Abstract
Western honey bee (Apis mellifera L.) populations face declines commonly attributed to pesticide, pathogen, and parasite stress. One way beekeepers combat these stressors is by providing supplemental protein diets to honey bee colonies to ensure adequate colony nutrition. However Nosema spp., a microsporidian parasite of the honey bee, is thought to be associated closely with a colony’s nutritional intake, thus possibly negating any benefit the bees otherwise would have received from a nutritional supplement. Through three objectives, we examined how adult bees’ consumption of wildflower pollen or commercial pollen substitute diets affected Nosema levels in the bees’ midguts. For our first objective, we investigated how method of inoculation with Nosema affects infection levels in inoculated bees. Bees were infected with spores of Nosema four days after emergence. On day 15, bees were collected from the cages and Nosema spores were quantified. We found that inoculation through the pollen diet resulted in the highest Nosema levels in inoculated bees. In our second and third objectives, we provided the test diets to caged, newly emerged bees for a period of 15 days. Bees consuming pollen and a sucrose solution had more Nosema in their midguts than did bees consuming the sucrose solution alone (control). The overall volume of diet consumed by the bees did not correlate with the level of Nosema in their midguts. The level of Nosema was higher in bees fed certain commercial pollen substitute diets than in bees fed wildflower pollen. Our study illustrates how providing nutritional supplements to adult honey bees can impact the intensity of Nosema in their midguts.
Introduction
The Western honey bee, Apis mellifera (Linnaeus, 1758), provides crucial pollination services for much of the world’s crops [1,2]. In the United States, the value of crops dependent on honey bees is in excess of $14.6 billion [3]. Honey bee colony populations have declined at a high annual rate over the last decade [4]. The exact cause for this decline is unknown and has been attributed to pesticide exposure, inadequate nutrition, parasites, pathogens, climate change, management practices, and other stressors [5–17].
Good colony nutrition, such as adequate protein and carbohydrate stores, is believed to help bees to resist or tolerate many of the stressors associated with modern apiculture [18–20]. Honey bees are highly dependent on foodstuffs stored within the hive. Worker bees do not have substantial protein reserves in their bodies; therefore, they require a daily diet of about 3.4–4.3 mg of pollen, depending upon their age, to make up this nutritional deficiency. A typical 10-frame colony consumes between 13.4 and 17.8 kg of pollen annually [21]. Nearly all protein and vitamins needed by bees are derived from pollen stored as bee bread inside the hive when pollen otherwise is not available in the environment [18,22].
Beekeepers in the U.S. routinely feed colonies pollen substitute diets when they believe bees are experiencing a nutrition dearth or if the incoming resources are believed to be of low or insufficient quality. For example, substitute pollen diets, which commonly consist of a protein source derived from soy, wheat, or lentils and is fortified with essential vitamins, often are fed to migratory colonies when the colonies are being used to pollinate crops. These diets help serve as a vital protein source to colonies [23] and offset the poor nutritional conditions frequently associated with agricultural landscapes [24,25]. Feeding colonies pollen substitutes increases annual honey yield, brood health, worker longevity, and worker development [22]. Though the primary protein source utilized by colonies is pollen [18], beekeepers feed colonies pollen substitutes because the diets are more widely available and affordable than is natural, bee-collected pollen [26].
Colonies with inadequate nutrition have a higher risk of experiencing negative effects associated with other stressors in the colony, such as pesticide exposure [27–29] and pathogen infection. Regarding the latter, Nosema spp (Nosema) is a pathogen of particular interest because of its close association with the honey bee midgut and bee nutrient adsorption. Nosema spp. are obligate intracellular fungal parasites of insects [30,31] and they have a worldwide distribution [32,33]. Nosema apis [34] and Nosema ceranae are two species that commonly affect the western honey bee. Nosema attacks the epithelial lining of the bee’s midgut. There, the pathogen multiplies and is spread throughout the colony via normal bee trophallaxis and uncontrolled defecation [22,35]. A Nosema infection is most problematic to colony health in the winter and early spring [36]. Nosema infection prevents adequate nutrient digestion and absorption in a bee’s midgut [37] contributing to an increase in appetite and a reduction in activity [20].
Beekeepers feed pollen substitutes to colonies to increase colony strength and reduce colony susceptibility to pathogens, such as Nosema. This practice may, however, be counterproductive given that Nosema competes with bees for nutrition, possibly leading to increased Nosema levels in bees provided pollen substitute diets. Thus, we hypothesized that Nosema infections may be worse in bees that have fed on natural pollen or beekeeper-provided pollen substitute diets than in bees that have not. To better explore the influence of diet on Nosema infections in bees, we first determined if the method of inoculating bees with Nosema affects overall Nosema levels in the inoculated bees. While there are standardized methods for Nosema inoculation [38], it is not known how the inoculation medium (i.e. sucrose solution or pollen) influences Nosema pathogenicity. Second, we determined the contribution of the amount of pollen consumed by a bee to the level of Nosema infection it had. Porrini et al. [39] demonstrated that bees fed pollen had higher Nosema levels than those fed less nutritious diets consisting of high fructose corn syrup and soy derived protein. Consequently, we hypothesized that Nosema levels in bees would correlate with increased pollen consumption and increase linearly relative to the amount of pollen the bees ingested. Finally, we determined the contribution of the consumption of pollen substitute diets by bees to Nosema levels in the bees during the fall and spring seasons. We hypothesized that bee consumption of diets with differing nutritional content would result in different Nosema spore numbers in the bees since Porrini et al. [39] showed Nosema levels varied between bees fed pollen and bees fed high fructose corn syrup containing a commercial mixture of amino acids and vitamins. By measuring the contribution of diet to the number of Nosema spores in a bee, we can guide beekeepers in choosing the most suitable pollen substitute diet for improving their colony health.
Materials and Methods
Obtaining honey bees
European-derived honey bees were obtained from the Bee Biology Research Unit at the University of Florida (29.627042N,-82.356373W) during fall 2013 and spring 2014. Frames of capped brood from multiple hives were collected and maintained in a reach-in incubator (Percival 136VS) at 34.5°C and 65% relative humidity (RH). After a period of 24 hours, newly emerged workers were pooled from the collected frames and 15 adult bees were placed into each bioassay cage.
Bioassay cages
Bioassay cages (S1 Fig Bioassay cage with adult honey bees), modified from Williams et al. [40], were constructed of a clear 295 mL cup (Amscan Big Party Pack, Simply Unforgettable Party Shop). The cup was inverted so that the bottom of the cup faced upwards. Two feeder holes were inserted in the plastic cup using a brass cork borer (size 7, 12.5 mm in outside diameter) that was heated over a Bunsen burner. One of the holes was inserted in the top of the cage (bottom of the cup) to accommodate a feeder containing 50% sucrose solution (1:1 sucrose: water, w/v) while the other hole was inserted on the side to accommodate a water feeder. The feeders were constructed of 1.5 ml Eppendorf centrifuge tubes with two holes (1.2 mm) drilled into the tubes for the bees to acquire the respective solution. One circular ventilation hole (2.3 cm) was inserted through the side of each cage using a heated cork borer. A piece of charcoal-colored, fiberglass screen, size #5 (Phifer, Lowes), was affixed over the hole using a hot glue gun. The top portion of a 100 mm × 15 mm culture plate (Fisherbrand) was placed under the bottom of the cage and secured to the cage using a rubber band placed longitudinally around the cage. A hole was inserted (using a heated, size 7 cork borer) into the center of the culture plate to accommodate base mount queen-rearing cups (JZ_BZ, Mann Lake LTD.) that were used to deliver the pollen or pollen substitute diet to the bees. A 6.5 cm × 5 cm piece of wax foundation (Walter T. Kelley Co.) was placed on the inside of the cage and secured using five, 1.27 cm long brass fasteners (Walmart). Five holes were inserted into the side of the plastic cup using a soldering iron to accommodate the brass fasteners used to secure the wax to the side of the cage.
Nosema quantification
Nosema spores for inoculation were collected by homogenizing, purifying, and quantifying spores as described in Fries et al. [38]. Forager bees were collected at the entrance of a hive infected with Nosema. The bee’s abdomens were removed and homogenized in 2 mL deionized (DI) H2O using a mortar and pestle during the fall studies and a FastPrep-24 with TeenPrep Adapter (MP Biomedicals) during the studies undertaken in spring. Ten mL of DI H2O was added to the homogenized tissue prior to filtering the homogenate. The homogenate was filtered through a two-step gravity filter with the first funnel lined with charcoal fiberglass screen, size #5 (Phifer, Lowe’s), and the second filter lined with sheer drapery (Batiste, JoAnn’s Fabric). The supernatant was centrifuged (Eppendorf 5810R) at 3000 RPM for 5 minutes. The supernatant was discarded and the resulting pellet was reconstituted in 10 mL DI H2O before being centrifuged a second time at 3000 RPM for 5 minutes to eliminate fat body fragments and other debris. After an additional round of reconstituting and centrifuging as above, the pellet was reconstituted in 5 mL DI H2O, thus creating an inoculation stock. To quantify the inoculum, 10 μL of inoculum was diluted with 90 μL DI H2O. 10 μL of the dilution was pipetted onto a hemocytometer (INCYTO C-CHIP). Spores were counted within the 25 squares in the innermost grid at 400× magnification using a phase contrast microscope (Leica).
The impact of inoculation route on Nosema levels in bees
A cage study was established to determine whether inoculation route and pollen intake impacts Nosema levels in bees. Cages were established with 15 newly emerged bees (<24 hours old) and received one of four treatments with five replications of each treatment. Bees in cages were fed (1) sucrose solution only with no Nosema inoculum (control), (2) sucrose solution only with Nosema inoculum in the sucrose solution, (3) sucrose solution and pollen with Nosema inoculum in the sucrose solution, or (4) sucrose solution and pollen with Nosema inoculum in the pollen. The Nosema inoculum was added directly to the “pollen mixed with 50% w/v sucrose solution” (denoted as “pollen” hereafter) or 50% sucrose solution and fed to the bees at a concentration of 200,000 spores per bee when bees were four days old. When appropriate, the inoculum was delivered through 0.15 g of wildflower pollen provided to bees in each cage (pollen inoculum) or through 125 μL of 50% sucrose solution (w/v) provided to bees in each cage (sucrose inoculum). For each cage, the inoculum, whether delivered through pollen or sucrose, was replaced after a period of 24 hours with fresh diet containing no spores. Bees in treatments receiving pollen were given 0.4 g wildflower pollen every four days. Bees in all cages were provided with 50% (w/v) sucrose ad libitum. Bioassay cages were kept in an incubator (Binder model # BF-400-UL) at 34.5°C and 40% RH. The humidity was maintained using a saturated sodium chloride solution. Dead bees were removed daily.
All living bees were collected at 15 days old and anesthetized by placing the cages in a -20°C freezer. Once the bees were anesthetized, abdomens were collected from 10 bees per cage and homogenized in 2 mL deionized (DI) H2O using a mortar and pestle. Nosema levels were quantified. We conducted a one-way analysis of variance (ANOVA) recognizing treatment (method of inoculation) as the independent variable and spore count as the dependent variable to determine whether there were differences in the Nosema spores between the treatment groups (JMP 10, SAS, Cary, NC).
The impact of pollen consumption on Nosema levels in bees
Honey bees were fed different amounts of wildflower pollen over a 15 day period to determine the contribution of pollen consumption to their Nosema levels. Cages were established with 15 newly emerged bees (<24 hours old) and grouped into one of six treatments with five cages per treatment. The caged bees were provided irradiated (to eliminate any potential pesticide contaminants) wildflower pollen (acquired from Straughn Farms, Waldo, Florida) in different quantities of 0 g (control), 0.1 g, 0.2 g, 0.3 g, 0.4 g, and 0.5 g of pollen per cage every four days such that the treatment groups received either 0 g, 0.4 g, 0.8 g, 1.2 g, 1.6 g, and 2.0 g of pollen per cage over the course of the study, according to treatment group designation. The caged bees were inoculated on day four with a concentration of 100,000 spores per bee delivered through 125 μL of 50% sucrose solution (w/v). Once the inoculated sucrose solution was consumed, the bees received 50% sucrose solution ad libitum throughout the remainder of the experiment while the pollen in the cages was replaced every four days. Dead bees were removed daily and the associated mortality was recorded. At the end of 15 days, the bees were anesthetized at -20°C. Two pooled samples of five abdomens per sample were homogenized separately from each cage. Nosema spores were purified and quantified. The two samples from each cage were averaged together to analyze the spores per bee from each cage. Treatment differences in Nosema levels, bee mortality, and diet consumption data were determined using a one way ANOVA recognizing treatment (amount of pollen provided) as the independent variable and Nosema level (spores/bee), bee mortality and diet consumption as dependent variables (JMP 10, SAS, Cary, NC).
The impact of bee consumption of commercial diets on Nosema levels
We established cages with 15 newly emerged workers (<24 hours old) to measure the impacts of commercial pollen substitute diets on Nosema spore levels in bees. Bees in ten cages per treatment, for a total of six treatments, were fed for a period of 15 days after establishment. Treatment groups consisted of bees fed sucrose solution and one of six possible diet regimens: (1) no pollen diet provided (control), (2) irradiated wildflower pollen (acquired from Straughn Farms, Waldo, Florida), (3) Ultra Bee powder containing 60% crude protein (Mann Lake), (4) Bee-Pro powder containing 48.5% crude protein and 3.8% crude fat (Mann Lake), (5) MegaBee powder containing 40% protein and 4% fat (Dadant), or (6) MegaBee Winter Patty containing 3% protein with carbohydrates and Honey Bee Healthy additive (Dadant). Each diet had the same amount of sucrose solution, but differing amounts of water to ensure a similar consistency across the diets. The diets were mixed according to the following ratios: 50:50 wildflower pollen (3 g pollen with 3 mL 50% sucrose solution, 1:2.5 Ultra Bee (3 g product powder with 3 mL 50% sucrose solution and 4.5 mL DI H2O), 1:2.5 Bee-Pro (3 g product powder with 3 mL 50% sucrose solution and 4.5 mL DI H2O), 1:2.5 MegaBee powder (3 g product powder with 3 mL 50% sucrose solution and 4.5 mL DI H2O), and 3:1 MegaBee Winter Patty (3 g patty with 1 mL 50% sucrose solution to increase malleability). 0.4 g of the respective diet was provided to bees in each cage and replaced every four days. The weight of the diet, indicative of diet consumption, was determined after it was removed for the cage. All cages were provided with 50% (w/v) sucrose ad libitum. Dead bees were removed daily. Bee mortality and diet consumption were recorded throughout the course of the experiment. Three days after cage establishment, bees were fed a Nosema inoculum at a concentration of 200,000 spores per bee in a 50% (w/v) sucrose solution. At the end of 15 days, the bees were anesthetized in a -20° C freezer. Two pooled samples of five abdomens per sample were homogenized separately from each cage. Nosema spores were purified and quantified. The two samples from each cage were averaged together to analyze the spores per bee for each cage. Differences in Nosema levels, bee mortality, and diet consumption (dependent variables) data were determined using a one-way ANOVA recognizing treatment (diet type) as the independent variable (JMP 10, SAS, Cary, NC).
This study was repeated in two seasons (study 1- fall 2013, study 2- spring 2014) to characterize any possible seasonal variations in diet impacts on Nosema levels in bees. The same pollen substitute diets were evaluated during both seasons. However, in spring 2014, the uninoculated wildflower pollen treatment was omitted and replaced with two other treatment groups fed only sucrose. One of the sucrose treatment groups received the Nosema inoculum in the sucrose, while the other sucrose treatment received no Nosema inoculum.
To confirm that Nosema was not present in the commercial diets prior to inoculation, caged bees were fed one of six treatments (sucrose only, wildflower pollen, Ultra Bee, Bee-Pro, MegaBee, MegaBee Winter Patty). The bees did not receive any Nosema inoculation. Five cages of 15 newly emerged (<24 hours old) bees per cage were established for each treatment group for a period of 15 days. Caged bees were fed 50% sucrose ad libitum and 0.4 g of the appropriate diet was placed in the respective treatment cages. Every four days, diets were replaced and bee mortality was recorded throughout the course of the experiment. At the end of 15 days, the bees were anesthetized in a -20° C freezer. Two pooled samples of five abdomens per sample were homogenized separately from each cage. Nosema spores were purified and quantified as previously described. The two samples from each cage were averaged together to determine the spores per bee from each cage. Differences in Nosema spore levels, bee mortality, and diet consumption (dependent variables) data were analyzed using a one-way ANOVA recognizing treatment (diet type) as the independent variable (JMP 10, SAS, Cary, NC).
Results
The impact of inoculation route on Nosema levels in bees
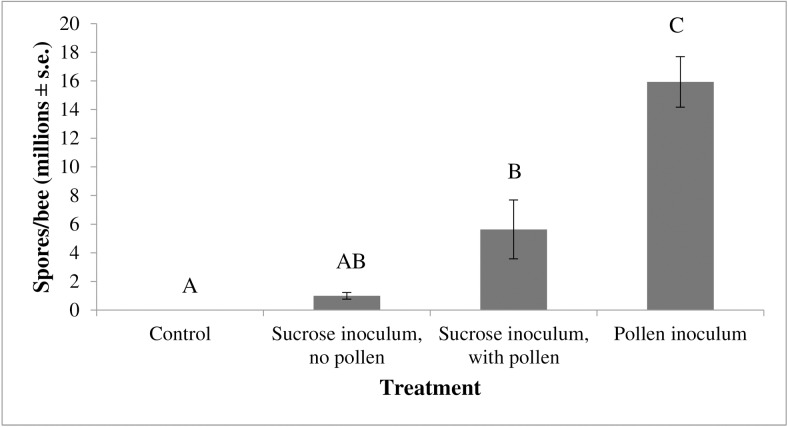
Bees receiving the inoculum in the pollen had significantly elevated spore levels (15.9 million spores ± 1.8 million spores (n = 4), average number spores ± s.e. (n)) than those receiving the inoculum in the sucrose solution (5.6 million ± 2 million (n = 5), ANOVA, F3,12 = 16.7, p<0.0001, Fig 1). The Nosema levels in sucrose-inoculated bees fed pollen was higher than in sucrose-inoculated bees not fed pollen (1 million ± 0.23 million (n = 3)). There was a significantly greater number of Nosema spores in bees inoculated through the pollen diet than in those inoculated through the sucrose solution (Fig 1). However, a sucrose delivery of Nosema was determined to be preferable to Nosema delivery through pollen because all sucrose-inoculated cages received a consistent application of sucrose that the bees consumed completely. The consumption of pollen by the bees varied between cages, thus leading to fears of unequal inoculation rates among bees in cages receiving the inoculum through pollen.
Fig 1. The impact of inoculation method on Nosema levels in bees.
Data are the average number of spores per bee (in millions) with the error bars denoting standard error. N = 17 for all treatment groups. The treatment groups are (1) control (bees fed a sucrose solution, no inoculum) (2) sucrose inoculum, no pollen (bees inoculated with Nosema through a sucrose solution and given no pollen), (3) sucrose inoculum, with pollen (bees inoculated with Nosema through a sucrose solution and given pollen), and (4) pollen inoculum, with sucrose solution (bees inoculated with Nosema through pollen). Treatment (method of inoculation) significantly affected Nosema levels in bees (ANOVA, F3,13 = 16.7, p<0.0001). Posthoc Tukey-HSD pairwise comparisons identified significant differences between treatments (data with the same letter are not different at α ≤ 0.05).
The impact of pollen consumption on levels in bees
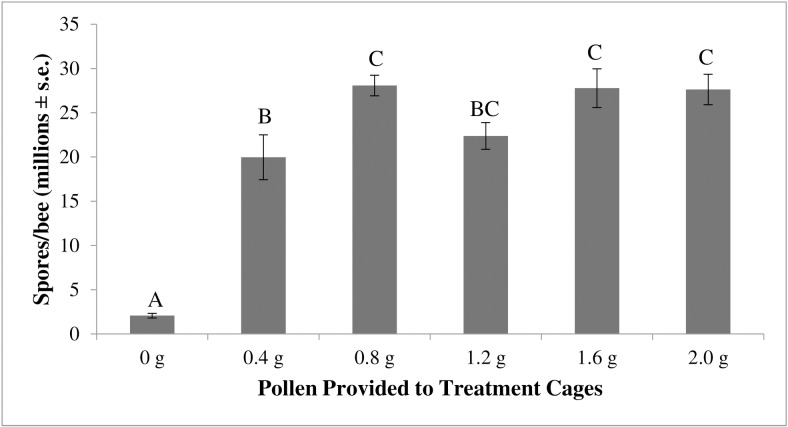
The amount of pollen provided to the bees had a significant effect on Nosema levels (average number of spores ± s.e. (n = 30)) in the bees (ANOVA F5,24 = 33.6, p<0.0001, Fig 2). There were significantly more Nosema spores in bees fed 0.8 g (28.1 million spores/bee ± 1.0 million spores (n = 5)), 1.6 g (27.8 million spores/bee ± 1.9 million (n = 5)), and 2.0 g (27.6 million spores/bee ± 1.5 million (n = 5)) of pollen than bees fed only 0.4 g of pollen (20.0 million spores/bee ± 2.3 million (n = 5)). Nosema levels in bees fed 1.2 g of pollen (22.4 million spores/bee ± 1.3 million (n = 5)) did not differ significantly from those in bees fed other pollen amounts. Bees fed any amount of pollen had significantly higher Nosema levels than bees fed no pollen at all (2.1 million spores/bee ± 0.24 million (n = 5)).
Fig 2. The effect of pollen consumption on Nosema levels in bees.
Data are the average number of spores per bee (in millions) with the error bars denoting standard error. N = 30 for all treatment groups. The treatment groups (x axis) represent the amount of pollen given to bees/cage. Treatment (amount of pollen provided to the bees) significantly affected the spore levels in bees (ANOVA F5,24 = 33.6, p<0.0001). Posthoc Tukey-HSD pairwise comparisons identified significance between treatments (data with the same letter are not different at α ≤ 0.05).
While there were no significant differences between treatments in Nosema levels in bees fed ≥0.8 g of pollen, we did find significant differences in total consumption of pollen between each of the treatment groups (ANOVA F5, 24 = 571.1, p<0.0001, Fig 3). The groups of bees provided with 2.0 g, 1.6 g, 1.2 g, 0.8 g, and 0.4 g of pollen consumed a total of 0.90 g ± 0.02 g, 0.81 g ± 0.02 g, 0.63 g ± 0.01 g, 0.51 g ± 0.02 g, and 0.29 g ± 0.01 g pollen respectively. Mortality averaged 0.7 bees/cage across the six treatments and was not significantly different (ANOVA F5,24 = 2.4324, p = 0.0642).
Fig 3. Consumption of pollen by bees across treatment groups.
Data are the average amount of pollen consumed by the bees (in grams) with the error bars denoting standard error. N = 30 for all treatment groups. The treatment groups (x axis) represent the amount of pollen given to the cage of bees. Bees receiving more pollen consumed significantly more pollen across all treatment groups (ANOVA F5, 24 = 571.1, p<0.0001). Posthoc Tukey-HSD pairwise comparisons identified significance between (data with the same letter are not different at α ≤ 0.05).
The impact of bee consumption of commercial diets on Nosema levels
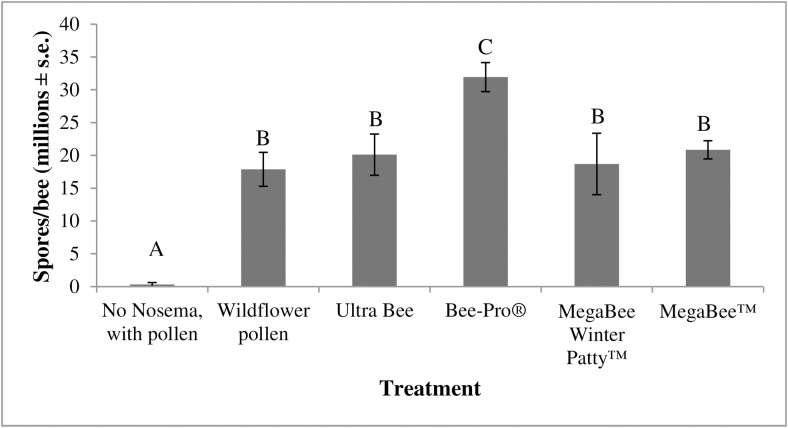
There was a significant difference in study 1 between the level of Nosema in bees consuming the various commercial diets (ANOVA, F5, 52 = 15.9, p<0.0001, Fig 4). Bees fed Bee-Pro (31.9 million spores/bee ± 2.2 million) had significantly more Nosema than those fed wildflower pollen (17.9 million spores/bee ± 2.6 million), Ultra Bee (20.1 million spores/bee ± 3.1 million), MegaBee (18.7 million spores/bee ± 4.7 million), and MegaBee Winter Patty (20.8 million spores/bee ± 1.4 million).
Fig 4. The impact of commercial pollen diet consumption by bees on Nosema levels in study 1 (fall 2013).
Data are the average number of spores per bee (in millions) with the error bars denoting standard error. N = 56 for all treatment groups. The treatment groups represent the various commercial pollen substitute diets provided to bees. Treatment (type of diet) significantly affected Nosema levels in bees (ANOVA, F5, 52 = 15.9, p<0.0001). Posthoc Tukey-HSD pairwise comparisons identified significance between treatments (data with the same letter are not different at α ≤ 0.05).
Similar to study 1, there was a significant difference in study 2 between Nosema levels in bees fed the various commercial diets (ANOVA, F5, 63 = 33.6, p<0.0001, Fig 5). There were significantly higher Nosema levels in bees fed Ultra Bee (21.4 million spores/bee ± 2.5 million) and MegaBee Winter Patty (21.1 million spores/bee ± 1.9 million) than those fed only sucrose solution (11.6 million spores/bee ± 1.7 million) or MegaBee (11.3 million spores/bee ± 1.9 million). Bee-Pro- (19.6 million spores/bee ± 3.4 million) and wildflower-fed (14.8 million spores/bee ± 1.1 million) bees had intermediate Nosema levels that were not significantly different from those in bees fed the other commercial diets. Bees fed BeePro in study 1 and 2 had different Nosema levels from one another (ANOVA F1,16 = 8.10, p = 0.0212, Table 1).
Fig 5. The impact of commercial pollen diet consumption by bees on Nosema levels in study 2 (spring 2014).
Data are the average number of spores per bee (in millions) with the error bars denoting standard error. N = 70 for all treatment groups. The treatment groups represent the various commercial pollen substitute diets provided to bees. Treatment (type of diet) significantly affected Nosema levels in bees (ANOVA, F5, 63 = 33.6, p<0.0001). Posthoc Tukey-HSD pairwise comparisons identified significance between treatments (data with the same letter are not different at α ≤ 0.05).
Table 1. The seasonal differences between Nosema levels across treatment diets in study 1 (fall 2013) and study 2 (spring 2014).
| Treatment | Year | N | Average number of Nosema spores ± s.e. (millions) | p-value |
|---|---|---|---|---|
| Wildflower | 2013 | 9 | 17.87 ± 2.60 | 0.2723 |
| 2014 | 10 | 14.75 ± 1.17 | ||
| Ultra Bee | 2013 | 10 | 20.11 ± 3.14 | 0.7594 |
| 2014 | 10 | 21.38 ± 2.61 | ||
| Bee-Pro | 2013 | 9 | 31.93 ± 2.23 | 0.0212* |
| 2014 | 10 | 19.65 ± 3.57 | ||
| MegaBee | 2013 | 8 | 18.69 ± 4.68 | 0.1396 |
| 2014 | 10 | 11.3 ± 2.06 | ||
| MegaBee Winter Patty | 2013 | 10 | 20.85 ± 1.38 | 0.9354 |
| 2014 | 10 | 21.05 ± 2.00 |
* denotes differences between years for spore numbers in bees for a given treatment at α ≤ 0.05.
The Nosema levels in uninoculated bees fed the various diets did not vary significantly and were negligible (F5, 24 = 0.9551, p = 0.4643, Table 2). Furthermore, bees consumed all diets equally (F4, 20 = 1.9294, p = 0.1448, Table 2). No single diet led to increased bee mortality (F5,24 = 1.3064, p = 0.2944, Table 2).
Table 2. Average levels of Nosema spores/bee, diet consumption per cage of bees, and bee mortality when fed the various diets in the absence of Nosema inoculum.
N = 5 for all data.
| Diet | Average number of Nosema spores/bee (millions ± s.e.) | Average amount of diet consumed per one cage of 15 bees (mg ± s.e.) | % bee mortality after 15 days (bees ± s.e.) |
|---|---|---|---|
| Sucrose only (control) | 0.27 ± 0.10 | N/A | 2.2 ± 0.86 |
| Wildflower | 0 | 0.84 ± 0.05 | 0.4 ± 0.25 |
| Ultra Bee | 0.67 ± 0.43 | 0.88 ± 0.05 | 1.6 ± 0.60 |
| Bee-Pro | 0.18 ± 0.08 | 0.89 ± 0.02 | 1.2 ± 0.73 |
| MegaBee | 0.03 ± 0.03 | 0.85 ± 0.04 | 2.2 ± 0.49 |
| MegaBee Winter Patty | 0.83 ± 0.62 | 0.75 ± 0.03 | 1.2 ± 0.49 |
Discussion
The impact of inoculation route on Nosema levels in bees
We suspected that the level of Nosema in bees would be impacted by the route of inoculum delivery. Our data show that Nosema levels were much greater in bees that fed on inoculated pollen than in bees fed inoculated sucrose solution. Although Nosema spores are quite resilient to environmental conditions, it is possible that spores experience greater mortality in sucrose solution due to differences in pH between nearly neutral sucrose solution and the rather acidic environment of the honey bee midgut [41]. Others have demonstrated a measurable difference in Nosema polar tube anatomy based upon environmental moisture content [42] and this may impact the virulence of the spore once it is ingested by the bee. While using sucrose solution to inoculate bees with Nosema is the “standard” [38], our data suggest that the method used for Nosema inoculation in honey bee bioassays may impact the resulting findings and conclusions drastically.
The impact of pollen consumption on Nosema levels in bees
Pollen is critical to the nutritional needs of the colony [43]. Our data suggest that Nosema does not replicate well in bees deprived of pollen. Inoculated bees provided at least 0.4 g of pollen per cage of 15 bees had over ten times higher Nosema levels than inoculated bees not receiving pollen, suggesting that bee nutrient intake is important to Nosema reproduction. Despite this, we did not find a positive correlation between Nosema levels and the amount of diet consumed by bees. Instead, our data suggest that any amount of pollen consumed by bees over a 0.8 g threshold produces statistically similar Nosema levels in bees.
Pollen consumption, even minute amounts, appears to contribute significantly to Nosema levels in bees. This finding is in agreement with similar studies in which the influence of diet on Nosema infection was evaluated [39]. Previous investigators showed that bees fed a diet containing pollen had six times more Nosema spores than bees fed a diet containing no pollen [39,44]. The researchers speculated that Nosema flourishes in a bee’s midgut when the bee is fed a more nutritious diet [39,44]. Alternatively it has been hypothesized that pollen consumption by bees could increase Nosema levels by increasing the surface area of the midgut, thus increasing the bee’s susceptibility to infection [39]. Since we did not see a linear increase in Nosema levels in bees fed more than 0.8g of pollen, we suspect that the level of Nosema in bees is dependent upon the nutritional content of the pollen or pollen substitute diet consumed by the bees and not an increase in the midgut internal surface area that results from bee consumption of diet.
The impact of bee consumption of commercial diets on Nosema levels
Companies frequently claim multiple benefits associated with feeding bees pollen substitute/supplement diets. These claims include greater brood rearing, increased hygienic behavior, increased queen acceptance, increased resistance to colony stressors (including pathogens, parasites, and pesticides), and increased pollination and honey production resulting from use of a particular diet. As a result of these claims, we determined how the consumption of four commercial pollen substitute diets widely available in the U.S. impacts the number of Nosema spores in bees. While we confirmed that Nosema infection likely is not derived from the diets themselves, bee consumption of the different diets did produce varying levels of Nosema in inoculated bees.
It is unclear, however, what components in the pollen substitute diets are contributing to an increase in Nosema levels in bees. We know from past studies [39,44] that protein can increase the level of Nosema in bees, but the protein level in the commercial diets we tested did not correlate with the Nosema levels seen in the bees. Diets that had very low amounts of protein (i.e. MegaBee Winter Patty at 3% crude protein) had comparable levels of Nosema infection to those of diets containing high levels of protein (i.e. Ultra Bee at 60% crude protein). Our data suggest that Nosema infection may be dependent on other dietary factors. Furthermore, it is unknown how seasonal patterns are contributing to Nosema infection. We found differences between the contribution of BeePro to Nosema levels in bees in the fall and spring seasons despite inoculating bees with the same number of spores and providing the same amount of diet both seasons. Nosema levels were ~50% higher in bees fed the Bee Pro treatment in the fall than in bees fed the Bee Pro treatment in spring; however, we did not observe the same significant increase in Nosema levels across the other protein diets. Furthermore, there were no significant differences in the Nosema levels produced in bees eating the commercial diets in the spring when compared with bees fed wildflower pollen. Further investigation is needed to understand how the differences in the nutritional needs and physiology throughout the seasons of the year can impact Nosema and other pathogen infections.
The consumption of even small quantities of pollen is positively correlated with increased levels of Nosema. Additionally, there is a relationship between some commercial diets and Nosema levels that warrants further investigation. Our data suggest that supplemental pollen diet feedings may increase the level of Nosema infection in the colony. However, increases in Nosema infection have not been predictive of colony mortality. Furthermore, there currently are no thresholds for Nosema infection that would indicate a reduction in colony health when thresholds are reached. Beekeepers commonly feed their colonies throughout the year to prevent malnutrition. While pollen consumption may lead to higher Nosema reproduction within honey bees, the negative cost of inadequate nutrition that would result from withholding pollen and pollen substitutes (such as reduced brood production, immunocompetence, and foraging efficacy) to safeguard against Nosema may preclude modifying current management practices. It is unknown if we would observe the same impact of commercial pollen substitute diets on Nosema levels at the field level. Thus, field level studies would be integral to furthering our understanding on how to promote colony strength while simultaneously protecting honey bees from increases in parasite and pathogen infections.
Supporting Information
(TIF)
Acknowledgments
We would like to thank Daniel Hahn for critical evaluations and discussions of the project and Hudson Vaner Ventura Tomé, Emily Helton, Ashley Mortensen, Jeanette Klopchin, and Dale Kelley for technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Gisder S, Möckel N, Linde A, Genersch E (2011) A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee‐pathogenic microsporidia. Environ Microbiol 13: 404–413. 10.1111/j.1462-2920.2010.02346.x [DOI] [PubMed] [Google Scholar]
- 2. Eilers EJ, Kremen C, Greenleaf SS, Garber AK, Klein A- M (2011) Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE 6: e21363 10.1371/journal.pone.0021363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morse RA, Calderone NW (2000) The value of honey bees as pollinators of US crops in 2000. Bee Culture 128: 1–15. [Google Scholar]
- 4. vanEngelsdorp D, Caron D, Hayes J, Underwood R, Henson M, Rennich K, et al. (2012) A national survey of managed honey bee 2010–11 winter colony losses in the USA: results from the Bee Informed Partnership. J Apicult Res 51: 115–124. [Google Scholar]
- 5. Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7: e40848 10.1371/journal.pone.0040848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins AM, Pettis JS, Wilbanks R, Feldlaufer MF (2004) Performance of honey bee (Apis mellifera) queens reared in beeswax cells impregnated with coumaphos. J Apicult Res 43: 128–134. [Google Scholar]
- 7. Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delègue M-H (2004) Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotox Environ Safe 57: 410–419. [DOI] [PubMed] [Google Scholar]
- 8. Decourtye A, Devillers J, Genecque E, Le Menach K, Budzinski H, Cluzeau S, et al. (2005) Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch Environ Con Tox 48: 242–250. [DOI] [PubMed] [Google Scholar]
- 9. Desneux N, Decourtye A, Delpuech J-M (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52: 81–106. [DOI] [PubMed] [Google Scholar]
- 10. Eiri DM, Nieh JC (2012) A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J Exp Biol 215: 2022–2029. 10.1242/jeb.068718 [DOI] [PubMed] [Google Scholar]
- 11. Frost EH, Shutler D, Hillier NK (2013) Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J Exp Biol 216: 2931–2938. 10.1242/jeb.086538 [DOI] [PubMed] [Google Scholar]
- 12. Haarmann T, Spivak M, Weaver D, Weaver B, Glenn T (2002) Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. J Econ Entomol 95: 28–35. [DOI] [PubMed] [Google Scholar]
- 13. Henry M, Beguin M, Requier F, Rollin O, Odoux J-F, Aupinel P,et al. (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336: 348–350. 10.1126/science.1215039 [DOI] [PubMed] [Google Scholar]
- 14. Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Pettis J (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5: e9754 10.1371/journal.pone.0009754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oldroyd BP (2007) What's killing American honey bees? PLoS Biol 5: e168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williamson SM, Wright GA (2013) Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216: 1799–1807. 10.1242/jeb.083931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu W, Schmehl DR, Mullin CA, Frazier JL (2014) Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 9: e77547 10.1371/journal.pone.0077547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brodschneider R, Crailsheim K (2010) Nutrition and health in honey bees. Apidologie 41: 278–294. [Google Scholar]
- 19. Hrassnigg N, Crailsheim K (2005) Differences in drone and worker physiology in honeybees (Apis mellifera). Apidologie 36: 255–277. [Google Scholar]
- 20. Naug D, Gibbs A (2009) Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 40: 595–599. [Google Scholar]
- 21. Crailsheim K, Schneider LHW, Hrassnigg N, Bühlmann G, Brosch U, Gmeinbauer R, et al. (1992) Pollen consumption and utilization in worker honeybees (Apis mellifera carnica): Dependence on individual age and function. J Insect Physiol 38: 409–419. [Google Scholar]
- 22. Mattila HR, Otis GW (2006) Influence of Pollen Diet in Spring on Development of Honey Bee (Hymenoptera: Apidae) Colonies. J Econ Entomol 99: 604–613. [DOI] [PubMed] [Google Scholar]
- 23. DeGrandi-Hoffman G, Chen Y, Huang E, Huang MH (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J Insect physiol 56: 1184–1191. 10.1016/j.jinsphys.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 24. Naug D (2009) Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol Conserv 142: 2369–2372. [Google Scholar]
- 25. Roulston TH, Cane JH (2000) Pollen nutritional content and digestibility for animals. Plant Syst Evol 222: 187–209. [Google Scholar]
- 26. Standifer LN, Moeller FE, Kauffeld NM, Herbert EW, Shimanuki H (1977) Supplemental feeding of honey bee colonies. USDA Agr Inform Bull 413, 8p. [Google Scholar]
- 27. Wahl O, Ulm K (1983) Influence of pollen feeding and physiological condition on pesticide sensitivity of the honey bee Apis mellifera carnica. Oecologia 59: 106–128. 10.1007/BF00388082 [DOI] [PubMed] [Google Scholar]
- 28. Aliouane Y, el Hassani AK, Gary V, Armengaud C, Lambin M, Gauthier M (2009) Subchronic exposure of honeybees to sublethal doses of pesticides: Effects on behavior. Environ Toxicol and Chem 28: 113–122. [DOI] [PubMed] [Google Scholar]
- 29. Schmehl DR, Teal PEA, Frazier JL, Grozinger CM (2014) Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J Insect Physiol 71: 177–190. 10.1016/j.jinsphys.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 30. Ewald PW (1983) Host-parasite relations, vectors, and the evolution of disease severity. Annu Rev Ecol Syst 465–485. [Google Scholar]
- 31. Forsgren E, Fries I (2010) Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet Parasitol 170: 212–217. 10.1016/j.vetpar.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 32. Cantwell G (1970) Standard methods for counting Nosema spores. Am Bee J 110: 22–223. [Google Scholar]
- 33. Matheson A (1996) World bee health update. Bee World 77: 45–51. [Google Scholar]
- 34. Zander E (1909) Tierische Parasiten als Krankheitserreger bei der Biene, Münchener Bienenzeitung 31, 196–204. [Google Scholar]
- 35. Bigliardi E, Sacchi L (2001) Cell biology and invasion of the microsporidia. Microbes Infect 3: 373–379. [DOI] [PubMed] [Google Scholar]
- 36. Pickard R, El-Shemy A (1989) Seasonal variation in the infection of honeybee colonies with Nosema apis Zander. J Apicult Res 28: 93–100. [Google Scholar]
- 37. Malone LA, Gatehouse HS (1998) Effects of Nosema apis Infection on Honey Bee (Apis mellifera) Digestive Proteolytic Enzyme Activity. J Invertebr Pathol 71: 169–174. [Google Scholar]
- 38. Fries I, Chauzat M-P, Chen Y-P, Doublet V, Genersch E, Gisder S, et al. (2013) Standard methods for Nosema research. J Apicult Res 52: 1–14. [Google Scholar]
- 39. Porrini MP, Sarlo EG, Medici SK, Garrido PM, Porrini DP, Damiani N,et al. (2011) Nosema ceranae development in Apis mellifera: influence of diet and infective inoculum. J Apicult Res 50: 35–41. [Google Scholar]
- 40. Williams GR, Alaux C, Costa C, Csáki T, Doublet V, Eisenhardt D, et al. (2013) Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J Apicult Res 52: 1–35. [Google Scholar]
- 41. Vojvodic S, Rehan SM, Anderson KE (2013) Microbial Gut diversity of Africanized and European honey Bee larval instars. PLoS ONE 8: e72106 10.1371/journal.pone.0072106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olsen PE, Rice WA, Liu TP (1986) In vitro germination of Nosema apis spores under conditions favorable for the generation and maintenance of sporoplasms. J Invertebr Pathol 47: 65–73. [Google Scholar]
- 43. Haydak M (1935) Brood rearing by honeybees confined to a pure carbohydrate diet. J Econ Entomol 28: 657–660. [Google Scholar]
- 44. Rinderer TE, Dell Elliott K (1977) Worker honey bee response to infection with Nosema apis: influence of diet. J Econ Entomol 70: 431–433. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All relevant data are within the paper.