Abstract
The crucial role of cancer stem cells (CSCs) in the pathology of malignant diseases has been extensively studied during the last decade. Nestin, a class VI intermediate filament protein, was originally detected in neural stem cells during development. Its expression has also been reported in different tissues under various pathological conditions. Specifically, nestin has been shown to be expressed in transformed cells of various human malignancies, and a correlation between its expression and the clinical course of some diseases has been proved. Furthermore, the coexpression of nestin with other stem cell markers was described as a CSC phenotype that was subsequently verified using tumorigenicity assays. The primary aim of this review is to summarize the recent findings regarding nestin expression in CSCs, its possible role in CSC phenotypes, particularly with respect to capacity for self-renewal, and its utility as a putative marker of CSCs.
Nestin has been shown to be expressed in transformed cells of various human malignancies, and a correlation between its expression and the clinical course of some diseases has been proved. Furthermore, the co-expression of nestin with other stem cell markers was described as a CSC phenotype that was subsequently verified using tumorigenicity assays. The primary aim of this review is to summarize the recent findings regarding nestin expression in CSCs, its possible role in CSC phenotypes, particularly with respect to capacity for self-renewal, and its utility as a putative marker of CSCs.
Keywords: Cancer stem cells, cytoskeleton, intermediate filaments, nestin, tumor markers
The role of cancer stem cells (CSCs) in the pathology of malignant diseases has been extensively studied during the last decade. At present, it is widely accepted that CSCs participate in the processes of tumor initiation, progression, metastasis, and relapse.1,2 The intermediate filament (IF) protein nestin is an extensively studied marker of neural stem cells that is a putative marker of the CSC phenotype, as its expression has been identified in many human malignancies.3,4 The primary aim of this review is to summarize the recent findings in this interesting field of tumor biology.
Characteristics and Detection of CSCs
Cancer stem cells are defined as a small subpopulation of undifferentiated cells in tumor tissue that are characterized by their capacity for self-renewal and differentiation into various lineages and clones that generate tumor masses. Due to their ability to form a continuously growing tumor, the synonymous terms “tumor-initiating” or “tumorigenic” have been used to describe these cells.5,6
Published reports suggest three primary hypotheses regarding the origin of CSCs. The first hypothesis is that CSCs develop from tissue-specific adult stem cells, which show the same capacity for self-renewal and differentiation and survive in the organism for an extended period, rendering these cells as vulnerable to the accumulation of oncogenic mutations. Furthermore, CSCs showed similar primitive phenotypes, such as the expression of specific stem cell markers.7 The second hypothesis for the origin of CSCs is based on the transformation and dedifferentiation of mature or differentiating cells, which have been reported to reacquire stem cell properties as a consequence of transforming mutations.8 The third hypothesis is that CSCs and, consequentially, tumorigenesis originate as a result of an “aberrant deposit” of embryonic stem cells in developing tissues during ontogenesis.1
Cancer stem cells are typically characterized based on the presence and/or absence of several cellular markers, a combination of which is specific for the CSC phenotype in a respective tumor. These markers include cell surface or membranous proteins (CD15, CD24, CD44, CD133, CXCR4, NCAM, and ABC transporters), cytoplasmic proteins (nestin, Musashi-1, and aldehyde dehydrogenase) or nuclear proteins (Sox-2, Oct3/4, and Nanog) that carry out various structural or metabolic functions in the cell.
The immunodetection of CSC markers on the cell surface has facilitated the identification and isolation of selected CSC subpopulations using appropriate sorting methods (FACS or magnetic-activated cell sorting (MACS)). Other frequently used methods for the isolation of CSCs are based on the functional characteristics of CSCs, for example, the expression of cell adhesion molecules, cytoprotective enzymes, and membrane transporters.9 These characteristics can be identified using standardized in vitro functional assays: detection of the side population, sphere formation assays, and clonogenicity assays, for example. However, in vivo tumorigenicity assays using immunodeficient mice represent the gold standard for the detection of CSCs because this method provides direct evidence of self-renewal and of tumor-forming capacities in an organism. A positive result on this test is considered to confirm the CSC phenotype in the observed cell population.5
Characterization of Nestin
Nestin (neuronal stem cell protein) was originally identified using the Rat-401 monoclonal mouse antibody in 1985. This antibody displayed specificity to an antigen that was transiently expressed in specific regions of the developing central nervous system (CNS) and in non-neuronal cells in the peripheral nervous system.10 Subsequent analysis led to the classification of nestin as a class VI IF protein.11
In general, IF represent one of the three main components of cytoskeleton in animal cells. In contrast to microtubules and actin filaments, which consist exclusively of highly conserved globular proteins tubulin and actin, respectively, IF proteins are fibrous and their expression is tissue- or cell-specific. All IF proteins exhibit the same structural organization: a central α-helical rod domain flanked by N- and C-terminal tail domains;12 therefore, IF are homopolymers or heteropolymers formed of two or more IF proteins. Intermediate filament proteins are classified according their structure and localization as follows: classes I and II encompass acidic and basic cytokeratins; class III embraces vimentin, desmin, glial fibrillary acidic protein, syncoilin, and peripherin; class IV consists of neurofilaments and α-internexin; class V of lamins; and class VI of nestin and synemin.13 Intermediate filaments are responsible for mechanical integrity of the cell, they serve as an integrating scaffold for other cytoskeletal components and for some organelles. They are also involved in formation of tissue architecture and in the process of tissue regeneration.14
The human nestin gene (Fig.1) is located on the long (q) arm of chromosome 1 at position 23.1. Its promoter resides in a 5′-non-translated region containing two Sp-1-binding sites and lacks a functional TATA box.15 The nestin gene consists of four exons separated by three introns. Enhancer elements were found in the first and second introns.16 The enhancer located in the first intron specifically increases nestin expression in myogenic precursors; the mechanism underlying this regulation is likely based on the presence of two E-boxes within the enhancer sequence, to which the transcription factor MyoD cooperatively binds.17 The second intron contains two neural precursor-specific enhancers, identified as a pan-CNS enhancer and a midbrain-specific enhancer, both of which contain at least two regulatory elements.18 These two enhancer elements represent binding sites for different types of regulatory molecules, for example, nuclear hormone receptors and transcription factors belonging to the SOX or POU family.18,19 The expression of the nestin gene is also regulated by epigenetic mechanisms, that is, DNA methylation and histone acetylation. Specifically, histone acetylation appears to be the preferred mechanism of nestin regulation during neural differentiation.20
Fig 1.
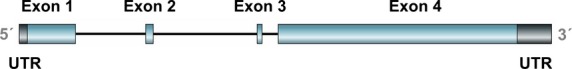
Exon/intron structure of the human nestin gene. Four exons are depicted in cyan color. The 5′-UTR (black) is located within the first exon; similarly, the 3′-UTR (black) is located within the fourth exon.
The human nestin protein (Fig.2) consists of 1621 amino acids and displays a predicted molecular weight of 177.4 kDa. However, nestin is typically detected by Western blotting at a higher apparent molecular weight, ranging from 200 to 240 kDa. This difference can be explained by the presence of multiple phosphorylation sites and glycosylated side chains.21 The structure of the nestin protein is similar to that of other IF proteins: a conserved 306-amino acid α-helical rod domain, which forms the protein core, 7-amino acid N-terminal and 1308-amino acid C-terminal tail domains. Within the rod domain, helical coils play an important role in dimerization and consecutive filament formation. Previous studies have suggested that nestin is able to form heterodimers only with other IF proteins, most favorably with vimentin.18,22
Fig 2.
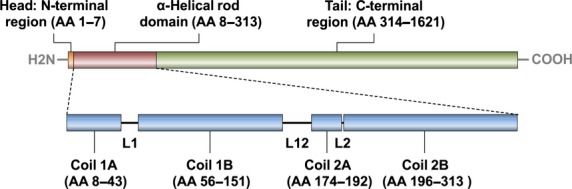
Domain structure of the human nestin protein. Three domains are depicted: “head” located in the N-terminal region (orange), α-helical rod domain (red), and “tail” in the C-terminal region (green). The rod domain consists of four coils (blue) separated by three linkers (L1, L12, and L2). Numbers of amino acids (AA) in the individual domains and coils are given in brackets.
Nestin Expression in Transformed Cells
Nestin expression in the developing and adult human organism under both physiological and pathological conditions has been well described.4,23–25 Although nestin is primarily regarded as a marker of neural stem/progenitor cells, its expression has also been shown in various embryonic cells and tissues, including skeletal muscle, cardiac muscle, umbilical cord blood cells, Sertoli and interstitial testicular cells, odontoblasts, hair follicle sheath cells, hepatic cells, and renal progenitors.23 In adults, nestin-positive cells are localized to tissue/organ-specific sites, where they serve as a quiescent resource of cells capable of proliferation, differentiation, and migration after their re-activation.23 The re-expression or upregulation of nestin was observed in various tissues during reparation processes after several types of injury, including the following: in reactive astrocytes after CNS damage,26 during the fibrotic response to ischemic heart disease,27 and in the context of mesangial cell repopulation after induced nephritis.28 Special attention has been paid to the detailed analyses of nestin expression in many types of human malignancies because nestin may serve as an important diagnostic or prognostic marker.
Increased nestin expression is typical for tumors generated from undifferentiated precursor cells or immature progenitors, which rapidly proliferate during neurogenesis.29 Although these precursor cells replace nestin with other IF proteins during neuronal differentiation, nestin expression can be transiently re-activated in transformed cells.25
In our previous detailed review, we summarized the current understanding of nestin expression in cancer cells and vascular endothelial cells from various types of solid tumors.24 Unsurprisingly, nestin was detected in neuroectodermal neuroepithelial tumors, including tumors of astrocytic, oligodendroglial, oligoastrocytic, ependymal, embryonic, neuronal, and mixed neuronal-glial origin. Furthermore, nestin expression was found in mesenchymal tumors (e.g. osteosarcoma, rhabdomyosarcoma, gastrointestinal stromal tumor), germ cell tumors (e.g. embryonal carcinoma, germinoma, choriocarcinoma, yolk sac tumor), and epithelial tumors (e.g. pancreatic adenocarcinoma, breast carcinoma, ovarian carcinoma, lung carcinoma).24,25,30,31
In some tumor types, increased nestin expression correlates with the tumor grade and indicates an immature and invasive phenotype of transformed cells.32 Moreover, a correlation between the levels of nestin expression in tumor tissue and the clinical course of the disease was reported for breast carcinoma, ovarian carcinoma, gastrointestinal tumors, germ cell tumors, osteosarcoma, ependymoma, and melanoma.33–39 Thus, nestin expression is considered to serve as a prognostic marker in these cancer types; however, its prognostic value should be verified in large cohorts of the patients. In contrast, no such relationship was found for pancreatic adenocarcinoma.40,41 Interestingly, the use of nestin as a predictive marker of a poor response to conventional therapy and to novel therapeutic agents was recently reported for multiple myeloma.42
The evidence regarding nestin expression in hematological malignancies is limited to a small number of studies of multiple myeloma. Nestin mRNA was detected in NCAM-positive cells of human myeloma cell lines and in primary myeloma cells.43 The nestin protein was immunodetected in mature plasma cells from multiple myeloma patients and in myeloma cell lines.44 Furthermore, an association between increased nestin expression and the pathogenesis of multiple myeloma has been reported.42
Nestin as a Putative Marker of CSCs
At present, there is no consensus concerning a “universal” marker for the identification of CSCs in various tumor types.9 Clearly, a tumor-specific CSC phenotype should be characterized by the co-expression of several markers, both intracellular and surface-associated. As mentioned above, one putative CSC marker is nestin, which is often co-expressed with other stem cell markers (Table1), such as CD133, Oct3/4 and Sox-2 (Fig.3).
Table 1.
Overview of known cancer stem cell (CSC) phenotypes in various human solid tumors and functional assays used for their identification
| Tumor type | Phenotype of CSCs | Functional assays | References | |
|---|---|---|---|---|
| In vitro | In vivo | |||
| Neurogenic tumors | ||||
| Medulloblastoma | Nestin, CD133 | Neurospheres, differentiation assay | Xenograft formation | 29,45 |
| Nestin, Sox2, CD133 | Neurospheres, differentiation assay, drug resistance | Xenograft formation | 46 | |
| Nestin, Sox2, CD133, β-catenin | Medullospheres | 47 | ||
| Ependymoma | Nestin, CD133 | Neurospheres, differentiation assay | 29 | |
| Nestin, Sox2, CD133 | Neurospheres, differentiation assay, drug resistance | Xenograft formation | 46 | |
| Glioma | Nestin, CD133 | Neurospheres, differentiation assay | Xenograft formation | 29,45 |
| Nestin, Sox2, CD133 | Neurospheres, differentiation assay, drug resistance | Xenograft formation | 46 | |
| Glioblastoma | Nestin, CD133, Musashi-1, Sox2 | Neurospheres, differentiation assay, clonogenic assay | Xenograft formation | 48 |
| CNS primitive neuroectodermal tumor | Nestin, Sox2, CD133 | Neurospheres, differentiation assay, drug resistance | Xenograft formation | 46 |
| Malignant peripheral nerve sheath tumors | CD133, Oct4, nestin, NGFR (CD271) | Spheres, differentiation assay | Xenograft formation | 49 |
| Mesenchymal tumors | ||||
| Rhabdomyosarcoma | Nestin, CD133 | Clonogenic assay | Xenograft formation | 53 |
| Osteosarcoma | Nestin, CD133 | 54 | ||
| CD133, Oct3/4, Sox2, Nanog, nestin | Sarcospheres, clonogenic test, in vitro tumorigenic assay, side population, differentiation assay | 55 | ||
| ABCA5, CBX3, ABCG2, ALDH | Spheres, clonogenic test, drug resistance | Xenograft formation | 56 | |
| Chondrosarcoma | CD133, Oct3/4, Sox2, Nanog, nestin | Sarcospheres, clonogenic test, in vitro tumorigenic assay, side population, differentiation assay | Xenograft formation | 55 |
| Fibrosarcoma | CD133, Oct3/4, Sox2, Nanog, nestin | Sarcospheres, clonogenic test, in vitro tumorigenic assay, side population | Xenograft formation | 55 |
| Epithelial tumors | ||||
| Ovarian carcinoma | Nestin, Oct4, Nanog | Spheroids, differentiation assay | Xenograft formation | 57 |
| Nestin, Nanog, Oct4, Sox2, ABCG2, CD133, CD117 | Spheres, drug resistance | Xenograft formation | 58 | |
| Nestin, Oct4, Nanog, Sox2, Bmi-1, CD133, CD44, CD24, ALDH1, CD117, ABCG2 | Spheroids, drug resistance | Xenograft formation | 59 | |
| Oral squamous cell carcinoma | Nestin, CD133, Oct4, Nanog, ABCG2, CD117 | Spheres, soft-agar assay, differentiation assay | Xenograft formation | 60 |
| Prostate carcinoma | CD49b, CD49f, CD44, deltaNp63, nestin, CD133, Nanog, Oct-4, Bmi-1, Jagged-1, Hes-1, Patched, Smoothened, CD201 | Prostaspheres, clonogenic assay | 61 | |
| CD117, ABCG2, Nanog, Oct4, Sox2, nestin, CD133 | Drug resistance | Xenograft formation | 62 | |
| Gallbladder carcinoma | CD133, nestin, Oct4, Nanog | Spheres, drug resistance, differentiation assay | Xenograft formation | 63 |
| Non-small-cell lung cancer | Nestin, CD133 | 64 | ||
| Lung cancers | CD44, CD90, Nanog, Oct4 | Spheres, irradiation resistance, clonogenic assay | Xenograft formation | 65 |
| Colon cancer | Nestin, Bmi1 | Spheres, soft agar colony formation assay, invasion assay, drug resistance | 68 | |
| Breast cancer | CD44, Oct4, nestin, CD24− | Mammospheres | 69 | |
| CD44, ESA, nestin, CD24− | Mammospheres, invasion assay | Xenograft formation | 70 | |
| Nanog, Oct3/4, nestin, Sox2, CD34 | 71 | |||
| Gastric adenocarcinoma | Nestin, CD44 | 72 | ||
| Pancreatic ductal adenocarcinoma | ALDH1A1, ABCG2, nestin | Spheres, invasion assay, side population, | 74 | |
| CD133, CD44, Oct4, nestin | Drug resistance | Xenograft formation | 76 | |
CNS, central nervous system.
Fig 3.
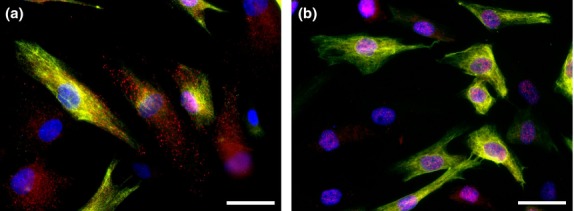
Co-expression of nestin and other putative cancer stem cells markers in the NSTS-11 rhabdomyosarcoma cell line. Representative double labeling for nestin and CD133 (a) and for nestin and Oct4 (b). CD133 (a) and Oct4 (b) were stained by indirect immunofluorescence using Alexa 568-labeled secondary antibody (red); nestin (a, b) was stained by the same method using Alexa 488-labeled secondary antibody (green); nuclei were counterstained with DAPI (blue). Bar = 25 μm.
Identification of nestin in CSCs of neurogenic tumors
The first study of nestin in CSCs revealed its expression in brain tumor stem cells (BTSCs) isolated from different types of human CNS tumors (medulloblastoma, astrocytoma, ependymoma, and ganglioglioma). All of these CSC populations were formed nestin-positive spheres, which were subsequently grown in vitro. However, nestin was downregulated during the induced differentiation of BTSCs. Moreover, BTSCs isolated from glioblastoma or medulloblastoma initiated tumor growth in the brains of NOD/SCID mice.29,45 Detailed analyses of cell lines derived from other types of pediatric brain tumors (i.e., ependymoma, medulloblastoma, glioma, and primitive neuroectodermal tumors) revealed that the number of nestin-positive CSCs is increased in spheres exhibiting a CSC phenotype based on functional assays. Furthermore, sphere-derived cells showed a higher capacity for multilineage differentiation and greater resistance to etoposide than monolayer-derived cells.46 Other evidence of increased nestin expression in spheres was described using glioblastoma and medulloblastoma cell lines47,48 and cells derived from peripheral nerve sheath tumor.49 This increase in nestin expression due to sphere formation appears to be a common phenomenon associated with the stemness of cells within the sphere. The studies mentioned above reported the co-expression of nestin with CD133 or both CD133 and Sox-2 as a common CSC phenotype in tumors of neurogenic origin.29,46–49
Nestin-positive cells in xenografts from human astrocytoma- and glioblastoma-derived CSCs showed significant co-expression of proliferating cell nuclear antigen (PCNA) indicating proliferation activity of these cells, as well as co-expression of vascular cell adhesion molecule-1, which may affect cell migration and spreading.50
In glioma-prone mice with nestin-GFP tagged transgene expressed both in neural stem cells and in relatively quiescent glioma cells, re-initiation of cell division and growth of nestin-positive glioma cells were observed after eradication of proliferating tumor cells by temozolomide.51 These results are in accordance with cancer stem cell theory concerning relapses after conventional chemotherapy aimed at proliferating tumor cells.
Furthermore, neural stem cells and “more stem-like glioma-initiating cells” showed similar biological features as well as gene expression profiles, particularly enrichment of Ca2+ signaling genes. High expression of Ca2+ channels (i.e., AMPA-selective glutamate receptor 1) correlated with expression of nestin and brain lipid-binding protein as well as with sensitivity to Ca2+ channel blockers in glioma-initiating cells. Nestin, together with brain lipid-binding protein and glutamate receptor 1, are thus considered a novel combination of glioma CSC markers and the enhanced sensitivity to Ca2+ could be included in functional tests of glioma CSCs.52
Identification of nestin in CSCs from mesenchymal tumors
Surprisingly, the coexpression of nestin with CD133, which was originally described as the CSC phenotype in neurogenic tumors, was also found in tumors of mesenchymal origin. The first evidence of nestin-/CD133-positive cells in rhabdomyosarcoma samples and cell lines (Fig.3a) was followed by verification of their tumorigenic potential based on functional assays.53 The same group also described the presence of nestin-/CD133-positive cells in osteosarcoma cell lines based on immunodetection and predicted the coexpression of these markers as a putative CSC phenotype.54 An additional study of osteosarcoma, chondrosarcoma, and fibrosarcoma cell lines indicated that nestin mRNA is expressed in sphere-forming subpopulations; however, adherent subpopulations of the same cell lines were identified as nestin-negative.55 Alternatively, one study of established osteosarcoma cell lines reported contrasting results: the same pattern of nestin expression (i.e., downregulation of nestin in adherent cells) was found in spheres and adherent populations of CHA59 cells based on real-time PCR and Western blotting, whereas a reverse pattern (i.e., downregulation of nestin in sarcospheres) was described in the HuO9 cell line.56 Interestingly, both spheres and adherent cells were detected as nestin-negative in the Saos-2 cell line,56 but strong nestin expression was previously identified in the same cell line based on immunofluorescence.54
Identification of nestin in CSCs from epithelial tumors
Cells coexpressing CD133 and nestin (a putative CSC phenotype) were also found in several types of epithelial tumors.
Early studies of ovarian adenocarcinoma, including high-grade serous ovarian carcinoma, identified a population of self-renewing CSCs that grew as sphere-forming clusters. Furthermore, these cells were tumorigenic in vivo, showing increased chemoresistance and expressed CSC markers, including nestin.57–59
Cultivation of oral squamous cell carcinoma cells in defined serum-free medium led to the formation of spheres enriched with cells exhibiting CSC phenotypes, including high expression of nestin and other stem cell markers (i.e., CD133, Oct-4, and Nanog), differentiation capacity, enhanced migratory capacity, and tumorigenicity. However, nestin was also found in sphere-like bodies of basal cells that showed limited proliferation potential and reduced expression of self-renewal genes.60
Similarly, stem-like prostate cancer cells expressing typical stem cell markers, such as Nanog, Oct4, Sox-2, CD133, and nestin, were isolated from prostate tumor tissue61 and from a prostate cancer cell line.62 In the first study, the obtained “prostaspheres” were maintained in vitro.61 The second study verified the proposed chemoresistance and tumorigenicity of these prostatic CSCs.62 Additionally, CSCs were obtained from human gallbladder carcinoma through sphere formation, and the expression of nestin, CD133, Oct-4, and Nanog was identified in these cells.63
Nestin/CD133-positive cells were also detected in paraffin blocks of 121 non-small-cell lung cancer samples based on immunohistochemistry and immunofluorescence; the co-localization of these two markers was detected in 17% of the samples, although the double-stained cells represented <1% of all positive cells. The authors concluded that CD133/nestin-positivity may serve as a marker of lung CSCs.64 Other relevant studies did not refer to nestin expression in lung CSCs65,66 and only mentioned the effect of nestin on cell proliferation.67
Treatment of an HCT-117 colon cancer cell line or primary colon cancer cells with interleukin-1β promoted sphere formation, upregulated the typical stemness markers nestin and Bmi1, and increased drug resistance. Moreover, the observed self-renewal status was accompanied by epithelial-mesenchymal transition (EMT), the upregulation of the EMT activator Zeb1, and the downregulation or loss of E-cadherin expression.68
Breast CSCs are typically isolated according to their ESA+/CD44+/CD24- phenotype. Interestingly, these cells also express Oct-4 and nestin and display increased tumorigenicity through the formation of mammospheres under in vitro conditions.69 The high expression of nestin is associated with a 100-fold increase in tumorigenicity in comparison with cells showing weak nestin expression and ESA+/CD44+/CD24- phenotype. Conversely, knockdown of nestin in breast CSCs led to cell cycle arrest, apoptosis induction, repression of cell migration, and inhibition of spontaneous EMT. On a molecular level, silencing of nestin disrupted Wnt/β-catenin activation, which is an important signaling pathway in breast CSCs.70 Furthermore, Oct-4/nestin-positivity in cancer tissues has been associated with younger age, malignant tumor grades, lymph node metastasis, and shorter survival.69,70 Co-expression of nestin with other established stem cell markers (e.g., Oct3/4 or Sox-2) was shown in circulating breast cancer cells.71
A correlation between the expression of nestin and CD44 was recently found in gastric adenocarcinoma; this study provided the first evidence of the nestin+/CD44+ phenotype in this type of tumor.72 In pancreatic ductal adenocarcinoma (PDAC), the expression of nestin as a putative marker of CSCs has recently been discussed.73,74 High levels of nestin together with ALDH1A1 and ABCG2 were detected in metastatic cells from human PDAC injected into NOG mice; moreover, these cells showed typical features of CSCs such as sphere formation and tumorigenicity. Downregulation of nestin by shRNA in these cells resulted in decreased capacity of both sphere formation in vitro and metastasizing in vivo.74 Nestin modulates nestin-positive cell invasion, EMT, and metastasis during the progression of PDAC. It was also shown that nestin expression in PDAC cells is regulated by the transforming growth factor-β1/Smad pathway: nestin expression is induced in a Smad4-dependent manner and under hypoxic conditions.75 Nestin was also detected in spheres together with CD133 and CD44,73 and the recently isolated Panc-1 stem-like cell line demonstrated coexpression of CD133, CD44, Oct4, and nestin.76 In contrast, no significant correlation between the CD133 and nestin expression patterns has been detected in PDAC tumor samples,41 and nestin expression did not correlate with the clinical course of PDAC.40
What is the Role of Nestin in CSCs?
In some tumor types, nestin expression correlates with aggressive growth, metastasis, and poor prognosis.25 Nevertheless, the role of nestin in transformed cells, especially CSCs, aside from its participation in cytoskeleton formation, remains unclear.
One primary hypothesis for the role of nestin in CSCs arose from an in vivo experiment: nestin knockout mice showed embryonic lethality and a reduced number of neuronal stem cells. Moreover, the remaining neuronal stem cells exhibited decreased self-renewal capacity and increased cell death, with no apparent defects in proliferation, differentiation, or cytoskeletal integrity.77 A proposed relationship between nestin expression and self-renewal is supported by the previous finding that the nestin promoter is activated by Notch, which binds to the second intron enhancer element of the nestin gene. The combined activation of Notch and KRAS led to the proliferation of nestin/PCNA-positive cells in lesions along the subventricular zone of mice, suggesting that these lesions may represent premalignant stages of tumorigenesis and that nestin/PCNA-positive cells may exhibit CSC phenotypes.78
It has been shown in neuroblastoma cells that E-box sequences in the regulatory second intron of the nestin gene are specific regions to which N-myc transcription factors can bind. Furthermore, an increase in N-myc protein levels subsequently enhances nestin expression and cell proliferation and motility.79 In addition, N-myc regulates the expression of several genes that encode stem-related factors, including lif, klf2, klf4, and lin28b.80 Thus, nestin unequivocally belongs to the group of stemness genes regulated by N-myc.
An additional mouse study indicated that nestin-positive CSCs localized to the perivascular niche of medulloblastoma tumor tissue and exhibited radioresistance through the activation of the AKT/PI3K and p53 signaling pathways. These characteristics are in contrast to those of the rest of the tumor mass, which undergoes apoptosis. In medulloblastoma displaying extensive nodularity, the loss of the PTEN gene in combination with Sonic hedgehog overexpression induced the formation of tumors from nestin-positive progenitor cells. Furthermore, the activation of the AKT signaling pathway using ionizing irradiation led to the proliferation of nestin-positive CSCs, whereas the majority of cells in the tumor mass were differentiated.81
A cytoprotective function of nestin was shown in neuronal stem cells, as overexpression of nestin inhibited oxidant-induced apoptosis, including the activity of cyclin-dependent kinase 5, which serves as an important regulator of neuronal development and function.82 However, this mechanism of nestin activity has not been demonstrated in CSCs.
The functions of many proteins are regulated by their cellular localization. Nestin is predominantly localized to the cytoplasm, where it participates in the formation of IFs as integral components of the cytoskeleton in animal cells. However, based on confocal microscopy and immunodetection using ultrathin sections by transmission electron microscopy, strong nestin signals were detected in the nuclei of the GM7 glioblastoma cell line;83 this cell line was later characterized as positive for both nestin and CD133.84 Subsequently, the nuclear localization of nestin was found in two neuroblastoma cell lines and in one medulloblastoma cell line out of a total of 11 neurogenic patient-derived cell lines. These results suggest that this phenomenon is not particularly rare in transformed cells. However, the percentage of cells displaying nestin-positive nuclei was approximately 10% in these cell lines, suggesting that these cells show no great advantage in clonal selection.85 A role for nestin in the nucleus is not yet clear, although previous cross-linking experiments revealed that nestin binds to DNA in N-myc-amplified N-type neuroblastoma cell lines.79
Recently, nestin was found on the cell surface of 14 glioma stem cell lines, and the positivity for nestin at the cell surface ranged from 1.4% to 70%. Isolated nestin-positive cells showed increased sphere-forming capacity and sphere size. The authors proposed that surface nestin underwent post-translational modification by γ-secretase and that, after plasma membrane exposure, nestin may be used as a marker for the isolation and characterization of CSCs in glioblastoma.86 Surface nestin was originally identified in murine glioma stem cells (GSCs) and it was reported as a ∼60-kDa N-terminal isotype of nestin. Surface nestin was detectable with GSC-targeting peptide with selective binding affinity to undifferentiated GSCs. Glioma stem cell-targeting peptide conjugated with fluorochrome is able to undergo internalization and its colocalization with nestin-positive GSCs was observed both in a glioma cell population maintained in vitro as well as in a murine glioblastoma model in vivo.87 Surface nestin thus seems to be a suitable target molecule for identification of CSCs in some types of brain tumors, especially in gliomas. In addition to the detection methods described above, the application of specific mAb against the N-terminal part of the nestin molecule or of GSC-targeting peptide could also be used for eradication of these cells because peptide- and antibody-based radiopharmaceuticals or cytotoxic conjugates are discussed as very promising strategies in cancer treatment.88–90
In conclusion, nestin is undoubtedly a putative CSC marker of both neurogenic tumors and tumors of epithelial or mesenchymal origin. The coexpression of nestin with other CSC markers, particularly CD133, Oct3/4, and Sox-2, should be considered as a specific CSC phenotype; however, the verification of this phenotype using functional assays is required for each tumor type. Some experimental results suggest that nestin expression is closely associated with self-renewal capacity, although the detailed mechanisms underlying this relationship remain unclear.
Acknowledgments
The authors thank Alena Nunukova for skillful technical assistance. This study was supported by grants from the Internal Grant Agency of the Czech Ministry of Healthcare (no. NT13443-4), the European Regional Development Fund, and the State Budget of the Czech Republic (RECAMO CZ.1.05./2.1.00/03.0101 and CEB OP VK CZ.1.07/2.3.00/20.0183).
Disclosure Statement
The authors have no conflict of interests.
References
- Ratajczak MZ. Cancer stem cells – normal stem cells “Jedi” that went over to the “dark side”. Folia Histochem Cytobiol. 2005;43:175–81. [PubMed] [Google Scholar]
- Fábián A, Barok M, Vereb G, Szöllosi J. Die hard: are cancer stem cells the Bruce Willises of tumor biology? Cytometry A. 2009;75:67–74. doi: 10.1002/cyto.a.20690. [DOI] [PubMed] [Google Scholar]
- Friedman GK, Gillespie GY. Cancer stem cells and pediatric solid tumors. Cancers (Basal) 2011;3:298–318. doi: 10.3390/cancers3010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Hagio M, Ishiwata T. Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol. 2013;19:42–8. doi: 10.3748/wjg.v19.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Neuzil J, Stantic M, Zobalova R, et al. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what’s in the name? Biochem Biophys Res Commun. 2007;355:855–9. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- McDonald SA, Graham TA, Schier S, Wright NA, Alison MR. Stem cells and solid cancers. Virchows Arch. 2009;455:1–13. doi: 10.1007/s00428-009-0783-1. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Rizzo P, Osipo C, Miele L. The Cancer Stem Cell Hypothesis. In: Bagley RG, Teicher BA, editors. Stem Cells and Cancer (Cancer Drug Discovery) New York: Humana Press; 2009. pp. 3–14. [Google Scholar]
- Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol. 2011;223:147–61. doi: 10.1002/path.2793. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–28. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Strelkov SV, Burkhard P, Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest. 2009;119:1772–83. doi: 10.1172/JCI38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc C, Etienne-Manneville S. Intermediate filaments in cell migration and invasion: the unusual suspects. Curr Opin Cell Biol. 2015;32:102–12. doi: 10.1016/j.ceb.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Dey P, Togra J, Mitra S. Intermediate filament: structure, function, and applications in cytology. Diagn Cytopathol. 2014;42:628–35. doi: 10.1002/dc.23132. [DOI] [PubMed] [Google Scholar]
- Cheng L, Jin Z, Liu L, et al. Characterization and promoter analysis of the mouse nestin gene. FEBS Lett. 2004;565:195–202. doi: 10.1016/j.febslet.2004.03.097. [DOI] [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Zhong H, Jin Z, Chen Y, et al. First intron of nestin gene regulates its expression during C2C12 myoblast differentiation. Acta Biochim Biophys Sin. 2008;40:526–32. doi: 10.1111/j.1745-7270.2008.00428.x. [DOI] [PubMed] [Google Scholar]
- Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. 2005;20:665–71. doi: 10.14670/HH-20.665. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H. Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol. 2004;24:8834–46. doi: 10.1128/MCB.24.20.8834-8846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Do JT, Araúzo-Bravo MJ, et al. Epigenetic hierarchy governing Nestin expression. Stem Cells. 2009;27:1088–97. doi: 10.1002/stem.43. [DOI] [PubMed] [Google Scholar]
- Messam CA, Hou J, Major EO. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol. 2000;161:585–96. doi: 10.1006/exnr.1999.7319. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, et al. Nestin expression – a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–22. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupkova O, Jr, Loja T, Zambo I, Veselska R. Nestin expression in human tumors and tumor cell lines. Neoplasma. 2010;57:291–8. doi: 10.4149/neo_2010_04_291. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol. 2011;17:409–18. doi: 10.3748/wjg.v17.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38:165–9. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- Calderone A. Nestin+ cells and healing the infarcted heart. Am J Physiol Heart Circ Physiol. 2012;302:H1–9. doi: 10.1152/ajpheart.00716.2011. [DOI] [PubMed] [Google Scholar]
- Daniel C, Albrecht H, Lüdke A, Hugo C. Nestin expression in repopulating mesangial cells promotes their proliferation. Lab Invest. 2008;88:387–97. doi: 10.1038/labinvest.2008.5. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- Veselska R, Skoda J, Neradil J. Detection of cancer stem cell markers in sarcomas. Klin Onkol. 2012;25:2S16–20. [PubMed] [Google Scholar]
- Sterlacci W, Savic S, Fiegl M, Obermann E, Tzankov A. Putative stem cell markers in non-small-cell lung cancer: a clinicopathologic characterization. J Thorac Oncol. 2014;9:41–9. doi: 10.1097/JTO.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Ehrmann J, Kolár Z, Mokry J. Nestin as a diagnostic and prognostic marker: immunohistochemical analysis of its expression in different tumours. J Clin Pathol. 2005;58:222–3. doi: 10.1136/jcp.2004.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar Z, Ehrmann J, Jr, Turashvili G, Bouchal J, Mokry J. A novel myoepithelial/progenitor cell marker in the breast? Virchows Arch. 2007;450:607–9. doi: 10.1007/s00428-007-0403-x. [DOI] [PubMed] [Google Scholar]
- He QZ, Luo XZ, Zhou Q, et al. Expression of nestin in ovarian serous cancer and its clinicopathologic significance. Eur Rev Med Pharmacol Sci. 2013;17:2896–901. [PubMed] [Google Scholar]
- Parfitt JR, McLean CA, Joseph MG, Streutker CJ, Al-Haddad S, Driman DK. Granular cell tumours of the gastrointestinal tract: expression of nestin and clinicopathological evaluation of 11 patients. Histopathology. 2006;48:424–30. doi: 10.1111/j.1365-2559.2006.02352.x. [DOI] [PubMed] [Google Scholar]
- Sakurada K, Saino M, Mouri W, Sato A, Kitanaka C, Kayama T. Nestin expression in central nervous system germ cell tumors. Neurosurg Rev. 2008;31:173–6. doi: 10.1007/s10143-007-0115-3. [DOI] [PubMed] [Google Scholar]
- Zambo I, Hermanova M, Adamkova Krakorova D, et al. Nestin expression in high-grade osteosarcomas and its clinical significance. Oncol Rep. 2012;27:1592–8. doi: 10.3892/or.2012.1687. [DOI] [PubMed] [Google Scholar]
- Nambirajan A, Sharma MC, Gupta RK, Suri V, Singh M, Sarkar C. Study of stem cell marker nestin and its correlation with vascular endothelial growth factor and microvascular density in ependymomas. Neuropathol Appl Neurobiol. 2014;40:714–25. doi: 10.1111/nan.12097. [DOI] [PubMed] [Google Scholar]
- Sabet MN, Rakhshan A, Erfani E, Madjd Z. Co-expression of putative cancer stem cell markers, CD133 and nestin, in skin tumors. Asian Pac J Cancer Prev. 2014;15:8161–9. doi: 10.7314/apjcp.2014.15.19.8161. [DOI] [PubMed] [Google Scholar]
- Lenz J, Karasek P, Jarkovsky J, et al. Clinicopathological correlations of nestin expression in surgically resectable pancreatic cancer including an analysis of perineural invasion. J Gastrointestin Liver Dis. 2011;20:389–96. [PubMed] [Google Scholar]
- Kim HS, Yoo SY, Kim KT, Park JT, Kim HJ, Kim JC. Expression of the stem cell markers CD133 and nestin in pancreatic ductal adenocarcinoma and clinical relevance. Int J Clin Exp Pathol. 2012;5:754–61. [PMC free article] [PubMed] [Google Scholar]
- Svachova H, Kryukov F, Kryukova E, et al. Nestin expression throughout multistep pathogenesis of multiple myeloma. Br J Haematol. 2014;164:701–9. doi: 10.1111/bjh.12689. [DOI] [PubMed] [Google Scholar]
- Liu S, Otsuyama K, Ma Z, et al. Induction of multilineage markers in human myeloma cells and their down-regulation by interleukin 6. Int J Hematol. 2007;85:49–58. doi: 10.1532/IJH97.06132. [DOI] [PubMed] [Google Scholar]
- Svachova H, Pour L, Sana J, Kovarova L, Raja KR, Hajek R. Stem cell marker nestin is expressed in plasma cells of multiple myeloma patients. Leuk Res. 2011;35:1008–13. doi: 10.1016/j.leukres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Hussein D, Punjaruk W, Storer LC, et al. Pediatric brain tumor cancer stem cells: cell cycle dynamics, DNA repair, and etoposide extrusion. Neuro Oncol. 2011;13:70–83. doi: 10.1093/neuonc/noq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini C, Ercole E, Mandili G, et al. Medullospheres from DAOY, UW228 and ONS-76 cells: increased stem cell population and proteomic modifications. PLoS One. 2013;8:e63748. doi: 10.1371/journal.pone.0063748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopino F, Angelucci C, Piacentini R, et al. Isolation of cancer stem cells from three human glioblastoma cell lines: characterization of two selected clones. PLoS One. 2014;9:e105166. doi: 10.1371/journal.pone.0105166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyra M, Kluwe L, Hagel C, et al. Cancer stem cell-like cells derived from malignant peripheral nerve sheath tumors. PLoS One. 2011;6:e21099. doi: 10.1371/journal.pone.0021099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnescu O, Brehar FM, Bleotu C, Gorgan RM. Co-localization of PCNA, VCAM-1 and caspase-3 with nestin in xenografts derived from human anaplastic astrocytoma and glioblastoma multiforme tumor spheres. Micron. 2011;42:793–800. doi: 10.1016/j.micron.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Niklasson M, Marinescu VD, et al. Selective calcium sensitivity in immature glioma cancer stem cells. PLoS One. 2014;9:e115698. doi: 10.1371/journal.pone.0115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana J, Zambo I, Skoda J, et al. CD133 expression and identification of CD133/nestin positive cells in rhabdomyosarcomas and rhabdomyosarcoma cell lines. Anal Cell Pathol. 2011;34:303–18. doi: 10.3233/ACP-2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselska R, Hermanova M, Loja T, et al. Nestin expression in osteosarcomas and derivation of nestin/CD133 positive osteosarcoma cell lines. BMC Cancer. 2008;8:300. doi: 10.1186/1471-2407-8-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirino V, Desiderio V, Paino F, et al. Human primary bone sarcomas contain CD133 + cancer stem cells displaying high tumorigenicity in vivo. FASEB J. 2011;25:2022–30. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- Saini V, Hose CD, Monks A, et al. Identification of CBX3 and ABCA5 as putative biomarkers for tumor stem cells in osteosarcoma. PLoS One. 2012;7:e41401. doi: 10.1371/journal.pone.0041401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- Ma L, Lai D, Liu T, Cheng W, Guo L. Cancer stem-like cells can be isolated with drug selection in human ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin. 2010;42:593–602. doi: 10.1093/abbs/gmq067. [DOI] [PubMed] [Google Scholar]
- He QZ, Luo XZ, Wang K, et al. Isolation and characterization of cancer stem cells from high-grade serous ovarian carcinomas. Cell Physiol Biochem. 2014;33:173–84. doi: 10.1159/000356660. [DOI] [PubMed] [Google Scholar]
- Chiou SH, Yu CC, Huang CY, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–95. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- Guzmán-Ramírez N, Völler M, Wetterwald A, et al. In vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissue. Prostate. 2009;69:1683–93. doi: 10.1002/pros.21018. [DOI] [PubMed] [Google Scholar]
- Liu T, Xu F, Du X, et al. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340:265–73. doi: 10.1007/s11010-010-0426-5. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Gao J, Wang M, et al. CD133(+) gallbladder carcinoma cells exhibit self-renewal ability and tumorigenicity. World J Gastroenterol. 2011;17:2965–71. doi: 10.3748/wjg.v17.i24.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janikova M, Skarda J, Dziechciarkova M, et al. Identification of CD133+/nestin+ putative cancer stem cells in non-small cell lung cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:321–6. doi: 10.5507/bp.2010.048. [DOI] [PubMed] [Google Scholar]
- Wang P, Gao Q, Suo Z, et al. Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS One. 2013;8:e57020. doi: 10.1371/journal.pone.0057020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alamgeer M, Peacock CD, Matsui W, Ganju V, Watkins DN. Cancer stem cells in lung cancer: evidence and controversies. Respirology. 2013;18:757–64. doi: 10.1111/resp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakuwa O, Maeno K, Kunii E, et al. Involvement of intermediate filament nestin in cell growth of small-cell lung cancer. Lung Cancer. 2013;81:174–9. doi: 10.1016/j.lungcan.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Cao X, Zhang Y, et al. Co-expression of Oct-4 and Nestin in human breast cancers. Mol Biol Rep. 2012;39:5875–81. doi: 10.1007/s11033-011-1398-6. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Lu P, Zhang H, et al. Nestin positively regulates the Wnt/β-catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Res. 2014;16:408. doi: 10.1186/s13058-014-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou P, Toloudi M, Chatziioannou M, Ioannou E, Papasotiriou I. Cancer stem cells stemness transcription factors expression correlates with breast cancer disease stage. Curr Stem Cell Res Ther. 2012;7:415–9. doi: 10.2174/157488812804484639. [DOI] [PubMed] [Google Scholar]
- Dhingra S, Feng W, Brown RE, et al. Clinicopathologic significance of putative stem cell markers, CD44 and nestin, in gastric adenocarcinoma. Int J Clin Exp Pathol. 2011;4:733–41. [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Yoshimura H, Naito Z, Ishiwata T. The roles and molecular mechanisms of nestin expression in cancer with a focus on pancreatic cancer. J Carcinog Mutagen. 2013;S9:002. [Google Scholar]
- Matsuda Y, Yoshimura H, Ueda J, Naito Z, Korc M, Ishiwata T. Nestin delineates pancreatic cancer stem cells in metastatic foci of NOD/Shi-scid IL2Rγ(null) (NOG) mice. Am J Pathol. 2014;184:674–85. doi: 10.1016/j.ajpath.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HT, Weng CC, Hsiao PJ, et al. Stem cell marker nestin is critical for TGF beta1-mediated tumor progression in pancreatic cancer. Mol Cancer Res. 2013;11:768–79. doi: 10.1158/1541-7786.MCR-12-0511. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhu H, Zhu Y, et al. CD133(+)/CD44(+)/Oct4(+)/Nestin(+) stem-like cells isolated from Panc-1 cell line may contribute to multi-resistance and metastasis of pancreatic cancer. Acta Histochem. 2013;115:349–56. doi: 10.1016/j.acthis.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Park D, Xiang AP, Mao FF, et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells. 2010;28:2162–71. doi: 10.1002/stem.541. [DOI] [PubMed] [Google Scholar]
- Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8:1072–82. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SK, Messam CA, Spengler BA, Biedler JL, Ross RA. Nestin is a potential mediator of malignancy in human neuroblastoma cells. J Biol Chem. 2004;279:27994–9. doi: 10.1074/jbc.M312663200. [DOI] [PubMed] [Google Scholar]
- Cotterman R, Knoepfler PS. N-Myc regulates expression of pluripotency genes in neuroblastoma including lif, klf2, klf4, and lin28b. PLoS One. 2009;4:e5799. doi: 10.1371/journal.pone.0005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–8. doi: 10.4161/cc.7.10.5954. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808–19. doi: 10.1038/sj.emboj.7601366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselska R, Kuglik P, Cejpek P, et al. Nestin expression in the cell lines derived from glioblastoma multiforme. BMC Cancer. 2006;6:32. doi: 10.1186/1471-2407-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loja T, Chlapek P, Kuglik P, et al. Characterization of a GM7 glioblastoma cell line showing CD133 positivity and both cytoplasmic and nuclear localization of nestin. Oncol Rep. 2009;21:119–27. [PubMed] [Google Scholar]
- Krupkova O, Jr, Loja T, Redova M, et al. Analysis of nuclear nestin localization in cell lines derived from neurogenic tumors. Tumour Biol. 2011;32:631–9. doi: 10.1007/s13277-011-0162-9. [DOI] [PubMed] [Google Scholar]
- Jin X, Jin X, Jung JE, Beck S, Kim H. Cell surface Nestin is a biomarker for glioma stem cells. Biochem Biophys Res Commun. 2013;433:496–501. doi: 10.1016/j.bbrc.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Beck S, Jin X, Yin J, et al. Identification of a peptide that interacts with Nestin protein expressed in brain cancer stem cells. Biomaterials. 2011;32:8518–28. doi: 10.1016/j.biomaterials.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Okarvi SM. Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer. Cancer Treat Rev. 2008;34:13–26. doi: 10.1016/j.ctrv.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Brown KC. Peptidic tumor targeting agents: the road from phage display peptide selections to clinical applications. Curr Pharm Des. 2010;16:1040–54. doi: 10.2174/138161210790963788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, et al. Tumor immunotargeting using innovative radionuclides. Int J Mol Sci. 2015;16:3932–54. doi: 10.3390/ijms16023932. [DOI] [PMC free article] [PubMed] [Google Scholar]


