Abstract
♦ Objectives:
We aimed to prospectively compare the incidence of catheter-related complications and catheter survival for straight (SCs) and coiled (CCs) Tenckhoff catheters in peritoneal dialysis (PD) patients.
♦ Methods:
This open prospective randomized trial recruited 189 PD patients with end-stage renal disease from the department of nephrology, The First Affiliated Hospital of Sun Yat-sen University from 6 November 2007 to 27 August 2008. The patients were randomized to a SC (n = 99) or a CC (n = 90) and were then followed for 2 years. All catheter placements were performed by two designated experienced nephrologists who used a standardized institutional placement protocol. The primary study outcomes were catheter-related complications and catheter survival at 1 and 2 years.
♦ Results:
We observed no significant differences in clinical and demographic characteristics between the groups at baseline. The overall incidence of catheter dysfunction was higher in the CC group than in the SC group (17.8% vs 7.1%, p = 0.03), and most of the events occurred 4 weeks or more after the catheters were implanted. Catheter tip migration and omental wrapping were the most common causes of catheter dysfunction. Surgical catheter rescue was more common in patients with CCs than in patients with SCs (9 vs 3 patients respectively, p = 0.05). No significant differences were observed in other catheter-related complications, including dialysate leaks, hernias, and PD-related infections (peritonitis, exit-site, and tunnel infections). Catheter survival rates in the SC and CC groups were similar at 1 year (96.7% ± 1.9% vs 96.5% ± 2.0%, p = 0.98) and at 2 years (95.3% ± 2.3% vs 92.4% ± 3.6%, p = 0.76).
♦ Conclusions:
The incidence of PD catheter–related complications is probably higher with CCs than with SCs. The results of our study suggest that a SC is the better option to reduce subsequent catheter complications.
Keywords: Tenckhoff catheter, coiled catheters, straight catheters, catheter-related complications
Peritoneal dialysis (PD) is an effective, safe, and convenient modality of renal replacement therapy for the patients with end-stage renal disease (ESRD). As a result, PD is considered the first-choice modality in many places worldwide. A functional and reliable peritoneal catheter is an important factor in ensuring successful and long-term PD therapy (1), and efforts to develop better PD catheters have therefore aimed to improve rates of infection or mechanical failure. Currently, there are many variations on the Tenckhoff catheter, with the structure of the intra-abdominal segment (straight vs coiled) being a design focus.
A coiled intra-abdominal segment is generally believed to reduce inflow pain and catheter migration; however, the results of previous prospective randomized studies comparing straight (SCs) and coiled catheters (CCs) have been controversial. Akyol et al. (2) and Johnson et al. (3) found no significant differences in migration between SCs and CCs, but Nielsen et al. (4) reported that, in percutaneous implantation, migration tended to occur less often with CCs than with SCs. In addition, Lo et al. (5) and Stegmayr et al. (6) reported no advantage of CCs over SCs in catheter function. Interestingly, a recent meta-analysis of swan-neck catheter use (7) indicated that CCs may be more prone to migration and mechanical dysfunction. To summarize, the results of earlier studies comparing the incidences of catheter-related complications and survival in PD patients receiving SCs and CCs have been controversial, and sample sizes have been small. As a result, the International Society for Peritoneal Dialysis (ISPD) does not provide definitive guidelines for choice of catheter (8,9).
The purpose of the present study was to evaluate the incidence of catheter-related complications and rates of catheter survival for groups receiving SCs and CCs in a large cohort of PD patients.
Methods
Patient Allocation
All ESRD patients 18 years of age or older who underwent a first PD catheter placement at The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, were eligible for enrollment in the study. Exclusion criteria were any of acute renal failure; referral for renal transplantation evaluation within 3 months; acute myocardial infarction within 3 months; acute heart failure, acute respiratory failure, or acute respiratory distress syndrome at the time of enrollment; malignant disease; and psychiatric disease.
The study protocol was reviewed and approved by the Ethics Committee of The First Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from all patients at the time of enrollment. The study enrolled 189 consecutive patients who received their first PD treatment at the Peritoneal Dialysis Center of The First Affiliated Hospital between November 6, 2007, and August 27, 2008.
Of the 189 patients, 99 were randomized to the SC group, whose members all received a 41-cm double-cuffed SC catheter with a 15.5-cm intraperitoneal segment with a straight tip (Covidien, Mansfield, MA, USA). The other 90 patients were randomized to the CC group, whose members all received a 62-cm double-cuffed catheter with a 17-cm intraperitoneal segment with a coiled tip (Covidien).
Catheter Implantation
All PD catheters were placed in an operating room using the standard institutional placement protocol. All placements were performed by one of two designated experienced nephrologists, each of whom had performed more than 100 PD catheter placements and who had worked as full-time PD nephrologists for more than 5 years.
The open surgical procedure was performed under local anesthesia with lidocaine. A 4-cm to 6-cm paramedian incision 2 – 4 cm below the umbilical level and 2 cm to the right of the linea alba was performed through the subcutaneous tissue and anterior rectus fascia. The rectus muscle fibers were separated bluntly, and the posterior rectus fascia and peritoneum were opened with a 5-mm incision. The intraperitoneal segment of the Tenckhoff catheter was positioned toward the pouch of Douglas using a wire introducer, and catheter function was tested by instilling and draining 0.5 – 1.0 L of a 0.9% saline solution. Any sign of a fluid leak was corrected. Proper catheter position was confirmed after streamlined drainage was observed. The inner cuff was fixed to the peritoneal membrane using a purse-string suture. The cuff was then invaginated by the posterior sheath of rectus muscles and fixed with a second purse-string suture. The anterior sheath of the rectus muscles was closed using continuous sutures, and the catheter was tunneled through the subcutaneous tissue toward the cutaneous exit site. The outer cuff was placed in the subcutaneous tissue 2 cm from the exit, with the external part of the catheter directed downward and laterally.
A prophylactic 2nd or 3rd-generation cephalosporin was administered intravenously 1 hour before the catheter placement procedure. Patients underwent PD therapy immediately after the successful catheter placement and transited to continuous ambulatory PD 7 days later. All patients used the twin-bag disconnect system and 1.5%, 2.5%, or 4.25% dextrose PD solution (Dianeal: Baxter, Shanghai, China) at a dose of 6 – 8 L daily.
Primary and Secondary Outcomes
The primary outcomes were catheter-related complications and 1-year and 2-year catheter survival rates. The secondary outcomes were death, transfer to hemodialysis, kidney transplantation, refusal of PD therapy, or recovery of renal function. Catheter-related complications included early complications (those occurring ≤4 weeks after catheter insertion) and late complication (those occurring >4 weeks after catheter insertion). All patients were followed every 3 months for 2 years. All catheter dysfunction—including catheter migration, dialysate leak, hernia, hemoperitoneum, pleural leak, scrotal edema, prolapse of the uterus, peritonitis, exit-site infection, and tunnel infection—was recorded.
Peritonitis was diagnosed when two of the following conditions were present: abdominal pain; cloudy effluent with an effluent white cell count of more than 100/μL (≥50% polymorphonuclear neutrophils); or a positive effluent culture (8). Evaluation of the exit site was performed strictly following the ISPD exit-site scoring system. Exit-site infection was defined as erythema with or without skin induration and purulent discharge from the exit site (8). Tunnel infection was defined by erythema, edema, or tenderness over the subcutaneous pathway (8). Catheter dysfunction was defined needing as more than 20 minutes to instill 1.5 L dialysate into the abdominal cavity or more than 45 minutes to drain 1.5 L of effluent. When catheter dysfunction occurred, radiography was used to identify the position of the intraperitoneal portion of the catheter. If catheter function did not recover after removal of fibrin or blood clots, or after the application of other nonoperative conservative measures, patients were brought to the operating room for an assessment of catheter position and surgical correction if indicated.
Statistical Analysis
The cohort size was determined by statistical power analysis. Assuming a 15% attrition rate and a 1.80 population standard deviation, a sample size of 90 patients per group was required to obtain a 0.05 significance level with a power of 0.80.
Normally distributed data are presented as mean ± standard deviation, and the Student t-test was used to assess the significance of differences. Non-normally distributed data are presented as medians with interquartile range (IQR), and the Wilcoxon rank sum test was used to assess the significance of differences. For categorical data, the Pearson chi-square test was used to assess the significance of differences between groups. Catheter survival was compared using life-table analyses and Kaplan–Meier analyses with a log-rank test. A p value of less than 0.05 was considered significant. All analyses were performed using the SPSS software application (version 16.0: SPSS, Chicago, IL, USA).
Results
Patient Demographics
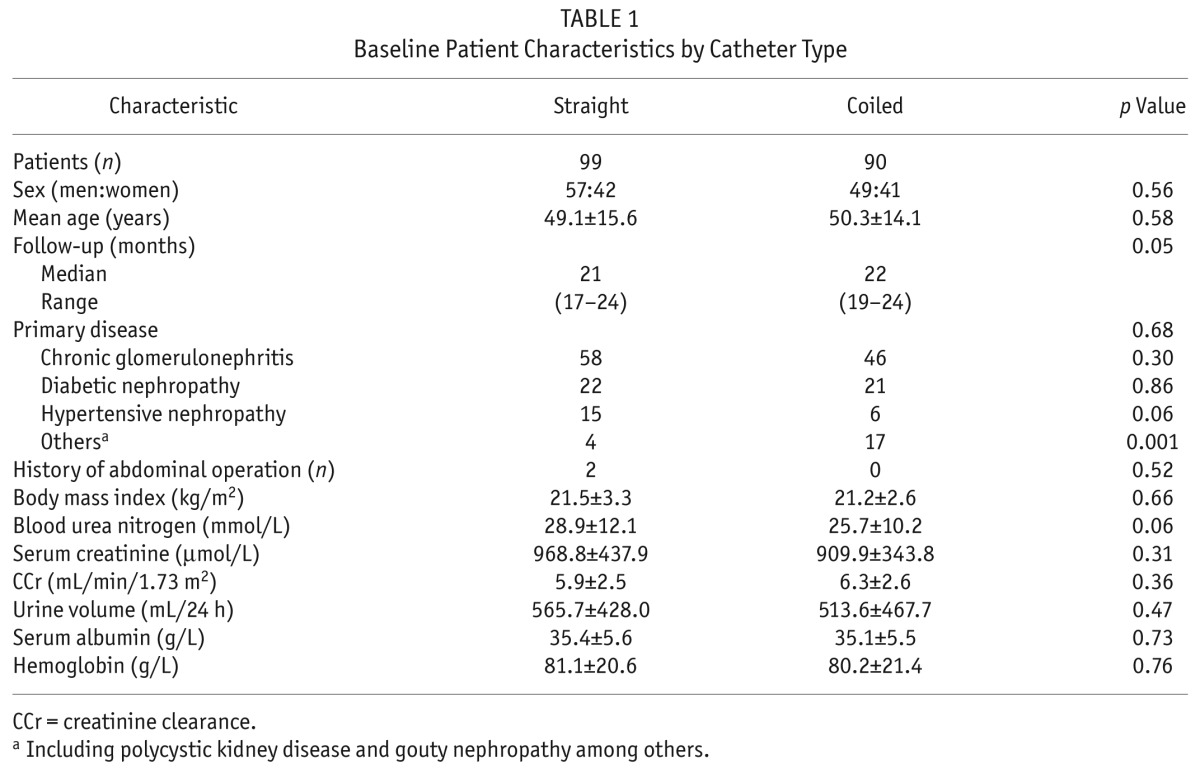
Table 1 shows the demographic and clinical characteristics of the 189 enrolled patients by catheter type. There were no significant differences between the SC and CC groups in terms of sex, age, diabetes mellitus and other primary diseases, body mass index, blood urine nitrogen, serum creatinine, endogenous creatinine clearance, 24-hour urine output, serum albumin, or hemoglobin. Two patients in the SC group, but none in the CC group, had previous abdominal surgeries. The cumulative period of catheter use was 1636 patient–months in the SC group and 1657 patient–months in the CC group. Mean observation time was 21 months (IQR: 17 – 24 months) in the SC group and 22 months (IQR: 19 – 24) in the CC group. None of the differences was statistically significant.
TABLE 1.
Baseline Patient Characteristics by Catheter Type
Catheter Dysfunction
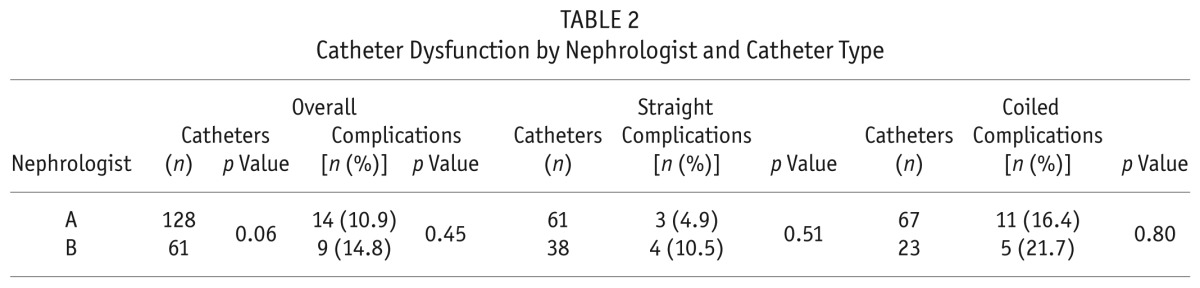
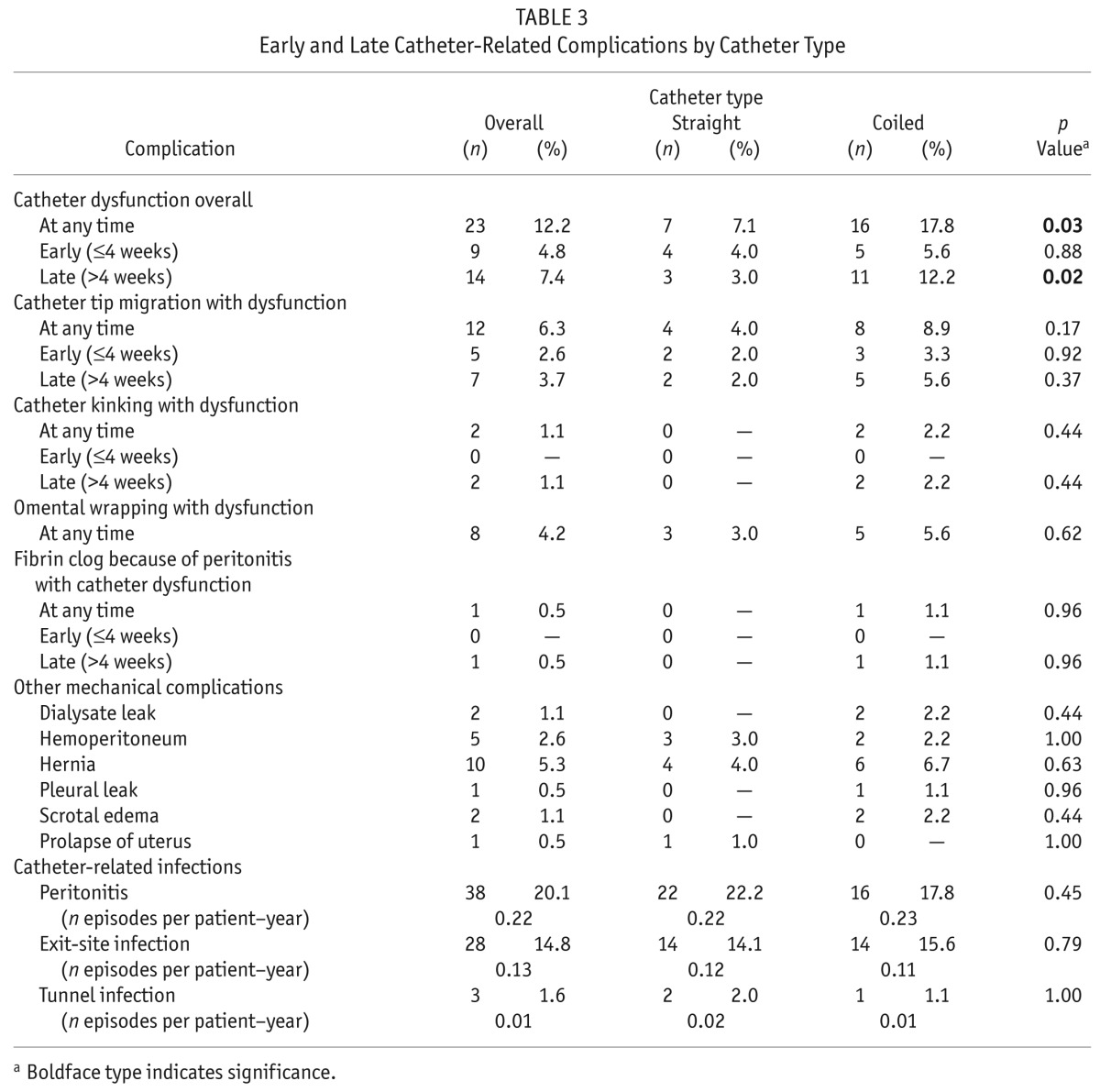
Catheter dysfunction was the most common noninfectious complication, occurring in 23 of the 189 patients (12.2%). Catheter dysfunction was significantly more common in the CC group than in the SC group [16 of 90 patients (17.8%) vs 7 of 99 patients (7.1%), p = 0.03]. The SC and CC groups both had similar incidences of early catheter dysfunction: 4 patients in the SC group (4%) and 5 patients in the CC group (5.6%, p = 0.88). However, the CC group had a significantly higher incidence of late catheter dysfunction (>4 weeks): 11 patients (12.2%) compared with 3 patients in the SC group (3%, p = 0.02). Catheter tip migration and omental wrapping were the most common causes of catheter dysfunction. The slightly higher incidence of combined early and late catheter dysfunction in the CC group was a result of catheter tip migration, kinking, omental wrapping, and fibrin-induced obstruction during episodes of peritonitis, but the difference between the groups was nonsignificant. Catheter dysfunction that required surgical rescue was more frequent in the CC group: 9 of 90 patients (10%) compared with 3 of 99 patients in the SC group (3.0%, p = 0.05). As Table 2 shows, the incidence of catheter dysfunction was not significantly different between the catheters inserted by the two nephrologists (p > 0.05).
TABLE 2.
Catheter Dysfunction by Nephrologist and Catheter Type
Other Catheter-Related Mechanical Complications
Other mechanical complications were not significantly different between the two groups (Table 3), including dialysate leaks (0% SC vs 2.2% CC, p = 0.44), hemoperitoneum (3.0% SC vs 2.2% CC, p = 1.0), hernia incidence (4.0% SC vs 6.7% CC,p = 0.63), pleural leaks (0% SC vs 1.1% CC, p = 0.96), scrotal edema (0% SC vs 2.2% CC, p = 0.44), or prolapse of the uterus (1.0% SC vs 0% CC, p = 1.0). No patient experienced extrusion of the subcutaneous cuff.
TABLE 3.
Early and Late Catheter-Related Complications by Catheter Type
Catheter-Related Infectious Complications
In our cohort, the most common infectious complications were peritonitis [38 of 189 patients (20.1%)], with an overall incidence of 0.22 episodes per patient–year, and exit-site infection [28 of 189 patients (14.8%)], with an overall incidence of 0.13 episodes per patient–year. The incidence of peritonitis was not significantly different between the SC and CC groups; however, peritonitis occurred earlier in the SC group than in the CC group (6.5 ± 6.4 months vs 11.6 ± 7.5 months, p = 0.007). Furthermore, we observed no significant differences between the SC and CC groups in other complications overall [dialysate leaks: n = 2 (1.1%); hemoperitoneum: n = 5 (2.6%); hernia: n = 10 (5.3%); pleural leakas: n = 1 (0.5%); scrotal edema: n = 2 (1.1%); prolapse of the uterus: n = 1 (0.5%); tunnel infection: n = 3 (1.6%)].
Catheter Outcome and Catheter Survival
Catheters were removed in 9 patients (4.8%) because of peritonitis (n = 6, 3.2%), leaks (n = 2, 1.1%), and catheter tip migration (n = 1, 0.5%). The reasons for catheter removal were not significantly different between the SC and CC groups. A comparison of catheter survival rates in the two groups using Kaplan–Meier analysis indicated no significant differences in 1-year (SC: 96.7% ± 1.9%; CC: 96.5% ± 2.0%; p = 0.98) or 2–year catheter survival (SC: 95.3% ± 2.3%; CC: 92.4 ± 3.6%; p = 0.76; Figure 1).
Figure 1 —
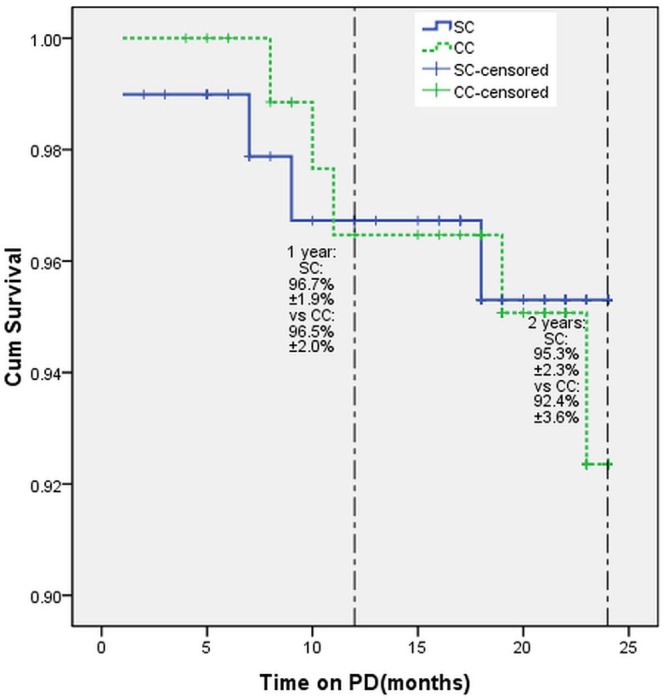
Cumulative catheter survival of straight (SC) and coiled (CC) peritoneal catheters. PD = peritoneal dialysis.
Patient Survival and Technique Survival
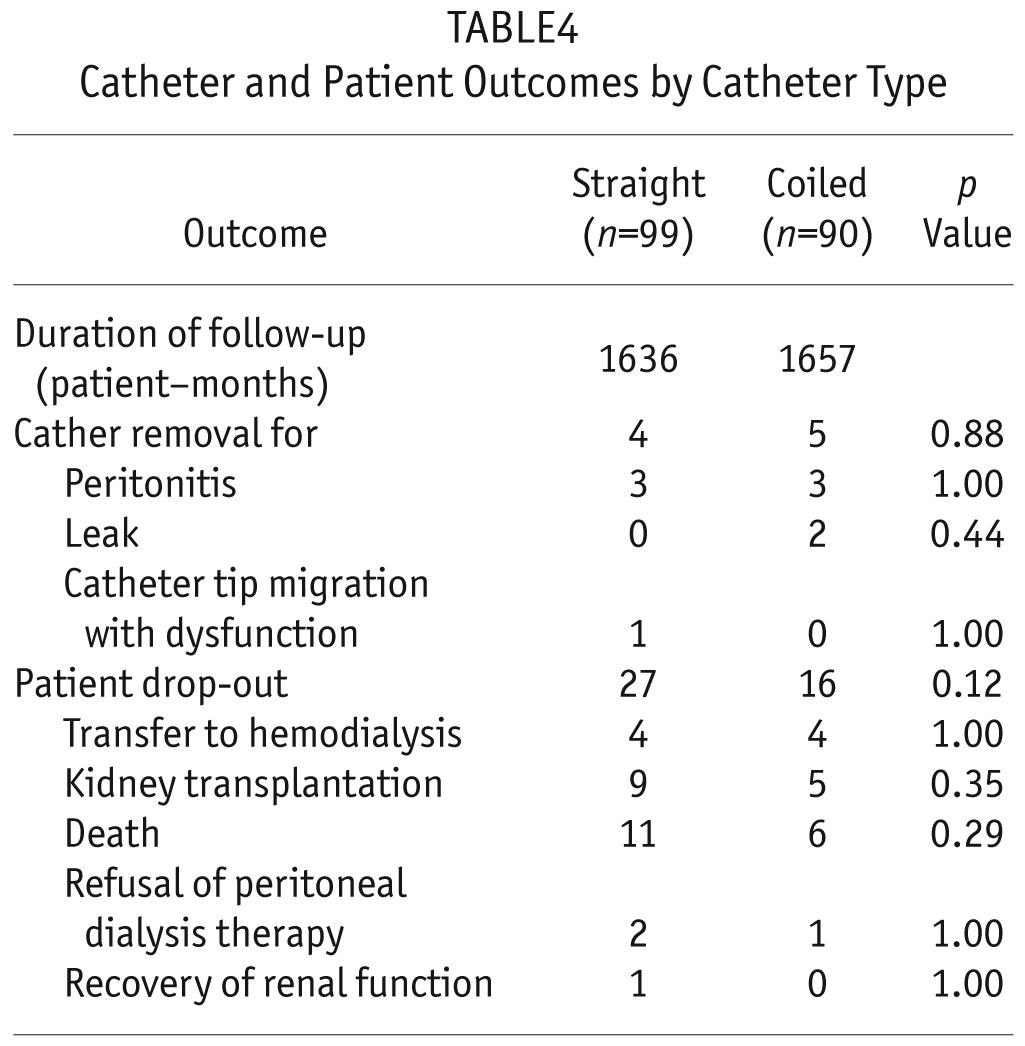
During the 2-year follow-up, 43 patients (22.8%) did not complete the study (Table 4). The overall drop-out rate was not significantly different in the two groups: 27 patients in the SC group (27.3%) and 16 patients in the CC group (17.8%). The causes of drop-out were also not different: 8 because of transfer to hemodialysis, 14 because of renal transplantation, 17 because of death, 3 because of refusal of PD therapy, and 1 because of recovery of renal function.
TABLE4.
Catheter and Patient Outcomes by Catheter Type
Discussion
Previous studies on catheter-related complications with straight or coiled Tenckhoff catheters are contradictory and limited by small sample sizes. The uniqueness of the present study is its prospective and randomized nature. To our knowledge, our study also assembled the largest cohort to date for an assessment of PD catheter–related complications.
In previous radiographic and laparoscopic studies, the main causes of catheter migration were omental wrapping, fibrous adhesions, and a kinked catheter (10). In our study, catheter tip migration and omental wrapping were also the main reasons for catheter dysfunction, which was confirmed by both radiography and surgical findings.
The omentum is a potential site for encapsulating inflammation and pus formation. Stegmayr et al. (6) proposed that a peritoneal catheter could initiate a nonspecific inflammatory reaction in the pelvic cavity, resulting in the catheter being wrapped by the omentum. Stegmayr and colleagues also noted that the surface area of a CC was greater than that of a SC and that the intraperitoneal segments were shorter in CCs than in SCs so that the catheter could be placed only in the superficial layer of the pelvic cavity (6). Those two characteristics mean that a CC more readily contacts the omentum and intestine, thereby reducing the probability of migration. In our study, the distance between the inner cuff and the catheter tip was 15.5 cm for SCs and 17 cm for CCs. The length of the CC was therefore sufficient to reach the pelvic cavity. Our results suggest that the length and surface of the CC are not likely to be the cause of a higher incidence of catheter migration as previously observed by Stegmayr and colleagues.
Tissue in the abdominal cavity—including the omentum and intestines—can potentially come into contact with the catheter and lead to increasing outflow resistance. That phenomenon, together with the accompanying greater outflow resistance because of a longer intraperitoneal segment, is a potential reason for the high incidence of omental wrapping or catheter obstruction seen with CCs.
Compared with implantation of a SC, implantation of a CC is, in general, more technically challenging. We believe that technical variations in the surgery might be one of the reasons for the higher incidence of CC migration. When the wire introducer is extracted from a CC, the coiled portion recovers its shape because of retraction. If the wire introducer is extracted too rapidly, the vertical depth from the catheter tip to the Douglas fold might be more superficial than the operator intends. At our center, we used the following standard procedure to overcome that problem: When the tip of the CC approached the vesicouterine or rectouterine pouch, the wire introducer was fixed, and the catheter was advanced slowly until the inner cuff was positioned outside the peritoneal membrane. As a result, catheter dysfunction requiring manipulation or catheter replacement in our study proved to be less than 20%, which exceeds the ISPD standard (8).
In China, most ESRD patients, because of cultural or economic concerns, do not want to begin dialysis at an early stage. For example, they do not like to undergo an early operation to create an arteriovenous fistula or insert a PD catheter; they wait until the situation is urgent. At our PD center, more than 98% of ESRD patients started PD therapy urgently and initiated PD immediately after the PD catheter was implanted. We therefore more often needed to use a purse-string suture at the peritoneum and rectus fascia to prevent dialysate leaks, hemoperitoneum, and hernia because PD therapy was going to immediately follow catheter placement.
The incidence of infectious complications in our study was similar to that in previous studies (2–6). Our overall incidence of peritonitis was 0.22 episodesper patient–year, which is compatible with the standard established by the ISPD [<0.67 episodes per patient–year (8)]. In addition, our catheter survival rates at 1 year (SC: 96.7% ± 1.9%; CC: 96.5% ± 2.0%) and at 2 years (SC: 95.3% ± 2.3%; CC: 92.4% ± 3.6%) were superior to the minimally acceptable level defined in the European best practice guidelines for peritoneal catheter survival [80% at 12 months (11)]. Peritonitis was the main reason for catheter failure in our study, which required catheter removal in some cases. Our results indicate that the catheter survival rate might be further improved by lowering the risk for infectious complications. A strict training protocol implemented by the nursing staff, good wound care, and proper sterile procedures are thus most likely to help reduce the incidence of catheter failure in PD patients.
Conclusions
Our study indicates that catheter dysfunction is more common with CCs than with SCs, although no differences in patient and technique survival were seen with either catheter type. Catheter-related complications were not reduced in patients with CCs. We therefore conclude that SCs appear to be the better option to reduce subsequent catheter-related complications in PD patients.
Disclosures
The authors of this study have no financial conflicts of interest to declare.
REFERENCES
- 1. Dell'Aquila R, Chiaramonte S, Rodighiero MP, Spano' E, Loreto P, Kohn CO, et al. Rational choice of peritoneal dialysis catheter. Perit Dial Int 2007; 27(Suppl 2):S119–25. [PubMed] [Google Scholar]
- 2. Akyol AM, Porteous C, Brown MW. A comparison of two types of catheters for continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1990; 10:63–6. [PubMed] [Google Scholar]
- 3. Johnson DW, Wong J, Wiggins KJ, Kirwan R, Griffin A, Preston J, et al. A randomized controlled trial of coiled versus straight swan-neck Tenckhoff catheters in peritoneal dialysis patients. Am J Kidney Dis 2006; 48:812–21. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen PK, Hemmingsen C, Friis SU, Ladefoged J, Olgaard K. Comparison of straight and curled Tenckhoff peritoneal dialysis catheter implanted by percutaneous technique: a prospective randomized study. Perit Dial Int 1995; 15:18–21. [PubMed] [Google Scholar]
- 5. Lo WK, Lui SL, Li FK, Choy BY, Lam MF, Tse KC, et al. A prospective randomized study on three different peritoneal dialysis catheters. Perit Dial Int 2003; 23(Suppl 2):S127–31. [PubMed] [Google Scholar]
- 6. Stegmayr BG, Wikdahl AM, Bergström M, Nilsson C, Engman U, Arnerlöv C, et al. A randomized clinical trial comparing the function of straight and coiled Tenckhoff catheters for peritoneal dialysis. Perit Dial Int 2005; 25:85–8. [PubMed] [Google Scholar]
- 7. Xie J, Kiryluk K, Ren H, Zhu P, Huang X, Shen P, et al. Coiled versus straight peritoneal dialysis catheters: a randomized controlled trial and meta-analysis. Am J Kidney Dis 2011; 58:946–55. [DOI] [PubMed] [Google Scholar]
- 8. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31. [PubMed] [Google Scholar]
- 9. Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30:424–9. [DOI] [PubMed] [Google Scholar]
- 10. Yilmazlar T, Kirdak T, Bilgin S, Yavuz M, Yurtkuran M. Laparoscopic findings of peritoneal dialysis catheter malfunction and management outcomes. Perit Dial Int 2006; 26:374–9. [PubMed] [Google Scholar]
- 11. Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, et al. European best practice guidelines for peritoneal dialysis. 3 Peritoneal access. Nephrol Dial Transplant 2005; 20(Suppl 9):ix 8–12. [DOI] [PubMed] [Google Scholar]


