Abstract
Neuroblastoma is a pediatric solid tumor that originates from embryonic neural crest cells. The MYCN gene locus is frequently amplified in unfavorable neuroblastomas, and the gene product promotes the progression of neuroblastomas. However, the molecular mechanisms by which MYCN amplification contributes to stem cell-like states of neuroblastoma remain elusive. In this study, we show that MYCN and its cis-antisense gene, NCYM, form a positive feedback loop with OCT4, a core regulatory gene maintaining a multipotent state of neural stem cells. We previously reported that NCYM is co-amplified with the MYCN gene in primary human neuroblastomas and that the gene product promotes aggressiveness of neuroblastoma by stabilization of MYCN. In 36 MYCN-amplified primary human neuroblastomas, OCT4 mRNA expression was associated with unfavorable prognosis and was correlated with that of NCYM. The OCT4 protein induced both NCYM and MYCN in human neuroblastoma cells, whereas NCYM stabilized MYCN to induce OCT4 and stem cell-related genes, including NANOG, SOX2, and LIN28. In sharp contrast to MYCN, enforced expression of c-MYC did not enhance OCT4 expression in human neuroblastoma cells. All-trans retinoic acid treatment reduced MYCN, NCYM, and OCT4 expression, accompanied by the decreased amount of OCT4 recruited onto the intron 1 region of MYCN. Knockdown of NCYM or OCT4 inhibited formation of spheres of neuroblastoma cells and promoted asymmetric cell division in MYCN-amplified human neuroblastoma cells. These results suggest that the functional interplay between MYCN, NCYM, and OCT4 contributes to aggressiveness of MYCN-amplified human neuroblastomas.
Keywords: MYCN, NCYM, neuroblastoma, OCT4, transcriptional regulation
Neuroblastoma is a pediatric solid tumor that arises in sympatho-adrenal tissues.1 Amplification of the MYCN oncogene is frequently observed in unfavorable neuroblastomas,2 and aberrant expression of MYCN contributes to neuroblastoma progression.3 The transcription factor MYCN regulates a wide variety of biological phenomena, including cell-cycle progression, apoptosis, differentiation, and stemness.4,5 MYCN transgenic mice spontaneously develop neuroblastomas,3 but unlike human MYCN-amplified neuroblastomas, the mice rarely have metastatic tumors. Recently, we reported that NCYM, a MYCN cis-antisense gene, encodes a protein that functions as an onco-promoting factor.6 The coding sequence of NCYM is not evolutionally conserved in mice, and the NCYM gene is co-amplified with MYCN in human primary neuroblastomas.6 The MYCN protein directly targets NCYM for transcriptional activation, whereas NCYM stabilizes MYCN protein, forming a positive autoregulatory loop.6,7 Expression of NCYM caused metastatic tumors in MYCN/NCYM double transgenic mice and inhibited apoptotic cell death.6 However, these results do not rule out the possibility that NCYM is involved in other cellular phenotypes to promote the aggressiveness of neuroblastoma.
Neuroblastomas originate from neural crest cells that differentiate into multiple cell lineages.8 Some neuroblastoma cells retain multipotency and highly express stem cell-related genes, such as OCT49 and LIN28.10 Intermediate (I)-type neuroblastoma cells highly express OCT4 and differentiate into neuroblastic (N)-type or substrate adherent (S)-type cells in response to retinoic acid or BrdU treatment, respectively.11 OCT4+/Tenascin C+ neuroblastoma cells were reported to serve as progenitors of tumor-derived endothelial cells, promoting neovascularization of the tumors.9 Furthermore, OCT4 is expressed in side-population cells of neuroblastoma.12 Despite these correlations between OCT4 expression and the stem cell-like state of neuroblastomas, the functional roles of OCT4 in neuroblastoma pathogenesis remain unclear. In this study, we investigated the biological and clinical significance of OCT4 in neuroblastomas and found that the newly evolved network between MYCN, NCYM, and OCT4 regulates aggressiveness of human neuroblastomas.
Materials and Methods
Immunofluorescence analysis
BE(2)-C and SK-N-AS cells were grown on coverslips and transfected with indicated shRNAs. Cells were fixed in 4% paraformaldehyde for 15 min at room temperature, blocked in 3% BSA, stained with the indicated antibodies, and examined with a laser scanning confocal microscope (DMI 4000B; Leica, Wetzlar, Germany).
Asymmetric cell division assay
We tested whether neuroblastoma cells showed asymmetric distribution of nuclear mitotic apparatus protein (NuMA). Asymmetric distributions of NuMA to one side of the cell were counted during mitotic stages. The spindle apparatus were also stained with anti-tubulin-α antibody to avoid false results caused by uneven dyeing. The antibodies used were anti-NuMA (Novus Biologicals, Littleton, CO, USA), and anti-tubulin-α (Thermo Fisher Scientific, Wilmington, DE, USA).
Statistical analysis
All data were presented as the mean ± standard deviation and were obtained from three independent experiments. Statistical significance in the clinical data was calculated using the log–rank test, χ2-test, and Student’s t-test. Hazard ratios were calculated using univariate and multivariate Cox regression analysis. Statistical analyses were undertaken using JMP 9 (SAS Institute Japan, Tokyo, Japan). Statistical significance was set at P < 0.05.
More detailed descriptions of the Material and Methods are described in Document S1.
Results
High expression of OCT4 associated with poor prognoses in MYCN-amplified human neuroblastomas
To examine the prognostic significance of OCT4 mRNA expression in human neuroblastoma, total RNA was extracted from 36 MYCN-amplified and 67 MYCN-non-amplified primary neuroblastomas and subjected to quantitative real-time RT-PCR. MYCN amplification was examined as previously described [13]. Kaplan–Meier analysis showed that high levels of OCT4 mRNA expression were significantly associated with poor outcomes in MYCN-amplified human neuroblastomas (Fig.1a), but not in MYCN-non-amplified human neuroblastomas (Fig.1b).
Fig 1.
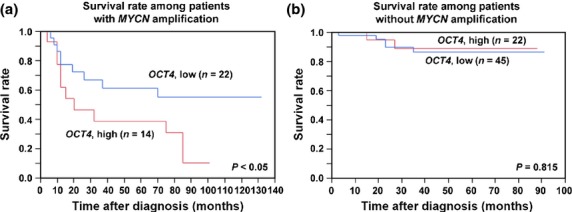
High OCT4 expression correlates with unfavorable prognosis in human MYCN-amplified neuroblastomas. (a) Overall survival of patients with MYCN-amplified neuroblastomas according to relative OCT4 expression levels (n = 36; high, n = 14; low, n = 22). P-value by log–rank test. (b) Overall survival of patients with MYCN-non-amplified neuroblastomas according to relative OCT4 expression levels (n = 67; high, n = 22; low, n = 45). P-value by log–rank test. OCT4 mRNA expression designated high or low according to the average value.
Expression levels of OCT4 correlated with prognostic factors
We next checked the relationship between the expression of OCT4 and prognostic factors. The expression levels of OCT4 were significantly correlated with International Neuroblastoma Staging System (INSS) stage, Shimada pathology, and expression of NCYM and MYCN in MYCN-amplified primary neuroblastomas (Table1). In addition, univariate Cox regression analysis of 36 MYCN-amplified primary neuroblastomas indicated that high levels of OCT4 mRNA expression tended to correlate with poor prognosis (Table S1). Multivariate Cox regression analysis also revealed that OCT4 mRNA expression was not independent of NCYM and MYCN mRNA expression in MYCN-amplified primary neuroblastomas (Table S2).
Table 1.
Prognostic significance of OCT4 expression and other clinical factors in MYCN-amplified neuroblastomas (χ2-test)
| Factor | OCT4 mRNA expression | P-value | ||
|---|---|---|---|---|
| Low (n = 22) | High (n = 14) | |||
| Age, months | <18 (n = 22) | 11 | 11 | 0.079 |
| ≥18 (n = 14) | 11 | 3 | ||
| INSS stage (3 or 4) | 3 (n = 9) | 8 | 1 | <0.05 |
| 4 (n = 27) | 14 | 13 | ||
| Tumor origin | Adrenal gland (n = 32) | 19 | 13 | 0.534 |
| Others (n = 4) | 3 | 1 | ||
| Shimada classification | Favorable (n = 6) | 6 | 0 | <0.001 |
| Unfavorable (n = 30) | 16 | 14 | ||
| MYCN mRNA expression | Low (n = 25) | 18 | 7 | <0.05 |
| High (n = 11) | 4 | 7 | ||
| NCYM mRNA expression | Low (n = 26) | 21 | 5 | <0.001 |
| High (n = 10) | 1 | 9 | ||
INSS, International Neuroblastoma Staging System.
NCYM induced OCT4 via induction of MYCN
We next examined the factors that predict OCT4 expression in primary neuroblastomas by multiple regression analysis (Table S3). The expression levels of NCYM, NANOG, KLF4, and c-MYC and MYCN amplification significantly contributed to the prediction of OCT4 expression in primary neuroblastomas (Table S3). Furthermore, the expression levels of NCYM mRNA were positively correlated with those of OCT4 and NANOG, whereas KLF4 expression was inversely correlated with that of MYCN and NCYM (Table S4).
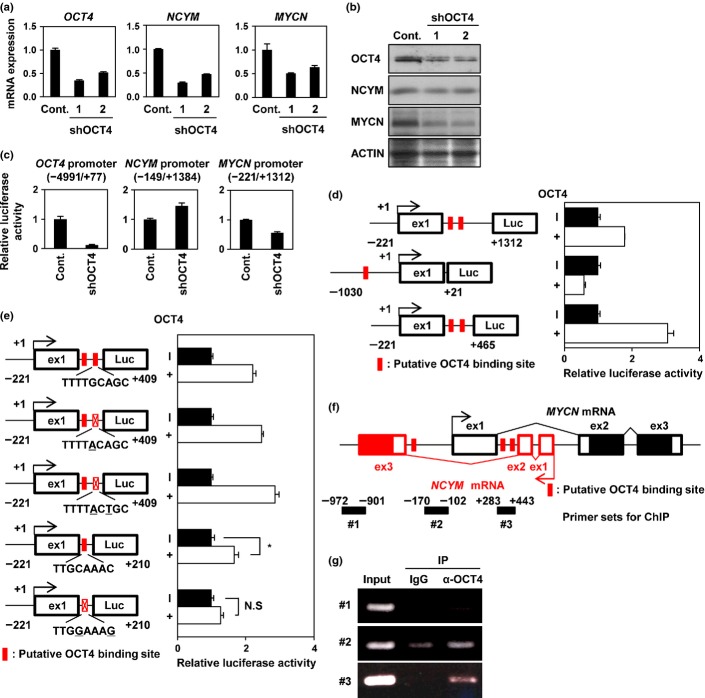
These results prompted us to assess whether NCYM regulates OCT4 as well as stem cell-related genes in human neuroblastoma cells. Overexpression of NCYM or MYCN, but not c-MYC, induced OCT4 mRNA expression (Figs S1,S2) as well as NANOG, LIN28, and SOX2, whereas neither NCYM nor MYCN enhanced c-MYC or KLF4 (Fig. S1). Knockdown of NCYM decreased OCT4 and MYCN expression at both mRNA and protein levels (Fig.2a,b), and suppressed their promoter activities (Fig.2c). In addition, the expression levels of a stem cell-related protein CD133 were also downregulated by NCYM knockdown (Fig. S3A). A previous report suggested that MYCN is directly recruited onto the distal enhancer of human OCT4.14 We thus checked the recruitment of MYCN onto putative E-box sites found in human OCT4 enhancer regions (Fig.2d). Endogenous MYCN protein was recruited onto the distal enhancer region (Fig.2d,e, #1), but not in the proximal enhancer region (Fig.2d,e, #2). Knockdown of NCYM diminished MYCN binding to the distal enhancer of OCT4 (Fig.2e). Together, these results suggest that NCYM regulates OCT4 transcription by induction of MYCN.
Fig 2.
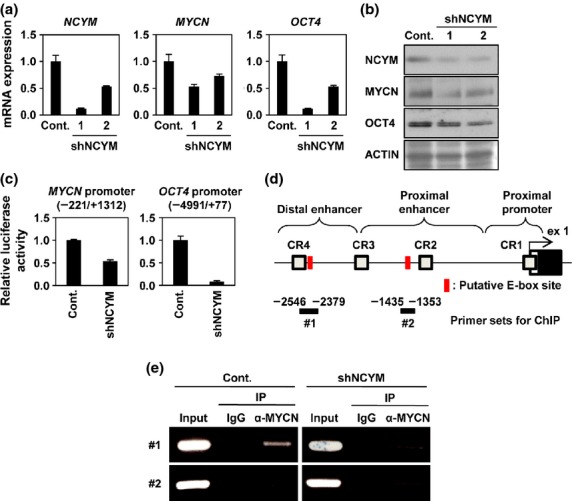
NCYM regulates OCT4 through the recruitment of MYCN onto the OCT4 promoter region in neuroblastoma cells. (a) Quantitative real-time RT-PCR analyses of NCYM, MYCN, and OCT4 in NCYM shRNA-transfected BE(2)-C intermediate (I)-type neuroblastoma cells. Seventy-two hours after infection, mRNA expression levels were measured by real-time RT-PCR with β-actin as an internal control (Cont.). (b) Western blot analyses of NCYM, MYCN, and OCT4 proteins in NCYM shRNA-transfected BE(2)-C I-type neuroblastoma cells. Seventy-two hours after infection, cells were subjected to Western blot analyses. ACTIN was used as loading control. (c) Luciferase activity of MYCN and OCT4 reporters in NCYM shRNA-transfected BE(2)-C I-type neuroblastoma cells. Seventy-two hours after infection, cells were subjected to luciferase reporter assay. Data are shown as the fold change in the luciferase activity. The activities were standardized by control cells. (d) Schematic depiction of the OCT4 promoter region. The OCT4 promoter is divided into three regions (distal enhancer, distal promoter, and proximal promoter). Each conserved region (CR1–4) and exon 1 of human OCT4 (ex1) are boxed. The gray, white, and black boxes indicate the conserved region, 5′-UTR, and coding region, respectively. The locations of the ChIP primers are indicated by bold lines. The putative E-box sites are shown in red boxes. (e) Identification of the MYCN binding region in the OCT4 promoter by ChIP assays. BE(2)-C I-type neuroblastoma cells were transfected with control shRNA or NCYM sh-1. Seventy-two hours after infection, cells were subjected to ChIP assay. Genomic DNA was amplified by PCR by specific primer sets as shown by bold black lines #1 and #2 in panel (d). The PCR bands indicated in panel #1 indicate amplification of the distal enhancer region; PCR bands indicated in panel #2 indicate amplification of the proximal enhancer region. IP, Immunoprecipitation.
In sharp contrast to human neuroblastoma cells, overexpression of NCYM in mice did not induce stem cell-related genes either in vitro (Fig. S4) or in vivo (Fig. S5). Furthermore, the E-box at the distal enhancer region of OCT4 is not evolutionally conserved among species (Fig. S6A).
Transcription of MYCN in human neuroblastoma directly stimulated by OCT4
OCT4, SOX2, and NANOG form core networks in embryonic stem (ES) cells by their mutual transcriptional regulations.15 We thus examined whether OCT4 regulates MYCN/NCYM transcription in human neuroblastoma cells. In BE(2)-C MYCN-amplified neuroblastoma cells, shRNA-mediated knockdown of OCT4 downregulated MYCN at both the mRNA and protein levels (Fig.3a,b). CD133 was also suppressed by OCT4 knockdown (Fig. S3B). Although OCT4 knockdown decreased NCYM mRNA expression, it showed marginal effects on the expression of NCYM protein (Fig.3a,b). In MYCN non-amplified SK-N-AS cells, overexpression of OCT4 induced the expression and promoter activities of MYCN and NCYM (Fig. S7), as well as the expression of NANOG and SOX2 (Fig. S8A). In BE(2)-C cells, OCT4 knockdown suppressed the promoter activities of OCT4 and MYCN, whereas it did not affect NCYM promoter activity (Fig.3c). These results suggest that OCT4 may not directly affect NCYM transcription at the endogenous expression level. Overexpression of OCT4 enhanced activities of MYCN reporter constructs containing the intron 1 region of MYCN (Fig.3d). We found two putative OCT4 binding sites within the intron 1 region, and generated luciferase reporter constructs harboring mutations in the OCT4 binding sites (Fig.3e). Mutations in the upstream OCT4 binding sequence diminished OCT4-mediated enhancement of MYCN promoter activity, whereas MYCN promoter constructs containing the WT upstream OCT4 site sustained the response to OCT4 overexpression (Fig.3e). Chromatin immunoprecipitation assay showed that OCT4 was directly recruited onto the intron 1 region of MYCN (Fig.3f,g, #3). We also observed the recruitment of OCT4 to the promoter of MYCN (Fig.3f,g, #2), although the OCT4 site in the MYCN promoter was not responsible for OCT4-mediated enhancement of MYCN promoter activity (Fig.3e). We next checked the conservation of the OCT4 binding site within intron 1 among species, and found that it is mostly conserved among primates, but not in mice (Fig. S6B).
Fig 3.
OCT4 induces transcription of MYCN in neuroblastoma cells. (a) Quantitative real-time RT-PCR analysis of NCYM, MYCN, and OCT4 in OCT4 shRNA-transfected BE(2)-C intermediate (I)-type neuroblastoma cells. Seventy-two hours after infection, mRNA expression levels were measured by real-time RT-PCR with β-actin as an internal control (Cont.). (b) Western blot analyses of NCYM, MYCN, and OCT4 proteins in OCT4 shRNA-transfected BE(2)-C I-type neuroblastoma cells. Seventy-two hours after infection, cells were subjected to Western blot analyses. ACTIN was used as loading control. (c) Luciferase activity of OCT4, NCYM, and MYCN reporters after OCT4 shRNA-transfected BE(2)-C I-type neuroblastoma cells. Seventy-two hours after infection, cells were subjected to luciferase reporter assay. Data are shown as the fold change in the luciferase activity. The activities were standardized by control cells. (d) Luciferase activity of MYCN (−221/+1312, −1030/+21, and −221/+465) reporters after OCT4 transfection of SK-N-AS neuroblastoma cells. Forty-eight hours after transfection, cells were subjected to luciferase reporter assay. Data are shown as the fold change in luciferase activity. The activities were standardized by control cells. (e) Luciferase activity of MYCN reporters (−221/+409, −221/+409 mutant 1, −221/+409 mutant 2, −221/+210 mutant, and −221/+409 mutant) after OCT4 transfection of SK-N-AS neuroblastoma cells. Forty-eight hours after transfection, cells were subjected to luciferase reporter assay. Data are shown as the fold change in the luciferase activity. The activities were standardized by control cells. The putative OCT4 binding sites are indicated in red boxes. Statistical significance determined by the Student’s t-test, $P < 0.05. (f) Schematic of the MYCN/NCYM promoter and coding region, divided into three exons (ex 1–3). Each translated region is boxed. The red and black boxes indicate NCYM and MYCN regions, respectively. Locations of the ChIP primers are indicated by the bold line. Putative OCT4 binding sites are indicated by red boxes. (g) Identification of the OCT4 binding region in the MYCN/NCYM region by ChIP assays in BE(2)-C I-type neuroblastoma cells. Seventy-two hours after infection, cells were subjected to ChIP assay. Genomic DNA was amplified by PCR using the primer sets shown in panel (f). IP, Immunoprecipitation.
OCT4 is downregulated on differentiation of neuroblastoma cells
BE(2)-C I-type neuroblastoma cells are stem cell-like cells that show the ability to differentiate into N-type cells in response to retinoic acid treatment11 and MYCN expression is downregulated during the differentiation.11 We assessed the expression of NCYM and OCT4 in BE(2)-C I-type cells treated with all-trans retinoic acid (ATRA) (Fig.4a,b). As reported previously,11 BE(2)-C I-type cells differentiated into N-type cells with marked neurite extensions (Fig.4a,b), accompanied by a rapid decrease of MYCN expression (Fig.4c,d). The decrease of MYCN was followed by the downregulation of NCYM, OCT4, NANOG, and SOX2 (Figs4c,d,S8B,C), whereas no significant changes were observed in the expression levels of NANOG mRNA (Fig. S8B). A neural maker GAP43 was induced in the ATRA-treated neuroblastomas cells (Fig. S8C). In good accordance with the strong correlation in primary tumors (Table S4), NCYM and OCT4 expression showed similar expression patterns in ATRA-treated BE(2)-C cells (Fig.4c,d). Furthermore, ATRA treatment decreased MYCN binding to the distal enhancer of OCT4 (Fig.4e) and OCT4 binding to the intron 1 region of MYCN (Fig.4f). Therefore, retinoic acid-induced neuronal differentiation abrogated the positive autoregulatory loops formed by MYCN, NCYM, and OCT4 through the simultaneous downregulation of their expression.
Fig 4.
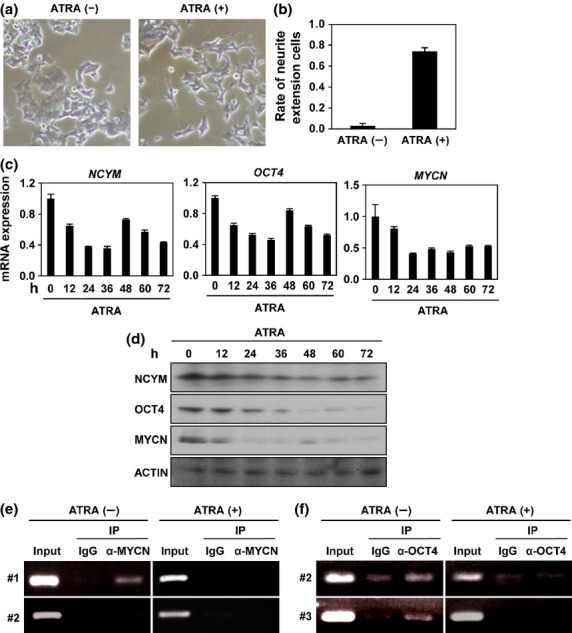
All-trans retinoic acid (ATRA)-induced neuronal differentiation abrogates the positive autoregulatory loops formed by MYCN, NCYM, and OCT4. (a) Morphology of BE(2)-C intermediate (I)-type neuroblastoma cells treated with or without ATRA. (b) Percentage of BE(2)-C I-type neuroblastoma cells with marked neurite extensions relative to control with or without ATRA. Error bars represent SEM from three independent experiments. (c) Quantitative real-time RT-PCR analysis of NCYM, MYCN and stem cell-related genes in ATRA-treated BE(2)-C I-type neuroblastoma cells. mRNA expression levels were measured by real-time RT-PCR with β-actin as an internal control. (d) Western blot analyses of NCYM, MYCN, and OCT4 proteins in ATRA-treated BE(2)-C I-type neuroblastoma cells. ACTIN was used as loading control. (e) Identification of the MYCN-binding region in the OCT4 promoter by ChIP assays. BE(2)-C I-type neuroblastoma cells were treated with or without ATRA. (f) Identification of the OCT4 binding region in the MYCN/NCYM promoter by ChIP assays. BE(2)-C I-type neuroblastoma cells were treated with or without ATRA.
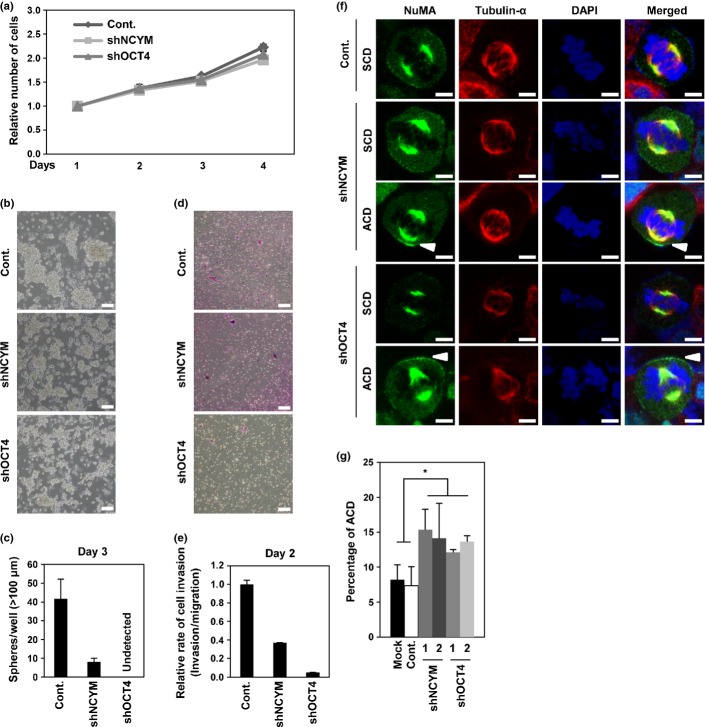
Self-renewal of neuroblastoma cells maintained by OCT4 and NCYM
We next examined whether OCT4 and NCYM contributes to self-renewal of neuroblastoma cells. Knockdown of NCYM or OCT4 in BE(2)-C cells inhibited formation of spheres of neuroblastoma cells and cellular invasion, whereas the cell proliferation was not significantly changed within 3 days after shRNA transduction (Fig.5). Izumi et al.16 reported that neuroblastoma cells have stem cell-like characteristics showing both asymmetric and symmetric cell divisions in vitro and that MYCN suppresses the asymmetric cell division (ACD). Consistent with the previous report,16 immunocytochemistry analyses showed a high percentage of cells exhibiting ACD in SK-N-AS MYCN-non-amplified cells compared with BE(2)-C MYCN-amplified cells (Fig. S9). The shRNA-mediated knockdown of NCYM or OCT4 significantly increased the number of cells exhibiting ACD in BE(2)-C cells (Fig.5f,g). Collectively, these results suggest that NCYM and OCT4 maintain self-renewal of human neuroblastoma cells.
Fig 5.
NCYM and OCT4 control self-renewal of neuroblastoma cells. (a) Cell viability assay of BE(2)-C intermediate (I)-type neuroblastoma cells with NCYM or OCT4 shRNA-mediated knockdown. Cell proliferation was examined by WST assays at the indicated time points. (b) Sphere formation assay of BE(2)-C I-type neuroblastoma cells. Representative images show induction of sphere-forming activity after knockdown of NCYM or OCT4. Scale bar = 100 μm. (c) Quantification of sphere numbers from panel (b). The numbers of spheres were counted 72 h after infection. Error bars represent SEM from three independent experiments. (d) Invasion assay of BE(2)-C I-type neuroblastoma cells. Representative images show invasion activity after knockdown of NCYM or OCT4. Scale bar = 100 μm. (e) Quantification of BE(2)-C I-type neuroblastoma cells invading Matrigel relative to control (Cont.) migration after NCYM or OCT4 shRNA-mediated knockdown from panel (d). The numbers of spheres were counted 48 h after infection. Error bars represent SEM from three independent experiments. (f) Representative images of symmetric distribution of nuclear mitotic apparatus protein (NuMA) during the late stage of mitosis in shRNA-treated neuroblastoma cells. Tubulin-α is indicated in red, NuMA is green, and DNA is blue. Arrows show the distribution of NuMA on the cell cortex. Scale bar = 5 μm. (g) Quantification of cells with asymmetric cell division (ACD) in shRNA-transfected human neuroblastoma cells during late metaphase and anaphase. Error bars represent SEM from three experiments. Statistical significance determined by the Student’s t-test, $P < 0.05. SCD, Symmetric cell division.
Discussion
Here, we found that OCT4 promotes aggressiveness of MYCN-amplified neuroblastoma cells by forming a positive regulatory loop with MYCN/NCYM (Fig.6). Despite a correlation between OCT4 expression and a stem cell-like state of neuroblastomas, the clinical significance of OCT4 in neuroblastomas has remained elusive. In this study, we found that OCT4 was correlated with NCYM expression and undifferentiated pathological characteristics in Shimada pathology. Furthermore, the expression levels of OCT4 were associated with unfavorable outcomes in MYCN-amplified tumors, but not in MYCN-non-amplified tumors. Previous studies showed that MYCN expression was inversely correlated with c-MYC in neuroblastoma17 and that low expression levels of KLF4 mRNA were associated with poor neuroblastoma outcome.18 Our results showed that NCYM was positively correlated with NANOG expression and was inversely correlated with KLF4 and c-MYC. As overexpression of OCT4 induced NANOG mRNAs, the correlation between NANOG and MYCN/NCYM in neuroblastomas may be explained by their common upstream regulator, OCT4. In vitro experiments showed that overexpression of NCYM induced OCT4, SOX2, and NANOG, but not c-MYC or KLF4. Therefore, among stem cell-related genes, NCYM mainly regulated the transcription of genes related to maintenance of pluripotency of ES cells19–21 in human neuroblastoma cells. The NCYM protein stabilized MYCN to stimulate OCT4 transcription, whereas OCT4 induced NCYM and MYCN through direct transcriptional activation of MYCN. Therefore, MYCN, NCYM, and OCT4 cooperate to induce each other, resulting in keeping their own expression at high levels and maintaining self-renewal of cells in MYCN-amplified neuroblastomas. Differentiation-inducing therapy by retinoic acid treatment has improved the overall survival of patients with MYCN-amplified neuroblastomas,22 and ATRA treatment abrogated the mutual transcriptional regulations between MYCN, NCYM, and OCT4, inducing neuroblastoma cell differentiation. The ATRA treatment rapidly decreased NCYM mRNA within 24 hours, but the protein levels of NCYM were hardly downregulated compared with those of MYCN or OCT4. Therefore, the NCYM protein may be relatively more stable than MYCN or OCT4 proteins. Previous studies have shown that OCT4-positive neuroblastoma cells have resistant potency to conventional therapy12 and multipotency to differentiation.9 Thus, the functional interplay between MYCN/NCYM and OCT4 may contribute to maintenance of the multipotent status of OCT4-positive cells and the disruption of the MYCN/NCYM–OCT4 network could be a good therapeutic strategy for aggressive tumors.
Fig 6.
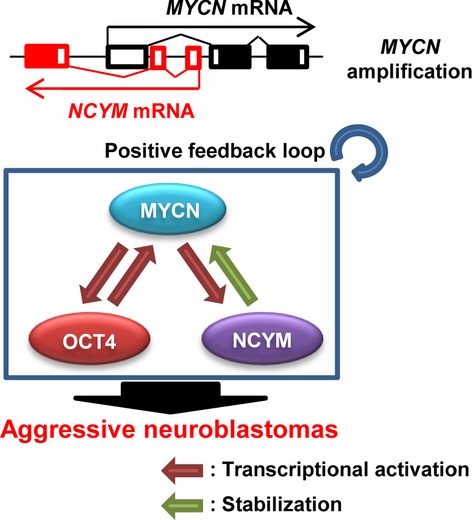
Schematic model of MYCN/NCYM–OCT4 networks in MYCN-amplified human neuroblastomas.
Pezzolo et al.9 reported that 2–30% of OCT4-positive cells were detected in approximately 90% of neuroblastoma samples (21 of 23). Thus, in contrast to other cancer stem cells,23 the stem cell-like populations of neuroblastomas may not be small. In addition, high OCT4 expression was correlated with poor prognoses in patients with MYCN-amplified neuroblastomas, but not MYCN-non-amplified tumors, although the expression levels of OCT4 in MYCN-non-amplified tumors were comparable to those in MYCN-amplified tumors. These results indicate that OCT4 requires MYCN amplification to promote aggressiveness of neuroblastomas. As NCYM inhibits apoptosis in MYCN-amplified tumors,6 NCYM may be required for efficient proliferation of multipotent OCT4-positive cells. Therefore, variable amounts of OCT4-positive cells in MYCN-amplified tumors may reflect the different percentages of proliferative stem cell-like cells, influencing the prognoses of patients.
Previous reports have shown the physiological roles of OCT4 in the transcriptional regulation of MYC family members in various species.24–26 OCT4 stimulates MYC transcription for cell proliferation in human and mouse ES cells24,25 and activates myc transcription for cell survival during zebrafish gastrulation.26 In the present study, we found the pathological significance of OCT4 for MYCN transcription in human neuroblastoma cells. The OCT4 binding sequence in MYCN intron 1 is not present in mice, but it is mostly conserved in other mammals. Although the E-box responsible for MYCN-mediated MYCN/NCYM transcription is highly constrained in mammals (Fig. S10), NCYM coding sequences are conserved only in humans and monkeys.6 Therefore, the transcriptional regulation of MYCN by OCT4 and the positive autoregulation of MYCN may have evolved before the emergence of the NCYM gene, and NCYM strengthens the MYCN–OCT4 network by stabilizing MYCN, thereby inducing OCT4 transcription. NCYM is positively selected during evolution;6 however, its physiological roles in normal stem cells have remained unknown. Because new genes have been reported to rapidly become essential after emergence,27,28 future studies will need to examine the physiological roles of the MYCN/NCYM–OCT4 networks in the maintenance of human normal stem cells.
Acknowledgments
This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labor and Welfare for the Third Term Comprehensive Control Research for Cancer, Japan (A.N.), a Grant-in-Aid from the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct), Ministry of Education, Culture, Sports, Science and Technology, Japan (A.N.), a Grant-in-Aid from Takeda Science Foundation (A.N.), a Grant-in-Aid for Scientific Research on Priority Areas (Japan Society for the Promotion of Science [JSPS] Kakenhi Grant No. 17015046) (A.N.), a Grant-in-Aid for Scientific Research (A) (JSPS Kakenhi Grant No. 24249061) (A.N.), a Fund for Health and Labor Sciences Research (Grant No. 26271201) (A.N.), a Grant-in-Aid for Research Activity Start-up (JSPS Kakenhi Grant No. 22890241) (Y.S.), and a Grant-in-Aid for Young Scientists (B) (JSPS Kakenhi Grant No. 24700957) (Y.S.).
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. NCYM and MYCN induce expression of embryonic stem cell-related genes.
Fig. S2. Overexpression of c-MYC does not enhance expression of OCT4, NCYM, or MYCN in SK-N-AS human neuroblastoma cells.
Fig. S3. Knockdown of NCYM or OCT4 decreases expression of CD133 in neuroblastoma cells.
Fig. S4. NCYM and MYCN do not induce mouse Oct4 in mouse neuroblastoma cells.
Fig. S5. Expression of OCT4 mRNA in tumors developed from MYCN and MYCN/NCYM transgenic mice.
Fig. S6. Conservation of OCT4 binding and E-box site in OCT4 promoter and MYCN/NCYM regions.
Fig. S7. OCT4 induces the expression of NCYM and MYCN in neuroblastoma cells.
Fig. S8. All-trans retinoic acid (ATRA) suppresses stem cell-related genes.
Fig. S9. Asymmetric cell division in human neuroblastoma cells.
Fig. S10. Conservation of the E-box site at the MYCN/NCYM region.
Table S1. Univariate Cox regression analysis using OCT4 mRNA expression level and clinical prognosis factors in MYCN-amplified neuroblastomas.
Table S2. Multivariate Cox regression analysis using OCT4 mRNA expression level and clinical prognosis factors in MYCN-amplified neuroblastomas.
Table S3. Multiple regression analysis for factors associated with OCT4 mRNA expression in neuroblastomas.
Table S4. Correlation between NCYM and MYCN mRNA expression and embryonic stem cell-related genes in neuroblastomas.
Doc. S1. Detailed descriptions of Materials and Methods.
References
- Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–4. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–95. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–66. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–90. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- Suenaga Y, Islam SM, Alagu J, et al. NCYM, a Cis-antisense gene of MYCN, encodes a de novo evolved protein that inhibits GSK3beta resulting in the stabilization of MYCN in human neuroblastomas. PLoS Genet. 2014;10:e1003996. doi: 10.1371/journal.pgen.1003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga Y, Kaneko Y, Matsumoto D, Hossain MS, Ozaki T, Nakagawara A. Positive auto-regulation of MYCN in human neuroblastoma. Biochem Biophys Res Commun. 2009;390:21–6. doi: 10.1016/j.bbrc.2009.09.044. [DOI] [PubMed] [Google Scholar]
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- Pezzolo A, Parodi F, Marimpietri D, et al. Oct-4+/Tenascin C+ neuroblastoma cells serve as progenitors of tumor-derived endothelial cells. Cell Res. 2011;21:1470–86. doi: 10.1038/cr.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar JJ, Domingo-Fernandez R, Ebus ME, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44:1199–206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- Ross RA, Spengler BA, Domenech C, Porubcin M, Rettig WJ, Biedler JL. Human neuroblastoma I-type cells are malignant neural crest stem cells. Cell Growth Differ. 1995;6:449–56. [PubMed] [Google Scholar]
- Hammerle B, Yanez Y, Palanca S, et al. Targeting neuroblastoma stem cells with retinoic acid and proteasome inhibitor. PLoS One. 2013;8:e76761. doi: 10.1371/journal.pone.0076761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira M, Oba S, Nakamura Y, et al. Expression profiling using a tumor-specific cDNA microarray predicts the prognosis of intermediate risk neuroblastomas. Cancer Cell. 2005;7:337–50. doi: 10.1016/j.ccr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Cotterman R, Knoepfler PS. N-Myc regulates expression of pluripotency genes in neuroblastoma including lif, klf2, klf4, and lin28b. PLoS One. 2009;4:e5799. doi: 10.1371/journal.pone.0005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–46. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- Izumi H, Kaneko Y. Evidence of asymmetric cell division and centrosome inheritance in human neuroblastoma cells. Proc Natl Acad Sci U S A. 2012;109:18048–53. doi: 10.1073/pnas.1205525109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann F, Muth D, Benner A, et al. Distinct transcriptional MYCN/c-MYC activities are associated with spontaneous regression or malignant progression in neuroblastomas. Genome Biol. 2008;9:R150. doi: 10.1186/gb-2008-9-10-r150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum CK, Lau ST, Tsoi LL, et al. Kruppel-like factor 4 (KLF4) suppresses neuroblastoma cell growth and determines non-tumorigenic lineage differentiation. Oncogene. 2013;32:4086–99. doi: 10.1038/onc.2012.437. [DOI] [PubMed] [Google Scholar]
- Kim J, Woo AJ, Chu J, et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–24. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–54. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Kotkamp K, Kur E, Wendik B, et al. Pou5f1/Oct4 promotes cell survival via direct activation of mych expression during zebrafish gastrulation. PLoS One. 2014;9:e92356. doi: 10.1371/journal.pone.0092356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang YE, Long M. New genes in Drosophila quickly become essential. Science. 2010;330:1682–5. doi: 10.1126/science.1196380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt JA, Wanjiru BM, Brant AT, Saelao P, Begun DJ, Jones CD. De novo ORFs in Drosophila are important to organismal fitness and evolved rapidly from previously non-coding sequences. PLoS Genet. 2013;9:e1003860. doi: 10.1371/journal.pgen.1003860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. NCYM and MYCN induce expression of embryonic stem cell-related genes.
Fig. S2. Overexpression of c-MYC does not enhance expression of OCT4, NCYM, or MYCN in SK-N-AS human neuroblastoma cells.
Fig. S3. Knockdown of NCYM or OCT4 decreases expression of CD133 in neuroblastoma cells.
Fig. S4. NCYM and MYCN do not induce mouse Oct4 in mouse neuroblastoma cells.
Fig. S5. Expression of OCT4 mRNA in tumors developed from MYCN and MYCN/NCYM transgenic mice.
Fig. S6. Conservation of OCT4 binding and E-box site in OCT4 promoter and MYCN/NCYM regions.
Fig. S7. OCT4 induces the expression of NCYM and MYCN in neuroblastoma cells.
Fig. S8. All-trans retinoic acid (ATRA) suppresses stem cell-related genes.
Fig. S9. Asymmetric cell division in human neuroblastoma cells.
Fig. S10. Conservation of the E-box site at the MYCN/NCYM region.
Table S1. Univariate Cox regression analysis using OCT4 mRNA expression level and clinical prognosis factors in MYCN-amplified neuroblastomas.
Table S2. Multivariate Cox regression analysis using OCT4 mRNA expression level and clinical prognosis factors in MYCN-amplified neuroblastomas.
Table S3. Multiple regression analysis for factors associated with OCT4 mRNA expression in neuroblastomas.
Table S4. Correlation between NCYM and MYCN mRNA expression and embryonic stem cell-related genes in neuroblastomas.
Doc. S1. Detailed descriptions of Materials and Methods.