Abstract
Aminopeptidase N (APN/CD13) is involved in tumor cell invasion and tumor angiogenesis and is considered a promising therapeutic target in the treatment of cancer. To develop a novel monoclonal antibody-based cancer therapy targeting APN/CD13, we established a fully humanized anti-APN/CD13 monoclonal antibody, MT95-4. In vitro, MT95-4 inhibited APN/CD13 enzymatic activity on the tumor cell surface and blocked tumor cell invasion. B16 mouse melanoma cells stably expressing human APN/CD13 were also established and were inoculated s.c. or injected i.v. into nude mice. We found that expression of human APN/CD13 in murine melanoma cells increased the size of subcutaneous tumors, extent of lung metastasis and degree of angiogenesis in the subcutaneous tumors; these tumor-promoting and angiogenesis-promoting characteristics were reduced by the i.p. administration of MT95-4. To further verify the specificity of MT95-4 for neutralization of APN/CD13 activity, MT95-4 was administered into NOD/SCID mice inoculated s.c. with H1299 or PC14 cells, which exhibit high expression of APN/CD13, or with A549 cells, which exhibit weak expression of APN/CD13. MT95-4 reduced tumor growth and angiogenesis in mice bearing H1299-derived and PC14-derived tumors, but not in mice bearing A549-derived tumors. These results suggested that the antitumor and anti-angiogenic effects of MT95-4 were dependent on APN/CD13 expression in tumor cells. Given that MT95-4 is the first fully humanized monoclonal antibody against APN/CD13, MT95-4 should be recognized as a promising candidate for monoclonal antibody therapy against tumors expressing APN/CD13.
Keywords: Aminopeptidase N, angiogenesis, fully humanized monoclonal antibody, invasion, tumor growth
Aminopeptidase N (APN/CD13) is a type II membrane-bound glycoprotein that acts as a zinc-binding metalloprotease.1 APN/CD13 consists of 967 amino acids with a short N-terminal cytoplasmic domain, a single transmembrane region, and a large extracellular C-terminal domain that cleaves N-terminal amino acid residues from small peptides.1–4 APN/CD13 is expressed by most cells of myeloid origin, including monocytes, macrophages and granulocytes, and is also abundantly expressed in the brush border of epithelial cells of the small intestine and renal tubules, synaptic membranes of the central nervous system, and endothelial cells.5 In hematopoietic cells, APN/CD13 is considered a marker of differentiation; however, its specific functions have not been fully elucidated. While APN/CD13 has been shown to play roles in the final digestion of peptides in the small intestine and in the degradation of neuropeptides in the synaptic membrane, its function in proximal tubular epithelial cells is less clear.1–5 Moreover, although APN/CD13 has been proposed to be involved in many physiological processes, mice deficient in the APN/CD13 gene are reported to develop normally without histological or physiological alterations.6 Notably, APN/CD13 has been reported to be expressed in various types of tumor cells5 and to play important roles in tumor growth by promoting tumor cell invasion7–9 and angiogenesis.10–12 Indeed, the expression of APN/CD13 is associated with a poor prognosis in patients with colon, pancreatic and lung cancers,13–15 and high levels of circulating APN/CD13 have been recognized as an independent prognostic factor in patients with non-small cell lung cancer.16 Based on these data, APN/CD13 can be regarded as a target molecule for cancer therapy. In previous studies, bestatin (Ubenimex), an inhibitor of APN/CD13, aminopeptidase B and leucine aminopeptidase, was shown to suppress tumor growth in xenograft tumor models.17,18 Moreover, in a clinical trial, adjuvant bestatin therapy prolonged survival in patients with resected stage I squamous cell lung carcinoma.19 These data support the potential and feasibility of cancer therapy targeting APN/CD13.
Previously, we established a murine monoclonal antibody against APN/CD13 (MH8-11) by immunizing mice with HT1080 human fibrosarcoma cells; this monoclonal antibody exhibited antitumor effects, inhibiting tumor cell invasion and angiogenesis.13 Therefore, we hypothesized that monoclonal antibody therapy targeting APN/CD13 would be useful as a treatment for tumors exhibiting APN/CD13 expression. Therefore, to promote the potential clinical application of our work, we aimed to establish a fully humanized monoclonal antibody for inhibition of APN/CD13 activity. In the current study, we raised fully humanized monoclonal antibodies by immunization of KM mice, which produce humanized antibodies,20 with HT1080 cells and, from the resulting antibodies, we selected a monoclonal antibody (named MT95-4) on the basis of its ability to inhibit APN/CD13 activity in vitro. To evaluate the antitumor effects of MT95-4 in vivo, we established subcutaneous tumor models and tail vein metastasis models using B16-F1 melanoma cells transfected with human APN/CD13 and human lung cancer cells, which exhibited high expression of APN/CD13, in immune-deficient mice. Tumor-bearing mice were then treated with MT95-4, and the tumor progression and angiogenesis were analyzed.
Materials and Methods
Cells and cell culture
HT1080, B16-F1 melanoma, H1299 and A549 cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). PC14 cells were obtained from Immuno-Biological Laboratories (Gunma, Japan). HT1080, B16-F1, and A549 cells were cultured in DMEM (Gibco, Grand Island, NY, USA), and H1299 and PC14 cells were cultured in RPMI 1640 Medium (Gibco) supplemented with 10% FBS and 1% penicillin-streptomycin. All cells were incubated at 37°C in an atmosphere containing 5% CO2.
A retroviral vector (pDON-5neoCD13; Takara Bio, Shiga, Japan) containing APN/CD13 cDNA and a control pDON-5neo vector were transfected into B16-F1 melanoma cells according to the manufacturer’s protocol. Transfectants resistant to 1 mg/mL G-418 (Promega, Madison, WI, USA) were selected and maintained in DMEM with 10% FBS and 1 mg/mL G-418. From these B16-F1 transfectants, one cell line that expressed high levels of APN/CD13 (APN-B16 cells) and another cell line that did not express APN/CD13 (control-B16 cells) were selected. The expression levels of APN/CD13 were determined by flow cytometry and real-time PCR.
Reagents and animals
Matrigel was purchased from BD Biosciences (Bedford, MA, USA). KM mice were kindly provided by Kyowa Hakko Kirin (Tokyo, Japan). Male BALB/c nude mice and NOD/SCID mice (6–8-weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA). Animals were maintained according to guidelines for the ethical use of animals in research at Hiroshima University.
Establishment of a fully humanized monoclonal antibody that inhibited APN/CD13 activity
Because the establishment of a murine monoclonal antibody against APN/CD13 had been successful by immunizing Balb/c mice with HT1080 human fibrosarcoma cells,13 KM mice, which lack endogenous genes encoding the immunoglobulin (Ig) H and L κ chains and instead carry human chromosome 14 fragments containing the entire human Ig H chain loci and the human κ L chain segments,20 were injected i.p. with HT1080 cells three times. Three days after the last injection, spleen cells from the mice were fused to SP2/O-AG14 myeloma cells (ATCC) to generate hybridomas. A clone that had an inhibitory effect on APN/CD13 activity assessed by aminopeptidase assay was selected (MT95-4).
Aminopeptidase assay
Cell-associated aminopeptidase activity was assessed by measuring the amount of 7-amino-4-methylcoumarin (AMC) liberated from l-alanine-4-methylcoumarin-7amide (Ala-MCA; Peptide Institute, Osaka, Japan), as previously described.8,9 A mixture containing 0.1 mM Ala-MCA, 5 × 103 HT1080 cells, and a raised antibody or human control IgG (Sigma, St. Louis, MO, USA) suspended in 200 μL of PBS was seeded into each well of a 96-well plate and incubated for 2 h at 37°C in an atmosphere containing 5% CO2. After incubation, 20 μL of 0.2 M EDTA was added to each well to terminate the reaction, and the level of AMC was then analyzed with a TriStar LB 941 Multimode microplate reader (excitation, 355 nm; emission, 460 nm; Berthold Technologies, Bad Wildbad, Germany).
Matrigel invasion assay
The invasive properties of tumor cells were assessed using Matrigel invasion chambers (24-well inserts, pore size: 8 μm [BD Biosciences]). Tumor cells were resuspended in medium containing 0.1% FBS. Cell suspensions with MT95-4 or control IgG were added to the upper chamber at a density of 2.5 × 104 cells/500 μL medium/well and incubated for 22 h at 37°C in an atmosphere containing 5% CO2. The cells invading to the lower surface of the membrane were stained with Diff-Quik stain after removing noninvading cells with cotton swabs. Images were captured using a microscope at a magnification of 200 × (model BZ-9000; Keyence, Osaka, Japan), and the number of invading cells was counted in five random microscopic fields per well.
Flow cytometric analysis
FACS was performed to assess the expression level of APN/CD13 on the cell surface. The cells were incubated for 30 min at 4°C with murine monoclonal anti-APN/CD13 antibodies (WM15; BD Biosciences) and isotype control. Cells were then washed with PBS and stained with Alexa-Fluor-488-conjugated goat anti-mouse secondary antibodies (Invitrogen, Carlsbad, CA, USA). The samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed using CELL Quest software (BD Biosciences).
Quantitative real-time PCR
Total RNA was isolated using an RNeasy Mini kit (Qiagen, Valencia, CA, USA). The isolated total RNA was reverse transcribed into cDNA using a High Capacity RNA-to-cDNA kit (Applied Biosystems, Framingham, MA, USA) following the manufacturer’s instructions. Quantitative real-time PCR was performed on an ABI Prism 7700 (Applied Biosystems) for detection and quantification of human APN/CD13 using beta-actin as a control housekeeping gene.
Western blot analysis
HT1080 cells were lysed with lysis buffer containing 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. The soluble fraction of the cell lysate was electrophoresed on 10% sodium dodecyl sulfate SDS-PAGE and transferred electrophoretically to membranes. The lower part of the membrane around 43 kDa was cut and incubated overnight with rabbit anti-β-actin antibodies (4976S; Cell Signaling Technology, Danvers, MA, USA). The remaining section of each membrane was evenly divided into two parts and incubated with rabbit anti-APN/CD13 antibodies (2972-1; Abcam, Cambridge, UK) or MT95-4. After washing, the membranes were incubated with HRP-conjugated anti-rabbit or anti-human antibodies (NA934 or NA933, respectively; GE Healthcare, Buckinghamshire, UK). The signals were detected using enhanced chemiluminescence reagent (GE Healthcare) followed by exposure to X-ray film.
Cell proliferation assays
Control-B16 and APN-B16 cells (5 × 103 cells/well) were incubated in 96-well plates, with 100 μL medium per well. After culturing for 1–4 days, 10 μL of Cell Counting Kit-8 reagent (Dojindo, Kumamoto, Japan) was added to each well according to the manufacturer’s protocol. The absorbance was measured at 450 nm using a microplate reader.
Subcutaneous tumor model
Control-B16 or APN-B16 cells (1 × 104) were inoculated s.c. into the right flanks of nude mice. H1299, PC14 or A549 cells (1 × 106) were inoculated into the right flanks of NOD/SCID mice. Tumor-bearing mice were injected i.p. with 1 mg/kg MT95-4 or control human IgG (Sigma) twice per week. The length and width of the tumors were measured using calipers, and the tumor volume was calculated using the formula: width2 × length × 0.5.21
Tail vein metastasis model
Control-B16 or APN-B16 (2 × 105) cells were injected into nude mice through the tail vein. H1299 cells (1 × 106) were injected into NOD/SCID mice. Tumor-bearing mice were injected i.p. with 1 mg/kg MT95-4 or control human IgG (Sigma) twice per week.
Evaluation of microvessel density in subcutaneous tumors
Frozen sections of subcutaneous tumors were incubated with rat polyclonal antibodies against mouse CD31 (550274; BD Biosciences) and then reacted for 30 min with a biotinylated rabbit anti-rat IgG antibody (Vector Laboratories, Burlingame, CA, USA). The immunoreaction was amplified with a Vectastain ABC Kit (Vector Laboratories) and visualized by incubation with a 3, 3-diaminobenzidine solution acting as a chromogen. The sections were then counterstained with hematoxylin and dehydrated. Images were captured using a microscope at a magnification of 200 × (model BZ-9000; Keyence), and the area of CD31-positive vessel-like structures was measured in five random microscopic fields per section using Dynamic Cell Count software (BZ-HIC; Keyence).
Statistical analysis
Statistical analyses were performed using Prism 5 (GraphPad software, San Diego, CA, USA). All the results are expressed as means ± SEM. Differences between the groups were evaluated using Student’s t-tests or Mann–Whitney U-tests. Differences with P-values of < 0.05 were considered statistically significant.
Results
A fully humanized monoclonal antibody (MT95-4) recognized APN/CD13 and inhibited invasion and aminopeptidase activity in HT1080 cells in vitro
By immunizing KM mice with HT1080 fibrosarcoma cells abundantly expressing APN/CD13, we established a fully humanized monoclonal antibody, MT95-4, which had inhibitory effects against aminopeptidase activity. To verify the specificity of MT95-4, western blotting analysis was performed using HT1080 cell lysates. As shown in Figure1a, MT95-4 detected a band at 150 kDa corresponding to the predicted size of APN/CD13, which coincided with the band from a commercially available anti-APN/CD13 antibody.
Fig 1.
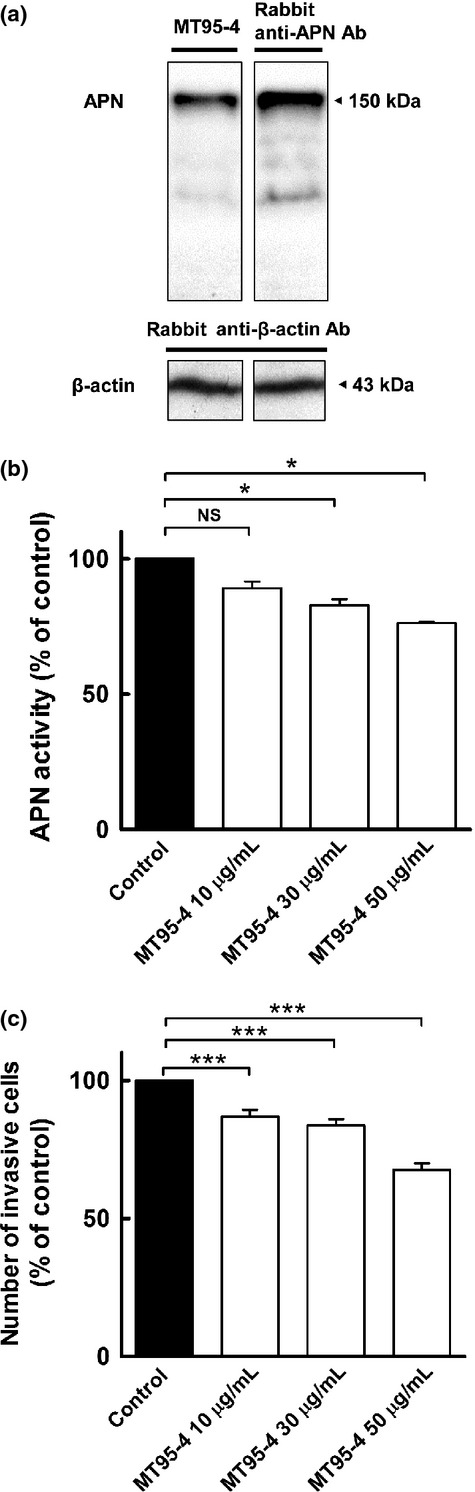
Function of a fully humanized monoclonal antibody, MT95-4, in vitro. (a) Evaluation of the specificity of MT95-4 by western blotting analysis using cell lysates from HT1080 cells. (b) Effects of MT95-4 on the aminopeptidase activity of HT1080 cells. The levels of AMC released from Ala-MCA were measured. Data represent the mean (± SEM) of triplicate samples. $P < 0.05 versus the control. NS, not significant. (c) Effects of MT95-4 on the invasion of HT1080 cells in a Matrigel invasion assay. Data represent the mean (± SEM) of five wells. $$$P < 0.001 versus the control.
To confirm the selection of MT95-4, we examined the inhibitory effects of MT95-4 on the aminopeptidase activity of HT1080 cells by measuring the level of AMC liberated from alanine-MCA. As shown in Figure1b, MT95-4 neutralized the aminopeptidase activity of HT1080 cells. We then evaluated whether MT95-4 affected the invasive properties of HT1080 cells using Matrigel invasion chambers. Compared to control IgG, MT95-4 inhibited the invasion of HT1080 cells (Fig.1c).
APN/CD13 promoted tumor growth and angiogenesis in vivo
We established two B16-F1-derived cell lines that differed in the expression levels of human APN/CD13 (control-B16 and APN-B16 cells). Quantitative real-time PCR and flow cytometric analysis revealed that APN-B16 cells expressed high levels of human APN/CD13, while control-B16 cells did not (Fig.2a,b). When the in vitro cell proliferation rates of the two cell lines were compared, no differences were observed (data not shown).
Fig 2.
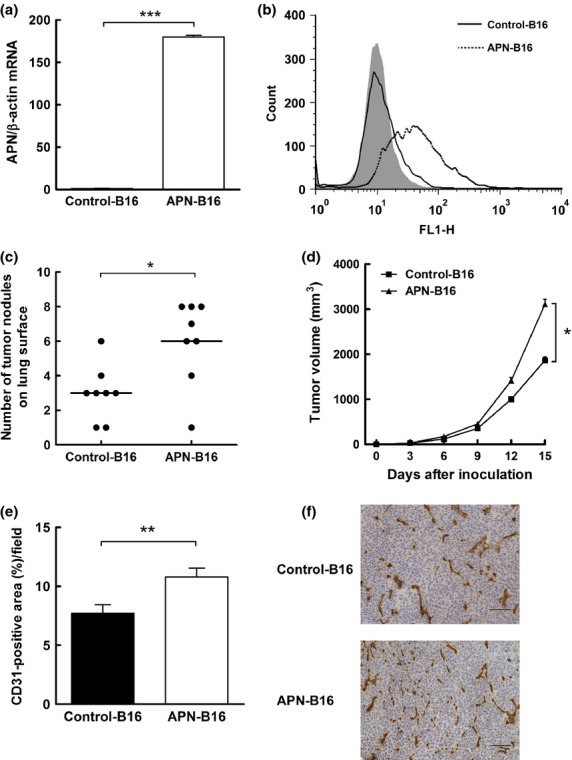
Effects of human APN/CD13 expression in murine B16-F1 melanoma cells. (a) Expression levels of human APN/CD13 mRNA in control-B16 and APN-B16 cells were evaluated by quantitative real-time PCR. (b) Expression levels of APN/CD13 on the surface of control-B16 and APN-B16 cells were assessed by flow cytometry. Data (a) represent the mean (± SEM) of triplicate samples. $$$P < 0.001. (c) Comparison of the numbers of lung surface nodules in the tail vein metastasis model between control-B16 and APN-B16 cells. The numbers of lung surface nodules in mice were counted 21 days after injection of control-B16 and APN-B16 cells. Each bar represents the mean number of nodules for eight mice per group. $P < 0.05. (d) Comparison of tumor volumes in a subcutaneous tumor model for tumors derived from control-B16 and APN-B16 cells. The sizes of subcutaneous tumors were measured twice a week for 2 weeks after inoculation of control-B16 and APN-B16 cells. The data represent the mean (± SEM) for eight mice per group. $P < 0.05. (e) Evaluation of angiogenesis in subcutaneous tumors derived from control-B16 and APN-B16 cells. The area containing CD31-positive vessels was measured. Data represent the means (± SEM) from eight mice per group. $$P < 0.01. (f) Immunohistochemical staining for CD31 in a subcutaneous tumor. Scale bar, 100 μm.
Next, to determine whether the expression of human APN/CD13 in murine B16-F1 melanoma cells affected tumor progression in vivo, control-B16 cells or APN-B16 cells were injected into nude mice through the tail vein or inoculated s.c. into right flanks of nude mice. As shown in Figure2c and d, mice bearing APN-B16 cells exhibited significantly increased numbers of lung surface nodules and larger subcutaneous tumors than mice bearing control-B16 cells. In addition, to confirm the role of APN/CD13 in tumor angiogenesis, the degrees of angiogenesis in these subcutaneous tumors were assessed by immunohistochemical staining with anti-CD31 monoclonal antibodies. The CD31-positive areas in subcutaneous tumors derived from APN-B16 cells were larger than those of tumors derived from control-B16 cells (Fig.2e,f).
MT95-4 reduced tumor progression and angiogenesis promoted by the expression of human APN/CD13 in murine B16-F1 melanoma cells
To confirm the neutralizing effect of MT95-4 against APN/CD13 in vivo, we established a tail vein metastasis model and a subcutaneous xenograft model using APN-B16 cells and administered MT95-4 (1 mg/kg) i.p. twice per week in tumor-bearing mice. As shown in Figure3a and b, administration of MT95-4 reduced the number of lung surface nodules and the size of subcutaneous tumors in mice bearing APN-B16 cells. In addition, the sections of subcutaneous tumors consisting of APN-B16 cells were immunohistochemically stained with anti-CD31 antibodies, and the areas of CD31-positive vessels were significantly smaller in the MT95-4-treated group than in the control group (Fig.3c,d).
Fig 3.
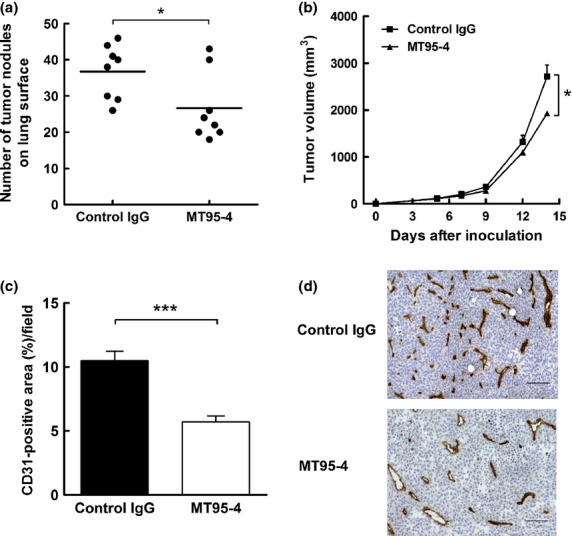
Effects of MT95-4 on tumor progression in the tail vein metastasis model and subcutaneous tumor model using APN-B16 cells. Mice were administered MT95-4 or control IgG (1 mg/kg) i.p. twice a week. (a) Evaluation of the number of lung surface nodules in the tail vein metastasis model following injection of APN-B16 cells. The number of tumor nodules on the lung surface was counted 21 days after injection of APN-B16 cells. Each bar represents the mean number of nodules from eight mice per group. $P < 0.05. (b) Evaluation of tumor volume in a subcutaneous tumor model using APN-B16 cells. The sizes of subcutaneous tumors were measured twice a week for 2 weeks after inoculation of APN-B16 cells. The data represent the means (± SEM) from eight mice per group. $P < 0.05. (c) Evaluation of angiogenesis in subcutaneous tumors derived from APN-B16 cells. The area of CD31-positive vessels was measured. Data represent the means (± SEM) from eight mice per group. $$$P < 0.01. (d) Immunohistochemical staining for CD31 in a subcutaneous tumor. Scale bar, 100 μm.
Antitumor and anti-angiogenic effects of MT95-4 were dependent on the expression of APN/CD13 in tumor cells
To further verify whether the antitumor effects of MT95-4 were associated with the expression of APN/CD13 in cancer cells, we administered MT95-4 to mice bearing human lung cancer cells abundantly or weakly expressing APN/CD13. As shown in Figure4a and b, real-time PCR analysis and flow cytometric analysis revealed that APN/CD13 was abundantly expressed in H1299 and PC14 cells but weakly expressed in A549 cells. Thus, we established subcutaneous tumor xenografts using H1299, PC14 and A549 cells in NOD/SCID mice, and MT95-4 or control IgG was administered i.p. The administration of MT95-4 reduced tumor volumes in mice bearing H1299 and PC14 cells (Fig.4c,f) but not in mice bearing A549 cells (Fig.4i). Moreover, the degree of angiogenesis in subcutaneous tumors derived from H1299 and PC14 cells was lower in the MT95-4-treated group than in the control group (Fig.4d,g); however, there were no differences in the degree of angiogenesis between MT95-4-treated and control IgG-treated mice bearing tumors derived from A549 cells (Fig.4j).
Fig 4.
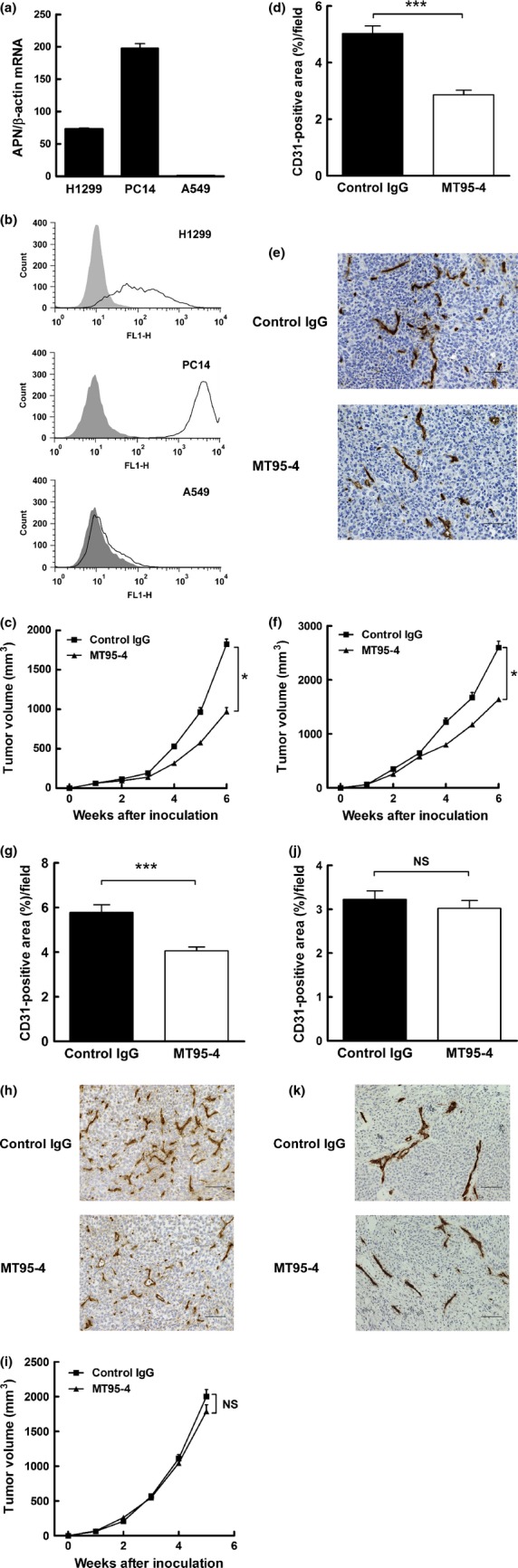
Effects of MT95-4 on tumor progression in the tail vein metastasis model and subcutaneous tumor model using human lung cancer cells. Mice were administered MT95-4 or control IgG (1 mg/kg) i.p. twice a week. (a) Expression levels of APN/CD13 mRNA in human lung cancer cells analyzed by real-time PCR. (b) Expression levels of APN/CD13 on the surface of human lung cancer cells, as analyzed by flow cytometry. (c), (f) and (i) Evaluation of tumor volumes in a subcutaneous tumor model using H1299 (c), PC14 (f) and A549 (i) cells. The sizes of subcutaneous tumors were measured once a week for 5 or 6 weeks after inoculation of tumor cells. The data represent the means (± SEM) for eight mice per group. $P < 0.05; NS, not significant. (d), (g) and (j) Evaluation of angiogenesis in subcutaneous tumors derived from H1299 (d), PC14 (g) and A549 (j) cells. The area of CD31-positive vessels was measured. Data represent the means (± SEM) for eight mice per group. $$$P < 0.001; NS, not significant. (e), (h) and (k) Immunohistochemical staining for CD31 in a subcutaneous tumor. Scale bar, 100 μm.
Discussion
In the present study, we established a fully humanized monoclonal antibody, MT95-4, that had the potential to neutralize human APN/CD13 activity. We also established two murine melanoma B16-F1-derived cell lines; one exhibited abundant expression of human APN/CD13, while the other did not exhibit expression of human APN/CD13. By inoculating these cells into nude mice, the expression of human APN/CD13 in murine melanoma cells was shown to promote tumor growth and angiogenesis in vivo. Interestingly, MT95-4 was able to reduce tumor growth and angiogenesis promoted by the expression of human APN/CD13 in vivo. In addition, we demonstrated that the ability of MT95-4 to inhibit tumor growth and angiogenesis was dependent on the expression of APN/CD13 in tumor cells. In vitro, MT95-4 dose-dependently inhibited the invasive properties of malignant tumor cells.
The most notable result from the current study was that we were able to establish a fully humanized monoclonal antibody, named MT95-4, that could neutralize human APN/CD13 activity. Given that MT95-4 recognized cell surface APN/CD13, we can speculate that the neutralization of APN/CD13 activity on the tumor cell surface by MT95-4 reduced tumor progression by inhibiting tumor cell invasion and angiogenic drive. Interestingly, MT95-4 could not recognize murine APN/CD13 (data not shown) and, thus, did not inhibit APN/CD13 activity in mouse endothelial cells. Therefore, the results of the present study suggested that the APN/CD13 activity of tumor cells promoted angiogenesis regardless of the APN/CD13 activity of endothelial cells, and this angiogenic drive from tumor cells was inhibited by MT95-4.
In the tumor microenvironment, APN/CD13 is expressed in stromal cells and endothelial cells, and the expression of APN/CD13 in these nonmalignant cells is involved in tumor growth and angiogenesis.10,22,23 In the present study, however, we could not affect the APN/CD13 activity of host murine cells in the tumor microenvironment because MT95-4 could not react with mouse APN/CD13. Interestingly, we confirmed that MT95-4 had the ability to inhibit in vitro tube formation by human umbilical vein endothelial cells (data not shown). Therefore, we can speculate that MT95-4 may inhibit APN/CD13 activity not only on tumor cells but also on endothelial cells in the human tumor microenvironment and may exert stronger antitumor effects in humans than in our mouse xenograft tumor models. In addition, we were not able to assess whether MT95-4 has antitumor effects related to antibody-dependent cellular cytotoxicity and/or complement-dependent cytotoxicity in the present study. Further experiments are required to evaluate these points.
APN/CD13 is considered a potential therapeutic target for the treatment of cancer. Currently, bestatin is clinically used as an APN/CD13 inhibitor. To the best of our knowledge, MT95-4 is the first fully humanized monoclonal antibody against APN/CD13 and has been shown to possess antitumor effects. Given that monoclonal antibodies against vascular endothelial growth factor or epidermal growth factor receptor are currently used in the clinical setting,24–26 we believe that MT95-4 may be a good candidate for monoclonal antibody-based anticancer therapy. Preparation for the clinical application of this antibody will begin in the near future.
In conclusion, we established a fully humanized monoclonal antibody against APN/CD13 (MT95-4) that inhibited the invasive properties of malignant tumor cells in vitro and prevented tumor progression and angiogenesis in vivo. These antitumor effects of MT95-4 were thought to be mediated by the neutralization of APN/CD13 activity at the tumor cell surface. Given that MT95-4 is a fully humanized antibody and may inhibit APN/CD13 activity in nonmalignant cells in the tumor microenvironment, which promote tumor growth, MT95-4 may be a promising candidate for monoclonal antibody-based anticancer therapy.
Acknowledgments
This work was carried out at the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- Look AT, Ashumun RA, Shapiro LH, Peiper SC. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest. 1989;83:1299–307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ship A, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82:1052–70. [PubMed] [Google Scholar]
- Luan Y, Xu W. The structure and main functions of aminopeptidase N. Curr Med Chem. 2007;14:639–47. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- Taylor A. Aminopeptidases: structure and function. FASEB J. 1993;7:290–8. doi: 10.1096/fasebj.7.2.8440407. [DOI] [PubMed] [Google Scholar]
- Dixon J, Kaklamanis L, Turley H, et al. Expression of aminopeptidase-n (CD 13) in normal tissues and malignant neoplasms of epithelial and lymphoid origin. J Clin Pathol. 1994;47:43–7. doi: 10.1136/jcp.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel R, Sun Y, Guzman-Rojas L, et al. Impaired angiogenesis in aminopeptidase N-null mice. Proc Natl Acad Sci USA. 2007;104:4588–93. doi: 10.1073/pnas.0611653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda J, Saiki I, Fujii H, Abe F, Kojima Y, Azuma I. Inhibition of tumor invasion and extracellular matrix degradation by ubenimex (bestatin) Clin Exp Metastasis. 1992;10:49–59. doi: 10.1007/BF00163576. [DOI] [PubMed] [Google Scholar]
- Saiki I, Fujii H, Yoneda J, et al. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54:137–43. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Nakajima M, Saiki I, Yoneda J, Azuma I, Tsuruo T. Human melanoma invasion and metastasis enhancement by high expression of aminopeptidase N/CD13. Clin Exp Metastasis. 1995;13:337–44. doi: 10.1007/BF00121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Kain R, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–7. [PMC free article] [PubMed] [Google Scholar]
- Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood. 2001;97:652–9. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K, Fujii H, Saitoh Y, et al. Aminopeptidase N (APN/CD13) is selectively expressed in vascular endothelial cells and plays multiple roles in angiogenesis. Cancer Lett. 2006;243:135–43. doi: 10.1016/j.canlet.2005.11.051. [DOI] [PubMed] [Google Scholar]
- Hashida H, Takabayashi A, Kanai M, et al. Aminopeptidase N is involved in cell motility and angiogenesis: its clinical significance in human colon cancer. Gastroenterology. 2002;122:376–86. doi: 10.1053/gast.2002.31095. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Nakajima Y, Tokuhara T, et al. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin Cancer Res. 2003;9:1503–8. [PubMed] [Google Scholar]
- Tokuhara T, Hattori N, Ishida H, et al. Clinical significance of aminopeptidase N in non-small cell lung cancer. Clin Cancer Res. 2006;12:3971–8. doi: 10.1158/1078-0432.CCR-06-0338. [DOI] [PubMed] [Google Scholar]
- Murakami H, Yokoyama A, Kondo K, Nakanishi S, Kohno N, Miyake M. Circulating aminopeptidase N/CD13 is an independent prognostic factor in patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:8674–9. doi: 10.1158/1078-0432.CCR-05-1005. [DOI] [PubMed] [Google Scholar]
- Inoi K, Goto S, Nomura S, et al. Aminopeptidase inhibitor ubenimex (bestatin) inhibits the growth of human choriocarcinoma in nude mice through its direct cytostatic activity. Anticancer Res. 1995;15:2081–7. [PubMed] [Google Scholar]
- Tsukamoto H, Shibata K, Kajiyama H, Terauchi M, Nawa A, Kikkawa F. Aminopeptidase N (APN)/CD13 inhibitor, Ubenimex, enhances radiation sensitivity in human cervical cancer. BMC Cancer. 2008;8:74. doi: 10.1186/1471-2407-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose Y, Genka K, Koike T, et al. Randomized double-blind placebo- controlled trial of bestatin in patients with resected stage I squamous-cell lung carcinoma. J Natl Cancer Inst. 2003;95:605–10. doi: 10.1093/jnci/95.8.605. [DOI] [PubMed] [Google Scholar]
- Ishida I, Tomizuka K, Yoshida H, et al. Production of human monoclonal and polyclonal antibodies in TransChromo animals. Cloning Stem Cells. 2002;4:91–102. doi: 10.1089/153623002753632084. [DOI] [PubMed] [Google Scholar]
- Worzalla JF, Bewley JR, Grindey GB. Automated measurement of transplantable solid tumors using digital electronic calipers interfaced to a microcomputer. Invest New Drugs. 1990;8:241–51. doi: 10.1007/BF00171833. [DOI] [PubMed] [Google Scholar]
- Guzman-Rojas L, Rangel R, Salameh A, et al. Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc Natl Acad Sci USA. 2012;109:1637–42. doi: 10.1073/pnas.1120790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Miyahara R, Takahashi R, et al. Stromal aminopeptidase N expression: correlation with angiogenesis in non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2009;57:591–8. doi: 10.1007/s11748-009-0445-x. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]


