Abstract
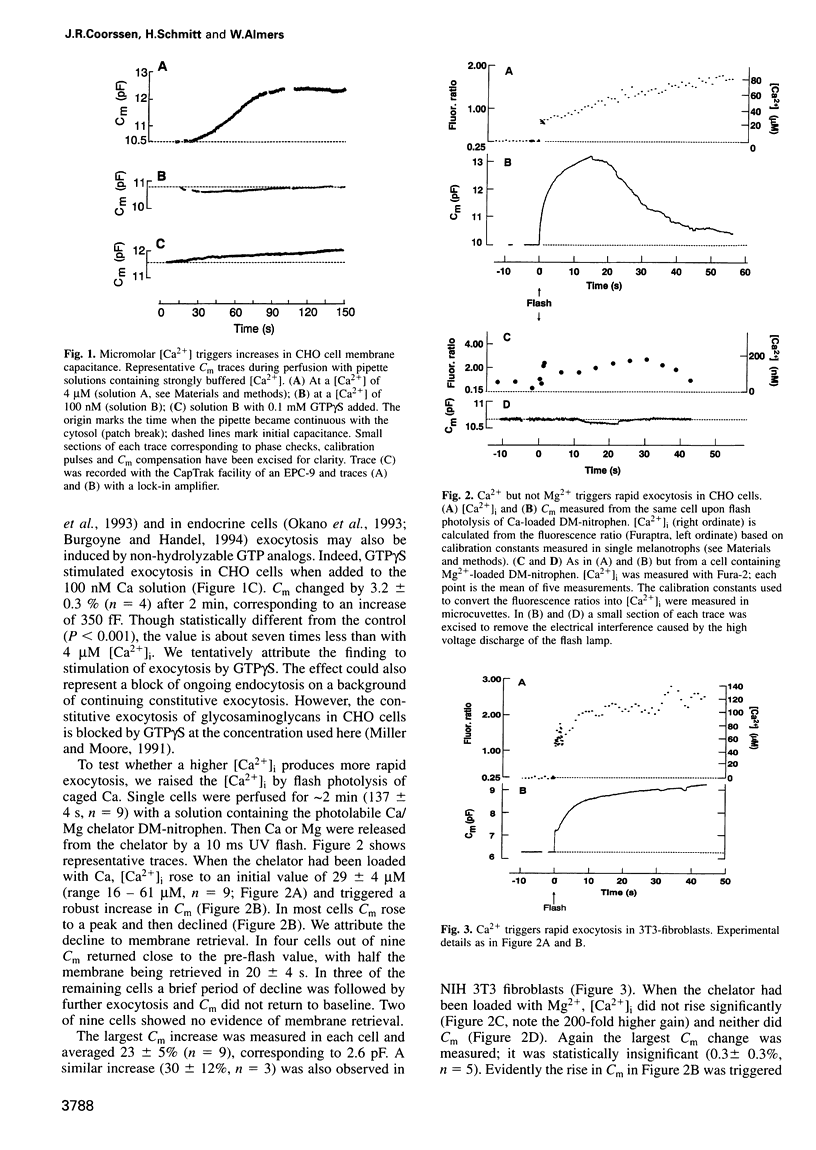
We have tracked the cell surface area of CHO cells by measuring the membrane capacitance, Cm. An increase in cytosolic [Ca2+], [Ca2+]i, increased the cell surface area by 20-30%. At micromolar [Ca2+]i the increase occurred in minutes, while at 20 microM or higher [Ca2+]i it occurred in seconds and was transient. GTPgammaS caused a 3% increase even at 0.1 microM [Ca2+]i. We conclude that CHO cells, previously thought capable only of constitutive exocytosis, can perform Ca2+-triggered exocytosis that is both massive and rapid. Ca2+-triggered exocytosis was also observed in 3T3 fibroblasts. Our findings add evidence to the view that Ca induces exocytosis in cells other than known secretory cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. J., Maurey K. M., Storrie B. Exocytosis of pinocytic contents by Chinese hamster ovary cells. J Cell Biol. 1982 Jun;93(3):632–637. doi: 10.1083/jcb.93.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Neher E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. J Physiol. 1987 May;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R., Huttner W. B. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993 Aug;5(4):628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Bi G. Q., Alderton J. M., Steinhardt R. A. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995 Dec;131(6 Pt 2):1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Handel S. E. Activation of exocytosis by GTP analogues in adrenal chromaffin cells revealed by patch-clamp capacitance measurement. FEBS Lett. 1994 May 16;344(2-3):139–142. doi: 10.1016/0014-5793(94)00361-0. [DOI] [PubMed] [Google Scholar]
- Coorssen J. R., Davidson M. M., Haslam R. J. Factors affecting dense and alpha-granule secretion from electropermeabilized human platelets: Ca(2+)-independent actions of phorbol ester and GTP gamma S. Cell Regul. 1990 Dec;1(13):1027–1041. doi: 10.1091/mbc.1.13.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y., Poo M. M. Quantal transmitter secretion from myocytes loaded with acetylcholine. Nature. 1992 Oct 22;359(6397):733–736. doi: 10.1038/359733a0. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994 Jun;125(5):1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Südhof T. C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994 Nov 18;79(4):717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Girod R., Popov S., Alder J., Zheng J. Q., Lohof A., Poo M. M. Spontaneous quantal transmitter secretion from myocytes and fibroblasts: comparison with neuronal secretion. J Neurosci. 1995 Apr;15(4):2826–2838. doi: 10.1523/JNEUROSCI.15-04-02826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. W., Birnbaum M. J. Identification of a nonneuronal isoform of synaptotagmin. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5895–5899. doi: 10.1073/pnas.92.13.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillova J., Thomas P., Almers W. Two independently regulated secretory pathways in mast cells. J Physiol Paris. 1993;87(3):203–208. doi: 10.1016/0928-4257(93)90031-n. [DOI] [PubMed] [Google Scholar]
- Li C., Ullrich B., Zhang J. Z., Anderson R. G., Brose N., Südhof T. C. Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995 Jun 15;375(6532):594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Nüsse O., Bennett J., Cromwell O. The membrane fusion events in degranulating guinea pig eosinophils. J Cell Sci. 1993 Jan;104(Pt 1):203–210. doi: 10.1242/jcs.104.1.203. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Moore H. P. Reconstitution of constitutive secretion using semi-intact cells: regulation by GTP but not calcium. J Cell Biol. 1991 Jan;112(1):39–54. doi: 10.1083/jcb.112.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., McNeil P. L. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995 Dec;131(6 Pt 2):1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T., Popov S., Buckley K. M., Poo M. M. Calcium-dependent transmitter secretion from fibroblasts: modulation by synaptotagmin I. Neuron. 1995 Sep;15(3):689–696. doi: 10.1016/0896-6273(95)90156-6. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Okano K., Monck J. R., Fernandez J. M. GTP gamma S stimulates exocytosis in patch-clamped rat melanotrophs. Neuron. 1993 Jul;11(1):165–172. doi: 10.1016/0896-6273(93)90280-5. [DOI] [PubMed] [Google Scholar]
- Parsons T. D., Coorssen J. R., Horstmann H., Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995 Nov;15(5):1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Al-Awqati Q. Regulation of transepithelial H+ transport by exocytosis and endocytosis. Annu Rev Physiol. 1986;48:153–161. doi: 10.1146/annurev.ph.48.030186.001101. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Bi G., Alderton J. M. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994 Jan 21;263(5145):390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Lee A. K., Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993 Jul;11(1):93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]



