Abstract
In patients with cancer and Parkinson’s disease, the DJ-1 protein may be secreted into the serum during the impaired response of the underlying cell-protective mechanisms. In order to determine the clinical significance of DJ-1 protein in the sera of breast cancer patients, we examined blood samples from a breast cancer group (n = 180) and a non-cancerous control group (n = 300). Higher levels of DJ-1 were detected in the breast cancer group (mean level, 42.7 ng/mL) than the control group (28.3 ng/mL) by ELISA (P = 0.019). Higher DJ-1 levels were significantly associated with advanced clinical grade, according to the TNM classification, negative hormone receptor status, and high Ki-67 labeling index, of biopsied materials; samples showed low DJ-1 protein expression despite upregulated DJ-1 mRNA. DJ-1 isoforms could be detected clearly in 17 blood samples (from 11 breast cancer patients, and 6 non-cancerous controls) by 2-D gel electrophoresis and immunoblot analysis. The isoform at the pI of 6.3 showed the highest intensity in all 11 cancer cases. Conversely, in the 6 non-cancerous cases, isoforms other than the pI 6.3 isoform were highly expressed, and there was a significant difference in the isoform pattern between breast cancer cases and controls (P = 0.00025). These data indicate that high levels of DJ-1, probably of isoform at pI 6.3, is a candidate serum marker of breast cancer.
Keywords: Biomarker, 2-D gel electrophoresis, ELISA, oxidative stress, Parkinson’s disease
Many epidemiological studies have indicated that natural antioxidants diminish the risk of cancer and prevent other life-threatening diseases such as neurodegenerative disorders, cardiovascular disease, stroke, and diabetes mellitus.1,2 Although the clinical characteristics of cancer are disparate from those of neurodegenerative diseases such as Parkinson’s disease, the underlying breakdown in prevention of excessive oxidative stress is comparable in both diseases. Recent advances in the understanding of the genetic basis of cancer and neurodegenerative disease have highlighted similar and overlapping pathways, including those that maintain an appropriate redox status.3 One protein involved in the development of both cancer and neurodegenerative disease is DJ-1.
The oncogene DJ-1 was initially discovered in 1997.4 It was subsequently found to be a causative gene in familial Parkinson’s disease and was named PARK7 in 2003.5 Physiologically, the DJ-1 protein acts as a sensor of various oxidative stresses, and plays an important role in determining whether a damaged cell will protect itself from oxidative stress or initiate apoptosis.6 Simultaneously, damaged cells secrete the DJ-1 protein in order to attenuate oxidative stress by initiating self-oxidization. Damaged neuronal cells secrete the DJ-1 protein, and indeed, high levels of DJ-1 are detected in the cerebrospinal fluid of patients with Parkinson’s disease.7
Breast cancer cells are also known to secrete DJ-1; Le Naour et al.8 reported high levels of DJ-1 protein in the sera of breast cancer patients and proposed the levels of DJ-1 protein in serum as a candidate biomarker for breast cancer. Our previous study confirmed that the triple-negative breast cancer cell line MDA-MB-231 secreted DJ-1.9 MDA-MB-231 cells expressed low levels of DJ-1 protein, and this expression pattern was detected in the cancer tissue by immunohistochemistry; in situ hybridization analysis detected the concurrent upregulation of DJ-1 mRNA. This expression pattern was found to be significantly associated with high levels of DJ-1 protein in the nipple discharge of breast cancer patients, suggesting that it was a secretion-related cancer expression pattern.10
In the current study, we have analyzed the clinical significance of DJ-1 levels in the sera of breast cancer patients, and its association with the expression pattern in breast cancer tissue in accordance with the TNM classification. Samples with highly elevated DJ-1 proteins in the sera were analyzed by 2-D gel electrophoresis and immunoblot analysis.
The 2-D gel electrophoresis pattern of DJ-1 protein in the sera was mainly examined in patients with Parkinson’s disease and Alzheimer’s disease. A meta-analysis of the DJ-1 protein pattern reported a common finding that DJ-1 oxidation correlates with neurodegenerative disorders and the acidic pI of DJ-1 isoforms increase in sera of patients with neurodegenerative disorders.11 In melanoma, expression of the acidic form of DJ-1 was higher than that of melanocyte.12 As for breast cancer, Le Naour et al.8 first showed a clear isoform pattern of the DJ-1 protein in both cancer cells and sera of breast cancer patients, although the difference in the isoelectric pattern between cancer and normal control was not determined. This study identified a cancer-specific DJ-1 isoform in sera that clearly distinguished breast cancer patients from non-cancerous subjects in 17 cases in which DJ-1 was highly elevated in the sera.
Subjects and Methods
Subjects
Subjects were divided into the breast cancer group (n = 180) and a control non-cancerous group (n = 300) for the purposes of this study. Breast cancer samples were collected from Tokyo Medical University Hospital (Tokyo, Japan), Ibaraki Medical Center of Tokyo Medical University (Ibaraki, Japan), and the National Defense Medical College (Saitama, Japan) from January 2011 to June 2013. One hundred and seventy three patients from whom the samples were obtained had received no specific medical examinations and had not been treated for the breast lesion. All subjects had received a pathological diagnosis of breast carcinoma obtained by needle biopsy specimens, which was confirmed by the examination of postoperative specimens. In the remaining 7 patients, no recurrent tumors had been detected for more than 2 years after the initial operation. In these cases, recurrent tumors were ascertained by medical examination after blood sampling. Blood samples were also collected from the control group, which comprised 300 Japanese women without breast cancer, who received a physical examination in Kasumigaura Institute for Health Evaluation (Ibaraki, Japan). Mammography screening detected abnormal findings in 55 out of 300 women. Breast examinations were carried out in the 55 individuals and no cancers were detected. There was no difference in age between the breast cancer group (mean age, 59.3 years; SD, 13.4) and the control group (mean age, 58.0 years; SD, 12.7). Clinical data were obtained from electronic medical records. Informed consent was obtained from all patients. The research ethics committees of Tokyo Medical University, the National Defense Medical College, and the Kasumigaura Institute for Health Evaluation approved this study.
Methods
Enzyme-linked immunosorbent assay
The DJ-1 concentration in blood samples was determined by the sandwich ELISA method; ELISA was carried out using the DJ-1/PARK7 ELISA kit (CircuLex, Ina, Japan) according to the manufacturer’s protocol. Each sample was diluted to 1:20, and was examined in triplicate. The mean value of triplicate experiments was recorded. These procedures were repeated three times.
Two-dimensional gel electrophoresis and immunoblot analysis
Protein precipitation was carried out by adding acetone to serum at a ratio of 1:10 (v/v). After a brief vortex, samples were allowed to stand at −20°C for 2 h, and were then centrifuged at 4°C for 30 min. Precipitated samples were dissolved in a buffer containing 2× sample buffer (7 M urea, 2 M thiourea, 4% CHAPS), 1% Immobilized pH gradient (IPG) buffer (GE Healthcare Japan, Tokyo, Japan), 0.2% DTT, and 0.0005% bromophenol blue on ice for 30 min. DJ-1 isoforms were analyzed using a 2-D gel electrophoresis system (GE Healthcare Japan). Briefly, two kinds of 13-cm long Immobiline DryStrip gels with a pH range from 3 to 10, and from pH 4 to 7, were used for isoelectric focusing; they were then applied to 12.5% acrylamide gels (14 × 16 cm), using the SE 600 Ruby Standard Dual Cooled vertical unit. DJ-1 isoforms were detected with an anti-human DJ-1 mouse mAb (3E8; Medical and Biological Laboratories, Nagoya, Japan) diluted to 1:10 000 and were visualized using chemiluminescence (Thermo Scientific, Rockford, IL, USA). In order to confirm the position of each isoform, blotted membranes were stained with gold-colloid stain (GE Healthcare Japan); 7 ng recombinant DJ-1 was loaded on the 2-D gel as a positive control in each experiment. Signals were detected using the LAS3000 image analyzer (Fuji film, Kanagawa, Japan) and DeCyder version 7.0 (GE Healthcare Japan), and detected spots were analyzed with the ImageMaster 2D Platinum Software version 7.0 and ImageQuant TL 8.1 software (GE Healthcare Japan).
In situ hybridization and immunohistochemistry of DJ-1
Needle-biopsy samples from 173 pretreatment subjects were analyzed by immunohistochemistry and in situ hybridization, as previously described.13 Briefly, tissue sections (4 μm) from the biopsy specimens were treated with the same primary anti-DJ-1 antibody used for the immunoblot analysis, and a secondary HRP-labeled anti-mouse IgG antibody (Dako EnVision+ System; Dako, Glostrup, Denmark). Immunostaining was detected by using an enzyme-labeled antibody system with 3′-diaminobenzidine tetrahydrochloride as the chromogen (Muto Pure Chemicals, Tokyo, Japan). Expression of DJ-1 protein was judged by using an image analyzer (WinROOF; Mitani, Tokyo, Japan). In situ hybridization was also carried out using 20 pmol of 3′-end biotin-labeled DJ-1 oligonucleotide probes per slide (sense primer, 5′-ATGACTTCCAAGCTGGCCGT-3′; antisense primer, 5′-CTTGTAAGAATCAGGCCGTCT-3′) (Nihon Gene Research Laboratories, Miyagi, Japan). The hybridization was carried out overnight at 45°C. After washing twice, signal amplification was carried out using the ABC Kit (Dako, Carpinteria, CA, USA). DJ-1 expression was detected with 5-bromo-4-chloro-3-indolyl phosphate (Sigma Chemicals, St. Louis, MO, USA). Expression of DJ-1 mRNA was determined by using the same image analyzer used for analyzing DJ-1 protein expression.
Histological subtyping
Histological evaluation was routinely carried out on slides stained with H&E according to the WHO’s Histological Typing of Breast Tumors.14 Pathological cancer stages were categorized by using the TNM classification affiliated with the International Union against Cancer.15 Immunohistochemistry was used to determine estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) status according to the American Society of Clinical Oncology/College of American Pathologists guidelines.16 Expression of HER2 was evaluated using HercepTest (Dako) according to the manufacturer’s instructions. Fluorescent in situ hybridization was used to confirm genomic amplification in equivocal cases of HER2 immunohistochemical staining (score 2+).17 The Ki-67 labeling index (%) was estimated by counting the number of positively stained cells per 500 cancerous cells in a high-powered field (×400). The cut-off level of the Ki-67 labeling index was set at 14%, according to the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011.18
Statistical analysis
Differences in the mean levels of DJ-1 between the breast cancer group and the non-cancerous group were analyzed using the Student’s t-test. Fisher’s exact test was applied to estimate the difference in DJ-1 isoform patterns between the two groups; software R (version 2.15.2, R Foundation of Statistical Computing, Vienna, Austria) was used for performing Fisher’s exact test. A P-value <0.05 was considered statistically significant.
Results
DJ-1 levels in serum
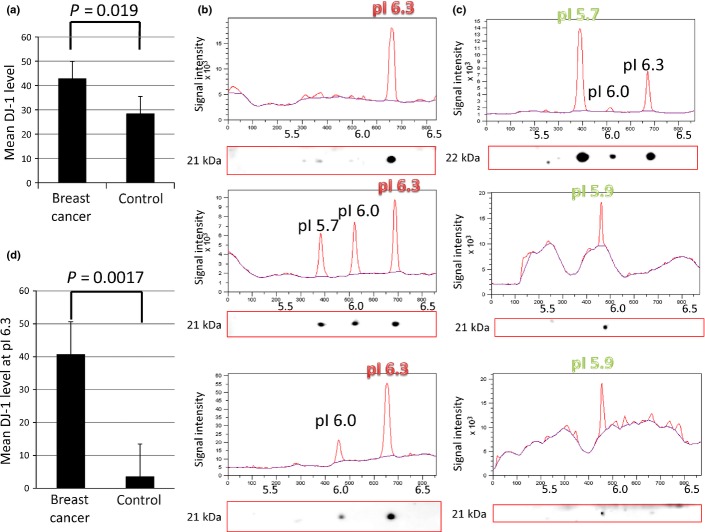
The mean level of DJ-1 in the breast cancer group was 42.7 ng/mL, whereas the mean level of DJ-1 in the non-cancerous group was 28.3 ng/mL; serum levels of DJ-1 were significantly different between the breast cancer and non-cancerous groups (P = 0.019) (Fig.1a). Table1 shows the association between DJ-1 levels and the pathological TNM classification in the breast cancer group. Significantly higher levels of DJ-1 were detected in cases with tumor sizes >5 cm, or where there was evidence of direct invasion to the chest wall or skin, irrespective of tumor size (T3 or T4), than in cases with tumor sizes ≤5 cm (T1 or T2) (P < 0.0001). The presence of both lymph node metastasis (P = 0.00017) and distant metastasis (P < 0.0001) were associated with significantly increased serum levels of DJ-1. These data suggest that serum levels of DJ-1 are elevated in the presence of cancer and correlate with the extent of clinical cancer. Table2 shows the association between biologic markers and DJ-1 levels. Higher levels of DJ-1 were significantly detected in negative estrogen receptor status (P = 0.012), negative progesterone receptor status (P = 0.013), and high Ki-67 labeling index (P = 0.022); however, DJ-1 levels did not correlate with HER2 status.
Fig 1.
Mean level of DJ-1 in serum, and the expression profile of its isoforms, in breast cancer and non-cancerous groups. (a) Mean levels of DJ-1 protein in sera from breast cancer and non-cancerous groups. In sera from the breast cancer group (n = 180), the mean level of DJ-1 protein was 42.7 ng/mL, whereas that in the non-cancerous group (n = 300) was 28.3 ng/mL. There was a significant difference in the mean levels between the two groups (P = 0.019). Data are means of three independent experiments with SE. (b) DJ-1 isoform patterns of three representative cancer cases. (c) Two-dimensional Western blot detection of DJ-1 isoforms shown as an LAS3000 image with molecular weight. Intensity of each isoelectric spot was semiquantified as a peak of signal intensity that was drawn by ImageQuant TL 8.1 software. The vertical axis shows signal intensity, and the horizontal axis shows the pI. The highest peak was detected at pI 6.3 in all cancer cases. (c) DJ-1 isoform patterns of three representative non-cancerous cases. DJ-1 isoform image and its semiquantified graph are shown in the same manner as (b). The vertical axis shows signal intensity, and the horizontal axis shows the pI. The highest peak was detected at the acidic side (pI 5.7, 5.9, 5.9) in each non-cancerous case. The y-axis is the signal intensity drawn by ImageQuant TL 8.1 software. (d) Mean levels of DJ-1 at pI 6.3 in the sera of abnormally high DJ-1 in breast cancer and non-cancerous groups. The mean level, calculated by the ratio of isoform peaks, of DJ-1 at pI 6.3 from sera of 11 cancerous cases (40.6 ng/mL) was significantly higher than that from 6 non-cancer cases (3.5 ng/mL) (P = 0.0017). Data are the mean of three independent experiments with SE.
Table 1.
Subjects from the breast cancer patient group, classified by TNM and serum DJ-1 levels
| Factor | No. | DJ-1, ng/mL | P-value |
|---|---|---|---|
| T | |||
| Tis | 14 | 19.5 | P < 0.0001 |
| 1 | 83 | 24.7 | |
| 2 | 51 | 26.0 | |
| 3 | 13 | 103.5 | |
| 4 | 12 | 165.7 | |
| N | |||
| 0 | 132 | 25.4 | P = 0.00017 |
| 1 | 31 | 75.3 | |
| 2 | 6 | 206.5 | |
| 3 | 4 | 15.4 | |
| M | |||
| 0 | 168 | 34.2 | P < 0.0001 |
| 1 | 5 | 247.6 | |
| Rec | 7 | 100.1 | |
| Total | 180 | 42.7 | |
Rec, recurrent tumor without treatment for more than 2 years.
Table 2.
Subjects from the breast cancer patient group, classified by biologic markers and serum DJ-1 levels
| Marker | No. | DJ-1, ng/mL | P-value |
|---|---|---|---|
| Estrogen receptor | |||
| Positive | 148 | 34.9 | P = 0.012 |
| Negative | 32 | 78.9 | |
| Progesterone receptor | |||
| Positive | 126 | 31.8 | P = 0.013 |
| Negative | 54 | 68.1 | |
| HER2 | |||
| Positive | 47 | 55.8 | NS |
| Negative | 133 | 38.1 | |
| Ki-67 labeling index | |||
| High | 121 | 53.3 | P = 0.022 |
| Low | 59 | 21.0 | |
| Total | 180 | 42.7 | |
HER2, human epidermal growth factor receptor 2; NS, not significant.
Association between expression of DJ-1 in breast cancer cells and DJ-1 levels in the serum
In situ hybridization of the needle biopsies revealed upregulated levels of DJ-1 mRNA in breast cancer tissue in 171 (98.8%) of 173 pre-therapeutic samples. At the protein level, low expression of DJ-1 was detected in 85 of 173 (49.1%) breast cancer cases by semiquantitative immunohistochemical analysis. Figure2 shows a breast cancer case with high DJ-1 mRNA levels and low expression of DJ-1 protein, whose expression pattern has been observed with secretion and with high DJ-1 levels in the nipple discharge, reported previously.9,10 In cases with low expression of DJ-1 protein, the mean level of DJ-1 protein in the sera was 67.0 ng/mL, significantly higher than that in cases with high expression of DJ-1 protein (23.5 ng/mL).
Fig 2.
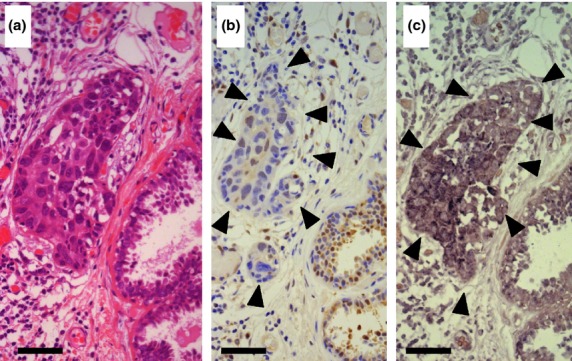
Expression of DJ-1 protein and mRNA in a breast cancer biopsy specimen. (a) The breast cancer nest is located in the center of the H&E stained specimen. Non-cancerous ducts are seen in the right lower corner. Bar = 50 μm (originally 400 high power fields). (b) Immunohistochemistry of DJ-1, in the same area as in (a). The immunoreactivity in the cancerous area is marked by arrowheads, and was analyzed by the image analyzer WinROOF. The average intensity of DJ-1 staining in the non-cancerous ducts located in the right corner was determined as the threshold value by applying the hue–lightness–saturation color space. The expression of DJ-1 in the cancerous area was judged as low because the intensity of DJ-1 staining was lower than the threshold value in the cancerous area. (c) In situ hybridization of DJ-1, in the same area as in (a, b). Upregulation of DJ-1 mRNA was detected in the cancerous lesion (arrowheads).
Two-dimensional gel electrophoresis and immunoblot analysis
Of 480 blood samples, distinct patterns of DJ-1 isoforms were detected in 17. Representative isoform patterns of both cancer and non-cancerous cases are shown in Figure1(b,c). Ten different isoforms of DJ-1 were detected, and all 11 breast cancer cases showed the highest intensity for the isoform at the pI of 6.3 (Table3). Conversely, no such isoform pattern was detected in the other 6 control cases (Fig.1c). Although one sample in the non-cancer group contained the isoform at pI 6.3, the level was no more than half of the highest level of the other isoform at pI 5.7. Fisher’s exact test showed a statistically significant difference in DJ-1 isoform distribution patterns between the breast cancer and non-cancerous groups (P = 0.00025). When the protein concentration was calculated by using the ratio of isoform peaks for the DJ-1 isoform at pI 6.3, a distinct difference was noted in the estimated DJ-1 level at pI 6.3 between breast cancer cases (40.6 ng/mL) and non-cancerous cases (3.5 ng/mL) (P = 0.0017) (Fig.1d).
Table 3.
Distribution of DJ-1 isoforms in sera of breast cancer and non-cancerous patients
| pI/MW, kDa | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.7 | 5.8 | 5.9 | 6.0 | 6.1 | 6.2 | 6.3 | 6.4 | 6.5 | 6.6 | 6.7 | 6.8 | 6.9 | 7.0 | 7.1 | 7.2 | 7.3 | 7.4 | 7.5 | |
| Cancer | 21 | ||||||||||||||||||
| 21 | |||||||||||||||||||
| 21 | |||||||||||||||||||
| 22 | |||||||||||||||||||
| 22 | 22 | 22 | |||||||||||||||||
| 22 | |||||||||||||||||||
| 21 | |||||||||||||||||||
| 21 | |||||||||||||||||||
| 21 | 21 | 21 | |||||||||||||||||
| 21 | 21 | ||||||||||||||||||
| 21 | |||||||||||||||||||
| Non-cancer | 21 | 22 | |||||||||||||||||
| 21 | 21 | 21 | |||||||||||||||||
| 22 | 22 | 22 | |||||||||||||||||
| 22 | 22 | ||||||||||||||||||
| 21 | |||||||||||||||||||
| 22 | 22 | ||||||||||||||||||
The highest peak of DJ-1 isoforms observed in each case is shown in bold. MW, molecular weight.
Discussion
This study demonstrated high levels of DJ-1, and the isoform at pI 6.3, in the serum samples from breast cancer patients. The molecular weight and pI of the DJ-1 protein are 19.8 kDa and 6.33, respectively, as calculated from the protein sequence, indicating slight modifications in the pI 6.3 isoform observed in the sera of breast cancer patients. DJ-1 is known to be modified by oxidation. A previous study of Parkinson’s disease detected an increase in DJ-1 isoforms with acidic pI, with a concurrent decrease in isoforms with basic pI in affected neuronal cells, and the acidic isoform in whole blood was identified as a candidate biomarker of late-stage Parkinson’s disease.19 This acidic isoform was formed by cysteine oxidation, specifically C106 oxidization, which plays a crucial role in Parkinson’s disease. By contrast, we have identified that an increase in the isoform with basic pI can distinguish breast cancer patients from those with Parkinson’s disease and other neurodegenerative disorders.
Various human cancers overexpress DJ-1 mRNA during oncogenesis and cancer progression, and escape from PTEN-induced cell death.20 Findings from our previous and present studies have confirmed that DJ-1 mRNA expression is increased in almost all breast cancer tissues.9 However, approximately half the breast cancer cases revealed low expression of DJ-1 protein despite upregulation of mRNA levels. Surprisingly, low expression of DJ-1 protein was not detected in benign breast tumors, such as papilloma and fibroadenoma, which consistently express DJ-1 protein. Data from our previous studies suggest that breast cancer cells that show low expression of DJ-1 proteins are associated with DJ-1 protein secretion, although the precise mechanism for downregulation of the DJ-1 protein remains unclear. We propose that breast cancer cells secrete the DJ-1 isoform with pI 6.3 from the pool of intracellular DJ-1 into the serum, which may partly reflect the DJ-1 expression profile in these cancer cells.
As shown in Table1, lymph node metastasis was significantly associated with elevated serum levels of DJ-1. However, the mean level in the N3 group (n = 4) was lower than that of the N0 group (n = 132). The reason for low levels in the N3 group was unclear. However, we speculated two reasons. One is that tumor extension may be confined to the lymphatic system, because no distant metastasis or vessel involvement was detected in the N3 group. The other reason is that breast cancer might not secrete DJ-1 in the N3 cases, in which cancer cells did not express a secretion pattern. This issue may in part suggest the limitation of serum DJ-1 protein as a tumor marker.
The DJ-1 protein undergoes various post-translational modifications (PTMs); consequently, more than 17 isoforms of intracellular DJ-1 have been detected, and may partly explain its multifunctional role.11 Varying ratios of DJ-1 isoforms are considered to determine the differing cellular responses to many stimuli by normal cells, including the neuronal cell. Among the known PTMs of the DJ-1 protein, oxidation, phosphorylation, ubiquitination, and sumoylation cannot produce an isoform with pI 6.3, molecular weight 21–22 kDa, as these modifications would lead to a much larger shift from this point. One potential PTM is the O-linked N-acetylglucosamine, which promotes oncogenic activation by enhancing glycolytic activation in various cancer cells including MDA-MB-231 and non-malignant human breast cells.21,22 This modification exemplifies how DJ-1 regulates metabolic reprogramming and signaling in cancer cells. Thus, further study is needed to identify the PTM that creates the DJ-1 isoform with pI 6.3 and its pathological role in cancer promotion.
Persistent secretion of DJ-1 may be a clinical marker not only for worsening chronic disorders but also for tumor progression. A majority of cancer cells express high levels of DJ-1 to prevent apoptosis.20 High DJ-1 levels are downregulated by muscadine; this phytochemical is derived from grape-skin extract and inhibits prostate cancer growth in vitro.23 Findings from our previous study of ductal carcinoma in situ revealed that breast tumor cells might secrete DJ-1, which could be detected in nipple discharge.9,10 Certain ductal carcinoma in situ may not progress to invasive cancer if persistent stress is appropriately removed from the damaged mammary cells. The unique properties of DJ-1 in the serum suggest that it may be an important tool for further development of chemopreventive agents against breast cancer.
In the non-cancerous group, all six patients who expressed extraordinarily high DJ-1 levels had underlying chronic diseases including hypertension, hyperlipidemia, type 2 diabetes, chronic arthritis, or cedar pollinosis; all of these diseases are considered to occur as a result of oxidative stress. A deficiency or constitutive activation of DJ-1 has been implicated in mast cell-derived allergic diseases such as cedar pollinosis;24 DJ-1 participates in fatty acid synthesis, and a high-fat diet induces isoform changes of DJ-1 protein, suggesting that DJ-1 is a sensor involved in nutrition-induced outcomes.25 DJ-1 also plays a key role in glucose homeostasis, and is generally involved in age- and lifestyle-related human diseases.26 Detection of high levels of DJ-1 in serum could present an opportunity to ameliorate all these diseases at once. The development of oxidative stress inhibitors is rapidly progressing, and the monitoring of oxidative stress will become more important to improve quality of life and longevity. The level of DJ-1 in serum is a promising candidate for monitoring stress, and the expression of varying DJ-1 isoforms may be a biomarker for various cellular defects, including breast cancer.
In summary, levels of DJ-1 were elevated in the sera of patients with cancer, although the presence of other underlying chronic disorders could not identify the cause of high levels of DJ-1 in the serum. Detection of DJ-1 isoform at pI 6.3 could divide breast cancer status from the non-cancerous state when patients were found to have high levels of DJ-1 in sera.
Acknowledgments
This study was supported by grants from the Foundation for Promotion of Cancer Research and the Ministry of Defense of Japan, and a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (Kakenhi 24590457). We are grateful to Mrs. Hisae Arai for technical assistance.
Disclosure Statement
The authors have no conflict of interest.
References
- Surh YJ. Cancer Chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2004;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Wang T, Chun OK, Song WO. Plasma and dietary antioxidant status as cardiovascular disease risk factors: a review of human studies. Nutrients. 2013;5:2969–3004. doi: 10.3390/nu5082969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine MJ, Plun-Favreau H, Wood NW. Parkinson’s disease and cancer: two wars, one front. Nat Rev Cancer. 2011;11:812–23. doi: 10.1038/nrc3150. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, et al. DJ-1, a novel oncogene which transforms NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–13. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science. 2003;299:256–9. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Ariga H, Takahashi-Niki K, Kato I, et al. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/683920. : doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waragai M, Wei J, Fujita M, et al. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson’s disease. Biochem Biophys Res Commun. 2006;345:967–72. doi: 10.1016/j.bbrc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Le Naour F, Misek DE, Krause MC, et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7:3328–35. [PubMed] [Google Scholar]
- Tsuchiya B, Iwaya K, Kohno N, et al. Clinical significance of DJ-1 as a secretory molecule: retrospective study of DJ-1 expression at mRNA and protein levels in ductal carcinoma of the breast. Histopathology. 2012;61:69–77. doi: 10.1111/j.1365-2559.2012.04202.x. [DOI] [PubMed] [Google Scholar]
- Oda M, Makita M, Iwaya K, et al. High levels of DJ-1 protein in nipple fluid of patients with breast cancer. Cancer Sci. 2012;103:1172–6. doi: 10.1111/j.1349-7006.2012.02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale M, Bonino D, Consoli P, et al. A meta-analysis of two-dimensional electrophoresis pattern of the Parkinson’s disease-related protein DJ-1. Bioinformatics. 2010;26:946–52. doi: 10.1093/bioinformatics/btq073. [DOI] [PubMed] [Google Scholar]
- Caputo E, Maiorane L, Vasta V, et al. Characterization of human melanoma cell lines and melanocyte by proteome analysis. Cell Cycle. 2011;10:2924–36. doi: 10.4161/cc.10.17.17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Iwaya K, Kikuchi R, et al. DJ-1 protein expression as a predictor of pathological complete remission after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2013;139:51–9. doi: 10.1007/s10549-013-2523-0. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ. The World Health Organization Classification of Tumours of the Breast, Fourth Edn. Special Subtypes. Lyon: World Health Organization; 2011. pp. 39–76. [Google Scholar]
- Sobin LH, Gospodarowics MK, Wittekind C. TNM Classification of Malignant Tumours. 7th edn. Hoboken, NJ: Blackwell Publishing Ltd; 2010. [Google Scholar]
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Cook TJ, Zabetian CP, et al. DJ-1 isoforms in whole blood as potential biomarkers of Parkinson disease. Sci Rep. 2012;2:954–64. doi: 10.1038/srep00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Peter M, Jang YJ, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;8:263–73. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Ferrer CM, Lynch TP, Sodi VL, et al. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54:820–31. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y, Nam JM, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathway. J Clin Invest. 2014;124:367–84. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson TS, Hartle DK, Hursting SD, et al. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67:8396–405. doi: 10.1158/0008-5472.CAN-06-4069. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kim HS, Kim A-R, et al. DJ-1 regulates mast cell activation and IgE-mediated allergic responses. J Allergy Clin Immunol. 2013;131:1653–62. doi: 10.1016/j.jaci.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Yamane T, Takahashi-Niki K, et al. Transcriptional activation of low-density lipoprotein receptor gene by DJ-1 and effect of DJ-1 on cholesterol homeostasis. PLoS One. 2012;7:e38144. doi: 10.1371/journal.pone.0038144. . doi: 10.1371/journal.pone.0038144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain D, Jain R, Eberhard D, et al. Age- and diet-dependent requirement of DJ-1 for glucose homeostasis in mice with implications for human type 2 diabetes. J Mol Cell Biol. 2012;4:221–30. doi: 10.1093/jmcb/mjs025. [DOI] [PubMed] [Google Scholar]