Abstract
The UHRF1 protein is pivotal for DNA methylation and heterochromatin formation, leading to decreased expressions of tumor suppressor genes and contributing to tumorigenesis. However, the factors that modulate UHRF1 expression in colorectal cancer (CRC) remain unclear. Here we showed that, compared with corresponding normal tissues, UHRF1 was upregulated and microRNA-9 (miR-9) was downregulated in CRC tissues. The expression of UHRF1 was inversely correlated with overall survival rates of patients with CRC. Overexpression of miR-9 in CRC cell lines significantly attenuated CRC cell proliferation and promoted cell apoptosis. The expression of UHRF1 was markedly reduced in pre-miR-9 transfected CRC cells. Using luciferase reporter assay, we confirmed that miR-9 was a direct upstream regulator of UHRF1. Finally, analysis of miR-9 and UHRF1 levels in human CRC tissues revealed that expression of miR-9 was inversely correlated with UHRF1 expression. Collectively, our results offer in vitro validation of the concept that miR-9 could repress the expression of UHRF1, and function as a tumor-suppressive microRNA in CRC. It may serve as a prognostic and therapeutic marker for CRC.
Keywords: Colorectal cancer, miR-9, prognosis, tumorigenesis, UHRF1
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second most common in females worldwide.1 Despite substantial progress being made in CRC diagnosis and treatment, the clinical outcome and prognosis of patients with metastatic colorectal cancer still remains poor. Different genetic and epigenetic abnormalities are common in CRC, such as KRAS mutation, MLH1 methylation, and microsatellite instability.2–5 Epigenetic regulation could lead to the activation of oncogenes and/or the inactivation of tumor suppressor genes. Thus, exploring the underlying epigenetic mechanisms of CRC could benefit the understanding of tumorigenesis and improve the current therapeutic strategies of CRC.
Ubiquitin-like with plant homeodomain and ring finger domains 1 (UHRF1) also known as ICBP90, was characterized as a multidomain protein, which is emerging as a major player in DNA methylation and cell proliferation.5,6 Its multiple domains comprise five structural elements: an N-terminal ubiquitin-like domain, followed by a tandem tudor domain, a plant homeodomain, a SET- and RING-associated (SRA) domain, and a C-terminal RING domain.7 The plant homeodomain could bind to trimethylated histone H3 lysine 9 (H3K9me3) and the SRA domain recognizes hemimethylated DNA.5 By binding to recruiting DNA (cytosine-5-)-methyltransferase 1, UHRF1 could maintain genomic DNA methylation.8 UHRF1 is also linked with heterochromatin formation by binding to histone deacetylase 1 and H3K9me3.9,10 Recent research has elucidated that UHRF1 is upregulated in various cancers, including CRC, hepatocellular cancer, and prostate cancer,6,11,12 and knockdown of UHRF1 expression in cancer cells inhibits cell growth.13 Therefore, UHRF1 inhibition may provide a promising route to cancer therapy.
MicroRNAs (miRNAs), a class of approximately 19–25-nt non-coding RNA molecules, also exert a critical role in tumorigenesis. They could regulate protein-coding genes through direct interaction with the 3′-UTR of their target mRNA and leading to translational inhibition.14,15 A growing number of studies have shown that aberrant expression of miRNAs is tightly linked to the development of CRC, such as miR-9, miR-143, miR-145, miR-103-1, miR-191, miR-21, miR-221, miR-222, miR-135b, and miR-34a,16–20 and plays pivotal roles in CRC pathophysiology such as cell proliferation, differentiation, apoptosis, and metastasis.19–24 In CRC, miR-9 serves as a tumor suppressor.25 The expression of miR-9 in CRC tissue was downregulated compared with normal colonic mucosa.18,26 Ectopic expression of miR-9 could enhance the motility of RKO cells and change cells’ morphological appearance, resulting in promoting the metastatic process of CRC.27 By binding to the 3′-UTR, miR-9 has the potential to suppress Cdx2 and E-cadherin transcript.28,29 Overall, miR-9 is associated with the development of CRC and could be used for CRC molecular-targeted therapies.
Playing an important role in connecting DNA methylation and histone modifications, UHRF1 is implicated in CRC development and progression. However, little is known of the factors that modulate UHRF1 expression. In this study, we examined the expression of UHRF1 in CRC tissues. Then a miRNA was predicted and confirmed to modulate the expression of UHRF1. Our results revealed that UHRF1 is a target of miR-9, which has a vital role in regulating CRC cell proliferation and apoptosis.
Materials and Methods
Cells
HCT116 and HT29 cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cell lines were cultured in McCoy’s 5A (modified) medium supplemented with 10% FBS and 50 U/mL penicillin and streptomycin sulfate.
Tissue specimens
A total of 38 patients with CRC who underwent surgical resection between 2008 and 2009 at Nanjing First Hospital (Nanjing, China) were recruited to the study. Patients who received preoperative chemotherapy were excluded. Specimens of cancer tissues and adjacent normal tissues were collected from patients with informed consent. All patients had a pathological diagnosis of CRC, and each patient was classified according to the TNM system. The samples were freshly frozen in liquid nitrogen and stored at −80°C. The 38 patients with CRC had an average age of 69 years (range, 45–85 years). Postoperative follow-up data were obtained from all patients, with a median follow-up period of 46 months (range, 6–68 months).
Immunohistochemistry
Sections were incubated at 37°C with the primary antibodies anti-UHRF1 (1:50, Abcam, Cambridge, UK) for 2 h. After rinsing with PBS, slides were incubated with secondary antibody for 30 min and washed again with PBS for 10 min. The protein was visualized by EnVision Detection Systems (Dako, Glostrup, Denmark). Semiquantitative analysis of staining results of individual tissue array cores was carried out by two independent observers without prior information of the clinicopathological features. The frequency of UHRF1-positive staining cells was scored on the basis of the percentage of positive cells as: negative, 0%; +1, 1–25%; +2, 26–50%; and +3, >51%. The intensity of UHRF1 expression was scored as: 1, weak; 2, moderate; and 3, strong. The scores were calculated to evaluate the UHRF1 expression levels and the levels were classified as low (≤2) or high (>2).
Vector constructs and transfection
The miRNA expression vectors for pre-miR-9 were obtained from GeneChem (Shanghai, China). The human pre-miR-9 sequence was amplified from human genomic DNA using specific primers (forward, GGGCCCGCTCTAGACTCGAGATATTTGCATGTCGCTATGTG; reverse, CGCGGCCGCCTAATGGATCCAAAAAAGGCACAGTCGAGGCTGATC). The amplicon was cloned into the GV259 (GeneChem) at the XhoI and BamHI sites. All cloned constructs were fully sequenced. HCT116 and HT29 cells were infected with the pre-miR-9 lentiviruses or control viruses lacking the miR-9 sequence (MOI = 50). After being cultured for 72 h, cells were detected by fluorescence microscopy. A stable infection efficiency of approximately 100% was attained. Expression of the mature miR-9 was verified by TaqMan real-time PCR. Transient transfection of miR-9 inhibitor (Ambion, Austin, TX, USA) was performed using Lipofectamine 2000 reagent according to the manufacturer’s instructions (Invitrogen).
In situ hybridization
Sections of paraffin and formaldehyde-fixed tissue were cut from CRC patients. In situ hybridization was carried out using the microRNA Hybridization Kit (Foco, Guangzhou, China) according to the manufacturer’s instructions. Briefly, the sections were incubated with 4% (v/v) paraformaldehyde for 15 min at room temperature. Afterwards, the slides were washed and incubated with prehybridization solution for 2 h at 55°C. Digoxin-labeled miR-9 probe and U6 snRNA probe were added to each tissue section and hybridized for 24 h at 37°C. After washing with PBS, blocking was carried out for 1 h at 37°C, and then the anti-digoxigenin Rhodamine conjugate was added to the sections. To stain the cell nucleus, the sections were counterstained with 20 μL DAPI.
RNA extraction and quantitative real-time RT-PCR
Total RNA containing small RNA was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s recommendations. cDNA was synthesized with the Reverse Transcription System (Promega, Madison, USA). Quantitative RT-PCR analyses were carried out to detect mRNA expression using SYBR Premix Ex Taq II (TaKaRa, Dalian, China). GAPDH was used as a reference gene to normalize the results. Primers used in this experiment were: 5′-CCACATCGTCCTCACAGC-3′ (forward), and 5′-GGTCCACATCATCCTCATAGC-3′ (reverse) for UHRF1; and 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-TGGTGAAGACGCCAGTGGA-3′ (reverse) for GAPDH. The mature miR-9 expression was determined using TaqMan miRNA assays (Applied Biosystems, Foster City, CA, USA), and U6 small nuclear RNA was used as an internal control.
Western blot analysis
Cells were washed in PBS twice and then lysed in RIPA buffer 72 h after transfection. Total proteins were separated by 8% SDS-PAGE and then transferred to PVDF membranes (Millipore, Danvers, MA, USA). The membranes were blocked in 5% fat-free milk in Tris-buffered saline–Tween for 1 h at room temperature and incubated overnight at 4°C with rabbit anti-UHRF1 polyclonal antibody (1:50; Abcam). GAPDH staining with goat Anti-GAPDH antibodies (1:1000; Sigma-Aldrich, St. Louis, MO, USA) was used as loading control. After incubation with secondary anti-rabbit or anti-goat polyclonal antibodies conjugated to HRP, protein bands were visualized using an ECL kit (SuperSignal West Pico, Thermo Scientific, Rockford, lL, USA) on Kodak film. Protein band intensity was quantified using Gel-Pro Analyzer (Media Cybernetics, Warrendale, PA, USA) and normalized to the respective housekeeping protein.
Cell viability
Cell proliferation rates were measured using Cell Counting Kit-8 (Beyotime, Hangzhou, China). Cells (1 × 104 pre-miR-9 and pre-miR-control) cells were seeded in each 96-well plate for 24, 48, 72, or 96 h. Cell Counting Kit-8 (10 μL) was added to each well at each monitored time point. Cells were incubated for 1.5 h at 37°C and then the optical density values were determined by a microplate reader at 450 nm. All experiments were carried out in triplicate.
Colony formation assay
Cells (2000 per well) were placed in 6-cm culture dishes in triplicate and cultured for 2 weeks. The colonies were fixed with 4% paraformaldehyde for 5 min, and then stained with Giemsa solution (KeyGEN BioTech, Nanjing, China) for 15 min after being washed twice with PBS. The number of colonies in each well was counted.
Flow cytometry analysis
Using the BD FACSCalibur (BD Biosciences, San Jose, CA, USA), transfected cells were incubated with an annexin V/7-AAD kit (BioLegend, San Diego, CA, USA) for 15 min at room temperature to detect the apoptosis.
Luciferase reporter assay
The wild-type or mutant 3′-UTR of UHRF1 containing the putative miR-9 binding site were cloned into pGL3 promoter (GeneChem), individually. One microgram vectors were cotransfected into HCT116 and HT29 cells in 24-well plates, together with 100 nM miR-9 or miR-control using Lipofectamine 2000. After 48 h, luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega). Normalized luciferase activity was reported as firefly/Renilla luciferase activity. Each transfectant was assayed in triplicate.
Statistical analysis
Statistical analyses were carried out using spss 16.0 for Windows (SPSS, Chicago, IL, USA). Student’s t-test was used for comparison between the two groups. Survival curves were plotted by the Kaplan–Meier method and compared with the log–rank test. Spearman’s correlation analysis was carried out to determine the correlation between the expression levels of miR-9 and UHRF1. A difference was considered significant at P < 0.05.
Results
UHRF1 significantly overexpressed in human CRC tissues
The localization and expression of UHRF1 was determined by immunohistochemistry in 38 CRC samples. Strong staining was found mainly in nucleus. Immunohistochemistry showed that UHRF1 expression was more frequently present in CRC tissues than that in the adjacent normal tissues. Fourteen of 38 cancer lesions (36.8%) were stained positive and four adjacent normal tissues were positive (10.5%). Nine cases (23.7%) were scored as strong expression and five cases (13.2%) as moderate expression. Representative result of UHRF1 staining is shown in Figure1a. Kaplan–Meier survival analysis revealed that overall 5-year survival was significantly lower in patients with high UHRF1 expression as compared to patients with low UHRF1 expression (Fig.1b).
Fig 1.
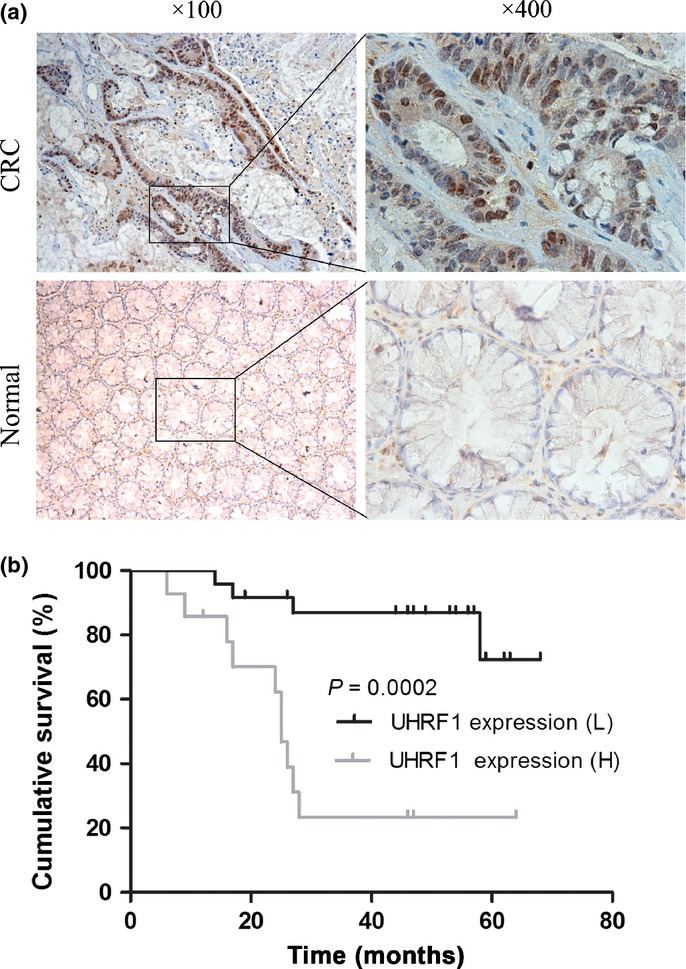
Expression of UHRF1 was investigated in patients with colorectal cancer (CRC). (a) Immunohistochemical analysis of UHRF1 in cancer tissues and adjacent normal tissues. (b) Kaplan–Meier overall survival analysis for CRC patients. H, high; L, low.
MicroRNA-9 expression is downregulated in CRC tissues and associated with prognosis in CRC
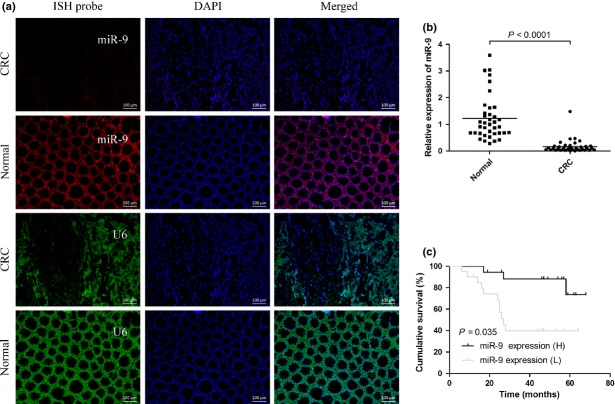
The expression of miR-9 was analyzed in CRC tissues (n = 38) and corresponding normal tissue samples by in situ hybridization and RT-PCR. Strikingly, in situ hybridization results showed that miR-9 expression level was remarkably reduced in CRC tissues compared with matched normal tissues, whereas U6 snRNA expression levels remained unchanged (Fig.2a). In agreement with the results of the in situ hybridization, the RT-PCR results showed that the expression levels of miR-9 were significantly lower in the CRC specimens than those in the adjacent normal specimens (P < 0.0001) (Fig.2b). Kaplan–Meier survival analysis suggested that patients with low miR-9 expression were significantly associated with a poor clinical outcome (Fig.2c).
Fig 2.
MicroRNA-9 (miR-9) expression levels are downregulated in colorectal cancer (CRC) tissues. (a) Representative in situ hybridization (ISH) images showing miR-9 (red) and control U6 (green) in CRC tissues and normal tissues, respectively. Scale bar = 100 μm. (b) Quantitation of miR-9 and control U6 expression in CRC tissues and matched normal tissues. (c) Kaplan–Meier analysis of the correlation between miR-9 expression and the overall survival of patients with CRC. H, high; L, low.
Overexpression of miR-9 inhibits proliferation of CRC cells in vitro
To determine whether miR-9 was involved in CRC tumorigenesis, stable HCT-116 and HT29 cell lines expressing pre-miR-9 were established. After pre-miR-9 transfection for 72 h in HCT-116 and HT29 cells, the expression levels of miR-9 were substantially increased, approximately 57-fold and 45-fold, respectively, compared to pre-miR control (Fig.3a,b). The cell proliferation assays suggested that upregulation of miR-9 can significantly inhibit CRC cell proliferation (P < 0.05; Fig.3c,d). Additionally, the plate colony formation assay was used to assess the effect of miR-9 expression on proliferation of CRC cell lines in vitro. Similarly, colony formation was significantly reduced in the miR-9 upregulated cells (P < 0.01; Fig.3e,f). Together, the results suggested that ectopic expression of miR-9 can significantly inhibit CRC cell proliferation.
Fig 3.
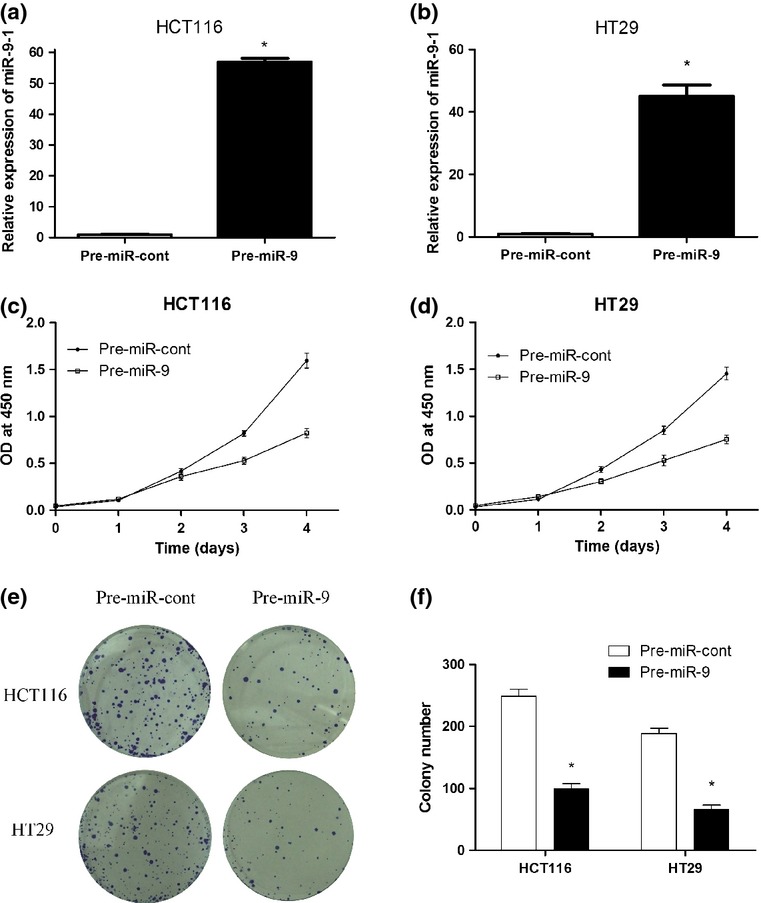
MicroRNA-9 (miR-9) inhibits human colorectal cancer cell proliferation and growth. (a, b) After transfection with pre-miR-9 and pre-miR-control (pre-miR-cont) for 72 h in HCT116 and HT29 cells, miR-9 expression was detected by RT-PCR. (c, d) Upregulation of miR-9 repressed cell proliferation as determined by CCK-8 assays. (e, f) Colony formation was carried out to analyze cell proliferation. $P < 0.05. OD, optical density.
MicroRNA-9 promotes CRC cells apoptosis in vitro
To detect the effects of miR-9 expression on HCT-116 and HT29 cell apoptosis, we used an annexin V/7-AAD kit to examine the apoptosis of these cells. After transfection with miR-9 for 72 h, the effect of miR-9 on apoptosis of HCT-116 and HT29 cells was evaluated by flow cytometry. It was found that the proportion of apoptotic cells was significantly increased in pre-miR-9-transfected CRC cells compared with control cells (Fig.4).
Fig 4.
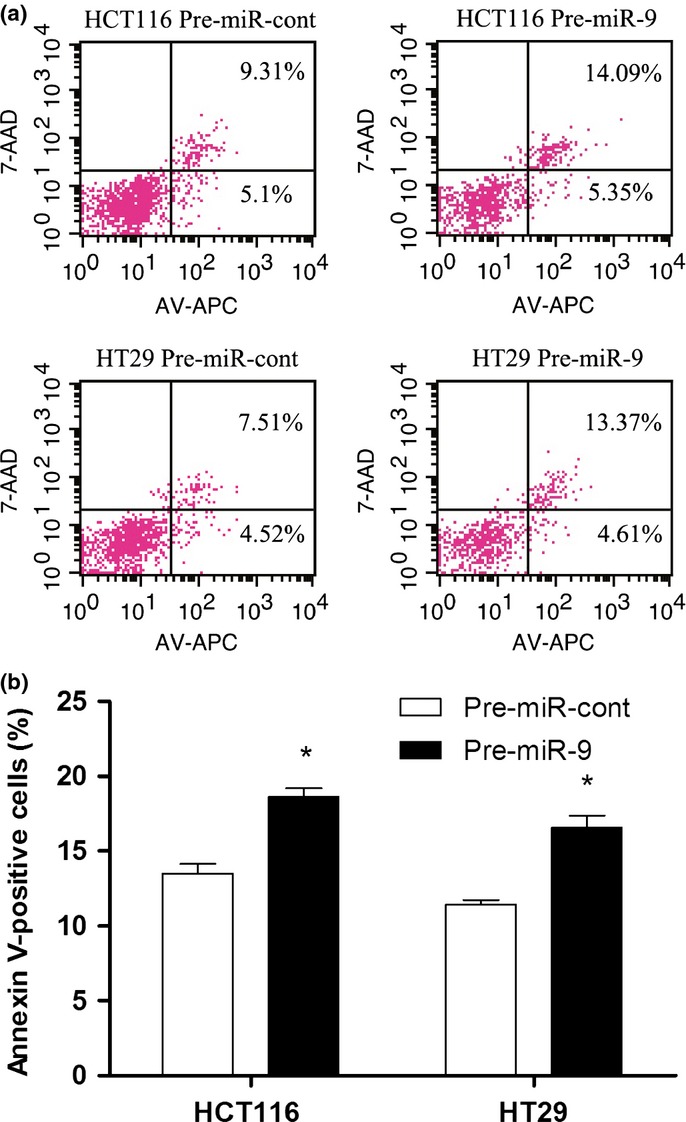
Ectopic expression of microRNA-9 (miR-9) promotes cell apoptosis. (a) The apoptosis cells were stained with annexin V (AV)/7-AAD and analyzed. The percentage at the upper right (UR) area and lower right (LR) area stands for the total apoptosis (UR + LR). (b) Quantitative analysis of total apoptosis cells in different groups.$P = 0.004.
UHRF1 is a direct target of miR-9
Next, the UHRF1 expression in pre-miR-9-infected CRC cells was determined. The results showed the expression of UHRF1 significantly decreased in stable pre-miR-9-transfected cells, whereas UHRF1 was upregulated after blocking expression of miR-9 with antagonistic oligonucleotide in both cell lines (Fig.5a,b). To further analyze the relationship between miR-9 and UHRF1, we used two computational algorithms (miRanDa and TargetScan) to predict the targets gene of miR-9. The bioinformatic methods suggested that miR-9 could target the 3′-UTR region of UHRF1. To validate the prediction, the wild-type or mutated 3′-UTRs of UHRF1 were cloned into luciferase reporter vector (Fig.5c). Dual luciferase reporter assay showed that, compared to miRNA control, miR-9 led to a significant relative luciferase activity reduction in the wild-type UHRF1 3′-UTR plasmid. However, luciferase activity was not reduced in the 3′-UTR with mutant binding sites (Fig.5d). These results showed that miR-9 may restrain UHRF1 expression by targeting its 3′-UTR.
Fig 5.
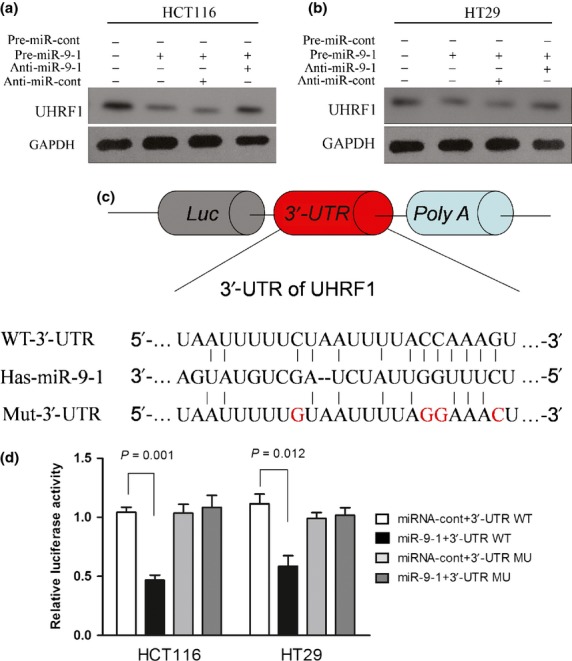
MicroRNA-9 (miR-9) directly targets the 3′-UTR of UHRF1. (a, b) Western blot analyses were carried out to determine the protein expression of UHRF1 in colorectal cancer cell lines transfected with lentivirus vectors and miR-9 inhibitors. (c) The schematic representation of UHRF1 3′-UTR luciferase constructs. The 3′-UTR of UHRF1 was cloned into GV259 vector (WT), and the mutant construct was obtained by site-directed mutagenesis by changing three base pairs of miR-9 seed-sequence (Mu) and inserting into the vector. (d) Relative Luc activity was assayed and calculated by the ratio of firefly/Renilla luciferase activity. Results are presented as mean ± SE from three duplicates.
Downregulation of UHRF1 inversely correlates with miR-9 expression in CRC
To further explore the relationship between miR-9 and UHRF1, we also used quantitative RT-PCR to analyze the expression of UHRF1 in 15 patients with CRC. These results showed that UHRF1 mRNA expression was higher in CRC tissues than that in corresponding normal tissues (Fig.6a). Spearman’s correlation analysis showed that the UHRF1 expression was inversely correlated with miR-9 expression in CRC (r = −0.606, P = 0.0004; Fig.6b).
Fig 6.
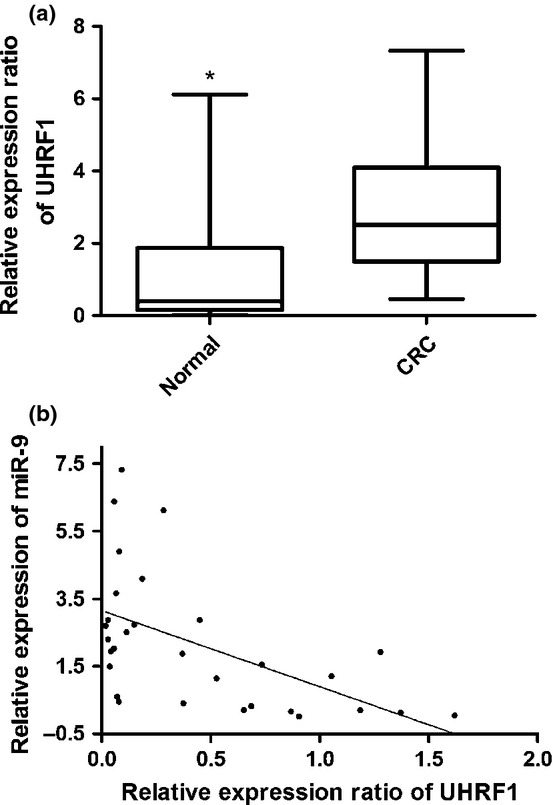
UHRF1 is upregulated in colorectal cancer (CRC) tissues and inversely correlated with microRNA-9 (miR-9) expression. (a) Average expression level of UHRF1 in cancerous tissues and corresponding normal tissues (n = 15). (b) Inverse correlation between miR-9 and UHRF1 mRNA level in CRC specimens.
Discussion
Epigenetic alteration, such as DNA methylation and histone modification, inactivates various tumor suppressor genes.30 It is generally agreed that UHRF1 is involved in gene epigenetic abnormalities and exerts its role as an oncogene in cancer development. Overexpressed UHRF1 could bind to methylated promoter regions of various tumor suppressor genes and suppress the transcription of these genes including p16INK4A, p14ARF, RARβ, FHIT, APC, DAPK, and p21.9,31 In the UHRF1 regulation axis, tumor suppressor p53 could downregulate UHRF1 through upregulation of p21WAF1 and subsequent deactivation of E2F1.32 Thus, due to the absence of intact p53 in most cancer cells, dysregulation of UHRF1 expression has been observed in several cancers. In addition, UHRF1 was reported to be associated with tumor stage and to predict poor prognoses.33,34 Consistent with previous studies, we also found that UHRF1 was upregulated in CRC. Patients with a high level of UHRF1 expression tended to have lower overall survival rates compared with those with low expression. The above results were obtained from only 38 CRC patients. To validate these results, more research on a larger population is warranted.
Increasing evidence has shown that miR-9 is confirmed to be downregulated in many types of cancers and considered to be an important mediator in cancer development through its ability to regulate different target genes. In oral squamous cell carcinoma, miR-9 could inhibit the proliferation of cancer cells by suppressing expression of CXC chemokine receptor 4.35 In acute myeloid leukemia, miR-9 exerts its effects through the cooperation with let-7 to restrain the oncogenic LIN28B/HMGA2 axis.36 In the present study, we showed that miR-9 was underexpressed in CRC tissues. Lentivirus-mediated overexpression of miR-9 could suppress the expression of UHRF1. Cell proliferation was also suppressed in pre-miR-9 transfected cells. Furthermore, restoration of miR-9 expression significantly induced cell apoptosis. Interestingly, the expression level of miR-9 was inversely correlated with UHRF1 expression in CRC tissues. Finally, we identified that UHRF1 is regulated by miR-9 in CRC by dual luciferase reporter assay.
In conclusion, our studies provide insight into the mechanism that miR-9 could function as a tumor-suppressive miRNA by repressing UHRF1 expression. Understanding the relationship between UHRF1 and miR-9 will not only increase our understanding of CRC tumorigenesis, but may also allow the development of a novel therapeutic strategy based on upregulation of miR-9.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21475063), Medicine Leading Talents of Jiangsu Province (LJ201131), the Jiangsu Health Department (H201325), and Jiangsu Provincial Special Program of Medical Science (BL2013036).
Disclosure Statement
The authors have no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174–9. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- Yun J, Rago C, Cheong I, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TM, Laird PW, Park PJ. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell. 2013;155:858–68. doi: 10.1016/j.cell.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick M, Kim JK, Esteve PO, et al. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–4. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- Wang F, Yang Y-Z, Shi C-Z, et al. UHRF1 promotes cell growth and metastasis through repression of p16ink4a in colorectal cancer. Ann Surg Oncol. 2012;19:2753–62. doi: 10.1245/s10434-011-2194-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Horton JR, Zhang X, et al. UHRF1, a modular multi-domain protein, regulates replication-coupled crosstalk between DNA methylation and histone modifications. Epigenetics. 2009;4:8–14. doi: 10.4161/epi.4.1.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achour M, Jacq X, Ronde P, et al. The interaction of the SRA domain of ICBP90 with a novel domain of DNMT1 is involved in the regulation of VEGF gene expression. Oncogene. 2008;27:2187–97. doi: 10.1038/sj.onc.1210855. [DOI] [PubMed] [Google Scholar]
- Unoki M, Nishidate T, Nakamura Y. ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG through its SRA domain. Oncogene. 2004;23:7601–10. doi: 10.1038/sj.onc.1208053. [DOI] [PubMed] [Google Scholar]
- Karagianni P, Amazit L, Qin J, et al. ICBP90, a novel methyl K9 H3 binding protein linking protein ubiquitination with heterochromatin formation. Mol Cell Biol. 2008;28:705–17. doi: 10.1128/MCB.01598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudbhary R, Hoshida Y, Chernyavskaya Y, et al. UHRF1 overexpression drives DNA hypomethylation and hepatocellular carcinoma. Cancer Cell. 2014;25:196–209. doi: 10.1016/j.ccr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbio F, Pistore C, Curti L, et al. The SRA protein UHRF1 promotes epigenetic crosstalks and is involved in prostate cancer progression. Oncogene. 2012;31:4878–87. doi: 10.1038/onc.2011.641. [DOI] [PubMed] [Google Scholar]
- Kumamoto K. UHRF1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncol Rep. 2012;28:1997–2002. doi: 10.3892/or.2012.2064. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Michael MZ, O’Connor SM, van Holst Pellekaan NG, et al. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–91. [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekaite L, Rantala JK, Bruun J, et al. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14:868–79. doi: 10.1593/neo.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri N, Braconi C, Gasparini P, et al. MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell. 2014;25:469–83. doi: 10.1016/j.ccr.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokavec M, Oner MG, Li H, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Investig. 2014;124:1853–67. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby O, Svoboda M, Michalek J, et al. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer. 2009;8:102. doi: 10.1186/1476-4598-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padi SK, Zhang Q, Rustum YM, et al. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology. 2013;145:437–46. doi: 10.1053/j.gastro.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DL, Wang ZQ, Zeng ZL, et al. Identification of microRNA-214 as a negative regulator of colorectal cancer liver metastasis by way of regulation of fibroblast growth factor receptor 1 expression. Hepatology. 2014;60:598–609. doi: 10.1002/hep.27118. [DOI] [PubMed] [Google Scholar]
- Lim L, Balakrishnan A, Huskey N, et al. MicroRNA-494 within an oncogenic microRNA megacluster regulates G1/S transition in liver tumorigenesis through suppression of mutated in colorectal cancer. Hepatology. 2014;59:202–15. doi: 10.1002/hep.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandres E, Agirre X, Bitarte N, et al. Epigenetic regulation of microRNA expression in colorectal cancer. Int J Cancer. 2009;125:2737–43. doi: 10.1002/ijc.24638. [DOI] [PubMed] [Google Scholar]
- Oberg AL, French AJ, Sarver AL, et al. miRNA expression in colon polyps provides evidence for a multihit model of colon cancer. PLoS One. 2011;6:e20465. doi: 10.1371/journal.pone.0020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Chen H, Zhou D, et al. MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med Oncol. 2012;29:1037–43. doi: 10.1007/s12032-011-9975-z. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Haraguchi T, Hiramatsu H, et al. Multiple microRNAs induced by Cdx1 suppress Cdx2 in human colorectal tumour cells. Biochem J. 2012;447:449–55. doi: 10.1042/BJ20120434. [DOI] [PubMed] [Google Scholar]
- Lu MH, Huang CC, Pan MR, et al. Prospero homeobox 1 promotes epithelial-mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin Cancer Res. 2012;18:6416–25. doi: 10.1158/1078-0432.CCR-12-0832. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Kim JK, Esteve PO, Jacobsen SE, et al. UHRF1 binds G9a and participates in p21 transcriptional regulation in mammalian cells. Nucleic Acids Res. 2009;37:493–505. doi: 10.1093/nar/gkn961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousli M, Hopfner R, Abbady AQ, et al. ICBP90 belongs to a new family of proteins with an expression that is deregulated in cancer cells. Br J Cancer. 2003;89:120–7. doi: 10.1038/sj.bjc.6601068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki M, Kelly JD, Neal DE, et al. UHRF1 is a novel molecular marker for diagnosis and the prognosis of bladder cancer. Br J Cancer. 2009;101:98–105. doi: 10.1038/sj.bjc.6605123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki M, Daigo Y, Koinuma J, et al. UHRF1 is a novel diagnostic marker of lung cancer. Br J Cancer. 2010;103:217–22. doi: 10.1038/sj.bjc.6605717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Liu K, Wu Y, et al. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/beta-catenin signaling pathway. Oncogene. 2014;33:5017–27. doi: 10.1038/onc.2013.448. [DOI] [PubMed] [Google Scholar]
- Emmrich S, Katsman-Kuipers JE, Henke K, et al. miR-9 is a tumor suppressor in pediatric AML with t(8;21) Leukemia. 2014;28:1022–32. doi: 10.1038/leu.2013.357. [DOI] [PubMed] [Google Scholar]