Abstract
Given the ever-increasing burden of end-stage renal disease (ESRD) in a global milieu of limited financial and health resources, interested parties continue to search for ways to optimize dialysis access. Government and payer initiatives to increase access to renal replacement therapies (RRTs), particularly peritoneal dialysis (PD) and hemodialysis (HD), may have meaningful impacts from clinical and health-economic perspectives; and despite similar clinical and humanistic outcomes between the two dialysis modalities, PD may be the more convenient and resource-conscious option. This review assessed country-specific PD-First/Favored policies and their associated background, implementation, and outcomes. It was found that barriers to policy-implementation are broadly associated with government policy, economics, provider or healthcare professional education, modality-related factors, and patient-related factors. Notably, the success of a given country's PD-Favored policy was inversely associated with the extent of HD infrastructure. It is hoped that this review will provide a foundation across countries to share lessons learned during the development and implementation of PD-First/Favored policies.
Keywords: Peritoneal dialysis, peritoneal dialysis first policy, peritoneal dialysis favored policy, global overview
An aging population and modifiable lifestyle risk factors accompanied by a decline in early-life infectious diseases have resulted in the rise of chronic diseases, particularly chronic kidney disease (CKD), as a predominant global health threat with profound socioeconomic and public health consequences (1,2). Clinically, CKD is characterized by progressive, irreversible kidney function deterioration culminating in end-stage renal disease (ESRD), the risk of which is mitigated by hemodialysis (HD) or peritoneal dialysis (PD) when kidney transplantation is unavailable or contraindicated.
Over the past 3 decades, many studies have compared outcomes associated with in-center HD (ICHD) vs PD using observational data. Clinically, although early studies consistently showed that patients initiating dialysis on PD had better earlier survival (3–6), contemporary data suggest that there is no significant difference in overall patient survival between PD and ICHD (7,8). These studies were found across the globe, including Canada (9), The Netherlands (5), Taiwan (10,11), Colombia (12), Australia and New Zealand (6), and the United States (13,14). In terms of quality of life (QoL), PD patients reported less illness intrusion, better renal care, higher satisfaction, and the ability to travel (15–18), while ICHD patients reported better staff and social interaction and less fear of isolation (19,20). Overall, there were no statistically significant differences in QoL between PD and ICHD patients, although PD patients tended to have higher QoL scores (the higher the better) (18,21–23). Economically, accumulating evidence demonstrated that PD has been a cost-saving therapy compared to ICHD in most developed countries and some developing countries (24–26). However, the provision of medical care to a large and growing dialysis patient population places a heavy economic burden on healthcare systems globally. In the US, for example, 1.3% of Medicare patients with ESRD accounted for 7.5% of Medicare spending in 2010 (27).
Despite the similar clinical and humanistic outcomes and the economic benefits associated with PD vs ICHD, 80% of prevalent dialysis patients received ICHD in 2010, with PD use ranging from 1.7% in Bangladesh to 76% in Hong Kong (27). The distribution of patients on PD globally does not reflect the view of nephrology professionals and the preference of patients and family caregivers (28–31). As one of the main dialysis modalities, PD is underutilized (32). Recognizing that patients who switch from ICHD to PD have a greater mortality risk (33) and incur more direct medical costs (34) vs patients initiated and maintained on PD, several countries have established “PD-First” (where PD is used as the first treatment modality for appropriate ESRD patients) or “PD-Favored” (where government policy on dialysis encourages the use of PD as the treatment choice while removing any existing disincentives) policies. In these countries, patient, provider, and payer incentives favor PD. In some other countries, “Home Dialysis-First” policies (including PD and home HD) are established as a complimentary strategy. These policies can be important in developing countries where resources are limited and must be optimized to meet disproportionally high ESRD rates (35).
Few studies have thoroughly compared PD-Favored policies regarding their background, implementation, and outcomes. Such information would help policy makers expand their knowledge on the applicability of these policies to their countries, learn from successful examples, and overcome potential barriers. Lessons learned from overcoming or failing to overcome barriers to PD-related policies can be shared among countries to facilitate the development of effective policies that generate better patient outcomes and mitigate healthcare expenditures. This review serves as a comprehensive assessment of country-specific PD-related policies so as to understand what worked well and what barriers hampered such efforts.
Global Policy Overview
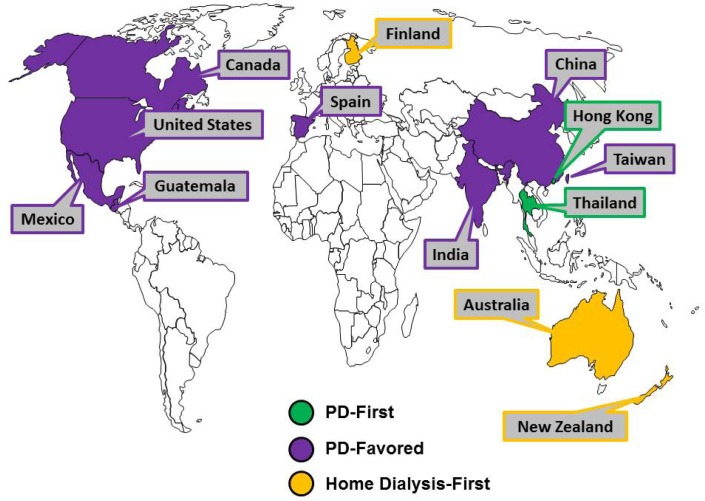
Through a targeted assessment of peer-reviewed literature, governmental and associated websites, Hong Kong and Thailand were identified as having PD-First policies, Canada, China, Guatemala, India, Mexico, Spain, Taiwan, and the United States were identified as having PD-Favored policies, and Australia, Finland, and New Zealand were identified as having Home Dialysis-First policies (Figure 1). The most frequently cited motivations for policy initiatives were to increase patient access to care, control costs through lower infrastructure and capital investments, empower patients, and optimize treatment provision.
Figure 1 —
Geographic summary of country-specific policy types. PD = peritoneal dialysis.
The timing and mechanism of the implementation of PD-First/Favored policies differed. Hong Kong is the first territory that has promoted PD-First policy among ESRD patients who required dialysis since 1985, and implemented the policy successfully with contributions from kidney-associated charity organizations (36). Starting in 2008, the Thailand National Health Insurance Fund implemented a PD-First policy by addressing important elements such as training and reimbursement (37). The Canada Ontario Ministry of Health and Long-Term Care established a provincial PD initiative in 2005 to increase use from 18% in 2005 to 30% in 2010 (38). In the United States, the Center for Medicare & Medicaid Services (CMS) implemented a prospective payment system that bundled most of the services provided to dialysis patients starting January 1, 2011 (39,40). As a result, the outcomes of these policies were different. The Hong Kong and Thailand PD-First programs are successful in terms of PD utilization rate, patient and technique survival, quality of life, and complications management (41–43). Ontario did not attain its 2010 goal of 30% PD use (38,44,45). Under the US bundled payment system, the utilization of PD has been growing at a much higher rate than the utilization of HD and the use of expensive drugs, especially erythropoiesis-stimulating agent (ESA), has declined substantially (46–48).
Tables 1, 2, and 3 provide details related to the background, implementation, and outcomes of PD-First, PD-Favored, and Home Dialysis-First dialysis policies, respectively.
TABLE 1.
Key Point Summary of PD-First Policies
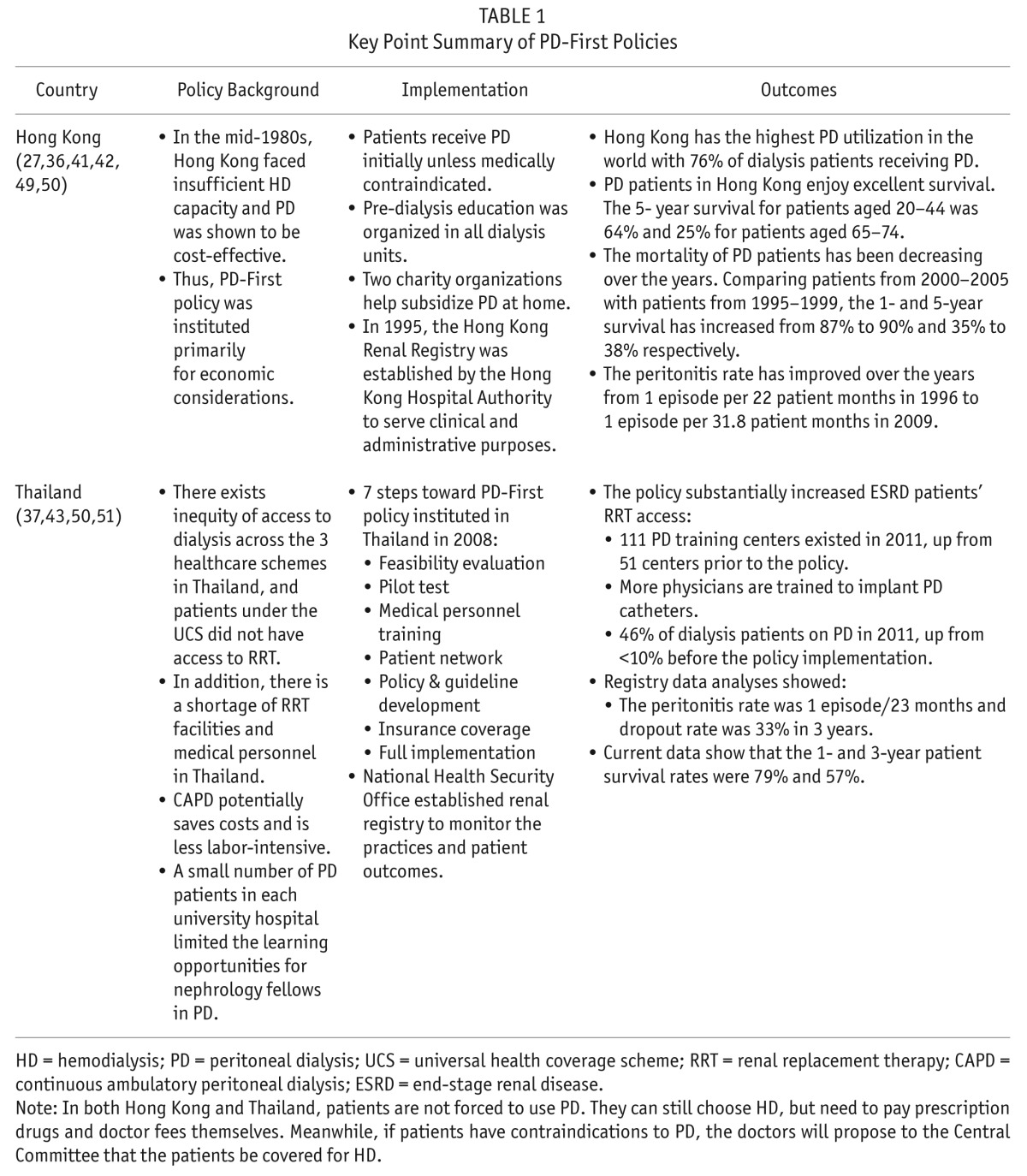
TABLE 2.
Key Point Summary of PD-Favored Policies
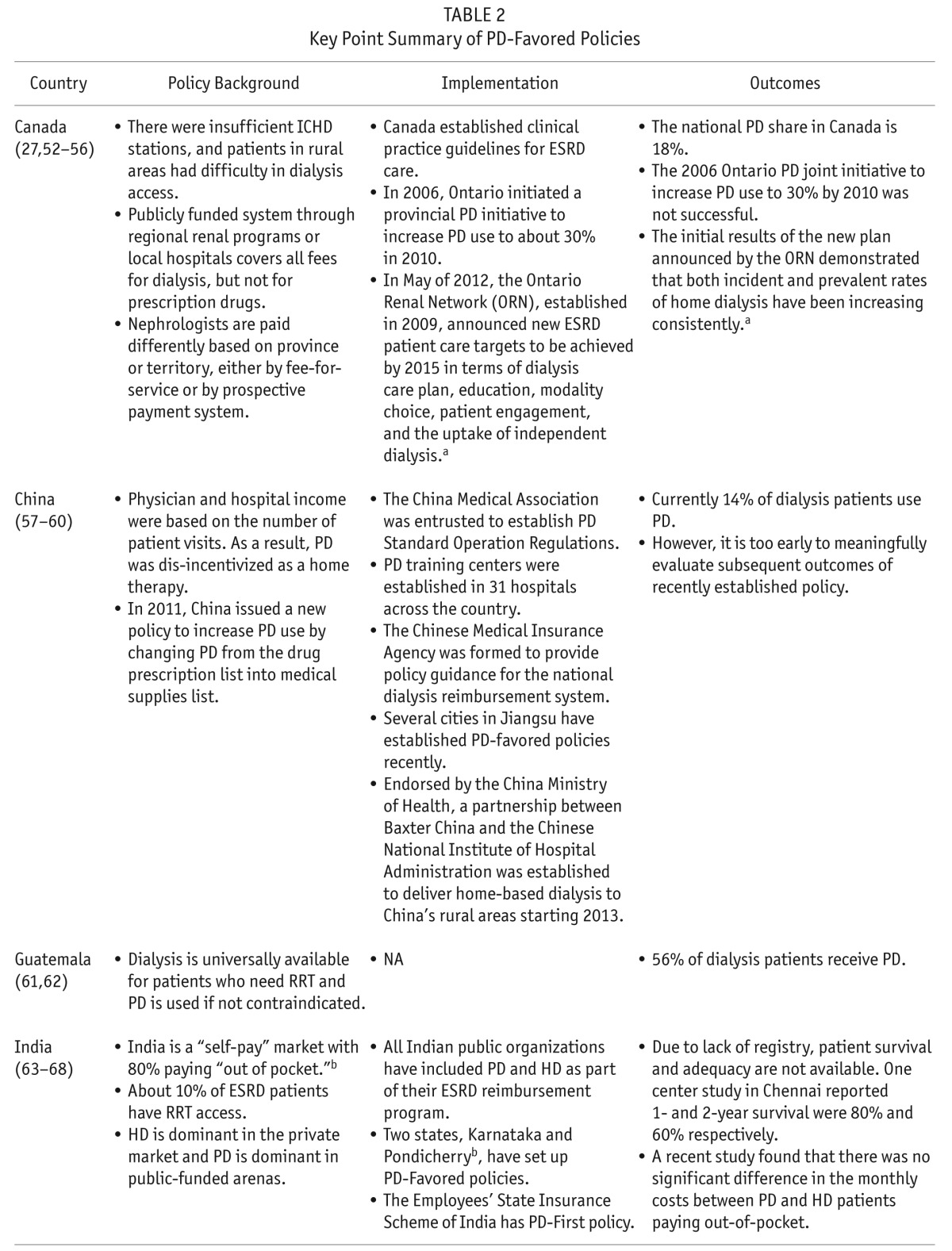
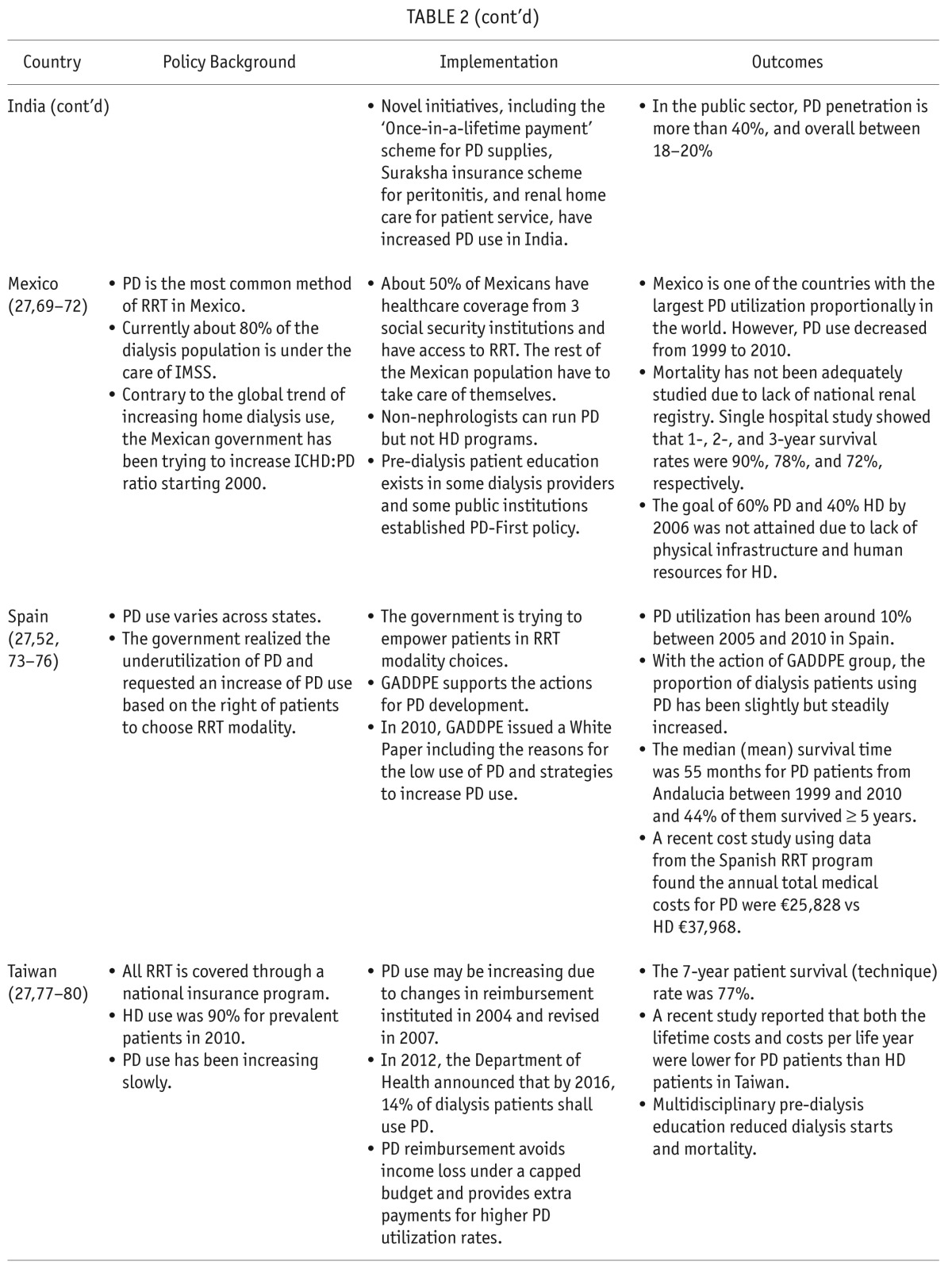
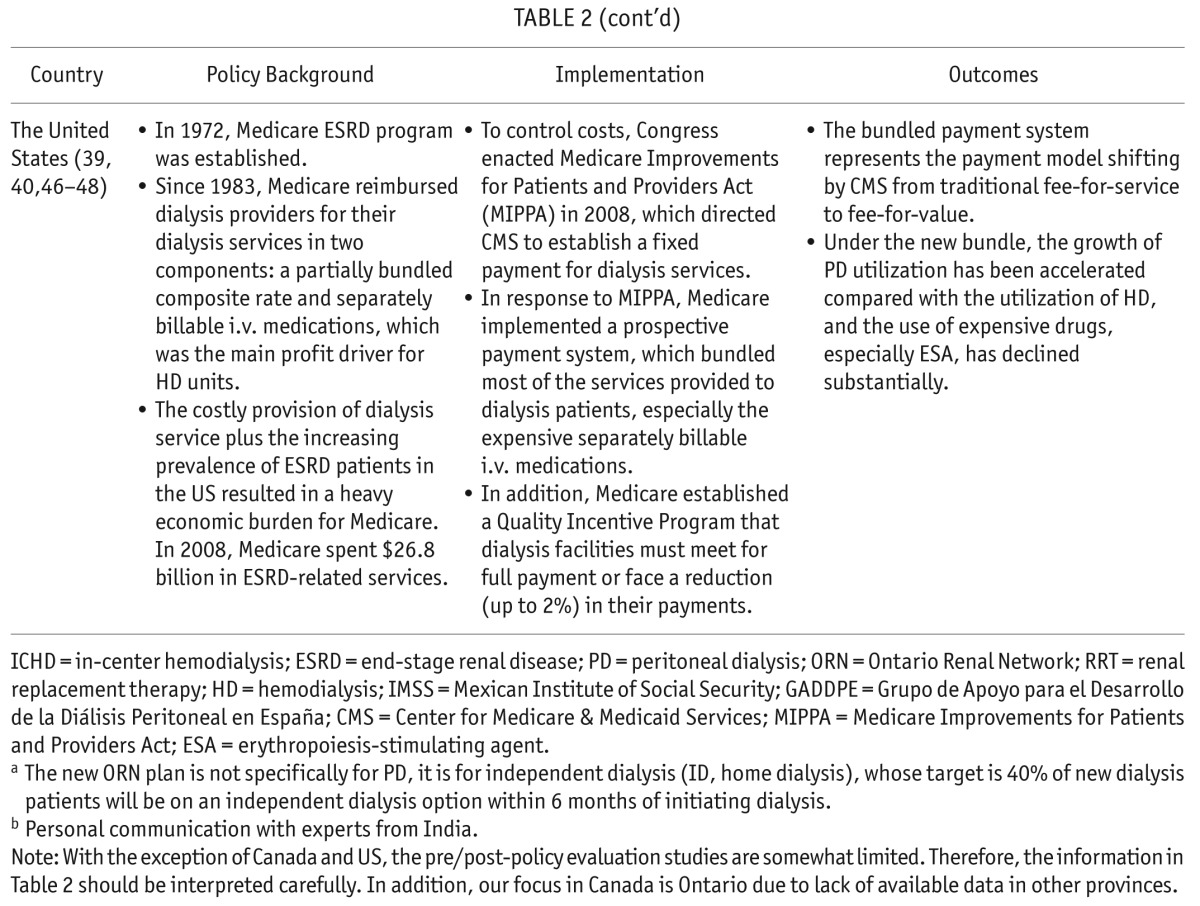
TABLE 3.
Key Point Summary of Home Dialysis-First Policies
Lessons Learned and Strategic Recommendations
Lessons on policy implementation were learned from the countries reviewed. Generally, for a PD-Favored policy to be implemented successfully, the country and/or region addressed five key elements systematically: government policies, economic factors, provider/healthcare professional education, modality-related factors, and patient-related factors.
Government Policies
Government reimbursement policies play a crucial role in cost containment for dialysis while ensuring the quality of care without compromising patient outcomes. A recent comparison of the dialysis reimbursement policies across 7 developed countries, including the United States and Ontario, Canada, found that the reimbursement systems were complex and varied significantly between countries (89). Different reimbursement policy could impact PD utilization dramatically. Policies to increase prevalent PD populations may be implemented without budgetary support and consequently be ineffective without adequate reimbursement (90). PD programs can also be dis-incentivized by government reimbursement policies favoring HD. On the other hand, policies with strong reimbursement incentives could be successful, which has been the case for the US's recently implemented bundled prospective payment system (48). Financial reimbursement policies are the most important non-medical factor contributing to modality selection worldwide (49) and pro-PD reimbursement policies have been associated with varying, yet positive, outcomes in many countries such as Thailand (100% PD reimbursement) and China/Taiwan (both incentivize PD with reimbursement).
Generally, PD rates are low in countries where provider reimbursement for PD is insufficient, when compared to countries where PD is encouraged through adequate reimbursement rates (91). Governments initiating dialysis programs in developing countries tend to invest in more expensive technology perceived to be more advanced (92). In countries with more private dialysis providers, a larger proportion of ESRD patients use ICHD instead of home dialysis (as has been the case in India). The trend is reversed for government policies utilizing public dialysis providers, for example in Mexico (92), where the same budget could provide RRT access to more patients through PD.
Strategic recommendations: Government policies should be developed with in-depth analysis of their background and involvement of all key stakeholders. They should also set benchmarks with detailed funding plans to facilitate patient-centered care, where patients are empowered to make modality choices. As the systematic policy change could have a dramatic impact, pilot tests should be conducted before the full implementation and continuous quality improvement should be maintained after the policy implementation.
Economic Factors
In developing countries, financial limitations constrain PD access (49,63,64,93). It was reported that the annual PD provision costs in those countries are much higher than the per capita gross national income. PD costs are higher than HD costs in some developing countries due to lower labor costs and the high price of importing PD fluids and disposables, although many of these studies did not mention the cost perspective (24,25), an important element in economic evaluation studies. Generally, costs to payers and providers may be more likely to affect practice patterns, whereas indirect costs are less influential. Indirect costs (e.g., transportation costs related to travel) are overlooked, but vital to accurate health-economic assessments from a societal perspective (94).
In the development of a national PD-First/Favored policy, it is critical to the policy's success to collect necessary economic evidence and involve all key stakeholders. The experience of Thailand in providing expensive RRT for patients under its universal health coverage scheme clearly demonstrated its importance (51). In addition, governing bodies might consider intra-country variability in economic and medical resources/infrastructure. Not doing so may result in disparate patient access. For example, in India, better access exists in wealthy urban centers while rural populations have insufficient access to dialysis care (95). Alternatively, the establishment of PD as the first dialysis modality in Thailand increased patient access to dialysis care without increasing the needs for HD infrastructure and staffing. Together with the volume-based negotiated price on PD fluid and PD-supportive program, the PD-First policy lowered costs and increased utilization.
Strategic recommendations: Country-specific cost data should be collected and analyzed using standardized cost and resource units. When possible, the economic evaluation should include information on patients' quality of life and employment status. For example, before the announcement of Thailand's PD-First policy, an economic evaluation was conducted to provide value-for-money information for policymakers to make decisions on whether to provide dialysis treatment for ESRD patients under the universal health coverage scheme (96).
Provider/Healthcare Professional Education
Peritoneal dialysis experience for nephrologists and dialysis centers is critical to a successful PD program implementation (97). Insufficient PD training for nephrology fellows has been a barrier to PD initiation and retention (92). Less experienced PD centers tend to deal with PD complications by switching the patients to HD (98,99) despite the potential benefits associated with PD, such as delaying the need for vascular access, preserving residual renal function, and bridging the gap to kidney transplantation (92).
Education played a critical role for the successful implementation of Hong Kong's PD-First policy whereby all dialysis units established pre-dialysis education programs (36). In Thailand and China, the barrier of insufficient provider education was overcome through routine provider visits to PD programs, the establishment of model-centers for PD-related care and rural satellite training centers, and institution-wide PD operation regulations (100). In Latin America, where there is a disproportionally large ESRD population, few nephrologists generally receive adequate PD training, and exposure to PD patients is limited (101). Consequently, non-nephrologists have been running PD clinics in Mexico (69,102).
Strategic recommendations: Countries looking to expand PD utilization should identify successful programs or centers of excellence and key subject experts and use these existing resources to train their fellow clinics and nephrology professionals, including training for nephrologists, nurses, and other dialysis staff. They could also form a functional hub-and-spoke service network as in Australia, where the hubs are usually tertiary centers and the spokes are satellite services (82).
Modality-Related Factors
A major challenge to PD is inadequate technique survival, which limits its initial survival benefit (8,103). The reasons for poor PD technique survival include infections, ultrafiltration failure, and volume overload (104). Thailand took vital steps to overcome modality-related factors specifically. First, patients and providers attended educational symposia addressing the relative benefits of PD vs HD. Second, anti-PD biases not scientifically grounded were identified and assuaged with continued education. Third, national registry data indicated the clinical success of PD-First. Together with registries, surveys (105) were also used to monitor patient outcomes and to identify programs not meeting established guidelines and/or those that are underperforming. Additionally, social support has been found to reduce the risk of technique failure in Hong Kong (106).
Strategic recommendations: All key stakeholders, especially patients and nephrology professionals, should attend educational programs with unbiased data on PD and HD. Anti-PD biases should be identified and appropriately addressed. Patient registry and other monitoring plans (e.g. surveys) should be established and data should be analyzed regularly. The results should be published through national or international conferences and peer-reviewed journals.
Patient Factors
Patient-specific barriers include patients' disinterest in home-based dialysis (107), patient burnout (104), a fear of peritonitis, burden on family members, lack of physicians' encouragement and lack of confidence in the quality of PD care (108). Participation in well-designed patient pre-dialysis educational programs has been shown to help patients choose their treatment modality with increased PD utilization and to improve patient outcomes (80,109–114) (e.g., Hong Kong). Education should preferably begin several months prior to dialysis initiation, although this may be difficult as patients are not regularly identified early and educational resources are insufficient.
However, patient-related barriers have been overcome in certain countries, such as Thailand and Hong Kong, through the development of clubs fostering PD advocacy and PD-related skill competency (shown to be associated with fewer dialysis initiations and lower mortality rates [115]), social activities, and rehabilitation services. Patient-centered/attended clubs and meetings may also ameliorate fears of medical complications. In addition, family support has been reported to be significantly associated with increased PD utilization among ESRD patients with barriers to self-care in Canada (116).
Strategic recommendations: National patient education guidelines and policies should be developed to address patient fears and cultural differences. Web-based and printed materials could also be valuable. Patient network opportunities should be facilitated for patients to educate each other in both medical and non-medical aspects.
Discussion and Conclusion
With the increasing economic burden associated with ESRD, several countries have realized the benefits of PD vs HD and implemented PD-First/Favored policies. To make the PD policies successful, policy makers should address 5 important elements systematically: reimbursement policy, economics, provider/healthcare professional education, modality-related factors, and patient-related factors. To facilitate patient-centered care, patient preference for dialysis modality selection should also be taken into account for patients without clinical contraindications in the design and implementation of PD-related programs. Studies have found that patient motivation was associated with improvement in dialysis practices and patient survival (117,118). In this case, comprehensive pre-dialysis education outlining options of all dialysis modalities is critical (32,119), especially for those in developed countries. In developing countries, on the other hand, the key challenge is to provide access to RRT for patients with ESRD. With limited resources and increasing demand, the provision of lower cost therapy of PD can extend patients' life while not compromising the quality of care and patient outcomes (120).
Thailand exemplifies a PD-First country that confronted each barrier successfully by having: 1) created a thorough, national, step-wise implementation plan; 2) set specific goals and milestones by which to evaluate the policy's progress; 3) established and maintained a national database of clinical, economic, and quality-of-life outcomes with which to populate comparative effectiveness analyses; 4) aligned the interests of relevant parties (i.e., patients, providers, and payers). The United States exemplifies a PD-Favored country that addressed the key non-medical barrier of reimbursement for dialysis modality selection by creating a financial incentive for increased use of lower cost dialysis therapies, mainly PD.
Meanwhile, the extent of current HD capacity also impacts the uptake of PD-favoring policies (121). One might look to the example of Canada (specifically Ontario) as an instance where the benefits associated with PD and corresponding reimbursement policy were not sufficient to overcome a pervasive HD infrastructure. Essentially, when HD capacity was there, the providers tended to maximize the use of their HD units because of the relatively low marginal cost of adding a new HD patient to an empty spot (92). Partially due to the situation, PD use was still around 18% in 2010 in Ontario despite its 2005 initiative to increase the use of PD to 30% by the end of 2010 (45,56).
Low PD utilization also appeared to correlate with wide socioeconomic variation due to partitioning by self-sufficient/governing provinces distributed over large areas geographically (e.g., Canada and India). This again reflects the importance of centralized alignment in establishing pro-PD policies and highlights how, in countries without existing national dialysis infrastructure, Thailand's model shows that pro-PD initiatives can be successfully implemented regardless of the economic or development status of the country. One should not overlook the importance a national data registry (representing vital components of comprehensive renal replacement therapy infrastructure and the associated economic factors) used to monitor outcomes and direct resources accordingly.
PD-Favored policies may be integrated into complimentary home dialysis-First policies (including both PD and home HD), as has been implemented in Australia, Finland, and New Zealand. Compared with ICHD, the benefits of home-based modalities include better control of health and treatment regimes, better patient quality of life, reduced travel for treatment, ability to work, and reduced non-dialysis related infection rates.
With regard to the aforementioned barriers and strategic recommendations, this review corresponds to previously published assessments of existing and/or potential PD and home dialysis-First policies (103,122–126). However, prior reviews have focused on a given territory, barrier(s) and solution(s) thereof. For example, as was recommended based on this review, Li et al. (103) provided a review of the PD-First policy in Hong Kong and its associated issues and suggested potential implementation strategies, including improving nephrologists' experience and expertise with PD, increasing patients' access to PD catheters, and providing social support to PD patients. Jose et al. (125) indicated that insufficient PD uptake results largely from poor patient education and that PD utilization and clinical outcomes/morbidity may improve though targeting this deficiency. Likewise, Gokal (124) noted that unbiased patient education is likely to result in increased PD uptake, but adds that PD utilization rates would benefit from providers being more thoroughly apprised of current PD-related outcomes and advances. Chaudhary et al. (123) offers similar sentiments in addition to suggesting economic solutions may be realized through the cost-effective bundling of PD services. From the perspective of this review, this is largely an issue related to reimbursement incentives and infrastructure as categorized by “Government Factors.” Burkhart (122) discussed the benefits of the US bundled payment system, along with the advantages of dialysis service consolidation, in particular, coordination and cooperation between government, provider, and patient entities, which may help avoid the biased reimbursement system. Ghaffari et al. (126) further pointed out the importance of government and healthcare system support, structured multidisciplinary pre-dialysis education programs, and training of healthcare professionals to the success of a PD-First policy model in the US dialysis setting.
Each country has a unique background in terms of its government role, health insurance coverage (who is covered and what is covered), health system finance (private vs public or mixed), economic status, clinical practice, and patients' view of quality of care (127). To support future design and implementation of PD-related programs, there are insufficient data that any of the specific PD policies has been or can be transferred to another country directly. However, the experience of these reviewed PD-First/Favored policies did show the importance of collecting data, identifying the key barriers, involving key stakeholders in all phases of the program, pilot testing, and objectively evaluating the patient outcomes following the implementation of the program.
As is frequent in literature/policy reviews, this work was limited by a restricted scope. For example, not all countries with PD-Favored policies (e.g., the United Kingdom [128]) were included. Additionally, this review was limited by the restricted availability of outcome data in countries where centralized RRT data registries do not exist. It is likely that the understanding of outcomes, policies, and implications will continue to evolve along-side the capacities for robust data registries. Finally, the PD-First/Favored policies were usually implemented in an uncontrolled manner and therefore the lessons learned were not tested with scientific rigor. As a result, it is important to do pilot tests and collect data to objectively measure the progress and timely revise the program if necessary.
Nevertheless, the details presented herein reflect opinions and findings published in the dialysis-related literature, and the barriers and recommendations appear relevant despite unique socioeconomic, demographic, and infrastructural factors. It is hoped, therefore, that governments, renal associations (including healthcare professional associations), patient groups, and other policy-making groups will learn from this assessment of country-specific dialysis-focused policies, which took into account PD's capacity to improve clinical outcomes as well as its positive impact on limited healthcare resources.
Disclosures
The funding for this study was provided by Baxter Healthcare Corporation, Deerfield, IL. FXL is an employee and stockholder of Baxter Healthcare Corporation. GI and AY were employees of Baxter Healthcare Corporation at the time of the development of this manuscript. XG is an employee of Pharmerit International, which received a consulting fee from Baxter Healthcare Corporation related to the development of this manuscript. PC is a non-paid consulting nephrologist for this manuscript from Thailand. RPF did not get paid for the development of this manuscript, but received research grants from Baxter for investigator-driven projects through the Clinical Evidence Council and also research grants from Fresenius Medical Care.
Acknowledgments
The authors gratefully acknowledge John Carter, MS, from Pharmerit International, Bethesda, MD, and Steve Guest, MD, from Baxter Healthcare Corporation, Deerfield, IL, in helping with the development of this manuscript.
REFERENCES
- 1. Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract 2011; 118(3):c269–77. [DOI] [PubMed] [Google Scholar]
- 2. Ayodele OE, Alebiosu CO. Burden of chronic kidney disease: an international perspective. Adv Chronic Kidney Dis 2010; 17(3):215–24. [DOI] [PubMed] [Google Scholar]
- 3. Khawar O, Kalantar-Zadeh K, Lo WK, Johnson D, Mehrotra R. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol 2007; 2(6):1317–28. [DOI] [PubMed] [Google Scholar]
- 4. Heaf JG, Lokkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 2002; 17(1):112–7. [DOI] [PubMed] [Google Scholar]
- 5. Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC. Comparison of hemodialysis and peritoneal dialysis survival in the Netherlands. Kidney Int 2007; 71(2):153–8. [DOI] [PubMed] [Google Scholar]
- 6. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20(1):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiu Y-W, Jiwakanon S, Lukowsky L, Duong U, Kalantar-Zadeh K, Mehrotra R. An update on the comparisons of mortality outcomes of hemodialysis and peritoneal dialysis patients. Semin Nephrol 2011; 31(2):152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies SJ. Peritoneal dialysis—current status and future challenges. Nat Rev Nephrol 2013; 9(7):399–408. [DOI] [PubMed] [Google Scholar]
- 9. Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 2012; 27(9):3568–75. [DOI] [PubMed] [Google Scholar]
- 10. Lee CC, Sun CY, Wu MS. Long-term modality-related mortality analysis in incident dialysis patients. Perit Dial Int 2009; 29(2):182–90. [PubMed] [Google Scholar]
- 11. Huang CC, Cheng KF, Wu HD. Survival analysis: comparing peritoneal dialysis and hemodialysis in Taiwan. Perit Dial Int 2008; 28(Suppl 3):S15–20. [PubMed] [Google Scholar]
- 12. Sanabria M, Munoz J, Trillos C, Hernandez D, Latorre C, Diaz CS, et al. Dialysis outcomes in Colombia (DOC) study: a comparison of patient survival on peritoneal dialysis vs hemodialysis in Colombia. Kidney Int 2008; 73(Suppl 108):S165–72. [DOI] [PubMed] [Google Scholar]
- 13. Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 2010; 21(3):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171(2):110–8. [DOI] [PubMed] [Google Scholar]
- 15. Brown EA, Johansson L, Farrington K, Gallagher H, Sensky T, Gordon F, et al. Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant 2010; 25(11):3755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubin HR, Fink NE, Plantinga LC, Sadler JH, Kliger AS, Powe NR. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA 2004; 291(6):697–703. [DOI] [PubMed] [Google Scholar]
- 17. Bohlke M, Nunes DL, Marini SS, Kitamura C, Andrade M, Von-Gysel MP. Predictors of quality of life among patients on dialysis in southern Brazil. Sao Paulo Med J 2008; 126(5):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodlad C, Brown E. The role of peritoneal dialysis in modern renal replacement therapy. Postgraduate Med J 2013; 89(1056):584–90. [DOI] [PubMed] [Google Scholar]
- 19. Juergensen E, Wuerth D, Finkelstein SH, Juergensen PH, Bekui A, Finkelstein FO. Hemodialysis and peritoneal dialysis: patients' assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin J Am Soc Nephrol 2006; 1(6):1191–6. [DOI] [PubMed] [Google Scholar]
- 20. McLaughlin K, Manns B, Mortis G, Hons R, Taub K. Why patients with ESRD do not select self-care dialysis as a treatment option. Am J Kidney Dis 2003; 41(2):380–5. [DOI] [PubMed] [Google Scholar]
- 21. Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG. Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health 2007; 10(5):390–7. [DOI] [PubMed] [Google Scholar]
- 22. Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 2012; 9(9):e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frimat L, Durand PY, Loos-Ayav C, Villar E, Panescu V, Briancon S, et al. Impact of first dialysis modality on outcome of patients contraindicated for kidney transplant. Perit Dial Int 2006; 26(2):231–9. [PubMed] [Google Scholar]
- 24. Just PM, Riella MC, Tschosik EA, Noe LL, Bhattacharyya SK, de Charro F. Economic evaluations of dialysis treatment modalities. Health Policy 2008; 86(2–3):163–80. [DOI] [PubMed] [Google Scholar]
- 25. Liu FX, Quock TP, John B, Noe L, Inglese G. Economic evaluations of peritoneal dialysis and hemodialysis: 2004–2012. F1000Research 2013; 2-273:1–10. [Google Scholar]
- 26. Klarenbach S, Manns B. Economic evaluation of dialysis therapies. Semin Nephrol 2009; 29(5):524–32. [DOI] [PubMed] [Google Scholar]
- 27. United States. Department of Health and Human Services. National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases. US Renal Data System (USRDS) USRDS 2012 Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD, USRDS; 2012. Available from: http://www.usrds.org/adr.aspx. [Google Scholar]
- 28. Ledebo I, Ronco C. The best dialysis therapy? Results from an international survey among nephrology professionals. NDT Plus 2008; 1(6):403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morton RL, Tong A, Howard K, Snelling P, Webster AC. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. Brit Med J (Clinical research ed) 2010; 340:c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, et al. Dialysis modality preference of patients with CKD and family caregivers: a discrete-choice study. Am J Kidney Dis 2012; 60(1):102–11. [DOI] [PubMed] [Google Scholar]
- 31. Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in the Netherlands. Am J Kidney Dis 2004; 43(5):891–9. [DOI] [PubMed] [Google Scholar]
- 32. Jiwakanon S, Chiu YW, Kalantar-Zadeh K, Mehrotra R. Peritoneal dialysis: an underutilized modality. Curr Opin Nephrol Hy 2010; 19(6):573–7. [DOI] [PubMed] [Google Scholar]
- 33. Koc Y, Unsal A, Basturk T, Sakaci T, Ahbap-Dal E, Sinangil-Arar A, et al. Is there impact of mortality prior hemodialysis therapy in peritoneal dialysis patients? Nefrologia 2012; 32(3):335–42. [DOI] [PubMed] [Google Scholar]
- 34. Chui BK, Manns B, Pannu N, Dong J, Wiebe N, Jindal K, et al. Health care costs of peritoneal dialysis technique failure and dialysis modality switching. Am J Kidney Dis 2013; 61(1):104–11. [DOI] [PubMed] [Google Scholar]
- 35. Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011; 80(12):1258–70. [DOI] [PubMed] [Google Scholar]
- 36. Yu AW, Chau KF, Ho YW, Li PK. Development of the “peritoneal dialysis first” model in Hong Kong. Perit Dial Int 2007; 27(Suppl 2):S53–5. [PubMed] [Google Scholar]
- 37. Sirivongs D, Kasemup V, Wangsiripaisal A, Jamma J. Facts about PD first policy in Thailand. National Health Security Office of Thailand Nonthaburi, Thailand: Sahamitrprinting and Publishing Company, Ltd.; 2011. [Google Scholar]
- 38. Oreopoulos DG, Coleman S, Doyle E. Reversing the decreasing peritoneal dialysis (PD) trend in Ontario: a government initiative to increase PD use in Ontario to 30% by 2010. Perit Dial Int 2007; 27(5):489–95. [PubMed] [Google Scholar]
- 39. Iglehart JK. Bundled Payment for ESRD—Including ESAs in Medicare's dialysis package. New Engl J Med 2011; 364(7):593–5. [DOI] [PubMed] [Google Scholar]
- 40. Golper TA, Guest S, Glickman JD, Turk J, Pulliam JP. Home dialysis in the new USA Bundled Payment Plan: implications and impact. Perit Dial Int 2011; 31(1):12–6. [DOI] [PubMed] [Google Scholar]
- 41. Li PK, Szeto CC. Success of the peritoneal dialysis programme in Hong Kong. Nephrol Dial Transplant 2008; 23(5):1475–8. [DOI] [PubMed] [Google Scholar]
- 42. Ho YW, Chau KF, Choy BY, Fung KS, Cheng YL, Kwan TH, et al. Hong Kong Renal Registry Report 2010. Hong Kong J Nephrology. 2010; 12(2):81–98. [Google Scholar]
- 43. Dhanakijcharoen P, Sirivongs D, Aruyapitipan S, Chuengsaman P, Lumpaopong A. The “PD First” policy in Thailand: three-years experiences (2008–2011). J Med Assoc Thai 2011; 94(Suppl 4):S153–61. [PubMed] [Google Scholar]
- 44. Quinn RR, Laupacis A, Hux JE, Moineddin R, Paterson M, Oliver MJ. Forecasting the need for dialysis services in Ontario, Canada to 2011. Healthc Policy 2009; 4(4):e151–61. [PMC free article] [PubMed] [Google Scholar]
- 45. Canadian Institute for Health Information Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2001 to 2010 Ottawa, Ontario: Canadian Institute for Health Information; 2010. Available from: https://secure.cihi.ca/free_products/2011_CORR_Annua_Report_EN.pdf. [Google Scholar]
- 46. Maddux FW. Impact of the bundled end-stage renal disease payment system on patient care. Blood purif 2012; 33(1–3):107–11. [DOI] [PubMed] [Google Scholar]
- 47. Hornberger J, Hirth RA. Financial implications of choice of dialysis type of the revised Medicare payment system: an economic analysis. Am J Kidney Dis 2012; 60(2):280–7. [DOI] [PubMed] [Google Scholar]
- 48. Hirth RA, Turenne MN, Wheeler JR, Nahra TA, Sleeman KK, Zhang W, et al. The initial impact of Medicare's new prospective payment system for kidney dialysis. Am J Kidney Dis 2013; 62(4):662–9. [DOI] [PubMed] [Google Scholar]
- 49. Li PK, Chow KM. The cost barrier to peritoneal dialysis in the developing world—an Asian perspective. Perit Dial Int 2001; 21(Suppl 3):S307–13. [PubMed] [Google Scholar]
- 50. Lo WK. Peritoneal dialysis in the far East—an astonishing situation in 2008. Perit Dial Int 2009; 29(Suppl 2):S227–9. [PubMed] [Google Scholar]
- 51. Tantivess S, Werayingyong P, Chuengsaman P, Teerawattananon Y. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. Brit Med J (Clinical research ed) 2013; 346. [DOI] [PubMed] [Google Scholar]
- 52. Manns BJ, Mendelssohn DC, Taub KJ. The economics of end-stage renal disease care in Canada: incentives and impact on delivery of care. Int J Health Care Finance Econ 2007; 7(2–3):149–69. [DOI] [PubMed] [Google Scholar]
- 53. Blake PG, Bargman JM, Brimble KS, Davison SN, Hirsch D, McCormick BB, et al. Clinical practice guidelines and recommendations on peritoneal dialysis adequacy. Perit Dial Int 2011; 31(2):218–39. [DOI] [PubMed] [Google Scholar]
- 54. Provincial Peritoneal Dialysis Coordinating Committee Provincial peritoneal dialysis joint initiative, strategy on increasing peritoneal dialysis (PD) use in Ontario 2006 [updated 2006; cited 2013 July 15]. Available from: http://orp.renalnetwork.on.ca/common/pages/UserFile.aspx?fileId=100547.
- 55. Ontario Renal Network Ontario Renal Network Plan 2012–2015: Ontario Renal Network; 2012 [updated 2012; cited 2012 August 29]. Available from: http://orp.renalnetwork.on.ca/.
- 56. Blake PG. Peritoneal dialysis in North America: 2013 update. [PowerPoint Presentation] In press 2013. [Google Scholar]
- 57. Thaizhou Human Resources and Social Scurity Bureau Notice on strengthening the management of medical insurance peritoneal dialysis Thaizhou City, China 2012 [updated 2012; cited 2012 September 21]. Available from: http://www.jstz.lss.gov.cn.
- 58. Kidney Care Network The peritoneal dialysate home delivery policy recently introduced in Wuxi, China 2012 [updated 2012; cited 2012 September 12]. Available from: www.shentouxi.com.cn.
- 59. ChinaBio Today Baxter Teams with China Officials to Bring Dialysis to Rural China. China Bio Today [Internet] 2013. September 7 Available from: http://www.chinabiotoday.com/articles/20130403. [Google Scholar]
- 60. The Central People's Government of the People's Rebulic of China Peritoneal dialysis policy in China – the Office of the Ministry of Health 2012 [updated 2012; cited 2012 March 26]. Available from: http://www.gov.cn/gzdt/2011-06/13/content_1882823.htm.
- 61. Guatemala Public Health and Social Security Unidad Nacional de Atención al Enfermo Renal Crónico 2012 [updated 2012; cited 2012 May 14]. Available from: http://unaerc.gob.gt/.
- 62. Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012; 23(3):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abraham G, Pratap B, Sankarasubbaiyan S, Govindan P, Nayak KS, Sheriff R, et al. Chronic peritoneal dialysis in South Asia—challenges and future. Perit Dial Int 2008; 28(1):13–9. [PubMed] [Google Scholar]
- 64. Agarwal SK, Srivastava RK. Chronic kidney disease in India: challenges and solutions. Nephron Clin Pract 2009; 111(3):c197–203. [DOI] [PubMed] [Google Scholar]
- 65. Jeloka TK, Upase S, Chitikeshi S. Monthly cost of three exchanges a day peritoneal dialysis is same as of thrice a week hemodialysis in self-paying Indian patients. Indian J Nephrol 2012; 22(1):39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reddy YNV, Abraham G, Mathew M, Ravichandran R, Reddy YNV. An Indian model for cost-effective CAPD with minimal man power and economic resources. Nephrol Dial Transplant 2011. October; 26(10):3089–91. [DOI] [PubMed] [Google Scholar]
- 67. Rajendran S. Good news for kidney patients, pilot project on peritoneal dialysis in Bangalore: The Hindu; 2013. [cited 2013 December 30] Available from: http://www.thehindu.com/news/national/karnataka/good-news-for-kidney-patients-pilot-project-on-peritoneal-dialysis-in-bangalore/article5258226.ece.
- 68. Jha V. Current status of end-stage renal disease care in South Asia. Ethn Dis 2009; 19(Suppl 1):S1–27–32. [PubMed] [Google Scholar]
- 69. Cueto-Manzano AM, Rojas-Campos E. Status of renal replacement therapy and peritoneal dialysis in Mexico. Perit Dial Int 2007; 27(2):142–8. [PubMed] [Google Scholar]
- 70. National Institute of Statistics and Geography National Information by State and Municipalities 2013 [cited 2013 July 16] Available from: http://www3.inegi.org.mx/sistemas/mexicocifras.
- 71. Paniagua R, Ramos A, Fabian R, Lagunas J, Amato D. Chronic kidney disease and dialysis in Mexico. Perit Dial Int 2007; 27(4):405–9. [PubMed] [Google Scholar]
- 72. Trevino-Becerra A. The mexican peritoneal dialysis model: a personal reflection. Artif Organs 2007; 31(4):249–52. [DOI] [PubMed] [Google Scholar]
- 73. Registro Español de Enfermos Renales Dialysis and transplant report in Spain, 2006. Nefrologia 2009; 29(6):525–33. [DOI] [PubMed] [Google Scholar]
- 74. Arrieta J, Bajo MA, Caravaca F, Coronel F, Garcia-Perez H, Gonzalez-Parra E, et al. Guidelines of the Spanish Society of Nephrology. Clinical practice guidelines for peritoneal dialysis. Nefrologia 2006; 26(Suppl 4):1–184. [PubMed] [Google Scholar]
- 75. Quiros-Ganga PL, Remon-Rodriguez C. Achieving better results for peritoneal dialysis in recent years. Nefrologia 2012; 32(5):587–96. [DOI] [PubMed] [Google Scholar]
- 76. Villa G, Rodriguez-Carmona A, Fernandez-Ortiz L, Cuervo J, Rebollo P, Otero A, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant 2011; 26(11):3709–14. [DOI] [PubMed] [Google Scholar]
- 77. Chen TW, Li SY, Chen JY, Yang WC. Training of peritoneal dialysis patients—Taiwan's experiences. Perit Dial Int 2008; 28(Suppl 3):S72–5. [PubMed] [Google Scholar]
- 78. Kao TW, Chang YY, Chen PC, Hsu CC, Chang YK, Chang YH, et al. Lifetime costs for peritoneal dialysis and hemodialysis in patients in Taiwan. Perit Dial Int 2013; 33(6):671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Peng YS, Chiang CK, Hung KY, Chang CH, Lin CY, Yang CS, et al. Comparison of self-reported health-related quality of life between Taiwan hemodialysis and peritoneal dialysis patients: a multi-center collaborative study. Qual Life Res 2011; 20(3):399–405. [DOI] [PubMed] [Google Scholar]
- 80. Wu IW, Wang SY, Hsu KH, Lee CC, Sun CY, Tsai CJ, et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality—a controlled cohort study based on the NKF/DOQI guidelines. Nephrol Dial Transplant 2009; 24(11):3426–33. [DOI] [PubMed] [Google Scholar]
- 81. Queensland Government Queensland Statewide Renal Health Services Plan, 2008–17, Part 2 2008 [updated 2008; cited 2003 July 16]. Available from: http://pandora.nla.gov.au/pan/108521/20091015-1137/www.health.qld.gov.au/publications/qh_plans/QS_renal_plan_part2.pdf.
- 82. Queensland Government Queensland Statewide Renal Health Services Plan, 2008–17, Part 1 Queensland, Australia 2008 [updated 2008; cited 2013 July 16]. Available from: http://pandora.nla.gov.au/pan/108521/20091015-1137/www.health.qld.gov.au/publications/qh_plans/QS_renal_plan_part1.pdf.
- 83. Australia and New Zealand Dialysis and Transplant Registry ANZDATA Registry 2010 Report: Australia and New Zealand Dialysis and Transplant Registry; 2012 [updated 2012; cited 2013 July 16]. Available from: http://www.anzdata.org.au/v1/report_2010.html.
- 84. Governments CoA National Healthcare Agreement Australia: Council of Australian Governments; 2011 [updated August 2011; cited 2012 September 1]. Available from: http://www.federalfinancialrelations.gov.au/content/npa/healthcare/national-agreement-superseded-Jul12.pdf.
- 85. NSW Department of Health NSW Renal Dialysis Service Plan to 2011 Sydney, Australia 2007 [updated 2007; cited 2013 July 16]. Available from: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155059/nswrenalplan_jan2007_final.pdf.
- 86. Honkanen EO, Rauta VM. What happened in Finland to increase home hemodialysis? Hemodial Int 2008; 12(Suppl 1):S11–5. [DOI] [PubMed] [Google Scholar]
- 87. Helantera I, Haapio M, Koskinen P, Gronhagen-Riska C, Finne P. Employment of patients receiving maintenance dialysis and after kidney transplant: a cross-sectional study from Finland. Am J Kidney Dis 2012; 59(5):700–6. [DOI] [PubMed] [Google Scholar]
- 88. Ashton T, Marshall MR. The organization and financing of dialysis and kidney transplantation services in New Zealand. Int J Health Care Finance Econ 2007; 7(4):233–52. [DOI] [PubMed] [Google Scholar]
- 89. Vanholder R, Davenport A, Hannedouche T, Kooman J, Kribben A, Lameire N, et al. Reimbursement of dialysis: a comparison of seven countries. J Am Soc Nephrol 2012; 23(8):1291–8. [DOI] [PubMed] [Google Scholar]
- 90. Laplante S, Vanovertveld P. A renal policy and financing framework to understand which factors favour home treatments such as peritoneal dialysis. In: Krediet RT, ed. Progress in Peritoneal Dialysis. Rijeka: Intech; 2011:115–32. [Google Scholar]
- 91. Just PM, de Charro FT, Tschosik EA, Noe LL, Bhattacharyya SK, Riella MC. Reimbursement and economic factors influencing dialysis modality choice around the world. Nephrol Dial Transplant 2008; 23(7):2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lameire N, Van BW. Epidemiology of peritoneal dialysis: a story of believers and nonbelievers. Nat Rev Nephrol 2010; 6(2):75–82. [DOI] [PubMed] [Google Scholar]
- 93. Correa-Rotter R. The cost barrier to renal replacement therapy and peritoneal dialysis in the developing world. Perit Dial Int 2001; 21(Suppl 3):S314–7. [PubMed] [Google Scholar]
- 94. Lamas Barreiro JM, Alonso SM, Saavedra Alonso JA, Gandara MA. Costs and added value of haemodialysis and peritoneal dialysis outsourcing agreements. Nefrologia 2011; 31(6):656–63. [DOI] [PubMed] [Google Scholar]
- 95. Jha V. Peritoneal dialysis in India: current status and challenges. Perit Dial Int 2008; 28(Suppl 3):S36–41. [PubMed] [Google Scholar]
- 96. Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health 2007; 10(1):61–72. [DOI] [PubMed] [Google Scholar]
- 97. Fang W, Qian J, Lin A, Rowaie F, Ni Z, Yao Q, et al. Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a Chinese centre. Nephrol Dial Transplant 2008; 23(12):4021–8. [DOI] [PubMed] [Google Scholar]
- 98. Plantinga LC, Fink NE, Finkelstein FO, Powe NR, Jaar BG. Association of peritoneal dialysis clinic size with clinical outcomes. Perit Dial Int 2009; 29(3):285–91. [PMC free article] [PubMed] [Google Scholar]
- 99. Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 2009; 29(3):292–6. [PubMed] [Google Scholar]
- 100. Thaiyuenwong J, Mahatanan N, Jiravaranun S, Boonyakarn A, Rodpai S, Eiam-Ong S, et al. Nationwide peritoneal dialysis nurse training in Thailand: 3-year experience. J Med Assoc Thai 2011; 94(Suppl 4):S162–6. [PubMed] [Google Scholar]
- 101. Pecoits-Filho R, Campos C, Cerdas-Calderon M, Fortes P, Jarpa C, Just P, et al. Policies and health care financing issues for dialysis in Latin America: extracts from the roundtable discussion on the economics of dialysis and chronic kidney disease. Perit Dial Int 2009; 29(Suppl 2):S222–6. [PubMed] [Google Scholar]
- 102. Pecoits-Filho R, Abensur H, Cueto-Manzano AM, Dominguez J, Divino Filho JC, Fernandez-Cean J, et al. Overview of peritoneal dialysis in Latin America. Perit Dial Int 2007; 27(3):316–21. [PubMed] [Google Scholar]
- 103. Li PK, Chow KM. Peritoneal dialysis-first policy made successful: perspectives and actions. Am J Kidney Dis 2013; 62(5):993–1005. [DOI] [PubMed] [Google Scholar]
- 104. Chaudhary K. Peritoneal dialysis drop-out: causes and prevention strategies. Int J Nephrol 2011; 2011:434608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kanjanabuch T, Chancharoenthana W, Katavetin P, Sritippayawan S, Praditpornsilpa K, Ariyapitipan S, et al. The incidence of peritoneal dialysis-related infection in Thailand: a nationwide survey. J Med Assoc Thai 2011; 94(Suppl 4):S7–12. [PubMed] [Google Scholar]
- 106. Szeto CC, Chow KM, Kwan BC, Law MC, Chung KY, Leung CB, et al. The impact of social support on the survival of Chinese peritoneal dialysis patients. Perit Dial Int 2008; 28(3):252–8. [PubMed] [Google Scholar]
- 107. Zhang AH, Bargman JM, Lok CE, Porter E, Mendez M, Oreopoulos DG, et al. Dialysis modality choices among chronic kidney disease patients: identifying the gaps to support patients on home-based therapies. Int Urol Nephrol 2010; 42(3):759–64. [DOI] [PubMed] [Google Scholar]
- 108. Tungsanga K, Kanjanabuch T, Mahatanan N, Praditpornsilp K, Avihingsanon Y, Eiam-Ong S. The status of, and obstacles to, continuous ambulatory peritoneal dialysis in Thailand. Perit Dial Int 2008; 28(Suppl 3):S53–S8. [PubMed] [Google Scholar]
- 109. Marron B, Ortiz A, de Sequera P, Martin-Reyes G, de Arriba G, Lamas JM, et al. Impact of end-stage renal disease care in planned dialysis start and type of renal replacement therapy—a Spanish multicentre experience. Nephrol Dial Transplant 2006; 21(Suppl 2):ii51–5. [DOI] [PubMed] [Google Scholar]
- 110. Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 2005; 68(1):378–90. [DOI] [PubMed] [Google Scholar]
- 111. Wuerth DB, Finkelstein SH, Schwetz O, Carey H, Kliger AS, Finkelstein FO. Patients' descriptions of specific factors leading to modality selection of chronic peritoneal dialysis or hemodialysis. Perit Dial Int 2002; 22(2):184–90. [PubMed] [Google Scholar]
- 112. Klang B, Bjorvell H, Clyne N. Predialysis education helps patients choose dialysis modality and increases disease-specific knowledge. J Adv Nurs 1999; 29(4):869–76. [DOI] [PubMed] [Google Scholar]
- 113. Manns BJ, Taub K, Vanderstraeten C, Jones H, Mills C, Visser M, et al. The impact of education on chronic kidney disease patients' plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005; 68(4):1777–83. [DOI] [PubMed] [Google Scholar]
- 114. Walker RC, Marshall MR. Increasing the uptake of peritoneal dialysis in New Zealand: a national survey. J Renal Care 2013; 40(1):40–8. [DOI] [PubMed] [Google Scholar]
- 115. World Health Organization The world health report: health systems financing: the path to universal coverage. Geneva, Switzerland: WHO Press; 2010. Available from: http://www.who.int/whr/2010/en/index.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Oliver MJ, Garg AX, Blake PG, Johnson JF, Verrelli M, Zacharias JM, et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant 2010; 25(8):2737–44. [DOI] [PubMed] [Google Scholar]
- 117. Schatell D, Alt PS. How understanding motivation can improve dialysis practices. Nephrol News Issues 2008; 22(10):32–3, 36. [PubMed] [Google Scholar]
- 118. Stack AG, Martin DR. Association of patient autonomy with increased transplantation and survival among new dialysis patients in the United States. Am J Kidney Dis 2005; 45(4):730–42. [DOI] [PubMed] [Google Scholar]
- 119. Ludlow MJ, Lauder LA, Mathew TH, Hawley CM, Fortnum D. Australian consumer perspectives on dialysis: first national census. Nephrology 2012; 17(8):703–9. [DOI] [PubMed] [Google Scholar]
- 120. Van Biesen W, Vanholder RC, Veys N, Dhondt A, Lameire NH. An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol 2000; 11(1):116–25. [DOI] [PubMed] [Google Scholar]
- 121. Komenda P, Sood MM. The economics of home dialysis: acting for the individual while planning responsibly for the population. Adv Chronic Kidney Dis 2009; 16(3):198–204. [DOI] [PubMed] [Google Scholar]
- 122. Burkart J. The future of peritoneal dialysis in the United States: optimizing its use. Clin J Am Soc Nephrol 2009; 4(Suppl 1):S125–31. [DOI] [PubMed] [Google Scholar]
- 123. Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol 2011; 6(2):447–56. [DOI] [PubMed] [Google Scholar]
- 124. Gokal R. Peritoneal dialysis in the 21st century: an analysis of current problems and future developments. J Am Soc Nephrol 2002; 13(Suppl 1):S104–16. [PubMed] [Google Scholar]
- 125. Jose MD, Johnson DW, Mudge DW, Tranaeus A, Voss D, Walker R, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology (Carlton) 2011; 16(1):19–29. [DOI] [PubMed] [Google Scholar]
- 126. Ghaffari A, Kalantar-Zadeh K, Lee J, Maddux F, Moran J, Nissenson A. PD First: peritoneal dialysis as the default transition to dialysis therapy. Semin Dialysis 2013; 26(6):706–13. [DOI] [PubMed] [Google Scholar]
- 127. The Commonwealth Fund International Profiles of Health Care Systems, 2012. Available from: http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2012/Nov/1645_Squires_intl_profiles_hlt_care_systems_2012.pdf.
- 128. National Institute for Health and Clinical Excellence NICE Clinical Guideline 125: Peritoneal dialysis in the treatment of stage 5 chronic kidney disease. In: National Health Services , ed. UK: 2011. Available from: http://guidance.nice.org.uk/CG125/NICEGuidance/pdf/English Accessed on 10 March 2014. [PubMed] [Google Scholar]