies have evaluated the effects of HMB supplementation on protein synthesis and degradation in the neonatal model. Therefore, in this study we hypothesized that supplementation of HMB would 1) stimulate protein synthesis through activation of the mTOR signaling pathway, and 2) reduce protein degradation through inhibition of the ubiquitin-proteasome and autophagy-lysosome pathways in skeletal muscle of neonates.
MATERIALS AND METHODS
Animals.
Four multiparous crossbred sows (Yorkshire × Landrace × Hampshire × Duroc; Agriculture Headquarters, Texas Department of Criminal Justice, Huntsville, TX) were housed, fed, and managed as previously described (15, 16). Piglets remained with the sow following birth and were studied at 5–7 days of age. Indwelling catheters were placed in the carotid artery and internal jugular under general anesthesia at 2 days of age (4). Piglets were allowed to recover postanesthesia and then were returned to the sow, where they remained until studied. The Animal Care and Use Committee of Baylor College of Medicine approved all experimental procedures. This study was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Treatments and infusions.
Prior to infusion study, piglets were removed from the sows and feed deprived for 12–14 h in a heated room (84°F). They were then randomly assigned to one of four treatment groups: HMB 0, HMB 20, HMB 100, or HMB 400 (n = 5–6/treatment group). Infusions were administered parenterally over 1 h. Piglets in the HMB 0, HMB 20, HMB 100, and HMB 400 groups received a priming infusion of either 0, 12, 60, or 240 μmol/kg body wt (BW) for 10 min prior to initiation of a 50-min infusion cycle during which they received either 0, 20, 100, or 400 μmol·kg BW−1·h−1 of their respective treatment. Blood samples were collected at the end of the 60-min infusion to measure blood glucose (YSI 2300 STAT Plus, Yellow Springs Instruments), insulin, HMB, amino acid, and keto acid concentrations.
Plasma and tissue HMB, α-keto acid, amino acid, and insulin concentrations.
Plasma α-keto acids were analyzed by high-performance liquid chromatography (HPLC) as described elsewhere (34). Plasma HMB was analyzed by gas chromatography-mass spectrometry (GC-MS) as previously described (32). Muscle biopsies were analyzed for HMB content by a modification to the plasma HMB assay. Briefly, muscle tissue acidified homogenates were extracted with ethyl ether. The HMB was then back-extracted into 0.1 M phosphate buffer, dried, and analyzed by GC-MS. An HPLC method (PICO-TAG reverse-phase column; Waters, Milford, MA) was used for analysis of the individual plasma amino acid concentrations (2). Plasma radioimmunoreactive insulin concentrations were analyzed using a porcine insulin radioimmunoassay kit (EMD Millipore, Billerica, MA).
Tissue protein synthesis in vivo.
A flooding dose of l-[4-3H]phenylalanine (1.5 mmol/kg BW, 0.5 mCi/kg; American Radiolabeled Chemicals, St. Louis, MO) was injected 30 min before organ collection to measure fractional protein synthesis rate (Ks) (21). Upon completion of the 1-h infusion, pigs were euthanized, and samples were obtained from the longissimus dorsi (LD), gastrocnemius, soleus, and diaphragm muscles, brain, duodenum, spleen, pancreas, kidney, skin, liver, left heart, lung, stomach, colon, and jejunum. Tissue samples were immediately frozen in liquid nitrogen and then stored at −70°C until analyzed as previously described (5).
Protein immunoblot analysis.
Proteins were separated on polyacrylamide gel electrophoresis (PAGE). For each assay, analysis of the samples from all treatments was performed at one time on triple-wide gels (C.B.S. Scientific, San Diego, CA). Proteins were electrophoretically transferred to polyvinlidene difluoride transfer (PVDF) membranes (Pall, Jersey Village, TX), which were incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody as previously described (8). For determination of the protein phosphorylations as well as the protein abundances, the data were corrected with β-actin. Primary antibodies that were used in the immunoblotting were: β-actin (Cell Signaling Technology, Danvers, MA), S6K1 Thr389 (Millipore, Bedford, MA), 4E-BP1 Thr46 (Invitrogen, Carlsbad, CA), eIF2α Ser51 (Cell Signaling Technology), eukaryotic elongation factor 2 (eEF2 Thr56, Cell Signaling Technology), system L transporter (LAT1; Cell Signaling Technology), MuRF1 (R&D Systems, Minneapolis, MN), (atrogin-1; ECM Biosciences, Versailles, KY), SNAT2 (Viva System Biology, San Diego, CA), and microtubule-associated protein-1 light chain 3 (LC3, Cell Signaling Technology).
Quantification of eIF4E·eIF4G complex.
Anti-eIF4E monoclonal antibody (courtesy of Dr. Leonard Jefferson, Penn State University College of Medicine, Hershey, PA) was used to immunoprecipitate eIF4E·eIF4G complex from samples of fresh tissue. Amounts of eIF4G detected by antibody (Cell Signaling Technology) were corrected by the eIF4E (Cell Signaling Technology) recovered from the immunoprecipitate as previously described (14, 16, 27).
Calculations and statistics.
Fractional rates of protein synthesis (Ks, % protein mass synthesized in a day) were calculated as: Ks (%/day) = [(Sb/Sa) × (1,440/t)] × 100, as previously described (17). Data were analyzed by ANOVA for a Complete Randomized Design using statistical software (SPSS, version 19). When a significant effect was determined by ANOVA, means were compared using Fisher's protected least significant differences post hoc test. Data are presented as least square means ± SE, and differences were considered significant at P < 0.05.
RESULTS
Plasma and tissue substrates and hormone concentrations.
Plasma concentrations of HMB were significantly greater (P < 0.05) in the HMB 100 and HMB 400 group than in the HMB 0 group 1 h after the initiation of infusion (Table 1). HMB infusion increased HMB levels in the LD muscle and were greater in the HMB 100 than in the HMB 0 and 20 groups and highest in the HMB 400 group (P < 0.05). The circulating concentrations of the α-keto acids α-ketoisocaproic acid (KIC), α-ketoisovalerate (KIV), and α-ketomethylvalerate (KMV) were not altered by HMB infusion (Table 1). Infusion of HMB had no effect on the concentrations of leucine, BCAA, essential amino acids (EAA), or nonessential amino acids (NEAA) (Table 1). Plasma glucose concentrations were slightly increased (P < 0.05) in the HMB 20 and HMB 400 compared with the HMB 0 group (Table 1). Plasma insulin concentrations were not significantly different among treatment (Table 1).
Table 1.
Plasma and longissimus dorsi muscle HMB concentrations and plasma α-keto acid, amino acid, glucose, and insulin concentrations in response to 1-h infusion of parenteral HMB1
| Measurement | HMB 0 | HMB 20 | HMB 100 | HMB 400 |
|---|---|---|---|---|
| Plasma HMB2 | 8.8 ± 85.6a | 89.6 ± 85.6ab | 316.1 ± 78.2b | 1,400.1 ± 85.6c |
| Muscle HMB3 | 11.4 ± 14.2a | 38.4 ± 15.3a | 132.3 ± 15.3b | 439.2 ± 14.2c |
| Plasma KIV2 | 21.8 ± 3.4 | 28.7 ± 3.4 | 24.6 ± 3.1 | 23.3 ± 3.1 |
| Plasma KMV2 | 28.3 ± 4.9 | 37.4 ± 4.9 | 31.0 ± 4.5 | 30.0 ± 4.5 |
| Plasma KIC2 | 39.5 ± 7.3 | 44.0 ± 7.3 | 44.7 ± 6.7 | 41.8 ± 6.7 |
| Plasma leucine2 | 145 ± 15.6 | 174 ± 15.6 | 176 ± 14.2 | 160 ± 14.2 |
| Plasma BCAA2 | 558 ± 58.0 | 647 ± 58.0 | 627 ± 53.0 | 614 ± 53.0 |
| Plasma EAA2 | 1,062 ± 97.0 | 1,158 ± 97.0 | 1,118 ± 88.0 | 1,146 ± 88.0 |
| Plasma NEAA2 | 1,890 ± 126.0 | 1,960 ± 126.0 | 2,114 ± 115.0 | 2,133 ± 115.0 |
| Plasma glucose4 | 81.5 ± 3.4a | 102 ± 3.4c | 90.0 ± 3.1ab | 94.9 ± 3.1bc |
| Plasma insulin5 | 1.4 ± 1.1 | 2.7 ± 1.2 | 2.2 ± 1.2 | 1.8 ± 1.1 |
Data are given as means ± SE; n = 5–6.
Plasma and longissimus dorsi muscle concentrations in piglets infused with 0, 20, 100, or 400 μmol·kg−1·h−1 β-hydroxy-β-methylbutyrate (HMB) for 1 h.
KIV, α-ketoisovalerate; KMV, α-ketomethylvalerate; KIC, α-ketoisocaproic acid; BCAA, branched-chain amino acids; EAA, essential amino acids; NEAA, nonessential amino acids.
Plasma concentrations in nmol/ml.
Skeletal muscle concentrations in nmol/g tissue.
Plasma concentrations in mg/dl.
Plasma concentrations in μU/ml. Labeled means in a row without a common letter differ, P < 0.05.
Protein synthesis.
The fractional rates of protein synthesis in the LD, gastrocnemius, soleus, and diaphragm muscles were greater (P < 0.05) in the HMB 20 than in the HMB 0 baseline group (Fig. 1, A–D). Protein synthesis rate in the LD muscle was greater in the HMB 100 than in the HMB 0 group (P < 0.05), but in the gastrocnemius, soleus, and diaphragm muscles no differences between groups were found. Protein synthesis rates in skeletal muscles did not differ between HMB 400 and HMB 0.
Fig. 1.
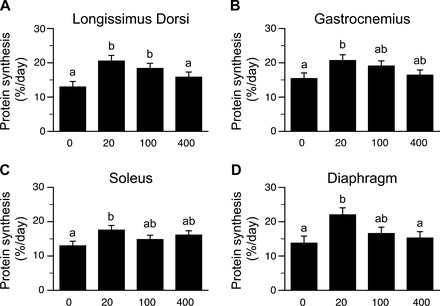
Fractional rates of protein synthesis in longissimus dorsi (LD), gastrocnemius, soleus, and diaphragm muscles of piglets infused with 0, 20, 100, or 400 μmol·kg−1·h−1 β-hydroxy-β-methylbutyrate (HMB) for 1 h. Values are means ± SE; n = 5–6 per treatment. a,bValues not sharing superscripts differ significantly (P < 0.05).
In the heart, liver, stomach, duodenum, jejunum, colon, pancreas, kidney, brain, and skin, protein synthesis rates did not differ between groups (Table 2). However, protein synthesis rates in the lung and spleen were higher (P < 0.05) in the HMB 20 than in the HMB 0 group (Table 2).
Table 2.
Visceral and vital organ tissue protein synthesis after 1-h infusion of HMB
| Tissue | HMB 0 | HMB 20 | HMB 100 | HMB 400 |
|---|---|---|---|---|
| Heart | 11.3 ± 0.69 | 12.2 ± 0.69 | 13.0 ± 0.63 | 13.0 ± 0.63 |
| Lung | 25.2 ± 1.06ab | 28.8 ± 1.06c | 23.7 ± 0.96a | 27.0 ± 0.96bc |
| Liver | 82.7 ± 5.34 | 92.5 ± 5.34 | 79.3 ± 4.88 | 79.0 ± 4.88 |
| Spleen | 21.3 ± 1.90a | 28.3 ± 1.90b | 23.1 ± 1.73ab | 25.4 ± 1.73ab |
| Stomach | 23.3 ± 3.02 | 26.6 ± 3.02 | 22.2 ± 2.76 | 24.0 ± 2.76 |
| Duodenum | 44.2 ± 4.45ab | 50.9 ± 4.45ab | 55.6 ± 4.06a | 41.8 ± 4.06b |
| Jejunum | 38.2 ± 3.96 | 33.3 ± 3.96 | 35.9 ± 3.62 | 30.3 ± 3.62 |
| Colon | 48.0 ± 4.71 | 47.8 ± 4.71 | 50.5 ± 4.30 | 57.6 ± 4.30 |
| Pancreas | 53.1 ± 3.92 | 51.4 ± 3.92 | 55.7 ± 3.58 | 56.3 ± 3.58 |
| Kidney | 43.1 ± 3.20ab | 35.8 ± 3.20a | 47.4 ± 2.92b | 50.1 ± 2.92b |
| Brain | 11.0 ± 0.90 | 11.4 ± 0.90 | 11.2 ± 0.82 | 11.3 ± 0.82 |
| Skin | 22.4 ± 3.39 | 32.5 ± 3.39 | 29.1 ± 3.10 | 27.6 ± 3.10 |
Data are given as means ± SE; n = 5–6. Protein synthesis (%/day) in piglets infused with 0, 20, 100, or 400 μmol·kg−1·h−1 HMB for 1 h. Labeled means in a row without a common letter differ, P < 0.05.
Intracellular signaling components.
Phosphorylation of S6K1 and 4EBP1 (Figs. 2 and 3, A–C) as well as formation of the active eIF4E·eIF4G complex (Fig. 4, A–C) in the LD, gastrocnemius, and soleus muscles were greater (P < 0.05) in the HMB 20 and HMB 100 groups than in the HMB 0 group. Infusion of HMB 20, but not HMB 100, compared with HMB 0, increased (P < 0.05) formation of the active eIF4E·eIF4G complex as well as the phosphorylation of S6K1 and 4EBP1 in the diaphragm (Figs. 2–4, D). Phosphorylation of eIF2α and eEF2 in the LD, gastrocnemius, soleus, and diaphragm muscles were unaffected by HMB treatment (Table 3).
Fig. 2.
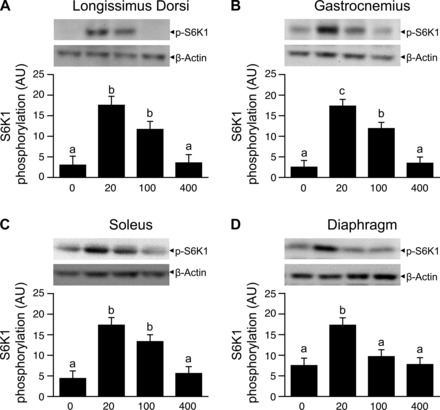
Phosphorylation of S6K1 in LD, gastrocnemius, soleus, and diaphragm muscles of piglets infused with 0, 20, 100, or 400 μmol·kg−1·h−1 HMB for 1 h. Values are means ± SE; n = 5–6 per treatment. a,b,cValues not sharing superscripts differ (P < 0.05).
Fig. 3.
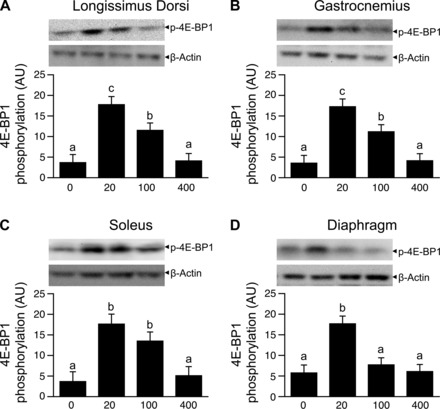
Phosphorylation of 4E-BP1 in LD, gastrocnemius, soleus, and diaphragm muscles of piglets infused with 0, 20, 100, or 400 μmol·kg−1·h−1 HMB for 1 h. Values are means ± SE; n = 5–6 per treatment. a,b,cValues not sharing superscripts differ (P < 0.05).
Fig. 4.
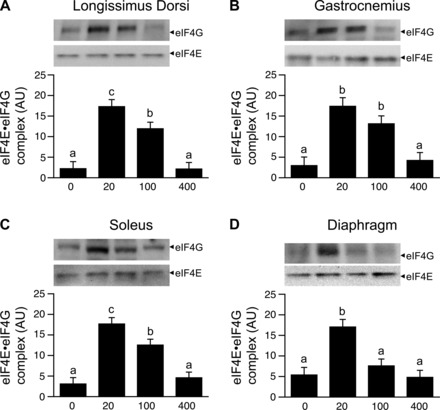
Abundance of eIF4E·eIF4G in LD, gastrocnemius, soleus, and diaphragm muscles of piglets infused with 0, 20, 100, or 400 μmol·kg−1·h−1 HMB for 1 h. Values are means ± SE; n = 5–6 per treatment. a,b,cValues not sharing superscripts differ (P < 0.05).
Table 3.
Phosphorylation of eIF2α and eEF2 in skeletal muscle of piglets infused with 0, 20, 100, or 400 μmol·kg−1·h −1 HMB for 1 h
| HMB 0 | HMB 20 | HMB 100 | HMB 400 | |
|---|---|---|---|---|
| eIF2α, AU | ||||
| LD | 0.83 ± 0.15 | 0.85 ± 0.15 | 0.89 ± 0.14 | 0.76 ± 0.14 |
| Gastrocnemius | 1.32 ± 0.21 | 1.42 ± 0.21 | 1.28 ± 0.19 | 1.25 ± 0.19 |
| Soleus | 0.79 ± 0.15 | 0.80 ± 0.15 | 0.81 ± 0.14 | 0.77 ± 0.14 |
| Diaphragm | 1.09 ± 0.18 | 0.91 ± 0.18 | 0.96 ± 0.16 | 1.00 ± 0.16 |
| eEF2, AU | ||||
| LD | 0.80 ± 0.14 | 0.86 ± 0.14 | 0.83 ± 0.13 | 0.74 ± 0.13 |
| Gastrocnemius | 1.04 ± 0.21 | 1.23 ± 0.21 | 1.12 ± 0.19 | 1.09 ± 0.19 |
| Soleus | 1.32 ± 0.31 | 1.60 ± 0.31 | 1.55 ± 0.29 | 1.41 ± 0.29 |
| Diaphragm | 0.81 ± 0.16 | 0.84 ± 0.16 | 0.87 ± 0.14 | 0.79 ± 0.14 |
Data are given as means ± SE; n = 5–6. AU, arbitrary units; LD, longissimus dorsi; eIF2α, eukaryotic initiation factor 2α; eEF2, eukaryotic elongation factor 2. Labeled means in a row without a common letter differ, P < 0.05.
The expressions of atrogin-1 and MuRF1 in the LD, gastrocnemius, soleus, and diaphragm muscles were unaffected by HMB treatment (Table 4). The LC3-II-to-total LC3 ratio in skeletal muscles was also unaffected by HMB treatment (Table 4). There was no effect of HMB on the abundance of the amino acid transporters LAT1 and SNAT2 in the LD, gastrocnemius, soleus, and diaphragm muscles (Table 5).
Table 4.
Protein degradation and autophagy signal activation following HMB supplementation in neonatal pigs
| HMB 0 | HMB 20 | HMB 100 | HMB 400 | |
|---|---|---|---|---|
| Atrogin-1, AU | ||||
| LD | 0.82 ± 0.15 | 0.85 ± 0.15 | 0.76 ± 0.14 | 0.77 ± 0.14 |
| Gastrocnemius | 1.09 ± 0.23 | 1.22 ± 0.23 | 0.99 ± 0.21 | 1.06 ± 0.21 |
| Soleus | 1.16 ± 0.28 | 1.16 ± 0.28 | 1.02 ± 0.26 | 1.23 ± 0.26 |
| Diaphragm | 1.30 ± 0.26 | 1.32 ± 0.26 | 1.54 ± 0.24 | 1.32 ± 0.24 |
| MuRF1, AU | ||||
| LD | 0.77 ± 0.12 | 0.82 ± 0.12 | 0.75 ± 0.11 | 0.82 ± 0.11 |
| Gastrocnemius | 0.69 ± 0.14 | 0.61 ± 0.14 | 0.67 ± 0.13 | 0.60 ± 0.13 |
| Soleus | 1.48 ± 0.29 | 1.34 ± 0.29 | 1.18 ± 0.26 | 1.13 ± 0.26 |
| Diaphragm | 1.22 ± 0.22 | 1.15 ± 0.22 | 1.00 ± 0.20 | 1.03 ± 0.20 |
| LC3-II/total LC3 ratio | ||||
| LD | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.15 ± 0.08 | 0.12 ± 0.08 |
| Gastrocnemius | 0.14 ± 0.03 | 0.15 ± 0.03 | 0.14 ± 0.03 | 0.17 ± 0.03 |
| Soleus | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.10 ± 0.02 | 0.09 ± 0.02 |
| Diaphragm | 0.25 ± 0.04 | 0.23 ± 0.04 | 0.22 ± 0.04 | 0.27 ± 0.04 |
Data are given as means ± SE; n = 5–6. Atrogin-1, muscle atrophy F-box; MuRF1, muscle RING finger 1 protein; LC3-II/LC3-I, microtubule-associated protein light chain 3. Labeled means in a row without a common letter differ, P < 0.05.
Table 5.
Amino acid transporters in muscle of neonatal pigs in response to 1-h HMB supplementation
| HMB 0 | HMB 20 | HMB 100 | HMB 400 | |
|---|---|---|---|---|
| LAT1, AU | ||||
| LD | 0.55 ± 0.07 | 0.52 ± 0.07 | 0.54 ± 0.07 | 0.58 ± 0.07 |
| Gastrocnemius | 0.73 ± 0.12 | 0.76 ± 0.12 | 0.70 ± 0.11 | 0.67 ± 0.11 |
| Soleus | 0.83 ± 0.14 | 0.77 ± 0.14 | 0.90 ± 0.14 | 0.82 ± 0.14 |
| Diaphragm | 0.60 ± 0.12 | 0.63 ± 0.12 | 0.54 ± 0.11 | 0.57 ± 0.11 |
| SNAT2, AU | ||||
| LD | 0.72 ± 0.11 | 0.75 ± 0.11 | 0.83 ± 0.10 | 0.76 ± 0.10 |
| Gastrocnemius | 0.88 ± 0.12 | 0.95 ± 0.12 | 0.90 ± 0.11 | 0.84 ± 0.11 |
| Soleus | 0.83 ± 0.15 | 0.91 ± 0.15 | 0.86 ± 0.14 | 0.82 ± 0.14 |
| Diaphragm | 0.62 ± 0.11 | 0.64 ± 0.11 | 0.59 ± 0.10 | 0.71 ± 0.10 |
Data are given as means ± SE; n = 5–6. LAT1, system L transporter 1; SNAT2, system A transporter 2. Labeled means in a row without a common letter differ, P < 0.05.
DISCUSSION
Neonates are highly responsive to anabolic stimulators such that even small variations in the systemic availability of these agents have significant effects on their muscle protein anabolism (11, 20). Amino acids, and particularly leucine, as well as insulin and glucose, have been identified as biochemical anabolic stimulants that result in increased protein synthesis in neonatal muscle (4, 7, 15, 35, 52). HMB, an active metabolite of the amino acid leucine, has been promoted as a nutritional supplement to enhance muscle mass and strength in adults (19, 30) and is commercially available enriched in nutritional supplements. However, there is a lack of evidence regarding HMB supplementation in the neonate. Since our previous work has shown that administration of either leucine (15, 16) or its α-keto acid KIC (17) can elicit a rapid increase in muscle protein synthesis in neonatal pigs, we hypothesized that HMB administration would induce a similar positive effect. The results in the current study reveal that HMB alone augments skeletal muscle protein synthesis in neonates in association with the activation of mTOR signaling.
In the present study, parenteral infusion of HMB stimulated protein synthesis in skeletal muscle of fasted animals compared with fasted controls. HMB is produced endogenously via leucine degradation in both animals and humans (31). Leucine supplementation to achieve circulating concentrations within the physiological postprandial range results in an increase in skeletal muscle protein synthesis (15). Leucine is unique in its ability to act as a nutrient signal that activates translation initiation (15), as supplementation with the other BCAA valine and isoleucine do not stimulate skeletal muscle protein synthesis (16). In the current study, 1-h infusion of HMB resulted in increased protein synthesis, similar to that in neonatal pigs infused with leucine (15, 17). Physiological levels of HMB typically range from 1 to 4 μmol/l but can increase 5- to 10-fold after leucine is fed (31, 33). In the current study, we achieved correspondingly higher circulating HMB levels that at our HMB 20 and HMB 100 doses were similar to those achieved in HMB supplementation studies in humans (54), but with leucine concentrations remaining in the fasting range. The maximal increase in protein synthesis in muscle was achieved at circulating HMB levels close to 100 nmol/ml (HMB 20), with higher circulating concentrations of ∼300 nmol/ml (HMB 100) not having an additional effect. Interesting, protein synthesis approached that seen in controls when circulating concentrations exceeded 1,000 nmol/ml at the HMB 400 dose. These results are supportive that HMB can be considered an alternative to leucine to augment muscle protein synthesis in the neonate, but HMB may be ineffective at very high doses.
In adult animals and humans, HMB has been extensively evaluated alone and in combination with other amino acids, with and without exercise, as a supplement to augment lean body mass and function (23, 25, 30, 31, 33, 37, 50, 54). Previous studies from our laboratory have shown that raising to fed levels either amino acids (7) or KIC (17) can independently stimulate skeletal muscle protein synthesis. In our study, muscle protein synthesis increased in response to HMB despite that circulating amino acids and KIC concentrations remained in the fasting range and were not altered during the 1 h HMB infusion. Since HMB is produced via transamination of leucine to KIC followed by irreversible KIC oxidation (31, 39, 48), an increase in KIC would not be expected in the current study. Although a slight increase in circulating glucose was noted in the HMB 20 and HMB 400 treatment groups, it is unlikely that these small changes in glucose concentrations contributed to the increase in protein synthesis, as HMB 400 infusion did not affect protein synthesis. On the basis of these results, we conclude that the increase in protein synthesis in response to HMB administration was not elicited by changes in amino acids, KIC, or glucose. In agreement with these findings, recent studies have shown that enteral supplementation of the free-acid form of HMB resulted in an increase in protein synthesis in young men (50) and that HMB supplementation of previously untrained males during resistance exercise training demonstrated a tendency to increase strength and lean body mass compared with placebo (30). Furthermore, in vitro HMB supplementation has been shown to stimulate protein synthesis in murine myotubes (12). In our study, circulating HMB levels of 100 nmol/ml increased protein synthesis in both glycolytic and oxidative skeletal muscles and in the diaphragm (a muscle of mixed composition). However, HMB supplementation did not alter protein synthesis in heart muscle after 60 min of exposure. Previously, we reported a stimulatory effect of acute parenteral leucine on protein synthesis in the heart (16); however, the response to leucine was less profound in the heart than in skeletal muscle and could reflect differences in the tissues' sensitivity to anabolic agents.
While leucine and HMB have been demonstrated to stimulate skeletal muscle protein synthesis, less is known regarding their effects on visceral tissues. Although enteral leucine supplementation has been shown to stimulate protein synthesis in visceral organs of neonatal (29, 45) and weaned pigs (53), acute parenteral administration of leucine does not stimulate protein synthesis in liver (15, 44). In the present study, HMB did not affect protein synthesis in visceral tissues, except for the lungs and the spleen in the HMB 20 and HMB 100 groups. Longer-term studies are needed to identify whether there is an effect of HMB on protein synthesis in visceral organs of the neonate.
In this study, the increase in skeletal muscle protein synthesis in response to HMB was associated with activation of mTOR-dependent translation initiation factors, as has been previously described for leucine (14–17). Infusion of HMB for 1 h resulted in augmented phosphorylation of S6K1 and 4EBP1 and increased abundance of the active eIF4E·eIF4G complex, events consistent with leucine's effects in skeletal muscle (14, 15, 43). The maximal increase in mTOR signaling was achieved at the HMB 20 dose, with the HMB 400 dose having no effect. Stimulation of mTOR-dependent translation by HMB has been shown recently in humans (50) and can be antagonized by the mTOR inhibitor rapamycin in myotubes (12).
Similarly to our previous study using short-term administration of leucine (44, 45), HMB supplementation had no effect on the phosphorylation of the tRNA-ribosome binding regulator eIF2α in skeletal muscle. Likewise, in the current study, HMB did not change the phosphorylation of eEF2, which regulates peptide elongation, consistent with our previous work with leucine in healthy neonatal pigs (44, 45, 51). In contrast, the phosphorylation of eEF2 has been shown to be attenuated by leucine in normal humans (9, 22), whereas HMB has been shown to antagonize the increase in the phosphorylation of eIF2α and eEF2 induced by cachectic stimuli in myotubes (12), suggesting a mechanism by which HMB attenuates depression of protein synthesis in catabolic settings.
The amino acid transporters SNAT2 and LAT1 modulate amino acid entry inside the cell and have been implicated in mTOR activation (46). Silencing of SNAT2 has been associated with decreased cellular levels of glutamine and leucine and thus linked to decreased mTOR activity and protein synthesis (18). Likewise, LAT1 abundance has been demonstrated to be positively associated with mTOR activation (42). In the current study, HMB did not affect SNAT2 and LAT1 abundances. The lack of effect of HMB on SNAT2 and LAT1 expression may be due to the short study period. Further investigation into the role of amino acid transporters and their effect on protein synthesis in response to HMB is thus certainly warranted.
In the current study, we did not identify effects of HMB on indexes of the ubiquitin-proteasome degradation pathway, the ubiquitin ligases atrogin-1 and MuRF1, or a marker of the autophagy-lysosome system, the LC3-II/total LC3 ratio. In adults, HMB supplementation has been suggested to antagonize muscle atrophy associated with cytokines (13), cancer (28), and acquired immunodeficiency syndrome (3), as well as preventing exercise-induced proteolysis and muscle damage during resistance exercise training in vivo and in vitro (30, 37). Enteral HMB in young human males has been shown to attenuate muscle protein breakdown (50). However, the lack of effect on HMB on protein degradation in the present study may have been due to the short period of HMB infusion. In addition, the neonatal pig has a high protein turnover and thus a high baseline expression of the ubiquitin ligases atrogin-1 and MuRF1, such that their expression cannot be further augmented by endotoxin, compared with older pigs (36). Therefore, the high muscle protein turnover in the neonatal pig may explain the lack of effect of HMB on protein degradation signaling. In this regard, in the neonate, muscle protein synthesis, not degradation, appears to be the major determinant of growth (11).
In summary, our results suggest that HMB is a promising parenteral agent for augmentation of protein synthesis and possibly growth in the neonate. In the neonatal piglet, HMB augments protein synthesis by activation of the mTOR-dependent translation signaling pathway, similarly to leucine stimulation, even in the presence of fasting circulating amino acids, leucine, and glucose. In contrast to leucine, the effects of HMB appear to be specific to appendicular skeletal muscle and diaphragm, and it does not affect protein degradation or autophagy signal activation.
The present data provide evaluation of the short-term effects of HMB on protein synthesis and degradation in the neonate. Additional work is required to determine whether comparable effects would be seen after longer-term exposure and in response to enteral supplementation. Nevertheless, the current data offer primary insight into the anabolic effects of HMB and its potential use to counteract poor growth in the neonate.
GRANTS
This project has been funded by Abbott Nutrition (T. A. Davis) and by the USDA/ARS under Cooperative Agreement 6250-510000-055 (T. A. Davis). This work is a publication of the United States Department of Agriculture/Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Abbott Nutrition. The contents of this publication do not necessarily reflect the views or politics of the U.S. Department of Agriculture, nor does the mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
DISCLOSURES
S. R. Davis is an employee of Abbott Nutrition.
AUTHOR CONTRIBUTIONS
Author contributions: S.M.W., S.R.D., and T.A.D. did the conception and design of research; S.M.W., S.W.E.-K., A.S., C.B., R.A.O., and H.V.N. performed experiments; S.M.W., A.S., H.V.N., and T.A.D. analyzed data; S.M.W., A.S., and T.A.D. interpreted results of experiments; S.M.W. prepared figures; S.M.W. drafted manuscript; S.M.W., S.W.E.-K., A.S., C.B., R.A.O., H.V.N., S.R.D., and T.A.D. edited and revised manuscript; S.M.W., S.W.E.-K., A.S., C.B., R.A.O., H.V.N., S.R.D., and T.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully thank M. L. Fiorotto for helpful discussions, R. D. Almonaci and S. J. Koo for expert technical assistance, J. C. Stubblefield for animal care, and L. Gullick and T. L. Leftely for secretarial assistance.
REFERENCES
- 1.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131: 856S–860S, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37: 593–599, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, Rathmacher JA. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. J Parenter Enteral Nutr 24: 133–139, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Davis TA, Fiorotto MF, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of sucking rats. Am J Physiol Regul Integr Comp Physiol 257: R1141–R1146, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12: 78–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279: E1226–E1234, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol 31: 48–55, 2007. [DOI] [PubMed] [Google Scholar]
- 11.El-Kadi SW, Suryawan A, Gazzaneo MC, Srivastava N, Orellana RA, Nguyen HV, Lobley GE, Davis TA. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab 302: E674–E686, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by β-hydroxy-β-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab 293: E923–E931, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-α and angiotensin II by β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab 295: E1417–E1426, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab 293: E1615–E1621, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288: E914–E921, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 140: 1418–1424, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol 18: 1426–1436, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 20: 445–451, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Frank JW, Escobar J, Nguyen HV, Jobgen SC, Jobgen WS, Davis TA, Wu G. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J Nutr 137: 315–319, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192: 719–723, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 140: 1970–1976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holecek M, Muthny T, Kovarik M, Sispera L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol 47: 255–259, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JD, Albritton WL, Sunshine P. Hyperammonemia accompanying parenteral nutrition in newborn infants. J Pediatr 81: 154–161, 1972. [DOI] [PubMed] [Google Scholar]
- 25.Kovarik M, Muthny T, Sispera L, Holecek M. Effects of beta-hydroxy-beta-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J Physiol Biochem 66: 311–319, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lin TA, Kong X, Haystead TA, Pause A, Belsham G, Sonenberg N, Lawrence JC., Jr PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science 266: 653–656, 1994. [DOI] [PubMed] [Google Scholar]
- 28.May PE, Barber A, D'Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg 183: 471–479, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140: 2145–2152, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC, Jr, Connelly AS, Abumrad N. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol 81: 2095–2104, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC., Jr Beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 130: 1937–1945, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Nissen S, Van Koevering M, Webb D. Analysis of beta-hydroxy-beta-methyl butyrate in plasma by gas chromatography and mass spectrometry. Anal Biochem 188: 17–19, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite b-hydroxy-b-methylbutyrate (HMB). J Nutr Biochem 8: 300–311, 1997. [Google Scholar]
- 34.Nissen SL, Van Huysen C, Haymond MW. Measurement of branched chain amino acids and branched chain alpha-ketoacids in plasma by high-performance liquid chromatography. J Chromatogr 232: 170–175, 1982. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284: E110–E119, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Orellana RA, Suryawan A, Wilson FA, Gazzaneo MC, Fiorotto ML, Nguyen HV, Davis TA. Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs. Am J Physiol Regul Integr Comp Physiol 302: R682–R690, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell ST, Tisdale MJ. Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem 330: 171–179, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31: 342–348, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Sabourin PJ, Bieber LL. Formation of beta-hydroxyisovalerate by an alpha-ketoisocaproate oxygenase in human liver. Metabolism 32: 160–164, 1983. [DOI] [PubMed] [Google Scholar]
- 40.Saigal S, Stoskopf BL, Streiner DL, Burrows E. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics 108: 407–415, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Sandri M. Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol 298: C1291–C1297, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Suryawan A, Davis TA. Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci 16: 1445–1460, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295: E868–E875, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suryawan A, Nguyen HV, Almonaci RD, Davis TA. Differential regulation of protein synthesis in skeletal muscle and liver of neonatal pigs by leucine through an mTORC1-dependent pathway. J Anim Sci Biotechnol 3: 3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71: 324–331, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor PM. Amino acid transporters: eminences grises of nutrient signalling mechanisms? Biochem Soc Trans 37: 237–241, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Thureen PJ, Melara D, Fennessey PV, Hay WW., Jr Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res 53: 24–32, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Van Koevering M, Nissen S. Oxidation of leucine and α-ketoisocaproate to β-hydroxy-β-methylbutyrate in vivo. Am J Physiol Endocrinol Metab 262: E27–E31, 1992. [DOI] [PubMed] [Google Scholar]
- 49.Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol 18: 631–635, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 591: 2911–2923, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140: 264–270, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab 275: E602–E609, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Yin Y, Yao K, Liu Z, Gong M, Ruan Z, Deng D, Tan B, Wu G. Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39: 1477–1486, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Zanchi NE, Gerlinger-Romero F, Guimaraes-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Seelaender M, Lancha AH., Jr HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids 40: 1015–1025, 2010. [DOI] [PubMed] [Google Scholar]


