Abstract
♦ Background and aims:
Oxidative stress plays an important role in the pathogenesis of cardiovascular disease (CVD). Central blood pressure (BP) is thought to be more relevant than peripheral BP for the pathogenesis of CVD. Advanced oxidation protein products (AOPP) are markers of oxidative stress. This study investigated the relationship between AOPP and central BP in peritoneal dialysis (PD) patients.
♦ Methods:
In a cross-sectional study of 75 PD patients (67% men), we analyzed two oxidative stress markers, AOPP (modified assay, mAOPP, correcting for the impact of triglycerides) and pentosidine, three inflammation markers, interleukin-6 (IL-6), tumor necrosis factor (TNF), and high-sensitivity C-reactive protein (hs-CRP). All patients underwent measurement of central systolic blood pressure (SBP) and diastolic blood pressure (DBP) by applanation tonometry.
♦ Results:
Patients with mAOPP levels above the median had a higher central SBP and DBP than those below the median values. In univariate analysis, the levels of mAOPP associated with central SBP and central DBP. Multiple regression analysis, adjusting for age, gender, diabetes, CVD, protein-energy wasting (PEW), hs-CRP and extracellular water by multi-frequency bioimpedance or N-terminal prohormone of brain natriuretic peptide (NT-proBNP), confirmed independent associations between mAOPP and central SBP and central DBP respectively.
♦ Conclusions:
The mAOPP level is independently associated with the central SBP and DBP in PD patients. This finding suggests that oxidative stress may be involved in the pathogenesis of hypertension or that hypertension itself or factors associated with hypertension such as fluid overload may have an additional effect on oxidative stress in PD patients.
Keywords: Oxidative stress, blood pressure, peritoneal dialysis
End-stage renal disease (ESRD) patients undergoing dialysis have a substantially increased risk of cardiovascular morbidity and mortality (1,2). Atherosclerotic and cardiovascular disorders are main causes of morbidity and mortality in dialysis patients and, while their development is multifactorial, hypertension is one of the most important factors. Therefore, lowering blood pressure (BP) could be considered a key target to reduce the high cardiovascular risk in this population (3). Central (i.e. aortic) BP, the BP exerted on the heart, brain, and kidney is more relevant than peripheral BP for the pathogenesis of cardiovascular disease (CVD) (4,5), and can be reliably and non-invasively assessed with recently developed techniques. The difference between peripheral and central BP is greater in young patients with compliant elastic arteries than in older patients with stiff and less compliant arteries (6,7).
A relationship between oxidative stress and BP was first suggested in the 1960s, but such association has been studied in detail only since the 1990s. Evidence from experimental and animal studies supports the role of oxidative stress in the pathogenesis of hypertension, although there is still no convincing proof that oxidative stress is a cause of human hypertension (8,9). In dialysis patients, oxidative stress and inflammation are associated with the development of CVD (10,11). The mechanisms are believed to involve both atherosclerosis and arteriosclerosis, with vascular calcification resulting in arterial stiffness leading to impaired vascular function; vascular dysfunction may in turn contribute to increased oxidative stress and inflammation, increasing the likelihood of morbidity and mortality due to CVD in dialysis patients (12,13).
Advanced oxidation protein products (AOPP) are markers of oxidative stress and mediators of inflammation, first detected in the plasma of chronic uremic patients in 1996 (14). Elevated plasma levels of AOPP have been detected both in patients on hemodialysis (HD) and peritoneal dialysis (PD), as well as in patients with coronary artery disease and diabetes (14–16). As plasma triglycerides interfere with the method of quantifying AOPP, leading to an over-estimation of AOPP values (17), a modified AOPP (mAOPP) assay, in which triglycerides are removed by precipitation before analysis, was developed and applied in uremic patients (18).
To the best of our knowledge, the relationship between AOPP and central BP has not yet been explored in humans. In the current study, we hypothesized that modified AOPP levels may correlate with central BP in PD patients.
Materials and Methods
Patients
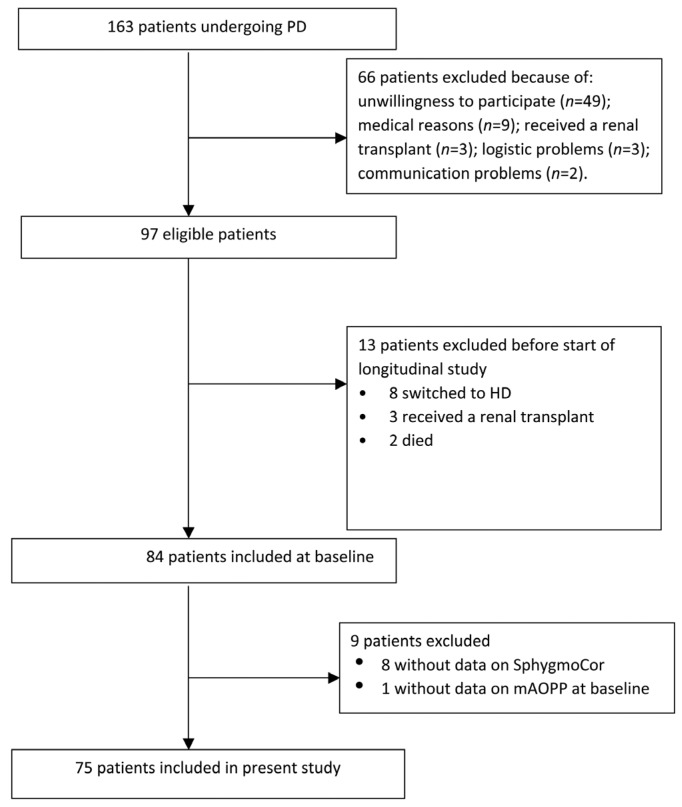
The current study is a cross-sectional study with a follow-up that originally aimed at evaluating variation in inflammatory markers in all prevalent PD patients who were being controlled at the Karolinska University Hospital and Danderyds Hospital in the Stockholm region. Patient recruitment occurred from March 2008 through April 2011. A total of 163 patients who were receiving regular PD treatment were invited to participate. Of these, 66 patients refused to participate or were precluded from entry into this study. Of the remaining 97 eligible patients, 13 patients did not start the study due to various reasons (including transfer to HD, transplantation and mortality). Thus, 84 patients started the study and were followed with blood samples obtained weekly during a period of 3 months. The current study is limited to baseline sampling of the 84 patients, among whom 8 additional patients with atrial fibrillation were excluded because no central BP measurements were available, and 1 more patient was excluded because of lack of mAOPP measurement. Thus, 75 prevalent patients with a median preceding time on PD of 11.5 (interquartile (IQ) range 5.9 – 28.8) months at baseline remained for inclusion in the analysis (Figure 1). The study protocol was approved by the Ethics Committee of Karolinska Institute at Karolinska University Hospital (Stockholm, Sweden) and each patient gave their written informed consent.
Figure 1 —
Flowchart describing the inclusion of participants in the study. The 75 patients who were included in this analysis had been treated by PD for a median of 11.5 (IQ range 5.9–28.8) months. PD = peritoneal dialysis; HD = hemodialysis; mAOPP = modified assay of advanced oxidation protein products; IQ = interquartile; SphygmoCor (Atcor Medical, Sydney, Australia).
Body mass index was calculated as weight in kilograms divided by the square of height in meters. Previous CVD was defined as history of any CVD as recorded in the Swedish Hospital Discharge Registry (International Classification of Diseases (ICD-8) codes 390 to 458 or ICD-9 codes 390 to 459). Diabetes was defined as fasting plasma glucose ≥ 7.0 mmol/L, 2-hour post-load glucose levels ≥ 11.1 mmol/L, or the use of oral hypoglycemic agents or insulin (19). Subjective global nutritional assessment (SGA) was used to evaluate the overall protein-energy wasting (PEW) status. Each patient was given a score that reflects the nutritional status as follows: 1 = normal nutritional status, 2 = mild wasting, 3 = moderate wasting and 4 = severe wasting. PEW was defined as a SGA score > 1 (20).
The majority (77%) of the PD patients were treated with continuous ambulatory peritoneal dialysis and 23% with automated peritoneal dialysis. All PD patients used glucose-based biocompatible solutions (Physioneal, Baxter Healthcare, Castlebar, Ireland; or Gambrosol Trio, Gambro Lundia AB, Lund, Sweden; or BicaVera, Fresenius Medical Care, Bad Homburg, Germany), some patients used amino-acid based solution (Nutrineal, Baxter Healthcare, Castlebar, Ireland) while for the long dwell, a majority of the patients used icodextrin-based solution (Extraneal, Baxter Healthcare, Castlebar, Ireland). The residual glomerular filtration rate (rGFR) of the PD patients was 3.2 ± 2.2 mL/min/1.73 m2, calculated as the average of renal creatinine and urea clearance from 24 hours' urine collection (21), while total (renal plus peritoneal) Kt/V was 2.2 ± 0.5. Each patient's medical chart was thoroughly reviewed by a nephrologist, who extracted data pertaining to previous CVD, comorbid conditions, and medications. Almost all (86%) of the patients were treated with antihypertensive drugs, including (57%) angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or both; calcium channel blockers (32%); β-blockers (70%); diuretics (86%); and 49 % of the patients used statins.
Laboratory Measurements
After an overnight oral fast, without interrupting the ongoing continuous treatment with PD, venous blood samples were taken; all blood samples were centrifuged immediately at 2,300 × g for 15 min at 4 °C and stored at −70 °C if not analyzed immediately. Serum interleukin-6 (IL-6) and tumor necrosis factor levels were measured by Immulite Automatic Immunoassay Analyzer (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) with assays manufactured for each analyzer. Pentosidine was measured as previously described (22). Since plasma pentosidine is mainly present as protein bound to albumin, and free pentosidine represents only 3 – 4% of total circulating pentosidine, the plasma pentosidine concentrations in picomol per liter were corrected for serum albumin and expressed as picomol per milligram of albumin. High sensitivity C-reactive protein (hs-CRP) calcium, phosphate, cholesterol, triglycerides, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and albumin were analyzed using routine methods.
Modified AOPP (MAOPP) Assay
The mAOPP assay included, in addition to the AOPP methodology (14), a sample preparation procedure to precipitate very low- and low-density lipoproteins in the plasma. As the HDL-cholesterol precipitating reagent (Thermo Electron Corporation, Vantaa, Finland) is no longer available, we modified the procedure and instead used, as precipitating reagent, 10 mg of dextran sulphate (molecular weight 500 KDa, Sigma-Aldrich, St Louis, MO, USA, product number D6001) and 246 mg magnesium sulphate X7H20 (molecular weight 246 KDa, Sigma-Aldrich, M5921) prepared in 1 mL distilled water. One part (20 uL) of this reagent was mixed with 10 parts (200 uL) of ethylenediaminetetraacetic acid (EDTA) plasma in an Eppendorf (Sarstedt, Nümbrecht, Germany) tube, vortexed, allowed to stand for 10 minutes, centrifuged at 1,500 × g for 30 minutes in a cold centrifuge (+4 °C), upon which the supernatant was carefully pipetted for further analysis. Then, mAOPP was immediately measured in the supernatant at 340 nm on a microplate spectrophotometer (SPECTRAmax 250, Molecular Devices Corporation, Sunnyvale, CA, USA) under acidic conditions and expressed as chloramine-T equivalents (18).
Assessment of Fluid Status by Bioimpedance
Multi-frequency bioimpedance (Xitron 4000B, Xitron Tech, San Diego, CA, USA) was used to assess total body water, extracellular water, and intracellular water at baseline in a subgroup of patients (n = 69) in whom such data were available.
Central BP Measurements
Measurements of central BP were performed using SphygmoCor hardware and software (Atcor Medical, Sydney, Australia) by one nurse who was trained to perform these measurements. Based on the operator index, which is an estimate of quality of the SphygmoCor derived waveforms, values below 80% were not taken into consideration.
Central BP measurement is based on the tonometer-recorded radial pulse waveform and a brachial BP. After obtaining sequential waveform series, the average peripheral and central arterial waveforms were recorded. The average of sequential radial waveforms was measured in real time with a validated transfer function algorithm using specially prepared software. The following parameters were recorded: estimated central (aortic) systolic and diastolic BP (SBP and DBP); peripheral (radial) SBP and DBP; central pulse pressure (PP) calculated as the difference between the estimated central SBP and DBP. Peripheral PP was calculated as the difference between the estimated peripheral SBP and DPB.
Statistical Analysis
Values were expressed as mean ± standard deviation (SD) for normally distributed continuous variables, or median (IQ range) for skewed variables, or percentage of total, as appropriate for categorical variables. Differences among the groups were analyzed by the Wilcoxon test or the Fisher's exact test. Spearman's rank correlation (rho) was used to determine correlations of mAOPP with hemodynamic variables. Multivariate regression models were applied to test the independent link between mAOPP and central and peripheral (radial) BP by adjusting for potential confounders including age, gender, the prevalence of three co-morbidities (diabetes, CVD, and PEW), hs-CRP and extracellular water by multi-frequency bioimpedance or NT-proBNP. Data are expressed as standard regression coefficients (β). Two-tailed 95% confidence intervals (CI) and p values are presented with p < 0.05 regarded as significant. Because p values were not adjusted for multiple testing, they have to be considered as descriptive. All statistical analyses were performed using statistical software STATA version 12 (Stata Corporation, College Station, TX, USA).
Results
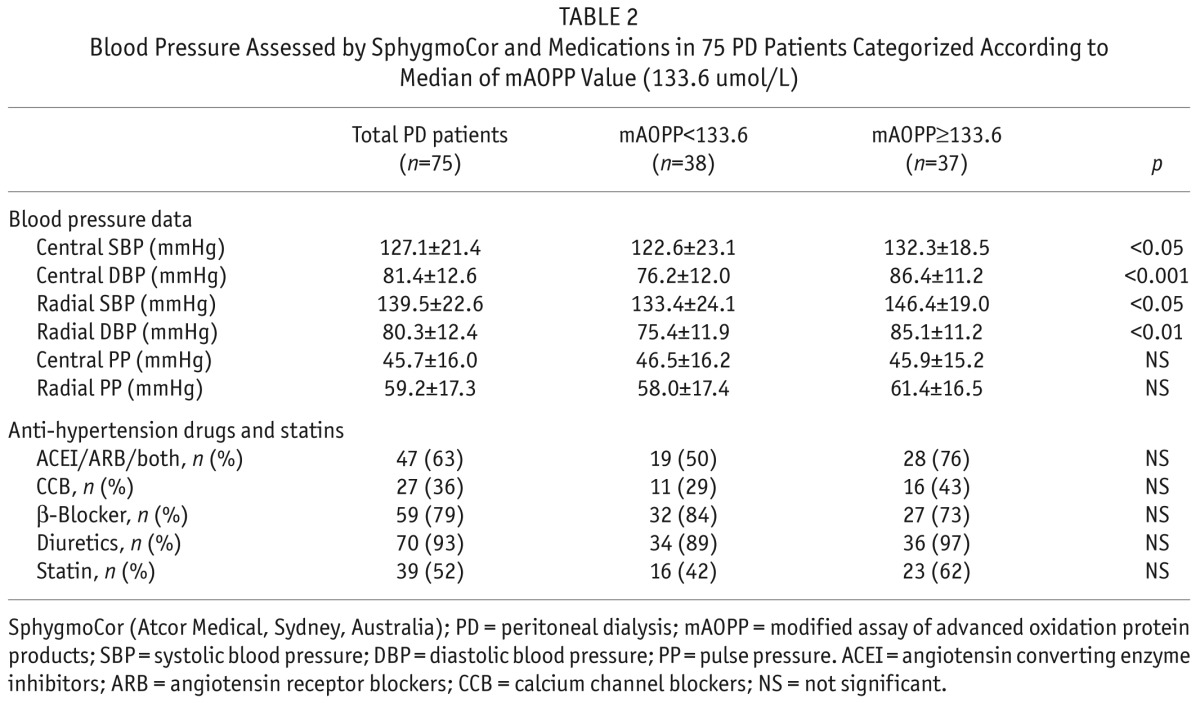
Demographic, clinical, biochemical and central hemodynamic characteristics of the study population, categorized according to whether mAOPP values were below or above the median mAOPP value (133.6 umol/L) are given in Table 1 and Table 2.
TABLE 1.
Demographic and Biochemical Data in 75 PD Patients Categorized According to Median of mAOPP Value (133.6 umol/L)
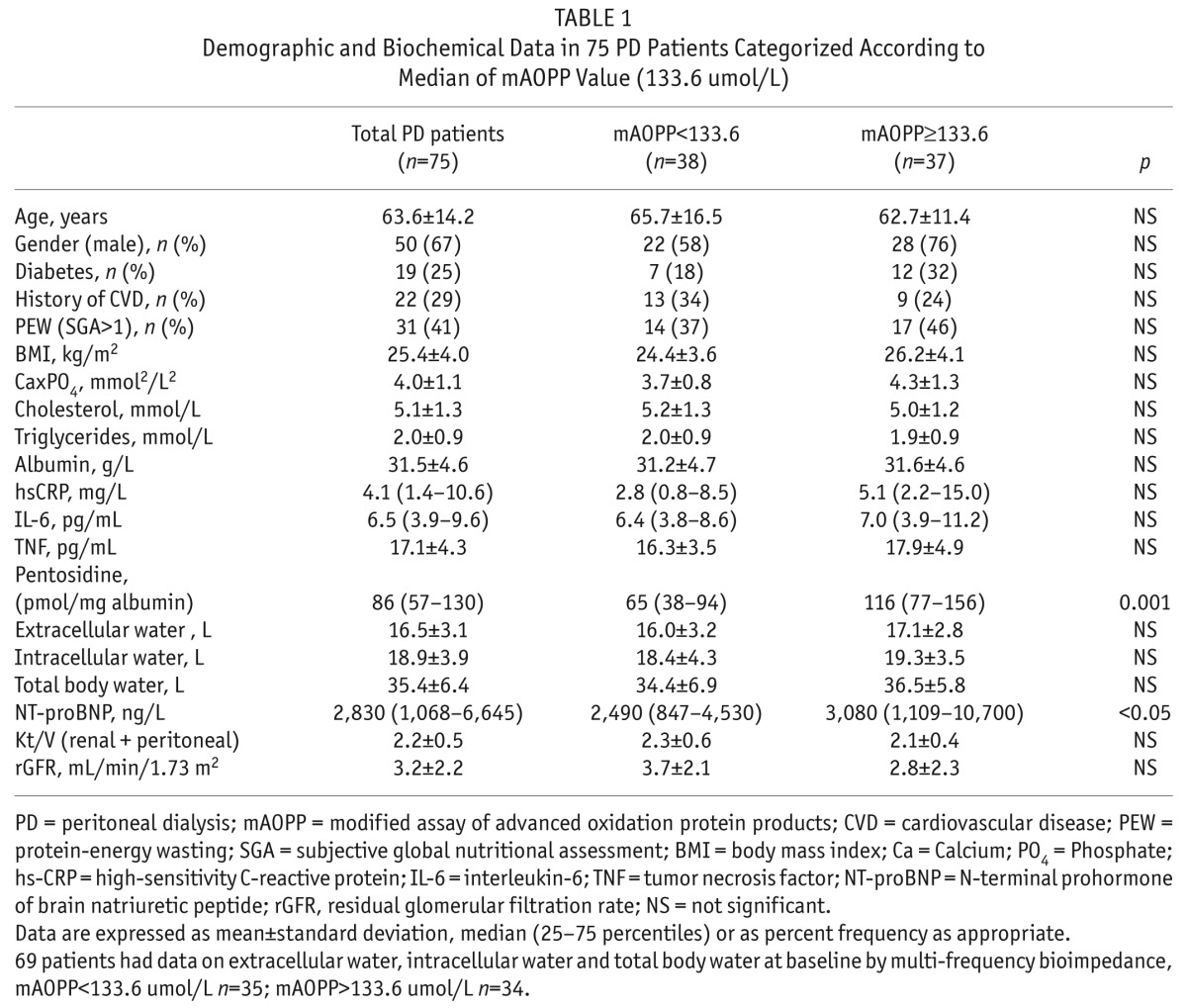
TABLE 2.
Blood Pressure Assessed by SphygmoCor and Medications in 75 PD Patients Categorized According to Median of mAOPP Value (133.6 umol/L)
Patients with high mAOPP levels had higher levels (p < 0.01) also of another biomarker of oxidative stress, pentosidine, expressed in relation to albumin levels, while the investigated inflammation markers, Kt/V, rGFR and other clinical and biochemical parameters, including serum albumin, did not differ between patients with mAOPP levels above or under median levels. In order to assess whether fluid overload—which can be expected to increase blood pressure—was associated also to the mAOPP concentrations, we performed in a subgroup of patients (n = 69) analysis of available measurements that had been obtained by multi-frequency bioimpedance on total body water, extracellular water, and intracellular water at baseline as well as data on circulating NT-proBNP at baseline. NT-proBNP (ng/L) was higher among the patients with mAOPP ≥ 133.6 umol/L (median level) than in those with mAOPP below this level (3,080 (1,109 – 10,700) versus 2,490 (847 – 4,530) ng/L; p < 0.05) whereas the bioimpedance data (total body water, extracellular water, and intracellular water) did not differ between the two mAOPP groups (Table 1).
Interestingly, central SBP, central DBP, peripheral SBP and peripheral DBP were all significantly higher in patients with mAOPP values above the median values. There were no statistically significant differences between the two mAOPP groups as regards the use of anti-hypertensive medications or statins (Table 2).
Bivariate Correlations
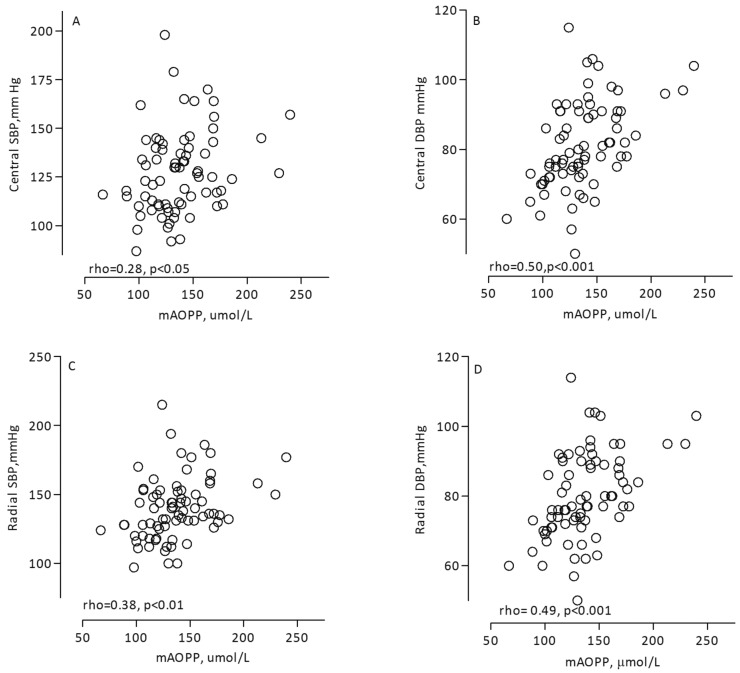
The circulating levels of mAOPP correlated positively with the hemodynamic parameters central SBP (rho = 0.28, p < 0.05, Figure 2A), central DBP (rho = 0.50, p < 0.001, Figure 2B), peripheral SBP (rho = 0.38, p < 0.01, Figure 2C), and peripheral DBP (rho = 0.49, p < 0.001, Figure 2D). Central and peripheral blood pressures were closely associated: central SBP correlated directly with peripheral SBP (rho = 0.94, p < 0.001), and central DBP correlated with peripheral DBP (rho = 0.99, p < 0.001).
Figure 2 —
Associations between mAOPP and hemodynamic parameters: central SBP (A), central DBP (B), peripheral SBP (C), and peripheral DBP (D) in 75 PD patients. mAOPP = modified assay of advanced oxidation protein products; SBP = systolic blood pressure; DBP = diastolic blood pressure; PD = peritoneal dialysis.
Another marker of oxidative stress, pentosidine (expressed in relation to serum albumin) also correlated with central SBP (rho = 0.22, p = 0.05) and central DBP (rho = 0.24, p < 0.05) as well as with mAOPP (rho = 0.51, p < 0.001). On the other hand, according to bivariate analysis, extracellular water (rho = 0.26, p < 0.05) as well as intracellular water (rho = 0.26, p < 0.05) and total body water (rho = 0.28, p < 0.05) correlated with mAOPP.
Multivariate Analysis
Multivariate regression models were used to determine if mAOPP was an independent predictor of central SBP and central DBP and the results showed that the correlations between mAOPP and both central SBP and central DBP remained significant after multiple adjustments including age, gender, diabetes, presence of CVD, presence of PEW, and hs-CRP. When we added extracellular water as a confounder in the model in addition to the previously used confounders, the results showed that the relations between mAOPP and SBP (r2 = 0.25; β = 0.25, p = 0.05) as well as DBP (r2 = 0.31; β = 0.41, p = 0.002) still remained significant (Table 3). Also, when, instead of extracellular water, we included NT-proBNP as a confounder, we got similar results for SBP (r2 = 0.27; β = 0.29, p = 0.02) and DBP (r2 = 0.30; β = 0.41, p = 0.002) (Table 4).
TABLE 3.
Multivariate Regression Models of mAOPP as a Predictor of Central Systolic and Diastolic BP in 75 PD patients
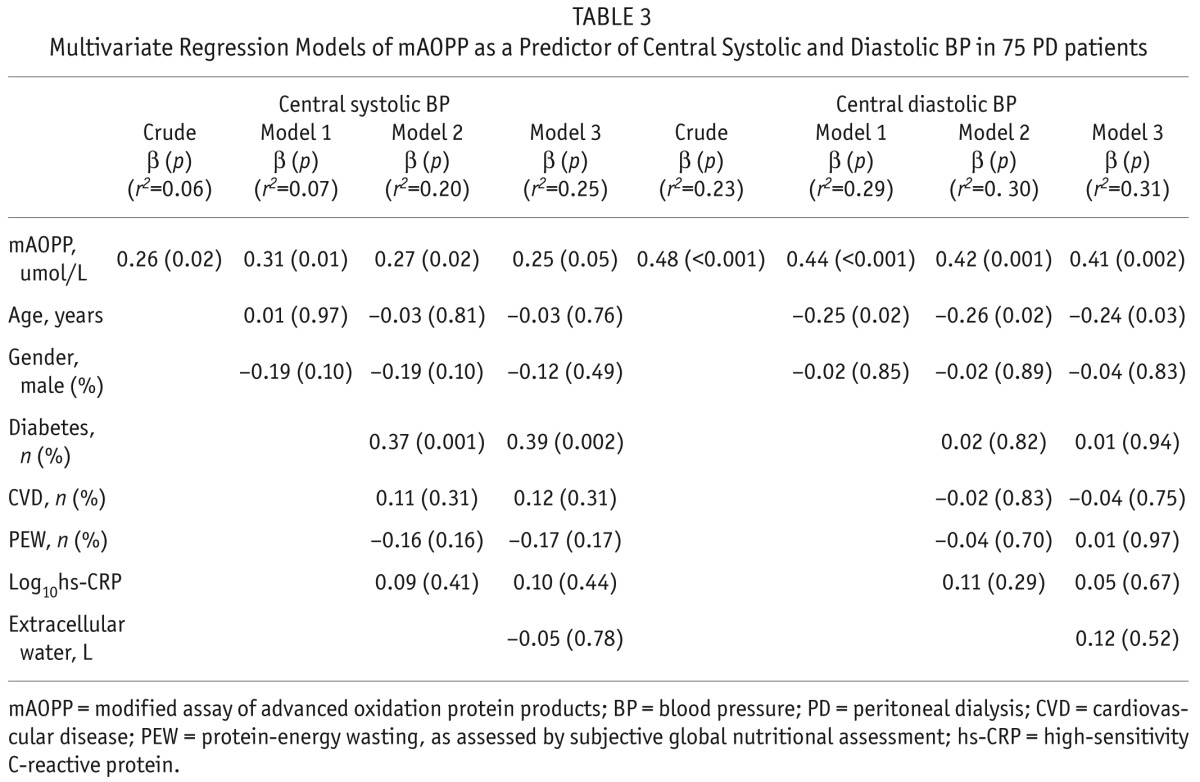
TABLE 4.
Multivariate Regression Models of mAOPP as a Predictor of Central Systolic and Diastolic BP in 75 PD Patients
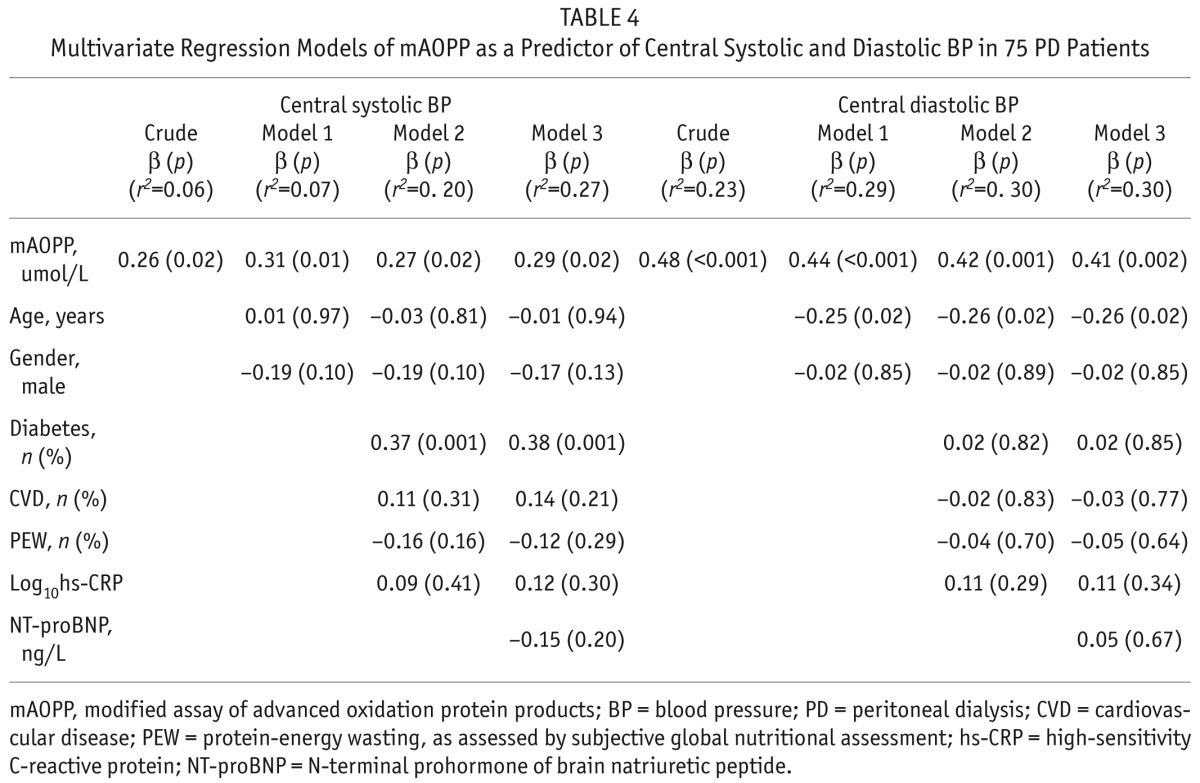
As the central and peripheral BP were strongly correlated, we applied the same models to determine mAOPP as an independent predictor of peripheral SBP and DBP, which were obtained simultaneously with measurements of central BP using the SphygmoCor device; the results were similar, i.e., mAOPP was an independent predictor also of peripheral blood pressure. In contrast, the correlation between pentosidine and central SBP and central DBP respectively lost its statistical significance after adjustment for the same confounders.
Discussion
The main finding of this study is that mAOPP, a marker of oxidative stress, is significantly associated with central (aortic) SBP and central DBP as well as with peripheral (radial) SBP and DBP in PD patients. The relationship between mAOPP and central BP was robust and independent of multiple potential confounders. These findings suggest that oxidative stress as measured by mAOPP is strongly linked with central blood pressure as assessed by applanation tonometry.
Starting in 1990, many studies have demonstrated the importance of oxidative stress in BP regulation. The number of PubMed hits with “hypertension” and “oxidative stress” as keywords of published articles has increased more than 10-fold in 20 years. Various experimental models of hypertension show evidence of oxidative excess. For example, mice deficient in reactive oxygen species (ROS)-generating enzymes have lower BP, and Angiotensin II infusion fails to induce hypertension in these mice (23,24). The role of oxidative stress in experimental hypertension is further strengthened by studies showing that treatment with antioxidant vitamins (vitamins C and E) and free radical scavengers attenuates or prevents the development of hypertension (25). Whereas there is evidence in animal models that oxidative excess is causally associated with BP (8,9,26,27), the evidence in humans is less convincing; thus, a causal link between oxidative stress and BP is not yet established. Factors implicated to counteract oxidative stress in human hypertension include decreased antioxidant activity, reduced levels of ROS scavengers, and reduced activation of ROS-generating enzymes (28). However, only a small number of clinical studies have shown positive BP-lowering effects of antioxidants (29), while most antioxidant clinical trials have failed to demonstrate BP reduction (30).
In PD patients, one study showed higher malondial-dehyde (MDA) levels, an indicator of lipid peroxidation, in hypertensive compared to normotensive patients and healthy controls; however, the levels of AOPP were not different between the hypertensive and normotensive PD patients (31). Another study showed that while oxidative stress was increased in dialysis patients compared to the healthy controls, there was no significant difference between the normotensive and hypertensive PD patients in terms of oxidative stress; however, all hypertensive patients were using antihypertensive medications such as angiotensin-converting enzyme inhibitors which could have reduced the levels of oxidative stress biomarkers (32). Thus, the results of the current study, showing that mAOPP, a marker of oxidative stress, was significantly associated with BP in PD patients, differ from those of previous reports. One possible reason for this discrepancy could be that almost all (86%) of our patients had hypertension. Furthermore, we measured the mAOPP level which is corrected for the impact of raised levels of triglycerides on AOPP measurements (18) and to the best of our knowledge there are no previous studies on mAOPP versus blood pressure in PD patients. In addition, the properties of mAOPP may differ from those of other markers of oxidative stress. Thus, in our study, while another oxidative stress marker, pentosidine, correlated with BP in univariate analysis, this association lost its statistical significance after adjustments for (multiple) confounders.
Recent studies have shown that central BP is a better predictor than peripheral BP of cardiovascular mortality (4,5). An increase of central BP is to a large extent related to changes in the properties of the large arteries that may influence the workload of the heart, and the risk for various complications such as left ventricular hypertrophy. It is plausible and supported by the literature that such changes could induce inflammation and oxidative stress (33). The introduction of improved methods for measurement of central BP are now available and this can now be measured easily, accurately, and non-invasively with relatively inexpensive devices (6,7), and, in the current study, allowed us to measure central BP by SphygmoCor (34).
The underlying mechanism by which there could be a link explaining the close association between AOPP and central BP in the current study is not clear. AOPP is one of many markers used to measure the presence and degree of oxidative stress. AOPP measures highly oxidized proteins, such as albumin and fibrinogen, and oxidized fibrinogen is thought to be a key molecule responsible for the AOPP reaction in human plasma (35). Oxidative damage to protein is caused by the action of free radicals and various other oxidizing compounds; a common term for these compounds is ROS. Interaction between ROS and fibrinogen results in the formation of oxidized fibrinogen, which can be detected as AOPP; AOPP is closely related to the basal production of ROS (36). Subcellular ROS stimulates mitogen-activated protein kinases (MAPK), tyrosine kinases, and transcription factors (such as NF kappa B, activator protein-1, and hypoxia-inducible factor-1), increases intracellular free Ca2+ concentration, and up-regulates pro-inflammatory gene expression and activity. At the vascular level, these alterations in intracellular signaling lead to endothelial dysfunction, reduced vasodilation, increased contraction, and structural re-modeling, causing increased peripheral resistance and elevated blood pressure (37). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-induced ROS in the hypothalamic and circumventricular parts of the brain are implicated in the central control of hypertension (8,9,26,27). We speculate that such a biological connection might be one explanation for our finding of a relationship between mAOPP and central BP.
Interestingly, higher mAOPP levels associated with signs of fluid overload as assessed both by higher extracellular water volume (by bioimpedance) and higher circulating levels of NT-proBNP. As higher blood pressure could be due to fluid overload, this could be one possible explanation for the observed link between mAOPP and higher central SBP and DBP in the current study. This observation is in line with a previous study in left ventricular hypertrophy patients showing an association between oxidative stress and NT-proBNP and in another study in which HD patients' oxidative stress associated with the degree of fluid overload (38,39). On the other hand, when we included in the multivariate regression models, in addition to the many other confounders already included in the model, extracellular water volume and NT-proBNP respectively as confounders, the mAOPP level remained independently associated with the central SBP and DBP. Although the exact mechanism(s) that could explain why mAOPP is associated to central blood pressure in our PD patients is unclear, we speculate that oxidative stress, possibly related at least to some extent to fluid overload, is a key mediator for this association.
Study Strengths and Limitations
Though this, to the best of our knowledge, is the first study addressing AOPP and central BP, and thus the first such study in PD patients, the results should be interpreted with caution and several limitations of the study should be noted. First, the cross-sectional nature of our observations precludes cause-effect inferences about the observed AOPP link with central BP. Second, the small sample size with only 75 patients is another limitation. Third, we did not perform an intervention to see whether changes in blood pressure resulted in changes in mAOPP levels, or if modifications of mAOPP levels using anti-oxidative medications could induce changes in central and peripheral BP. We have no clear understanding of the mechanisms by which mAOPP could be linked to central blood pressure in PD patients. Nevertheless, the close relation between, on the one hand, the physiological parameter central BP, and, on the other hand, a completely independent laboratory measurement of mAOPP is conspicuous and intriguing. Ideally, based on the hypothesis generated by the current study, a clinical trial should be performed testing the hypothesis that antioxidants could reduce mAOPP and concomitantly central BP levels in PD patients. PD patients may be more suitable than HD patients for such a study as BP control in HD patients is influenced by the impact of ultrafiltration during the HD session.
Conclusions
In summary, the mAOPP level is independently correlated with central and peripheral BP in PD patients. These results support the concept that oxidative stress may be involved in the pathogenesis of hypertension or that hypertension or factors associated with hypertension such as fluid overload may induce oxidative stress, or both. Further studies in larger cohorts of PD patients, and in other patient populations with a high prevalence of high central and peripheral BP and oxidative stress, are warranted to test the hypothesis that mAOPP is a valid and potentially modifiable predictor of central BP.
Disclosure
Bengt Lindholm is affiliated with Baxter Healthcare. None of the other authors have any conflicts of interest to declare.
Acknowledgments
The PD cohort study was supported by a grant from Amgen, Inc. This study was supported also by grants from the Swedish Medical Research Council, Osterman's, Martin Rind's and Westman's Foundations. Baxter Novum is the result of a grant from Baxter Healthcare Corporation to the Karolinska Institutet. HX was supported by Peking Union Medical College Hospital fund and a grant from Baxter Healthcare Corporation, China. ICR was supported by the Rio Hortega program, Instituto Carlos III, Spain. We thank Britta Hylander, Karolinska University Hospital Solna, Guna Germanis, Danderyds Hospital, and Åsa Lindé and the nurses at KBC for their excellent job in sampling and cohort collection.
REFERENCES
- 1. Eknoyan G. Cardiovascular mortality and morbidity in dialysis patients. Miner Electrolyte Metab 1999. Jan-Apr 25(1–2):100–4. [DOI] [PubMed] [Google Scholar]
- 2. Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 2009. December; 4(Suppl 1):S5–11. [DOI] [PubMed] [Google Scholar]
- 3. Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 2009. March 21; 373(9668):1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010. August; 31(15):1865–71. [DOI] [PubMed] [Google Scholar]
- 5. Nichols WW, Denardo SJ, Wilkinson IB, McEniery CM, Cockcroft J, O'Rourke MF. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens (Greenwich) 2008. April; 10(4):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng HM, Lang D, Tufanaru C, Pearson A. Measurement accuracy of non-invasively obtained central blood pressure by applanation tonometry: A systematic review and meta-analysis. Int J Cardiol 2012; 167(5):[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an N-point moving average method. J Am Coll Cardiol 2011. February 22; 57(8):951–61. [DOI] [PubMed] [Google Scholar]
- 8. Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann Med 2012. June; 44(Suppl 1):S2–16. [DOI] [PubMed] [Google Scholar]
- 9. Montezano AC, Touyz RM. Molecular mechanisms of hypertension—reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol 2012. May; 28(3):288–95. [DOI] [PubMed] [Google Scholar]
- 10. Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol 2004. September; 24(5):469–73. [DOI] [PubMed] [Google Scholar]
- 11. Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin Dial 2010. Sep-Oct 23(5):498–509. [DOI] [PubMed] [Google Scholar]
- 12. Guerin A, Pannier B, London G. Atherosclerosis versus arterial stiffness in advanced renal failure. Adv Cardiol 2007; 44:187–98. [DOI] [PubMed] [Google Scholar]
- 13. Brunet P, Gondouin B, Duval-Sabatier A, Dou L, Cerini C, Dignat-George F, et al. Does uremia cause vascular dysfunction? Kidney Blood Press Res 2011; 34(4):284–90. [DOI] [PubMed] [Google Scholar]
- 14. Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996. May; 49(5):1304–13. [DOI] [PubMed] [Google Scholar]
- 15. Kaneda H, Taguchi J, Ogasawara K, Aizawa T, Ohno M. Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis 2002. May; 162(1):221–5. [DOI] [PubMed] [Google Scholar]
- 16. Piwowar A, Knapik-Kordecka M, Warwas M. AOPP and its relations with selected markers of oxidative/antioxidative system in type 2 diabetes mellitus. Diabetes Res Clin Pract 2007. August; 77(2):188–92. [DOI] [PubMed] [Google Scholar]
- 17. Valli A, Suliman ME, Meert N, Vanholder R, Lindholm B, Stenvinkel P, et al. Overestimation of advanced oxidation protein products in uremic plasma due to presence of triglycerides and other endogenous factors. Clin Chim Acta 2007. April; 379(1–2):87–94. [DOI] [PubMed] [Google Scholar]
- 18. Anderstam B, Ann-Christin BH, Valli A, Stenvinkel P, Lindholm B, Suliman ME. Modification of the oxidative stress biomarker AOPP assay: application in uremic samples. Clin Chim Acta 2008. July 17; 393(2):114–8. [DOI] [PubMed] [Google Scholar]
- 19. Resnick HE, Harris MI, Brock DB, Harris TB. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes care 2000. February; 23(2):176–80. [DOI] [PubMed] [Google Scholar]
- 20. Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B, et al. Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int 1998. March; 53(3):773–82. [DOI] [PubMed] [Google Scholar]
- 21. Section I. Measurement of renal function, when to refer and when to start dialysis. Nephrol Dial Transplant 2002; 17(Suppl 7):7–15. [DOI] [PubMed] [Google Scholar]
- 22. Nascimento MM, Suliman ME, Bruchfeld A, Hayashi SY, Manfro RC, Qureshi AR, et al. The influence of hepatitis C and iron replacement therapy on plasma pentosidine levels in haemodialysis patients. Nephrol Dial Transplant 2004. December; 19(12):3112–6. [DOI] [PubMed] [Google Scholar]
- 23. Bhatia K, Elmarakby AA, El-Remessy AB, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2012. January 15; 302(2):R274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haque MZ, Majid DS. High salt intake delayed angiotensin II-induced hypertension in mice with a genetic variant of NADPH oxidase. Am J Hypertens 2011. January; 24(1):114–8. [DOI] [PubMed] [Google Scholar]
- 25. Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 2010. May; 126(2):119–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovasc Ther 2010. August; 28(4):e20–32. PubMed PMID: 20370791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am 2009. May; 93(3):621–35. [DOI] [PubMed] [Google Scholar]
- 28. Zalba G, San Jose G, Moreno MU, Fortuno A, Diez J. NADPH oxidase-mediated oxidative stress: genetic studies of the p22(phox) gene in hypertension. Antioxid Redox Signal 2005. Sep-Oct; 7(9–10):1327–36. [DOI] [PubMed] [Google Scholar]
- 29. Rodrigo R, Prat H, Passalacqua W, Araya J, Bachler JP. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin Sci (Lond) 2008. May; 114(10):625–34. [DOI] [PubMed] [Google Scholar]
- 30. Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Mol Interv 2010. December; 10(6):354–62. [DOI] [PubMed] [Google Scholar]
- 31. Demirci S, Sekeroglu MR, Noyan T, Koceroglu R, Soyoral YU, Dulger H, et al. The importance of oxidative stress in patients with chronic renal failure whose hypertension is treated with peritoneal dialysis. Cell Biochem Funct 2011. April; 29(3):249–54. [DOI] [PubMed] [Google Scholar]
- 32. Kocak H, Gumuslu S, Sahin E, Ceken K, Gocmen YA, Yakupoglu G, et al. Advanced oxidative protein products are independently associated with endothelial function in peritoneal dialysis patients. Nephrology (Carlton) 2009. April; 14(3):273–80. [DOI] [PubMed] [Google Scholar]
- 33. Mizuno Y, Jacob RF, Mason RP. Effects of calcium channel and renin-angiotensin system blockade on intravascular and neurohormonal mechanisms of hypertensive vascular disease. Am J Hypertens 2008. October; 21(10):1076–85. [DOI] [PubMed] [Google Scholar]
- 34. Weiss W, Gohlisch C, Harsch-Gladisch C, Tolle M, Zidek W, van der Giet M. Oscillometric estimation of central blood pressure: validation of the Mobil-O-Graph in comparison with the SphygmoCor device. Blood Press Monit 2012. June; 17(3):128–31. [DOI] [PubMed] [Google Scholar]
- 35. Selmeci L, Szekely M, Soos P, Seres L, Klinga N, Geiger A, et al. Human blood plasma advanced oxidation protein products (AOPP) correlates with fibrinogen levels. Free Radic Res 2006. September; 40(9):952–8. [DOI] [PubMed] [Google Scholar]
- 36. Selmeci L. Advanced oxidation protein products (AOPP): novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome. Free Radic Res 2011. October; 45(10):1115–23. [DOI] [PubMed] [Google Scholar]
- 37. Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009. June; 196(2):193–222. [DOI] [PubMed] [Google Scholar]
- 38. Scolletta S, Carlucci F, Biagioli B, Marchetti L, Maccherini M, Carlucci G, et al. NT-proBNP changes, oxidative stress, and energy status of hypertrophic myocardium following ischemia/reperfusion injury. Biomed Pharmacother 2007. Feb-Apr; 61(2–3):160–6. [DOI] [PubMed] [Google Scholar]
- 39. Celik G, Yontem M, Cilo M, Bilge M, Mehmetoglu I, Unaldi M. The relationship between glutathione peroxidase and bioimpedance parameters in nondiabetic hemodialysis patients. Hemodial Int 2012. April; 16(2):274–81. [DOI] [PubMed] [Google Scholar]