Abstract
Mammographic screening with clinical breast examination has been recommended in Japan since 2000. Although mammographic screening without clinical breast examination has not been recommended, its introduction is anticipated. The efficacies of mammographic screening with and without clinical breast examination were evaluated based on the results of randomized controlled trials. PubMed and other databases for studies published between 1985 and 2014 were searched. The study design was limited to randomized controlled trials to evaluate mortality reduction from breast cancer. Five studies were eligible for meta-analysis of mammographic screening without clinical breast examination. The relative risk for women aged 40–74 years was 0.75 (95% confidence interval, 0.67–0.83). Three studies evaluated the efficacy of mammographic screening with clinical breast examination. The relative risk for women aged 40–64 years was 0.87 (95% confidence interval, 0.77–0.98). The number needed to invite was always lower in mammographic screening without clinical breast examination than in mammographic screening with clinical breast examination. In both screening methods, the number needed to invite was higher in women aged 40–49 years than in women aged 50–70 years. These results suggest that mammographic screening without clinical breast examination can afford higher benefits to women aged 50 years and over. Although evidence of the efficacy of mammographic screening without clinical breast examination was confirmed based on the results of the randomized controlled trials, a Japanese study is needed to resolve local problems.
Keywords: Breast cancer, cancer screening, mammography, meta-analysis, review
Breast cancer is currently the most common cancer in Japan and accounts for 19.0% of all new cancers.1 The age-standardized rate has been reported to be 51.5 per 100 000 women. The incidence rate of breast cancer initially increased gradually between 1975 and 1999 and has risen steeply since 2000 when mammography was introduced for breast cancer screening. In North America and Europe, the incidence of breast cancer has increased according to age. In Japan, the highest incidence rate of breast cancer has been observed in women aged 45–49 years.1
Japan is the first among East Asian countries to introduce breast cancer screening, and it has a unique program for population-based screening. In 1987, the Japanese government approved the introduction of breast cancer screening in Japan.2 The first screening method was clinical breast examination with women aged 30 years and over as the target population. In 2000, mammographic screening was added for women aged 50 years and over, but clinical breast examination was used for women aged 30–49 years. Since 2004, a combination of mammography and clinical breast examination has been recommended for women aged 40 years and over as population-based screening. However, in most developed countries, mammographic screening without clinical breast examination has been the standard method for breast cancer screening. In the previous evidence report for cancer screening in Japan, it was not clearly specified why mammographic screening without clinical breast examination is not recommended.3 Although mammographic screening without clinical breast examination has not been recommended, its introduction to local communities is anticipated owing to limitations in specialists who can carry out clinical breast examination. To successfully introduce mammographic screening without clinical breast examination, the efficacy of mammography must be evaluated with and without clinical breast examination. However, most guidelines and evidence reports have combined the results of a meta-analysis for mammographic screening with and without clinical breast examination.4,5 There has been a lengthy discussion regarding the appropriateness of including women aged 40–49 years in the target population for breast cancer screening.4,5 In most European countries, the target age group is 50–69 years, excluding the 40–49 years age group.6
To confirm evidence of the effectiveness of the Japanese screening program and to identify the best available method for breast cancer screening in Japan, we carried out a systematic review and meta-analysis of randomized controlled trials (RCTs) with and without mammographic screening. The results of the systematic review and meta-analysis were used for the development of comprehensive guidelines for breast cancer screening published by the National Cancer Center, Japan.
Methods
Systematic review of published reports
To identify the individual efficacy of mammographic screening with and without clinical breast examination, we searched PubMed, Web of Science, Igaku-Cyuo zasshi, and J Dream databases for studies using search terms such as “breast cancer”, “mammography”, “clinical breast examination”, “physical breast examination”, or “mortality reduction”, published between January 1985 and April 2012. Additional references recommended were identified and included as needed. If the result from a branch of a large-scale RCT was published, the study was included. In addition, we searched for articles with revised results based on an extended follow-up and other RCTs regarding mammographic screening to evaluate mortality reduction from breast cancer from April 2012 to December 2014. The searches were limited to English language or Japanese language publications. Original articles published after peer review were included, whereas guidelines and evidence reports were excluded. The study design was limited to RCTs to evaluate mortality reduction from breast cancer. Modeling studies were not included. The RCTs for mammographic screening with and without clinical breast examination compared with a no screening group with the usual care were selected.
To select appropriate evidence for our research questions, we carried out a two-stage review: the title and abstract were initially checked and the full papers were subsequently reviewed. For the initial step, articles without an abstract were also excluded. Two reviewers screened the abstracts individually and subsequently reviewed the full papers of potentially relevant studies. To select appropriate evidence, a systematic review of the retrieved articles was carried out using the checklist according to the study design and the quality of the studies was defined.7 If the decision for the full paper review was inconsistent, the appropriateness of these studies was carefully discussed. Finally, adequate studies were selected and included in a meta-analysis.
Meta-analysis
Based on the results of the systematic review, we carried out a meta-analysis. Although the follow-up years were different among the studies, we cited the results of 13 years follow-up from the Cochrane review8 and original data from selected articles. Meta-analysis for RCTs of mammography with and without clinical breast examination was carried out for women of different age groups as follows: women aged 40–74 years (all age group), women aged 40–49 years, and women aged 50 years and over. For studies that reported cumulative count data, we carried out a Mantel–Haenszel fixed-effects meta-analysis to obtain the relative risk with the corresponding 95% confidence interval (CI). Statistical analyses were carried out using StatsDirect3 (StatsDirect, Altrincham, UK).
Comparison of benefit and harm
To compare benefit and harm, the number needed to invite (NNI) was calculated on the basis of the mortality risk from breast cancer in Japanese women. The NNI refers to the number needed to avoid one breast cancer death. The NNI can show the impact of the benefits of cancer screening, as well as suggest harms because unnecessary examinations increase with increasing number. To estimate the NNI in Japan, we used the prediction results for Japanese women9 and the meta-analysis results.
A high recall rate for diagnostic examination can also be considered as harm for mammographic screening participants owing to an increase in unnecessary examinations. We also calculated the number needed for diagnostic examination to avoid one breast cancer death on the basis of the recall rate of mammographic screening in communities.10 These results were compared between mammographic screening with and without clinical breast examination divided into different age groups from 40 to 70 years.
Results
Search of published works
The number of articles identified from the search using PubMed and other databases was 5270. After a two-stage review, 110 English articles were selected. From these 110 articles, six RCTs for mammographic screening without clinical breast examination were identified: Malmö study,11,12 Canadian study II,13–15 Swedish Two-County study,16–22 Stockholm study,23,24 Gothenburg study,25,26 and the UK Age trial.27 Three RCTs for mammographic screening with clinical breast examination were also identified as follows: New York HIP study,28 Edinburgh study,29 and Canadian study I.30,31 The Canadian studies consisted of two groups with different targets: women aged 50–59 years for Canadian study II,13–15 and women aged 40–49 years for Canadian study I.30,31 In Canadian study II, the screening method for the intervention group was mammography with clinical breast examination; clinical breast examination was also provided for the control group with the same frequency as that for the intervention group.13–15 In Canadian study I, the screening method for the intervention group was mammography with clinical breast examination; clinical breast examination was provided for the control group only at the first screening.30,31 Based on the inclusion criteria related to a comparator, we excluded Canadian study II from the evidence of mammography without clinical breast examination, and included Canadian study I as the evidence of mammography with clinical breast examination. From April 2012 to December 2014, although the revised results were reported in a Canadian study, there were no additional studies to evaluate mortality reduction from breast cancer.15
Evidence of mammographic screening with and without clinical breast examination
Mammographic screening without clinical breast examination
Five RCTs of mammographic screening without clinical breast examination were identified for mortality reduction from breast cancer (Table1).11–27 Each of these studies began in the 1980s, except the UK Age trial which started in 1991. Randomized allocation was performed at individual base except the Swedish Two-County study. Although the screening method was the same in these studies, the target age group, screening interval, and follow-up periods were different (Table1). Although the target age group was different among the five RCTs, all of these studies included women aged in their 40s as their target age group. In the UK Age trial, the study targets were limited to women aged 39–41 years years because the aim of the trial was evaluation of the efficacy of mammography for women aged in their 40s.27 The screening view was mainly one-view, but two-view was used at the first screening in the Malmö study, Gothenburg study, and UK Age trial. The screening interval for women aged 50 years and over was from 18 to 33 months. The results were analyzed using the intention to treat method in all studies.
Table 1.
Randomized controlled trials for evaluation of mammographic screening without clinical breast examination
| Malmö I and II | Swedish Two-County | Stockholm | Gothenburg | UK Age trial | |
|---|---|---|---|---|---|
| Starting year of the study | 1976 | 1977 | 1981 | 1982 | 1991 |
| Randomization | Individual | Cluster | Birthday | Birthday | Individual |
| Number | 60 076 | 133 065 | 60 800 | 52 222 | 160 921 |
| Target age | 45–69 years/43–49 years | 38–75 years | 39–65 years | 39–59 years | 39–41 years |
| Screening method | MMG | MMG+SBE | MMG | MMG | MMG |
| View | First, two-view Subsequent, one-view or two-view | One-view | One-view | First, two-view Subsequent, one-view or two-view | First, two-view Subsequent, one-view or two-view |
| Screening interval, months | 18–24 | 24 (40s)–33 (50s) | 24–28 | 18 | 12 |
| Screening frequency | 6–8 | 2–4 | 2 | 4–5 | 8–10 |
| Screening periods, years | 12 | 7 | 4 | 7 | 8 |
| Participation rate, % | 74 | 85 | 82 | 84 | 81 |
| Relative risk (95%CI) | 0.81 (0.61–1.07) | 0.68 (0.57–0.81) | 0.73 (0.50–1.06) | 0.75 (0.58–0.97) | 0.83 (0.66–1.04) |
Relative risk was based on the results of 13 years of follow-up based on the references 8 (Gøtzsche & Jørgensen, 2013) and 16 (Tabar et al., 1995). CI, confidence interval; MMG, mammography; SBE; self-breast examination.
Based on the outcome of 13 years of follow-up, the results suggest mortality reduction from breast cancer by mammographic screening without clinical breast examination, although significant results were also obtained in the Swedish Two-County study (0.68; 95%CI, 0.57–0.81) and Gothenburg study (0.75; 95%CI, 0.58–0.97).10 When the targets of these studies were limited to women aged in their 40s, significant results in terms of mortality reduction from breast cancer could not be obtained in all the studies.
Mammographic screening with clinical breast examination
Three RCTs of mammographic screening with clinical breast examination served as eligible evidence for mortality reduction from breast cancer (Table2).28–31 Compared with the studies related to mammographic screening without clinical breast examination, the starting years of these studies were early and detailed information was insufficient. The New York HIP study was the first RCT of this kind. It started in 1963 with the aim of evaluating the efficacy of mammographic screening.28 The other studies commenced around the 1980s. In the Edinburgh study, inappropriate randomization was suggested because of the different socio-economic classes between the intervention group and the control group.29 Although the screening method was the same in these studies, the control group in Canadian study I was initially provided clinical breast screening.30,31 Although the target age group was different among the three RCTs, all of these studies included women aged 40s as their target. Although two-view mammography was used for all the studies, the screening interval was different, that is, 12 months for the New York HIP study28 and Canadian study I,30,31 and 24 months for the Edinburgh study.29 The results were analyzed using the intention to treat method. The results of 13 years of follow-up for the New York HIP study and Canadian study I were obtained from the Cochrane review.8 The results of 14 years of follow-up for the Edinburgh study29 were directly obtained from the article. Although not statistically significant, these results suggest mortality reduction from breast cancer by mammographic screening with clinical breast examination. Similar results were suggested when the targets of these studies were limited to women aged 40–49 years.
Table 2.
Randomized controlled trials for evaluation of mammographic screening with physical examination
| New York HIP | Canada I | Edinburgh | |
|---|---|---|---|
| Starting year of study | 1963 | 1980 | 1978 |
| Randomization | Individual | Individual | Cluster |
| Subjects | |||
| Number | 62 000 | 89 835 | 54 654 |
| Target age | 40–64 years | 40–49 years | 45–64 years |
| Screening method | MMG+CBE | MMG+CBE+SBE | MMG+CBE |
| Mammography | |||
| View | Two-view | Two-view | First, two-view Subsequent, one-view or two-view |
| Screening interval, months | 12 | 12 | 24 |
| Screening frequency | 4 | 4–5 | 2–4 |
| Screening periods, years | 3 | 5 | 6 |
| Participation rate, % | 65 | 88 | 65 |
| Relative risk (95%CI) | 0.83 (0.70–0.99) | 0.97 (0.74–1.27) | 0.85 (0.68–1.05) |
Relative risk was based on the results of 13 years of follow-up for the New York HIP and Canada I studies (Gøtzsche & Jørgensen, 2013), and 14 years of follow-up for the Edinburgh study (Alexander et al., 1999). CBE, clinical breast examination; CI, confidence interval; MMG, mammography; SBE, self breast examination.
Meta-analysis
Mammographic screening without clinical breast examination
Five studies were eligible for the meta-analysis of mammographic screening without clinical breast examination programs (Table1). The overall relative risk for all the age groups was 0.75 (95%CI, 0.67–0.83) (Fig.1a). When the target age group was divided into two groups, the relative risks were 0.81 (95%CI, 0.68–0.96) for women aged 40–49 years and 0.71 (95%CI, 0.62–0.81) for women aged 50–74 years (Fig.1b,c).
Fig 1.
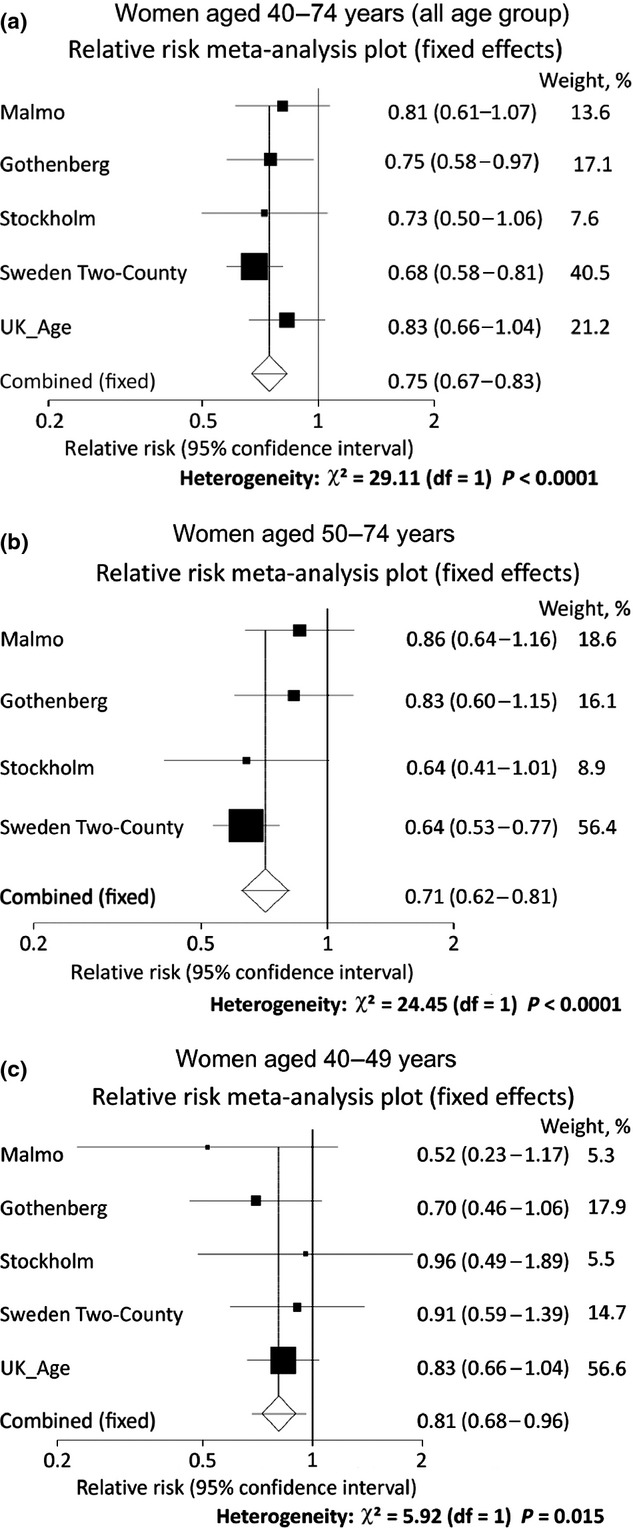
Meta-analysis of mammography without clinical breast examination. Five studies were eligible for the meta-analysis of mammographic screening without clinical breast examination programs: Malmö study,11,12 Swedish Two-County study,16–22 Stockholm study,23,24 Gothenburg study,25,26 and UK Age trial.27 Women were divided into three target age groups: 40–74 years (all age group) (a); 50–74 years (b); 40–49 years (c).
Mammographic screening with clinical breast examination
Three studies were selected to evaluate the efficacy of mammographic screening with clinical breast examination (Table2). The overall relative risk for all the age groups was 0.87 (95%CI, 0.77–0.98) (Fig.2a). When the target age group was divided into two groups, the relative risks were 0.87 (95%CI, 0.72–1.04) for women aged 40–49 years and 0.83 (95%CI, 0.70–0.99) for women aged 50–64 years (Fig.2b,c).
Fig 2.
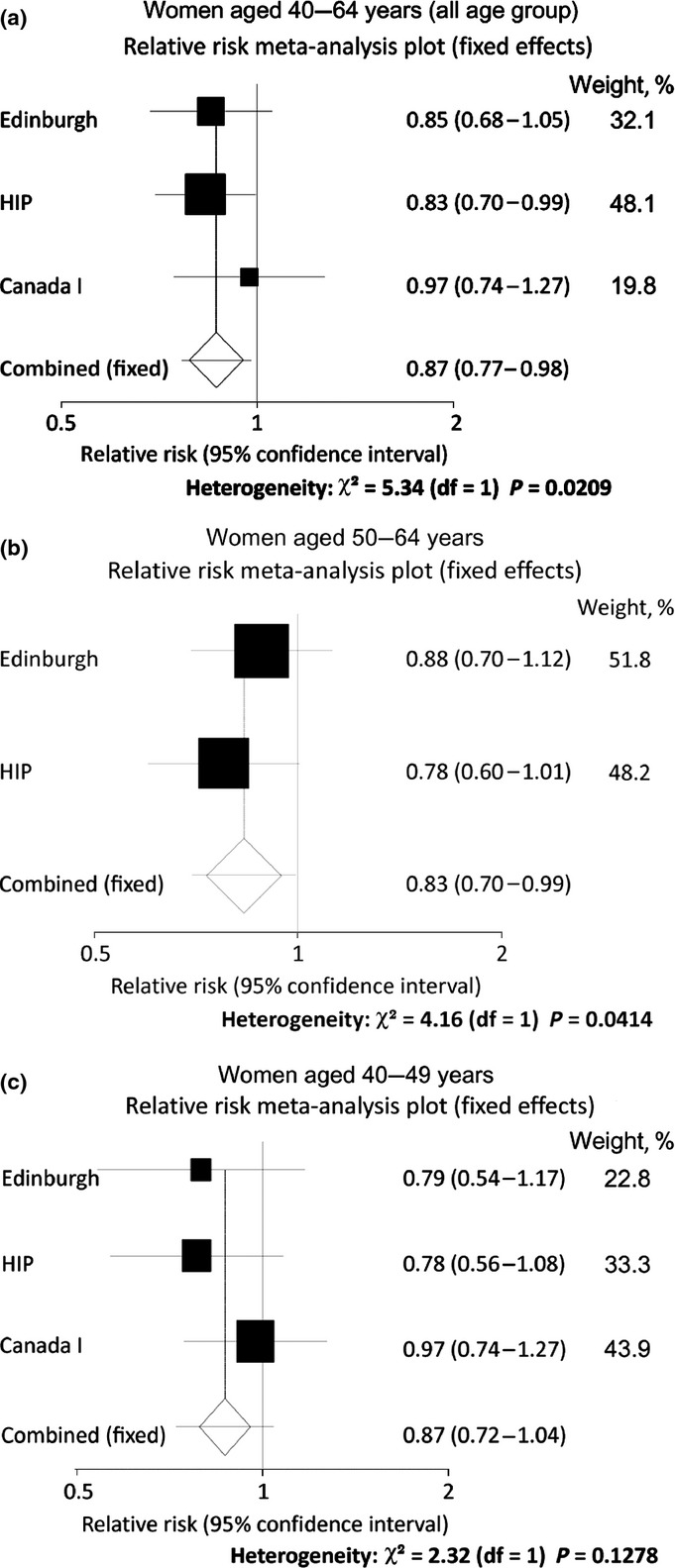
Meta-analysis of mammographic screening with clinical breast examination. Three randomized controlled trials were identified as eligible: New York HIP study,28 Edinburgh study,29 and Canadian study I.30,31 Women were divided into three target age groups: 40–64 years (all age group) (a); 50–64 years (b); 40–49 years (c).
Comparison of benefit and harm
The NNI and the number needed for diagnostic examination to avoid one breast cancer death were calculated for mammographic screening with and without clinical breast examination for women aged 40–70 years (Table3). The NNI was consistently lower in mammographic screening without clinical breast examination than in mammographic screening with clinical breast examination. In both screening methods, the NNI was higher in women aged 40–49 years than in women aged 50–70 years. Similar results were obtained for the number needed for recall of diagnostic examination to avoid one breast cancer death. These results suggest that mammographic screening without clinical breast examination could provide higher benefits for women aged 50 years and over.
Table 3.
Comparison of benefit and harm between mammographic screening with and without clinical breast examination (CBE)
| Screening method | Target age | ||||||
|---|---|---|---|---|---|---|---|
| 40 years | 45 years | 50 years | 55 years | 60 years | 65 years | 70 years | |
| Mammographic screening without CBE | |||||||
| Per 1000 women screened | |||||||
| Number of recalls | 77 | 77 | 67 | 67 | 53 | 53 | 53 |
| Per single death prevented | |||||||
| Number needed to invite | 2530 | 1713 | 864 | 777 | 782 | 807 | 833 |
| Number of recalls | 195 | 132 | 58 | 52 | 41 | 43 | 44 |
| Mammographic screening with CBE | |||||||
| Per 1000 women screened | |||||||
| Number of recalls | 99 | 99 | 76 | 76 | 62 | 62 | 62 |
| Per single death prevented | |||||||
| Number needed to invite | 3698 | 2504 | 1474 | 1325 | 1334 | 1376 | 1420 |
| Number of recalls | 366 | 248 | 112 | 101 | 83 | 85 | 88 |
Numbers needed to invite are expressed per 1000 women invited for 13-year follow-up.
Discussion
Although it has been 15 years since the Japanese government has recommended mammographic screening with clinical breast examination, mammographic screening without clinical breast examination has not yet been introduced. In the present study, individual efficacy could be confirmed for mammographic screening with and without clinical examination. The impacts of mortality reduction were different between both methods. The NNIs of mammographic screening without clinical breast examination were consistently lower than those of mammographic screening with clinical breast examination among women aged 40–70 years. In addition, the recall rate for diagnostic examinations was higher in mammographic screening with clinical breast examination than in mammographic screening without clinical breast examination.10 Compared with mammographic screening with clinical breast examination, mammographic screening without clinical breast examination could reduce harm. However, the NNIs were always higher in women aged 40–49 years than in women aged 50 years and over for both methods.
Clinical breast examination was introduced as the first screening method for breast cancer and it has been carried out with mammographic screening in Japan.2 In Japan, physicians perform clinical breast examinations, whereas in some countries, nurses can undertake that role. In the Canadian I and II studies, clinical breast examinations were carried out by trained nurses.13–15,30,31 The Edinburgh study also recommended clinical breast examinations be carried out by nurses.29 Although clinical breast examination alone was not recommended in developed countries, this method has been commonly used in developing countries.32 The positive efficacy of clinical breast examination has been suggested by the results of a previous RCT in India.33 Randomized controlled trials have been performed to evaluate the efficacy of clinical breast examination.33,34 The sensitivity of clinical breast examination was found to be higher in Japanese studies (50–70%) than in Indian studies.33,35–37 The results of a Japanese case–control study suggested mortality reduction when symptomatic women were excluded.38 Despite its advantages, there are serious problems with the continued use of clinical breast examination. Although several studies have reported that training programs could improve the accuracy of clinical breast examination,39,40 it is difficult to standardize the method because of a lack of an educational system at the national level. Moreover, insufficient human resources can also be a barrier for improving the participation rates of mammographic screening with clinical breast examination in communities. Because of the low accuracy of clinical breast examination, breast ultrasonography has been anticipated as an alternative method that can be combined with mammographic screening. The efficacy of a combination of mammography and ultrasonography in Japan has been evaluated.41
There has been significant discussion whether or not to include women aged 40–49 years in the target population of mammographic screening. In 2009, the US Preventive Services Task Force changed its policy for women aged in their 40s and stopped its recommendation of routine screening.4 The Task Force suggested that women aged in their 40s should have the individual autonomy to choose whether or not to participate in mammographic screening based on shared decision-making with their family physicians. In most European countries, women aged in their 40s have not been included in the target population for breast cancer screening.6 After the publication of the new guidelines of the US Preventive Services Task Force, the appropriateness of the target age group was carefully examined in previous studies.5,8,42,43 The results of these studies were similar with regard to women aged in their 40s, that is, not to include them in the target population. However, as the distribution of breast cancer incidence is different in East Asian countries, the same conclusion could not be easily obtained. Although the benefit of mammographic screening is lower in women aged in their 40s, the data for NNI calculation was based on the results of RCTs conducted in Western countries. The proportion of dense breast in women aged in their 40s is higher in Japan than in Western countries42 and this leads to a lower accuracy of mammographic screening. To resolve the local problem in Japan, a study evaluating mortality reduction from breast cancer among women aged in their 40s is required.
To effectively introduce population-based screening, the balance of benefits and harms of cancer screening must be considered.6 However, measurement methods for quantitative assessment have not yet been standardized to date. Although NNI is commonly used, the appropriate threshold for the balance of benefits and harms remains unclear. Even if the threshold can be defined, it can be changed considering the local context in terms of disease burden and medical resources. From previous studies, we attempted to evaluate the benefits and harms using the results of meta-analysis of RCTs and available Japanese data. In the Japanese situation, the benefits were always higher in women aged 50 years and over. As there is still no standard established in Japan, the appropriateness of including women aged in their 40s in the NNI cannot be ascertained.
There are additional limitations of this study. First, since most of the RCTs assessed were started before 1990, mammographic equipment use during that time might have been different from contemporary equipment. At present, even if clinical breast examination is not added, benefits can be obtained, especially with mammography alone. Second, to resolve our research questions, all RCTs using mammography with and without clinical breast examination were included in our analysis. The Edinburgh study is often excluded from the set of evidence because of its inadequate randomization. When this study was excluded, we could not obtain significant results for mammographic screening with clinical breast examination (relative risk = 0.87; 95%CI, 0.75–1.01). Third, Canadian study II was not included in a meta-analysis of mammographic screening without clinical breast examination because the control group underwent clinical breast examination for breast cancer screening. Most guidelines include mammographic screening with clinical breast examination for evaluating the efficacy of mammographic screening.4,5,8,43,44 The results of our study may show an overestimation of the efficacy of mammographic screening without clinical breast examination. Finally, although the efficacy of mammographic screening without clinical breast examination could be identified for women aged 40–74 years, the efficacy of mammographic screening with clinical breast examination was unclear for women aged 65–74 years because there was no study that included this age group for the target population.
In conclusion, the results of our analysis suggest that mammographic screening without clinical breast examination may afford higher benefits to women aged 50 years and over. Although evidence regarding the effectiveness of mammographic screening without clinical breast examination could be confirmed based on previous RCTs, a Japanese study is needed to resolve local problems, including identification of the appropriate target age group for Japanese women and taking into consideration the balance of benefits and harms.
Acknowledgments
We thank Ms. Kanoko Matsushima, Ms. Junko Asai, and Ms. Hiromi Sugiyama for secretarial work. We are also grateful to Dr. Edward F. Barroga, Associate Professor and Senior Medical Editor of Tokyo Medical University for the editorial review of the manuscript. This study was supported by the National Cancer Center, Tokyo, Japan (grant no. 26-A-30).
Disclosure Statement
The authors have no conflict of interest.
References
- National Cancer Center. Center for Cancer Control and Information Services. Estimation of cancer incidence. [Cited 26 Feb 2015.] Available from URL: http://ganjoho.ncc.go.jp/professional/statistics/index.html.
- Oshima A. A critical review of cancer screening programs in Japan. Int J Technol Assess Health Care. 1994;10:346–58. doi: 10.1017/s0266462300006590. [DOI] [PubMed] [Google Scholar]
- Hisamichi S. Guidelines for cancer screening programs. Tokyo: Japan Public Health Association; 2000. ; (in Japanese.) [Google Scholar]
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–37. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Canadian Task Force on Preventive Health Care. Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ. 2011;183:1991–2001. doi: 10.1503/cmaj.110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. 2008. Cancer screening in the European Union: report on the implementation of the Council Recommendation on cancer screening. Lyon.
- Hamashima C, Saito H, Nakayama T, Nakayama T, Sobue T. The standardized development method of the Japanese guidelines for cancer screening. Jpn J Clin Oncol. 2008;38:288–95. doi: 10.1093/jjco/hyn016. [DOI] [PubMed] [Google Scholar]
- Gøtzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;(6):CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Center. Center for Cancer Control and Information Services. Cancer Statistics 2012. [Cited26 Feb 2015.] Available from URL: http://ganjoho.jp/data/professional/statistics/backnumber/2012/cancer_statistics_2012.pdf.
- Kasahara Y, Tsuji I, Ichimura M, et al. Anuual Report 2011 on Breast Cancer Screening in Japan. J Jpn Assoc Breast Cancer Screening. 2012;21:48–58. [Google Scholar]
- Andersson I, Janzon L. Reduced breast cancer mortality in women under age 50: updated results from the Malmö Mammographic Screening Program. J Natl Cancer Inst Monogr. 1997;22:63–7. doi: 10.1093/jncimono/1997.22.63. [DOI] [PubMed] [Google Scholar]
- Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomized trials. Lancet. 2002;359:909–19. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ. 1992;147:1477–88. [PMC free article] [PubMed] [Google Scholar]
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst. 2000;92:1490–9. doi: 10.1093/jnci/92.18.1490. [DOI] [PubMed] [Google Scholar]
- Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar L, Fagerberg G, Chen HH, et al. Efficacy of breast-cancer screening by age – New results from the Swedish Two-County Trial. Cancer. 1995;75:2507–17. doi: 10.1002/1097-0142(19950515)75:10<2507::aid-cncr2820751017>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tabár L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomized trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1:829–32. doi: 10.1016/s0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- Duffy SW, Tabar L, Vitak B, et al. The Swedish Two-County Trial of mammographic screening: cluster randomization an d end point evaluation. Ann Oncol. 2003;14:1196–8. doi: 10.1093/annonc/mdg322. [DOI] [PubMed] [Google Scholar]
- Tabar L, Vitak B, Chen HH, Prevost TC, Duffy SW. Update of the Swedish Two-County Trial of breast cancer screening: histologic grade-specific and age-specific results. Swiss Surg. 1999;5:199–204. doi: 10.1024/1023-9332.5.5.199. [DOI] [PubMed] [Google Scholar]
- Tabar L, Duffy SW, Yen MF, et al. All-cause mortality among breast cancer patients in a screening trial: support for breast cancer mortality as an end point. J Med Screen. 2002;9:159–62. doi: 10.1136/jms.9.4.159. [DOI] [PubMed] [Google Scholar]
- Tabár L, Vitak B, Chen HH, Yen MF, Duffy SW, Smith RA. Beyond randomized controlled trials: organized mammographic screening substantially reduces breast carcinoma mortality. Cancer. 2001;91:1724–31. doi: 10.1002/1097-0142(20010501)91:9<1724::aid-cncr1190>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Duffy SW, Tabar L, Olsen AH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen. 2010;17:25–30. doi: 10.1258/jms.2009.009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisell J, Eklund G, Hellström L, Lidbrink E, Rutqvist LE, Somell A. Randomized study of mammography screening-preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat. 1991;18:49–56. doi: 10.1007/BF01975443. [DOI] [PubMed] [Google Scholar]
- Frisell J, Lidbrink E, Hellström L, Rutqvist LE. Follow-up after 11 years – update of mortality results in the Stockholm mammographic screening trial. Breast Cancer Res Treat. 1997;45:263–70. doi: 10.1023/a:1005872617944. [DOI] [PubMed] [Google Scholar]
- Bjurstam N, Björneld L, Duffy SW, et al. The Gothenburg Breast Screening Trial – first results on mortality, incidence, and mode of detection for women ages 39–49 years at randomization. Cancer. 1997;80:2091–9. [PubMed] [Google Scholar]
- Bjurstam N, Björneld L, Warwick J, et al. The Gothenburg Breast Screening Trial. Cancer. 2003;97:2387–96. doi: 10.1002/cncr.11361. [DOI] [PubMed] [Google Scholar]
- Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L Trial Management Group. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomized controlled trial. Lancet. 2006;368:2053–60. doi: 10.1016/S0140-6736(06)69834-6. [DOI] [PubMed] [Google Scholar]
- Habbema JD, van Oortmarssen GJ, van Putten DJ, Lubbe JT, van der Maas PJ. Age-specific reduction in breast cancer mortality by screening: an analysis of the results of the Health Insurance Plan of Greater New York study. J Natl Cancer Inst. 1986;77:317–20. [PubMed] [Google Scholar]
- Alexander FE, Anderson TJ, Brown HK, et al. 14 years of follow-up from the Edinburgh randomized trial of breast-cancer screening. Lancet. 1999;353:1903–8. doi: 10.1016/s0140-6736(98)07413-3. [DOI] [PubMed] [Google Scholar]
- Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ. 1992;147:1459–76. [PMC free article] [PubMed] [Google Scholar]
- Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med. 2002;137:305–12. doi: 10.7326/0003-4819-137-5_part_1-200209030-00005. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention. Breast Cancer Screening. Lyon: IARC Press; 2002. pp. 64–72. [Google Scholar]
- Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103:1476–80. doi: 10.1093/jnci/djr304. [DOI] [PubMed] [Google Scholar]
- Boulos S, Gadallah M, Neguib S, et al. Breast screening in the emerging world: high prevalence of breast cancer in Cairo. Breast. 2005;14:340–6. doi: 10.1016/j.breast.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Shibata A, Takahashi T, Ohuchi N, Fukao A. Evaluation of service screening for breast cancer by clinical breast examination using regional cancer registry data. Jpn J Public Health. 2005;52:128–36. [PubMed] [Google Scholar]
- Honjo S, Ando J, Tsukioka T, et al. Relative and combined performance of mammography and ultrasonography for breast cancer screening in the general population: a pilot study in Tochigi Prefecture, Japan. Jpn J Clin Oncol. 2007;37:715–20. doi: 10.1093/jjco/hym090. [DOI] [PubMed] [Google Scholar]
- Uchida K, Yamashita A, Kawase K, Kamiya K. Screening ultrasonography revealed 15% of mammographically occult breast cancers. Breast Cancer. 2008;15:165–8. doi: 10.1007/s12282-007-0024-x. [DOI] [PubMed] [Google Scholar]
- Kanemura S, Tsuji I, Ohuchi N, et al. A case control study on the effectiveness of breast cancer screening by clinical breast examination in Japan. Jpn J Cancer Res. 1999;90:607–13. doi: 10.1111/j.1349-7006.1999.tb00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DA, Donnelly MB, Plymale MA, et al. Improving residents’ clinical skills with the structured clinical instruction module for breast cancer: results of a multiinstitutional study. Breast Cancer Education Working Group. Surgery. 1997;122:324–33. doi: 10.1016/s0039-6060(97)90024-6. [DOI] [PubMed] [Google Scholar]
- Lee KC, Dunlop D, Dolan NC. Do clinical breast examination skills improve during medical school? Acad Med. 1998;73:1013–9. doi: 10.1097/00001888-199809000-00025. [DOI] [PubMed] [Google Scholar]
- Ishida T, Suzuki A, Kawai M, et al. A randomized controlled trial to verify the efficacy of the use of ultrasonography in breast cancerscreening aged 40–49 (J-START): 76 196 women registered. Jpn J Clin Oncol. 2014;44:134–40. doi: 10.1093/jjco/hyt199. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Sasa M, Yamaguchi T, Kondo H, Akaiwa H, Sagara Y. Breast cancer screening by mammography in women aged under 50 years in Japan. Anticancer Res. 2000;20:3689–94. [PubMed] [Google Scholar]
- Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108:2205–40. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci E1 EUROSCREEN Working Group. Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J Med Screen. 2012;19:S5–13. doi: 10.1258/jms.2012.012077. [DOI] [PubMed] [Google Scholar]


