Abstract
We carried out a multicenter dose-escalation phase I study of oral OPB-51602, a signal transducer and activator of transcription 3 phosphorylation inhibitor, in patients with relapsed or refractory hematological malignancies to evaluate the safety, maximum tolerated dose (MTD), pharmacokinetics, and preliminary antitumor activity. Twenty patients were treated with OPB-51602 at doses of 1, 2, 3, 4, and 6 mg in the “3 + 3” dose escalation design. The most common treatment-related adverse events included nausea (55%), peripheral sensory neuropathy (45%), and diarrhea (40%). The most frequently observed grade 3 or 4 drug-related adverse events were neutropenia (20%), leukopenia (15%), lymphopenia (10%), and thrombocytopenia (10%). The MTD was 6 mg, with dose-limiting toxicities of grade 3 lactic acidosis and increased blood lactic acid levels observed in one of three patients and grade 1–2 peripheral neuropathy in three of three patients. The recommended dose was determined to be 4 mg. OPB-51602 was rapidly absorbed, and exposure tended to increase in a dose-dependent manner. Accumulation of OPB-51602 was seen with 4 weeks of multiple treatments. No clear therapeutic response was observed. Durable stable disease was observed in two patients with acute myeloid leukemia and one with myeloma. In conclusion, the MTD of OPB-51602 was 6 mg. OPB-51602 was safe and well tolerated in a dose range of 1–4 mg. However, long-term administration at higher doses was difficult with the daily dosing schedule, and no response was seen. Therefore, further clinical development of OPB-51602 for hematological malignancies with a daily dosing schedule was terminated.
Keywords: Daily dosing schedule, hematological malignancy, OPB-51602, phase I study, STAT3 inhibitor
Activation of signal transducer and activator of transcription 3 (STAT3) has been reported in a wide range of human tumors.1–3 STAT3 is activated by at least three pathways including pathways mediated by cytokines, growth factors, and oncogenic non-receptor tyrosine kinase-activated signal transduction.4–14 Activated STAT3 translocates to the nucleus and controls expression of a set of target genes that regulates cell proliferation, survival, and angiogenesis.4,10 Numerous studies have shown that tumor growth is inhibited by blocking constitutively activated STAT3.14–18 Therefore, STAT3 is thought to be a promising therapeutic target for anticancer therapy.4,5,18
OPB-51602 is an orally active low molecular-weight compound with potent antitumor activity in numerous in vivo and in vitro models of solid and hematopoietic tumors, as shown in our non-clinical studies (Otsuka Pharmaceutical Co., Ltd, unpublished data). Non-clinical studies also have indicated that OPB-51602 inhibits tyrosine and serine phosphorylation of STAT3 with no notable changes in the amount of total STAT3 (Otsuka Pharmaceutical Co., Ltd, unpublished data). This effect is thought to contribute to the antitumor properties of OPB-51602, although the mechanism of action has not been fully elucidated.
We undertook an open-label, non-randomized, multicenter, dose-escalation phase I trial in patients with relapsed or refractory hematologic malignancies to determine the safety profile, maximum tolerated dose (MTD), pharmacokinetics, and preliminary antitumor activity of OPB-51602.
Patients and Methods
Patient selection
Enrolment criteria for patients included: (i) diagnosis of acute myeloid leukemia (AML), non-Hodgkin’s lymphoma, multiple myeloma (MM), or chronic myeloid leukemia; (ii) relapsed after or refractory to previous standard treatment; (iii) Eastern Cooperative Oncology Group performance status of 0–1; and (iv) aged 20–75 years. Adequate bone marrow, hepatic, and renal functions were mandatory and were defined as: hemoglobin, ≥8.0 g/dL; absolute neutrophil count, ≥1.5 × 109/L; platelet count, ≥75 × 109/L (not applicable for leukemia); bilirubin, ≤1.5× upper limit of normal (ULN); aspartate aminotransferase, ≤2.5× ULN; alanine aminotransferase, ≤2.5× ULN; and creatinine, ≤1.5× ULN. All patients signed written informed consent. The study was approved by the institutional review board at each participating institute.
Study design
The primary objective of this study was to determine the tolerability, safety profile, and MTD of OPB-51602 in patients with relapsed or refractory hematological malignancies. Secondary objectives included determination of pharmacokinetics and the preliminary antitumor activity of OPB-51602 in this patient population.
OPB-51602 was given orally once daily, continuously for 4 weeks per cycle, until disease progression or unacceptable toxicity was observed. The starting dose was 1 mg, and the dose was escalated to 2, 3, 4, and 6 mg. Dose escalation was based on the “3 + 3” design. Maximum tolerated dose was defined as the dose in which dose-limiting toxicities (DLTs) in the first treatment cycle were observed in two or more out of six patients. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. A DLT was defined as any of the following that were related to OPB-51602 during the first treatment cycle: grade 3 nausea, vomiting, or diarrhea despite the use of anti-emetic or anti-diarrheal drugs; any grade 3 non-hematologic toxicity, excluding alopecia; grade 4 neutropenia lasting ≥8 days (not applicable for leukemia); grade 3 febrile neutropenia or infection due to neutropenia (not applicable for leukemia); and grade 4 thrombocytopenia or grade 3 thrombocytopenia requiring platelet transfusion (not applicable for leukemia).
Assessment of the treatment response was evaluated according to internationally recognized response criteria for MM, non-Hodgkin’s lymphoma, AML, or chronic myeloid leukemia.19–22
Pharmacokinetics
Blood samples were collected for pharmacokinetic analysis in the first treatment cycle on days 1–4 and on days 28–31. The time course of the plasma concentration and pharmacokinetic parameters of OPB-51602 were determined. Pharmacokinetic parameters were estimated using non-compartmental methods with Phoenix WinNonlin 6.3.
Evaluation of levels of phosphorylated STAT
Immunostaining for pY705-STAT3 was carried out on formalin-fixed, paraffin-embedded bone marrow clotted samples or lymph node biopsy samples using anti-phospho-STAT3 (Tyr705) rabbit mAb (code number 9145; Cell Signaling Technology, 3 Trask Lane, Danvers, MA, 01923, United States). Samples were obtained from available patients prior to treatment and at the end of each treatment cycle. If at least one tumor cell showed staining in the nucleus, pY705-STAT3 staining was considered positive.
Results
Patients
From May 2011 to August 2013, a total of 20 patients were enrolled in this study and treated with oral OPB-51602 (1 mg, n = 4; 2 mg, n = 3; 3 mg, n = 4; 4 mg, n = 6; 6 mg, n = 3). Table1 shows a summary of demographic and other baseline characteristics for all patients, which included 13 males and 7 females with a median age of 64 years (range, 49–74 years). Primary diseases were AML in seven patients, diffuse large B-cell lymphoma in five patients, MM in four patients, follicular lymphoma in three patients, and angioimmunoblastic T-cell lymphoma in one patient. The median number of prior treatment regimens was 3.5 (range, 1–9).
Table 1.
Patients’ demographics and characteristics
| Number of enrolled patients | n = 20 |
|---|---|
| Age, years, median (range) | 64 (49–74) |
| Gender, n (%) (male/female) | 13 (65)/7 (35) |
| ECOG performance status, n (%) (0/1) | 20 (100)/0 |
| Prior chemotherapy | |
| Median number of prior treatment regimens (range) | 3.5 (1–9) |
| pY705-STAT3 | |
| Assessed patients, n | 16 |
| Positive, n (%) | 4 (25) |
| Tumor type, n (%) | |
| AML | 7 (35) |
| NHL | 9 (45) |
| DLBCL | 5 (25) |
| FL | 3 (15) |
| AITL | 1 (5) |
| MM | 4 (20) |
AITL, angioimmunoblastic T-cell lymphoma; AML, acute myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; MM, multiple myeloma; NHL, non-Hodgkin’s lymphoma; STAT3, signal transducer and activator of transcription 3.
Safety
The DLT analysis set consisted of 15 patients (three patients per dose) among the 20 patients enrolled in this study. The remaining five patients were excluded from the DLT analysis set because of early progression of disease or request from the patient to discontinue administration of OPB-51602 before completion of the first treatment cycle. No DLT was found in the 1- to 4-mg cohorts, but DLT did emerge in one of three patients in the 6-mg cohort. The DLT appeared as grade 3 lactic acidosis and an increased blood lactic acid level, which were observed on day 13 after the start of administration and quickly resolved with suitable medical intervention after discontinuation of OPB-51602. During the first treatment cycle, grade 1–2 peripheral sensory neuropathy was also found in three patients in the 6-mg cohort. The frequency of peripheral sensory neuropathy tended to exacerbate in a dose-dependent manner, and because it was thought to influence long-term administration of OPB-51602, dose escalation was discontinued without supplementing the 6-mg cohort with an additional three patients. The MTD of OPB-51602 for hematological malignancies was estimated to be 6 mg, and the recommended dose was determined to be 4 mg.
Treatment-related AEs were reported in 18 patients (90%). Table2 lists the treatment-related AEs that occurred in ≥20% of patients. The common treatment-related AEs were nausea (n = 11, 55%), peripheral sensory neuropathy (n = 9, 45%), diarrhea (n = 8, 40%), decreased appetite (n = 7, 35%), anemia (n = 6, 30%), malaise (n = 5, 25%), vomiting (n = 5, 25%), neutropenia (n = 5, 25%), leukopenia (n = 5, 25%), thrombocytopenia (n = 5, 25%), and fatigue (n = 4, 20%). Grade 3 or 4 drug-related AEs were neutropenia (n = 4, 20%), leukopenia (n = 3, 15%), lymphopenia (n = 2, 10%), thrombocytopenia (n = 2, 10%), anemia (n = 1, 5%), diarrhea (n = 1, 5%), increased blood lactic acid levels (n = 1, 5%), acidosis (n = 1, 5%), and hypophosphatemia (n = 1, 5%). Treatment-related AEs leading to discontinuation of treatment occurred in two patients (palpitations in one patient and acidosis, peripheral sensory neuropathy, and blood lactic acid increases in another patient) in the 6-mg cohort.
Table 2.
Treatment-related adverse events that occurred in ≥20% of patients with relapsed or refractory hematological malignancies treated with oral OPB-51602
| Treatment-related adverse events | n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg (n = 4) | 2 mg (n = 3) | 3 mg (n = 4) | 4 mg (n = 6) | 6 mg (n = 3) | Total (n = 20) | |||||||
| All Gr | Gr 3/4 | All Gr | Gr 3/4 | All Gr | Gr 3/4 | All Gr | Gr 3/4 | All Gr | Gr 3/4 | All Gr | Gr 3/4 | |
| Nausea | 3 (75) | 0 (0) | 1 (33) | 0 (0) | 4 (100) | 0 (0) | 2 (33) | 0 (0) | 1 (33) | 0 (0) | 11 (55) | 0 (0) |
| Peripheral sensory neuropathy | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 3 (75) | 0 (0) | 2 (33) | 0 (0) | 3 (100) | 0 (0) | 9 (45) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (25) | 0 (0) | 3 (50) | 0 (0) | 3 (100) | 1 (33) | 8 (40) | 1 (5) |
| Decreased appetite | 1 (25) | 0 (0) | 1 (33) | 0 (0) | 2 (50) | 0 (0) | 3 (50) | 0 (0) | 0 (0) | 0 (0) | 7 (35) | 0 (0) |
| Anemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 2 (33) | 1 (17) | 2 (67) | 0 (0) | 6 (30) | 1 (5) |
| Malaise | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (17) | 0 (0) | 3 (100) | 0 (0) | 5 (25) | 0 (0) |
| Vomiting | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 5 (25) | 0 (0) |
| Neutropenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 2 (33) | 2 (33) | 2 (67) | 2 (67) | 5 (25) | 4 (20) |
| Leukopenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 2 (33) | 1 (17) | 2 (67) | 1 (33) | 5 (25) | 3 (15) |
| Thrombocytopenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (50) | 1 (25) | 2 (33) | 1 (17) | 1 (33) | 0 (0) | 5 (25) | 2 (10) |
| Fatigue | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 4 (20) | 0 (0) |
Gr, grade.
Responses
No objective response, including complete response or partial response, was obtained. Durable stable disease (>6 months) was observed in three patients: two patients with AML in the 1- and 2-mg cohorts and one patient with MM in the 4-mg cohort. The patient with acute megakaryoblastic leukemia (AML-M7) in the 1-mg cohort continued OPB-51602 administration for 8 months (eight cycles). Another patient with acute myelomonocytic leukemia (AML-M4) in the 2-mg cohort continued OPB-51602 administration for 15 months (15 cycles). The patient with MM in the 4-mg cohort continued OPB-51602 administration for 8 months (eight cycles).
Pharmacokinetics
OPB-51602 was rapidly absorbed after both single and multiple administrations. The median time to reach the maximum plasma concentration (tmax) was between 2.0 and 4.5 h (Table3, Fig.1). For the lower dose groups (the 1- and 2-mg cohorts), terminal-phase elimination half-life (t1/2,z) was evaluable in a few patients due to low OPB-51602 plasma levels after single administration. The mean t1/2,z was 50.1–240.0 h after multiple administrations. Exposure (area under the plasma concentration–time curve from 0 to 24 h [AUC24 h] and maximum plasma drug concentration [Cmax]) tended to increase in a dose-dependent manner following multiple administrations across the dose range from 1 to 4 mg (Table3). We observed accumulation of OPB-51602 with a 2.6–10.2-fold increase in AUC24 h and a 1.4–4.6-fold increase in Cmax with doses of 1, 2, 3, and 4 mg.
Table 3.
Pharmacokinetic parameters (mean) of OPB-51602 after single and repeated dosing in patients with relapsed or refractory hematological malignancies
| Dose | 1 mg | 2 mg | 3 mg | 4 mg | 6 mg |
|---|---|---|---|---|---|
| Single administration: day 1 | |||||
| n | 3 | 2 | 4 | 4 | 2 |
| Cmax, ng/mL | 0.2 | 0.2 | 0.7 | 3.8 | 2.2 |
| tmax, h† | – | – | 4.5 | 3.0 | – |
| AUC24 h, h ng/mL | 0.8 | 1.0 | 3.0 | 18.0 | 10.6 |
| t1/2,z, h | – | – | 6.6‡ | 4.6¶ | – |
| Multiple administrations: day 28 | |||||
| n | 3 | 3 | 3 | 2 | 0 |
| Cmax, ng/mL | 1.1 | 2.0 | 2.2 | 4.7 | – |
| tmax, h† | 4.0 | 4.0 | 2.0 | – | – |
| AUC24 h, h·ng/mL | 10.9 | 20.7 | 27.3 | 50.8 | – |
| t1/2,z, h | 50.1 | 124.0 | 70.6§ | 240.0‡ | – |
†Median; ‡n = 1; §n = 2; ¶n = 3. –, Not calculated, plasma concentrations below the lower limit of quantification were set to 0 ng/mL; AUC24 h, area under the plasma concentration–time curve from 0 to 24 h; Cmax, maximum plasma drug concentration; n, number of patients; t1/2,z, terminal-phase elimination half-life; tmax, time to maximum plasma concentration.
Fig 1.
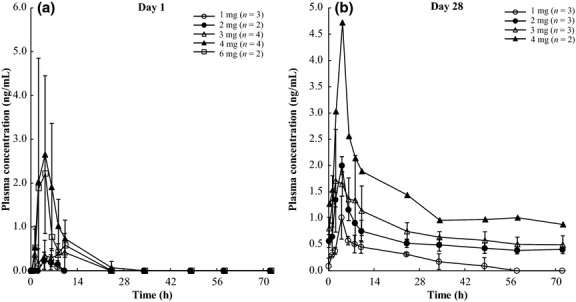
Time-course of the mean plasma con-centration of OPB-51602 after single administration (a) and multiple administrations on day 28 (b). Plasma concentrations below the lower limit of quantification were considered to be 0 ng/mL. Values are the mean ± SD.
Phosphorylated STAT levels
Pharmacodynamic effects of OPB-51602 on the expression of phosphorylated STAT3 at tyrosine 705 (pY705-STAT3) were measured using immunostaining. pY705-STAT3 was assessed in 16 patients (Table4). Four patients (follicular lymphoma, patient number 001S003; AML, patient numbers 003S003 and 003S009; and diffuse large B-cell lymphoma, patient number 004S003) were positive for pY705-STAT3 before treatment with OPB-51602. Among these four positive patients, the pY705-STAT3 status changed from positive to negative after OPB-51602 treatment in one patient with AML-M4 (patient number 003S003) in the 2-mg cohort. This patient experienced long-term stable disease. Immunostaining for pY705-STAT3 after treatment with OPB-51602 was not carried out in two patients (patient numbers 003S009 and 004S003) because these patients discontinued OPB-51602 administration within cycle 1 due to progressive disease or DLT. The relationship between pY705-STAT3 and efficacy (antitumor effect) was not investigated due to the lack of response.
Table 4.
Results of immunostaining for phosphorylated signal transducer and activator of transcription 3 (STAT3) in patients with relapsed or refractory hematological malignancies treated with oral OPB-51602
| Patient no. | Dose (mg) | Tumor type | Sampling point | Type of sample | Result of pSTAT3 |
|---|---|---|---|---|---|
| 002S001 | 1 | MM | Baseline | BMCLOT | Negative |
| 002S003 | 2 | AITL | Baseline | LN | Negative |
| Baseline | BMCLOT | Negative | |||
| 003S003 | 2 | AML-M4 | Baseline | BMCLOT | Positive |
| End of cycle 1 | BMCLOT | Negative | |||
| End of cycle 2 | BMCLOT | Negative | |||
| End of cycle 3 | BMCLOT | Negative | |||
| End of cycle 4 | BMCLOT | Positive | |||
| End of cycle 5 | BMCLOT | ND | |||
| End of cycle 6 | BMCLOT | Positive | |||
| End of cycle 7 | BMCLOT | Positive | |||
| End of cycle 8 | BMCLOT | Negative | |||
| End of cycle 10 | BMCLOT | Positive | |||
| End of cycle 11 | BMCLOT | Negative | |||
| End of cycle 12 | BMCLOT | Negative | |||
| End of cycle 13 | BMCLOT | Positive | |||
| End of cycle 14 | BMCLOT | Negative | |||
| End of cycle 15 | BMCLOT | Positive | |||
| End of cycle 16 | BMCLOT | Positive | |||
| 002S004 | 3 | DLBCL | Baseline | LN | Negative |
| Baseline | BMCLOT | Negative | |||
| 002S005 | 3 | DLBCL | Baseline | LN | Negative |
| Baseline | BMCLOT | Negative | |||
| 003S005 | 3 | AML-M2 | Baseline | BMCLOT | Negative |
| 004S001 | 3 | FL | Baseline | LN | Negative |
| Baseline | BMCLOT | Negative | |||
| 002S006 | 4 | MM | Baseline | BMCLOT | Negative |
| 002S007 | 4 | FL | Baseline | LN | Negative |
| Baseline | BMCLOT | N.D. | |||
| 003S006 | 4 | DLBCL | Baseline | LN | Negative |
| Baseline | BMCLOT | Negative | |||
| 003S007 | 4 | MM | Baseline | BMCLOT | Negative |
| 003S008 | 4 | AML-M2 | Baseline | BMCLOT | Negative |
| 003S009 | 4 | AML-M6 | Baseline | BMCLOT | Positive |
| 001S003 | 6 | FL | Baseline | LN | Negative |
| Baseline | BMCLOT | Positive | |||
| End of cycle 1 | BMCLOT | Positive | |||
| End of cycle 2 | BMCLOT | Positive | |||
| 004S003 | 6 | DLBCL | Baseline | LN | Positive |
| Baseline | BMCLOT | Negative | |||
| 006S001 | 6 | FL | Baseline | LN | Negative |
| Baseline | BMCLOT | Negative |
Positive pSTAT3 at baseline is shown in italics. AITL, angioimmunoblastic T-cell lymphoma; AML, acute myeloid leukemia; BMCLOT, bone marrow clotted sample; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LN, lymph node biopsy sample; MM, multiple myeloma; ND, not determined; NHL, non-Hodgkin’s lymphoma; pSTAT3, pY705-STAT3.
Discussion
The Jak–STAT pathway is activated in most hematopoietic tumors and is considered a good target for anticancer therapy.23,24 The Jak2-specific inhibitor ruxolitinib has been approved for myelofibrosis as an anticancer drug that targets the Jak–STAT pathway.25,26 In cancer, however, STAT3 is phosphorylated not only by Jak, but also by other growth factor signals and oncogenic non-receptor-type tyrosine kinases such as Src.4,5 STAT3 has both tyrosine and serine phosphorylation sites, and a tyrosine kinase inhibitor such as ruxolitinib can suppress tyrosine phosphorylation by Jak1/2, but not phosphorylation on serine.27 OPB-51602 is an inhibitor that targets STAT3 and can inhibit phosphorylation on both tyrosine and serine. Thus, OPB-51602 was expected to act as a STAT inhibitor with a different profile from the Jak inhibitors.
In the present phase I, multicenter, open-label, dose-escalation study of oral OPB-51602 as a single agent, OPB-51602 was reasonably well-tolerated with an oral dose of 1–4 mg in patients with relapsed or refractory hematologic malignancies. However, when patients were treated with OPB-51602 at 6 mg, none of the patients remained in the study for 28 days due to grade 3 or 4 treatment-related AEs including neutropenia, diarrhea, acidosis, and hypophosphatemia. These results indicate that long-term administration with OPB-51602 at higher than the recommended dose (4 mg) may be difficult. Goh et al. reported a phase I study of OPB-51602 against solid tumors using a regimen of 2 weeks of administration followed by a 1-week washout period. The main signs of toxicity were nausea/vomiting, diarrhea, peripheral neuropathy, and fatigue.28 Although the treatment schedule differed, the findings of this study are consistent with that report, and a similar safety profile was observed in patients with hematological malignancies.
In the above study, Goh et al.28 also reported that after administration of OPB-51602, the expression of pY705-STAT3 was significantly decreased in human peripheral monocytes of solid tumor patients. The expression of pY705-STAT3 was measured in tumor samples in this study, but almost no pY705-STAT3 expression was detected, and no clear decrease after administration was found in the measureable samples. A change in pY705-STAT3 expression after OPB-51602 administration was observed in one patient in the 2-mg cohort. Expression of pY705-STAT3 returned to positive during OPB-51602 administration in this patient. One reason may be that OPB-51602 did not completely inhibit pY705-STAT3 expression completely because the dose of OPB-51602 was low. As described in Goh et al.’s phase I trial, decreased pY705-STAT3 expression was observed in the 4-mg cohort. The details of the reason for this are not clear because no change in pY705-STAT3 expression was observed in the 4-mg or higher cohorts. No clear difference was observed in pharmacokinetic profiles between this study and Goh et al.’s phase I study. We have no data regarding the concentration of OPB-51602 in PBMCs or tumor cells from patients. However, in tumor-bearing mice, the concentrations of OPB-51602 in tumor tissue were higher than in plasma (Otsuka Pharmaceutical Co., Ltd, unpublished data). From those results and the range of concentrations in which OPB-51602 showed an inhibitory effect against pY705-STAT3 expression in vitro, the concentration of OPB-51602 in tumor cells of patients reached a concentration that is expected to decrease pY705-STAT3 expression. The possibility also remains that inhibition of STAT activation may not lead to antitumor efficacy. However, in order to clarify the effectiveness of OPB-51602, the study population may be required to be restricted to patients with an activated Jak-STAT pathway.
In conclusion, the MTD of OPB-51602 was considered to be ≥6 mg, and OPB-51602 was shown to be safe and well tolerated in a dose range of 1–4 mg. However, long-term administration at higher doses was difficult with a daily dosing schedule, and no therapeutic response was seen. Therefore, further clinical development of OPB-51602 for hematological malignancies with a daily dosing schedule was terminated. Questions still remain unanswered, such as the relationship between STAT inhibition and antitumor efficacy, the optimal patient population, and the optimal dose and schedule, but other STAT inhibitors are currently under development and are expected to clarify these details.
Acknowledgments
The authors would like to thank the patients, their family members, doctors, nurses, and staff members who participated in this trial. We are also grateful to Dr. Kiyoshi Kitamura, Dr. Noriko Usui, and Dr. Norio Komatsu for their helpful advice as members of the Independent Data and Safety Monitoring Board. This study was sponsored by Otsuka Pharmaceutical Co., Ltd.
Disclosure Statement
The authors have no conflict of interest.
References
- Sternberg DW, Gilliland DG. The role of signal transducer and activator of transcription factors in leukemogenesis. J Clin Oncol. 2004;22:361–71. doi: 10.1200/JCO.2004.10.124. [DOI] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311–9. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–54. [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211–7. doi: 10.1016/s0955-0674(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–73. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–72. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- Silva CM. Role of STATs as downstream signal transducers in Src family kinase mediated tumorigenesis. Oncogene. 2004;23:8017–23. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- Smithgall TE, Briggs SD, Schreiner S, Lerner EC, Cheng H, Wilson MB. Control of myeloid differentiation and survival by Stats. Oncogene. 2000;19:2612–8. doi: 10.1038/sj.onc.1203477. [DOI] [PubMed] [Google Scholar]
- Lin TS, Mahajan S, Frank DA. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene. 2000;19:2496–504. doi: 10.1038/sj.onc.1203486. [DOI] [PubMed] [Google Scholar]
- Danial NN, Rothman P. JAK-STAT signaling activated by Abl oncogenes. Oncogene. 2000;19:2523–31. doi: 10.1038/sj.onc.1203484. [DOI] [PubMed] [Google Scholar]
- Hayakawa F, Naoe T. SFK-STAT pathway: an alternative and important way to malignancies. Ann N Y Acad Sci. 2006;1086:213–22. doi: 10.1196/annals.1377.002. [DOI] [PubMed] [Google Scholar]
- Bowman T, Broome MA, Sinibaldi D, et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA. 2001;98:7319–24. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J, Ryan D, Kim JS, et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–55. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- Leong PL, Andrews GA, Johnson DE, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA. 2003;100:4138–43. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–9. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–53. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–67. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Kiladjian J-J, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Mesa RA, Gotlib J, et al. A double- blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovski U, Wu JY, Harris DM, et al. Stimulation of the B-cell receptor activates the JAK2/STAT3 signaling pathway in chronic lymphocytic leukemia cells. Blood. 2014;123:3797–802. doi: 10.1182/blood-2013-10-534073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh BC, Wong ALA, Soo RA, et al. Phase I study of OPB51602, a small molecule inhibitor of STAT3 phosphorylation, in patients with refractory solid malignancies. J Clin Oncol. 2012;30:173s. (abstr 3002) [Google Scholar]


