Abstract
We systematically examined by immune-histology the lungs of some widely used mouse models of asthma. These models include sensitization by multiple intraperitoneal injections of soluble ovalbumin (OVA) or of OVA with alum, followed by three intranasal or aerosol challenges 3 days apart. Within 24 hours after a single challenge there is fibrinoid necrosis of arterial walls with deposition of immunoglobulin and OVA and infiltration of eosinophilic polymorphonuclear cells that lasts for about 3 days followed by peribronchial B-cell infiltration and slight reversible goblet cell hypertrophy. After 2 challenges, severe eosinophilic vasculitis is present at 6 hours, increases by 72 hours and then declines; B-cell proliferation and significant goblet cell hypertrophy and hyperplasia (GCHTH) and bronchial smooth muscle hypertrophy recur more prominently. After 3 challenges, there is significantly increased induced bronchus associated lymphoid tissue (iBALT) formation, GCHTH and smooth muscle hypertrophy. Elevated levels of Th2 cytokines: IL-4, IL-5 and IL-13, are present in bronchial lavage fluids. Sensitized mice have precipitating antibody and positive Arthus skin reactions but also develop significant levels IgE antibody to OVA but only 1 week after challenge. We conclude that the asthma like lung lesions induced in these models is preceded by immune complex mediated eosinophilic vasculitis and iBALT formation. There are elevations of Th2 cytokines that most likely produce bronchial lesions that resemble human asthma. However, it is unlikely that mast cell activated atopic mechanisms are responsible as we found only a few presumed mast cells by toluidine blue and metachromatic staining limited to the most proximal part of the main stem bronchus, and none in the remaining main stem bronchus or in the lung periphery.
In 1992, Nakajima et al.1 introduced an experimental model of asthma in mice using two intraperitoneal (i.p). immunizations with ovalbumin (OVA) in alum followed by inhalation of aerosolized OVA. Since then many papers have been published using this model or variations of it [1,802 articles are listed in Pub Med under “Ovalbumin Mouse Models of Asthma”; see reviews by Cohn 2, Zosky and Sly 3, Nials and Uddin, 4 Kumar and Foster 5 and Mullane ad Williams 6]. As pointed out by Kumar et al. 7, “There is no single “classical” model, because numerous alternatives exist with respect to the choice of mouse strain, method of sensitization, route and duration of challenge and approach to assessing the host response.” The experimental approach that we utilized includes three phases: sensitization, resting and challenge. Sensitization usually consists of multiple i.p. injections of soluble OVA over a two or six week period, but may also be accomplished by subcutaneous or i.p. injection, with alum. Then the mice are “rested” for 7 to 40 days. Challenge is by intra-nasal injection or aerosol inhalation of soluble OVA, typically for 3 times with 3 days rest in between. Then 2 or 3 days after the last challenge chemical, physiologic or histologic analysis is used to evaluate the effects (for examples see Table 1). A prominent finding is increased airway resistance and enhanced respiratory pause after methacholine challenge (airway hyperresponsiveness, AHR) measured using a plethysmograph 8–10. Additional findings include: increased mononuclear cells and eosinophils in bronchial lavage fluid, goblet cell hypertrophy (GCHT) of the bronchial epithelium, peribronchial mononuclear cell infiltrates in the lung, and development of bronchus associated lymphoid tissue (BALT) 11, as well as production of circulating IgE antibody, a requirement for interleukin (IL)-4, IL-5 and IL-10 produced by CD4-Th2 cells 12, 13, and reduction of effects if treated with beta-blockers. Interestingly, mast cells, a critical mediator of atopic asthma in humans, are not required 13 and are not increased in lungs of affected mice14.
Table 1.
Summary of selected published results of experimental mouse models of ovalbumin (OVA)-induced asthma.
| Mouse strain | Sensitization | Challenge | Result | References |
|---|---|---|---|---|
| BALB/c | 1 μg OVA + alum, 2 x in 2 weeks. | 5% OVA aerosol 10–14 days later; variable sacrifice times. | Eosinophil infiltration of trachea, peak at 24 hrs; inhibition by anti CD4 and anti-IL-5 antibodies. | 1 |
| BALB/c | 10 μg i.p. OVA 7x in 2 weeks; no alum. | 1 or 8 aerosol challenges 4 weeks later; sacrifice 24 hrs after last challenge. | Immediate increase in airway responsiveness after challenge. | 15 |
| C57BL/6 IL-4-/- W/WV (mast cell def.); B6.Aa-/- | 10 μg OVA i.p. with alum; single injection. | Days 14–21 daily aerosol 1% OVA; euthanized 1 day later. | BAL eosinophilia; peribronchial mixed infiltrate (monocytes, lymphocytes and eosinophils). Goblet cell hyperplasia, IL-4 dependent; mast cells not required. Class II/T-cells required). | 13 |
| B6D2F1/J | 8 μg OVA in alum, i.p., days 0 and 5. | 0.5% OVA x 2 aerosol, 6–20 days later. Variable sacrifice times. | Blood and BAL fluid, eosinophilia in lung infiltrate, goblet cell hyperplasia, decreased by steroids. | 16 |
| C57BL/6J, RAG-1, P- selectin, CD4 and CD8 def. | 0.1 mg/mouse day 1. | 2% OVA aerosol 5 min, day 8; 1% aerosol, 20 min, days 15 to 21. | Early monocyte infiltrate followed by eosinophils and lymphocytes; Mature CD4+ T-cells required for lung eosinophilia. Goblet cell hyperplasia. | 17 |
| BALB/c | 10 μg OVA i.p. or s.c., 7x for 2 weeks, no alum. | 20 μg OVA i.t. days 40, 43, 46. Sacrificed 3 days later. | Mononuclear and eosinophil infiltration in lungs. Formation of BALT with IgE and IgA anti-OVA plasma cells. | 11 |
| BALB/c | 10 μg i.p. OVA 7x for 2 weeks; no alum. | 40 days after; i.t. 20 μg OVA; 1x, 3x, and 6x. | Eosinophils and monocytes in lung; hyperplasia of mucous cells before cells invade; fibrosis beneath basement membrane. | 18 |
| BALB/c | 100 μg OVA, 3 × 1 week dermal patch. | 1% OVA aerosol; single challenge; euthanized 24 hrs later. | Eosinophilia in BAL and airway hyperresponsiveness. | 19 |
| BALB/cj | 25 μg OVA s.c. in alum days 1, 7, 14. | 25 μg OVA i.n. days 21–25, sacrificed day 26. | Heterogeneous and increased airway responsiveness to methacholine challenge. | 20 |
| BALB/c C57BL/6J | 25 μg OVA i.p. days 1, 7, 14. | 25 μg OVA i.n. days 21–25, sacrificed day 26. | Chronic administration of beta-blockers reduces inflammation and mucous metaplasia. | 21 |
| BALB/c, FVB/N β2-AR-Null | 25 μg OVA i.p. days 1, 7, 14. | 25 μg OVA i.n. days 41–45; sacrificed on day 46. | Effects of β2-AR inverse agonists are caused by inhibition of β2-AR signaling pathway. | 22 |
| 129/BALB/c CCR3-/- | 100 μg OVA 3 × 1 week dermal patch. | 1% OVA aerosol, single challenge; euthanized 24 hrs later | CCR3 required for skin and lung eosinophilia; mast cell numbers did not increase in WT mice. | 14 |
| BALB/c | 10 μg OVA in alum i.p. | 1% OVA in aerosol 3 or 7 days for 1 or 6 weeks. | Eosinophilia in BAL, goblet cell hyperplasia, subepithelial collagen deposition, chronically. | 23 |
| BALB/c WT T-Bet-/- | 10 μg OVA i.p. 2x in alum for 2 weeks. | 1 week later; 100 μg OVA i.n. 1x daily for 5 days; euthanize after 24 hrs. | Eosinophils in lung, hyperplasia of mucous cells. IL-17 dependent. Histology of lung not described but pictures show BALT. | 24 |
| BALB/c | 100 μg OVA 2 x i.p. days 0 and 14 in alum. | 1% OVA 22–28 days; euthanized day 29; passive transfer of DC-CTLA4Ig. | DC-CTLA4Ig reduces inflammation and increases Th1 and Treg cells. | 25 |
| BALB/c | 50 μg OVA i.p. plus alum days 0, 14, 28. | 2% OVA i.t. days 35, 36, 37 and 40. | IL-10 produced by CD4+ T cells increased neutrophils. | 12 |
| BALB/c | 50 μg OVA i.p. plus alum days 0 and 12 | 5% OVA aerosol days 22, 26, 30; euthanized day 31. | A phosphodiesterase (PDE-4) inhibitor (PDE-423) reduces peribronchial inflammation. | 26 |
| C57Bl/6 | 10 μg OVA i.p. days 0, 7, 14, 21, 28 and 35. | 20 μg OVA i.n. days 40, 43, 46. | Inhibition of histamine-releasing-factor (HRF) reduces peribronchial inflammation. | 27 |
| BALB/c | 10 μg OVA i.p. in alum, 2x in 2 wks. | 1% OVA in aerosol, 3 days per week 1, 2 4, and 8 weeks. | Increased airway responsiveness. Increase in neutrophils,
eosinophils, etc. in BAL and tissues. Many eosinophils in lung inflammation. |
28 |
Abbreviations: def., deficient; i.n., intranasal; i.p., intraperitoneal; i.t., intratracheal; s.c., sub-cutaneous.
Many shortcomings of this model have been pointed out and pathologic descriptions of the pulmonary lesions reported2, 4–6. We now show for the first time that the major initiating pathologic change is immune complex mediated eosinophilic vasculitis followed by formation of induced bronchus-associated lymphoid tissue (iBALT). These changes precede pathologic changes in the bronchi consistent with asthma, including hyperplasia and hypertrophy of bronchial mucosa, peribonchial inflammation and smooth muscle hypertrophy. A basic principle of pathology is that it is usually not possible to determine how a lesion started by examination of the lesion at a late stage. We could not find a systematic study of the early changes in the lung after a single or double pulmonary challenge. We now report that the lesions of the experimental mouse models of asthma are not initiated by mast cell degranulation but by immune complex eosinophilic vasculitis. Sensitized mice have precipitating antibody and Arthus-type reactions when skin tested with OVA. In addition, although there are elevations of serum IgE antibody to OVA 1 week after the first challenge or after the second challenge, mast cells are only found in the most proximal part of the main stem bronchus and are not seen in the distal bronchus or peripheral lung of normal or sensitized and challenged mice.
Materials and Methods
Mice
Female BALB/c mice, 5–6 weeks old, were purchased from Taconic, Hudson, NY, and acclimatized and housed in the Wadsworth Center’s animal facility under controlled temperature and humidity in a 12 hour light/dark cycle. Purina lab chow and water were available ad lib. Mice were 7–8 weeks old at the start of each study. All experimental protocols were approved by the Wadsworth Center Animal Care Committee according to Animal Welfare Assurance Number A3183-01 of the National Institutes of Health. OVA was purchased from Sigma (catalogue number A5503).
Experimental design
Three different protocols of sensitization and challenge of mice with OVA were followed, based on methods described in the literature.
First method
A minor modification of the protocol of Hessel et al. 15 was used. Mice were sensitized by i.p. injections of 10 μg OVA (Sigma; catalogue number A5503) in 100 μL saline on alternate days for 2 weeks (seven injections) and then, 40 days after the beginning of sensitization, challenged intra-nasally with 25 μg OVA in 50 μL saline under anesthesia. Thirty-two mice were divided into three groups receiving 1, 2 or 3 challenges and 2 mice from each group euthanized at 6, 24, 72 hours and 1 and 2 weeks after challenge. Two control mice received 50 μL saline intra-nasally only. For skin testing, sensitized mice were injected in the shaved abdomen with saline on one side and 40 μg OVA (in 20 μL saline) on the other. Blood and bronchial-alveolar lavage (BAL) fluid were collected at euthanasia and then the mice were systemically perfused with 10 ml PBS. Lungs were instilled with 1 ml 4% paraformaldehyde (PFA) then immediately excised, placed in 4% PFA and dissected. Lungs and ovalbumin-injected skin were placed in one cassette; spleen, thymus, intestinal sections (Peyer’s patches) and saline-injected skin were placed in a second cassette. Blood was clotted at 4°C, spun down and serum collected and stored at −20°C. In addition in the second part of this experiment, 8 sensitized mice were injected intravenously (i.v.) with 500 μg OVA to determine if there might be a systemic reaction to antigen, such as anaphylactic shock. Anaphylactic shock was not elicited and no evidence of serum sickness was found.
Second method
For this model we followed the procedure published in the most recent report on the experimental asthma model that we could find 27, with slight modification. BALB/c mice, 8 weeks old at time of first sensitization, received 6 i.p. injections of ovalbumin (10 μg in 100 μL 0.9% saline) once per week for 6 weeks and divided into three groups. Two to 3 mice were euthanized 6, 24, 48, 72 hours and 1 and 2 weeks after a single; double or triple intranasal challenge with 25 μg OVA in 50 μL saline. All mice were skin tested 6 hours prior to euthanasia and the tissues collected and processed as described above.
Third method
This method was recently described as a short model that immunized with alum and did not use the extended sensitization period used in most of the models 29. Fourteen female BALB/c mice 6 weeks old were given a single i.p. sensitization of 100 μg of OVA in alum. On day 7 all mice were challenged intra-nasally with 100 μg of OVA. Two mice each were sacrificed at each of the following times after a single challenge: 6, 24, 48, 72 hours and 1 and 2 weeks. Controls included 2 sensitized mice that were not challenged and sacrificed at 24 hours and 2 non-sensitized, unchallenged mice. Second and third challenges were not given.
Blood, cell and tissue collection
Blood was collected from the facial vein under full anesthesia and individual drops were used to make smears on glass slides. The remaining blood was allowed to clot, then centrifuged and the serum removed and stored at −20 degrees C. The trachea was isolated, incised and an 18 gauge, blunt-ended needle was inserted to collect BAL cells, following three instillations (500 μL each) of 0.9% saline, 2.6 mM EDTA. The three samples were pooled, red blood cells lysed with ammonium chloride buffer and cytospins made on a Shandon cytocentrifuge. The systemic circulation was then perfused with 10 ml saline via the left ventricle. For tissue examination, the lungs and various organs from 4–6 mice per time point were collected and fixed in freshly prepared 4% PFA. Following automatic processing and embedding in paraffin, 5 μm sections were placed on conventional glass slides for hematoxylin and eosin staining. These slides were screened and select tissue blocks recut into serial sections for immunohistochemical analysis.
Blood Cytology
Blood smears were air-dried then stored at room temperature. Smears were fixed in methanol, serially stained with May-Grunwald (catalogue number 63550) and Giemsa (catalogue number 48900), both purchased from Sigma-Fluka Chemical Company (St. Louis, MO.) and differentiated with Sorensen’s buffer, pH 6.8. In addition, smears were stained with modified Wright stain on a Hematek slide stainer. Differential counts were done microscopically under oil immersion by a certified hematology technician.
Bronchoalveolar (BAL) Cells
To detect eosinophils, cytospins were dried overnight, fixed in methanol, serially stained with eosin and methylene blue/toluidine blue (0.06 and 0.04%, respectively), then rinsed in tap water and air dried. Mast cells were detected by a 1% toluidine blue solution (see below).
BAL Fluid Cytokine Analysis
Cytokines present in the BAL fluid were analyzed using a Luminex-based system. The panel of 6 cytokines (IL-2, IL-4, IL-5, IL-12, IL-13 and interferon-γ) was chosen to sample representative Th1 and Th2 cytokines and was purchased as a kit from R & D Systems (Minneapolis, MN). Following BAL lavage to collect cells, the pooled samples were centrifuged and the supernatants decanted into cryotubes and stored at −80 ° C until analysis. Plates were prepared according to manufacturer’s instructions using 50 μL of BAL fluid and cytokine levels read on a Luminex machine. Duplicate samples were analyzed from each animal. Data were pasted into an Excel file and graphed within Excel.
Histochemistry
For eosinophil detection in tissues, we followed the method of Albert et al. 30. Briefly, 5 μm paraffin sections were rehydrated, stained 1 hour in a 1% ethanolic solution of Congo Red (Sigma; catalogue # C6767), counterstained in hematoxylin, dehydrated and mounted in Permount (Fisher Scientific, Fair Lawn, NJ; catalogue # SP15). For mast cells in tissues, two methods were used. Sections were stained either with an ethanolic 1% toluidine blue (Polysciences Inc., Warrington, PA., catalogue # 01234) solution diluted 1:10 in 1% sodium chloride or by using the thionin technique, with thionin acetate (Sigma; catalogue # T7029). Mucicarmine and periodic acid Schiff staining followed standard techniques, using ingredients purchased from Sigma (periodic acid; product # P78750), J.T. Baker, St. Louis, MO., (carmine; product number 3-E380) and MCB, Norwood, Ohio (basic fuchsin; product # BX135).
Immunohistochemistry
Serial sections on charged glass slides were rehydrated through xylenes and ethanols to water, subjected to antigen retrieval and stained with various antibodies listed in Table 2 as described previously 31. Controls included sections treated with secondary peroxidase labeled antibody only. Signals were developed with Extravidin-peroxidase (Sigma; catalogue # E2886) and the chromogen was diaminobenzidine (Sigma; catalogue # D8001). Labeling was amplified using biotinylated tyramine, following the method of Kerstens 32. Images were captured on an Olympus microscope, model BX51 equipped with a digital camera and Optronics Version 1.2 software. All slides were evaluated by a board certified pathologist (Dr. Sell). Control immunoperoxidase staining is shown in Supplemental Fig. 1.
Table 2.
Antibodies used to label tissue sections.
| A. Primary antibodies | ||||||
|---|---|---|---|---|---|---|
| Antibody | Target | Source | Clone | Host | Concentration | Antigen retrieval |
| CD3 | T cells | Abcam ab5690 | Polyclonal | Rabbit | 1:500* | Tris HCl buffer |
| F4/80 | Tissue macrophages, Kupffer, Langerhans and dendritic cells. | AbD Serotec MCA497G | CLA3-1 | Rat | 1:5–10,000* | Proteinase K digestion |
| Ig (G + M) | Plasma cells, immunoglobulin | Jackson Immuno- Research # 115-065-044 | Polyclonal | Goat | 1:5,000* | Citrate buffer |
| Ovalbumin | Ovalbumin | Abcam Ab181688 | Polyclonal | Rabbit | 1:200 | Pronase |
| Pax-5 | B cells | Abcam ab109443 | 3852-1 | Rabbit | 1:10–20,000* | Citrate buffer |
| PPC | Type II pneumocyte | Abcam ab40879 | Polyclonal | Rabbit | 1:1,000 | Tris HCl buffer |
| B. Secondary Antibodies | |||
|---|---|---|---|
| Source | Host | Specificity | Label |
| Jackson ImmunoResearch # 112-065-167 | Goat | Rat | Biotin-SP* |
| Jackson ImmunoResearch # 705-065-147 | Donkey | Goat | Biotin-SP |
| Jackson ImmunoResearch # 111-065-144 | Goat | Rabbit | Biotin-SP |
, amplified with biotinylated tyramine (see text for details). PPC, prosurfactant protein C.
, d-Biotinyl-έ-aminocaproic acid-NHS-ester
Immunodiffusion
Gel preparation and staining followed the method of Hornbek 33. Ouchterlony plates were generated on conventional 1″ × 3″ glass slides, with an underlayer of 0.5% agar (Difco Noble agar; catalogue # 0142-01) and a top layer of 2%. Polyclonal rabbit anti-ovalbumin (catalogue # 1221) was purchased from Abcam, Cambridge, MA. Samples were pipetted into punch wells and the slides incubated 48 hours at room temperature in a humidified chamber. Gels were stained with 0.5% Coomassie Brilliant Blue R250 (Bio-Rad Laboratories, Richmond, CA.; catalogue number 161-0406), destained in 5% glacial acetic acid, 15% ethanol then air-dried and photographed.
IgE assay
IgE antibody to OVA in sera and BAL fluid was assayed by an ELISA kit for OVA-specific IgE (Chondrex Inc., Redmond, WA; kit number 3010), following the manufacturer’s directions. The OVA-specific antibody was a rat monoclonal, clone 77-1. A Bio-Tek micro-plate reader, model EL808 was used to read the plate at 450 nM.
Statistics
Statistical differences in means were determined by one way analysis of variance (ANOVA) with Tukey’s HSD (honest significant difference) test.
Results
Histologic grading of the lung lesions at 48 hours from total of 8 experiments using 3 different models is summarized in Table 3. There is no identifiable qualitative difference in the response to challenge after the various sensitization protocols, so that our observations are pooled for presentation.
Table 3.
Average histologic grade of reactions (0 to 3) at 48–72 hours.
| Exp. | Reference | Brief Description | Total # mice | Challenges | Fibrinoid Necrosis | PMNs | Perivascular Round Cells | Peribronchial Round Cells | iBALT |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 27 | Sensitization 1/week for 6 weeks; challenges 6, 9, 12 days later | 6 | 1 | 2.5 | 2.9 | 0.4 | 0.6 | 0 |
| 5 | 2 | 1.0 | 2.1 | 2.8 | 2.1 | 1.4 | |||
| 3 | 3 | 0.3 | 1.1 | 3.0 | 3.0 | 2.7 | |||
|
| |||||||||
| 2 | 15 | Sensitization 7/14 days; challenges 6, 9, 12, days later | 6 | 1 | 2.8 | 2.4 | 0.9 | 1.0 | 0 |
| 5 | 2 | 2.4 | 2.6 | 3.0 | 2.6 | 1.8 | |||
| 6 | 3 | 0.8 | 0.9 | 3.0 | 2.8 | 2.8 | |||
|
| |||||||||
| 3 | 29 | Sensitization 1 high dose with alum; challenge 7 days later | 4 | 1 | 2.5 | 2.8 | 0 | 1.0 | 0 |
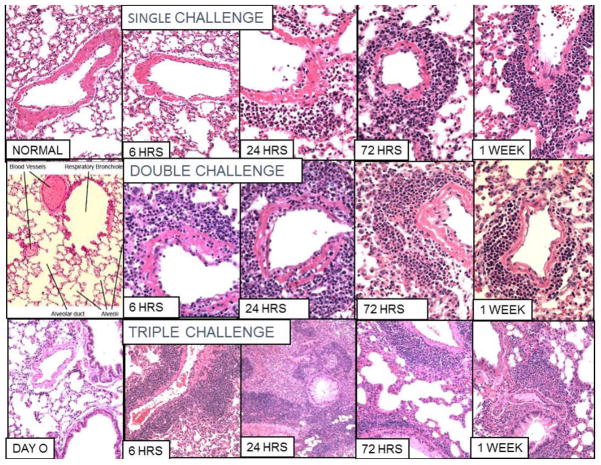
Vascular and peribronchial inflammation after single, double and triple challenge is shown by H&E staining in Fig. 1 (See also Table 4)
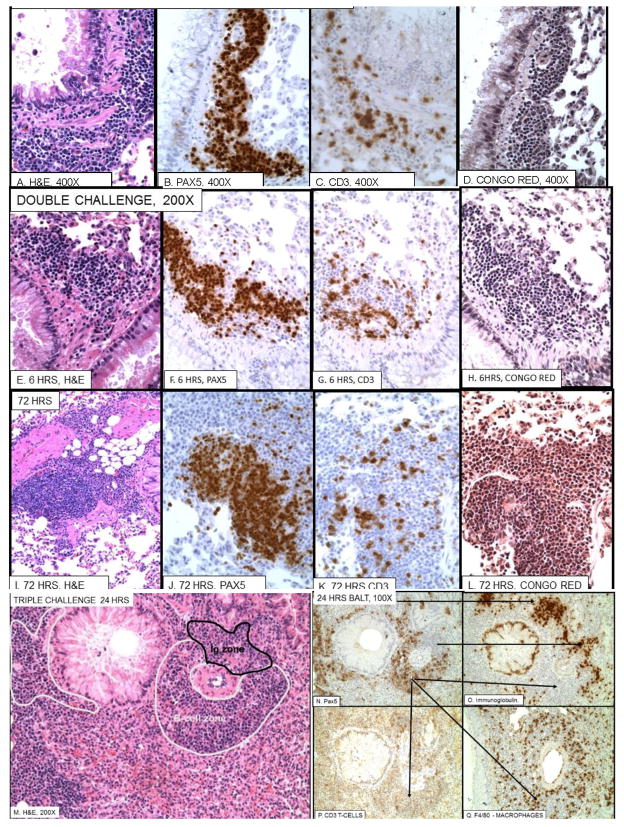
Figure 1. Vascular and bronchial inflammation in ovalbumin sensitized mice.
100X and 200X pictures of H&E stained microscopic slides after single (first row), double (second row), and triple (third row) intranasal ovalbumin (OVA) challenges of OVA sensitized mice. Normal and day 0 (before 3rd challenge) are shown in the first column for comparison. At 6 hours after a single challenge there is essentially no change in pulmonary arteries from normal. However after a second challenge there is severe fibrinoid necrosis and polymorphonuclear infiltrate. After 3 challenges there is little fibrinoid necrosis but a marked perivascular infiltrate of mononuclear cells. After 1 and 2 challenges the vasculitis resolves with slight residual round cell infiltrate. However, after three challenges there is extensive iBALT formation at 24–72 hours that then resolves over the next two weeks.
Table 4.
Histopathologic grading of lesions in lungs
| No. Challenges | Time after pulmonary challenge | 1 week | ||
|---|---|---|---|---|
| 6 hours | 24 hours | 72 hours | ||
| Necrotizing Vasculitis | ||||
|
| ||||
| 1 | 0 | + | ++ | + |
|
| ||||
| 2 | ++ | +++ | ++ | + |
|
| ||||
| 3 | + | + | + | 0 |
|
| ||||
| Perivascular Round Cell Infiltrate | ||||
|
| ||||
| 1 | 0 | 0 | + | + |
|
| ||||
| 2 | ++ | ++ | +++ | ++ |
|
| ||||
| 3 | +++ | ++++ | ++++ | +++ |
|
| ||||
| BALT Formation | ||||
|
| ||||
| 1 | 0 | 0 | + | + |
|
| ||||
| 2 | + | ++ | +++ | ++ |
|
| ||||
| 3 | ++ | +++ | ++++ | +++ |
|
| ||||
| Goblet Cell Hypertrophy and Hyperplasia (GCHHT) | ||||
|
| ||||
| 1 | 0 | 0 | + | 0 |
|
| ||||
| 2 | + | ++ | +++ | + |
|
| ||||
| 3 | +++ | ++++ | ++++ | ++ |
Note. H+E stained slides were coded and read by a board certified pathologist Grading was from 0 (normal) to +++++ (Highest level of infiltrate).
After a single challenge (Fig. 1, top row) vascular fibrinoid necrosis with polymorphonuclear cell (PMN) infiltrate appears at 24 hours and by 72 hours begins to change from eosinophilic PMN to mononuclear and shift from perivascular to peribronchial. By 2 weeks the inflammation has largely subsided, but a small cuff of peribronchial mononuclear cells can be seen (pictures not included). After double challenge (Fig. 1, second row) increased fibrinoid necrosis with eosinophilic PMNs is seen at 6 hours through 48 hours. By 72 hours, the infiltrate contains a majority of mononuclear cells with many fewer PMNs. Perivascular and then peribronchial mononuclear infiltration is greater than after a single challenge but is also resolving by 2 weeks. After triple challenge (Fig. 1, third row), there is little, if any, fibrinoid necrosis and very little vascular infiltrate with PMNs, Instead, the most striking change is perivascular and peribronchial mononuclear cell infiltration over 30 cells wide around vessels adjacent to bronchi as well as a mixed mononuclear infiltrate in the alveoli reaching a peak at 24 to 72 hours, then decreasing by 1 week.
Bronchial Lesions
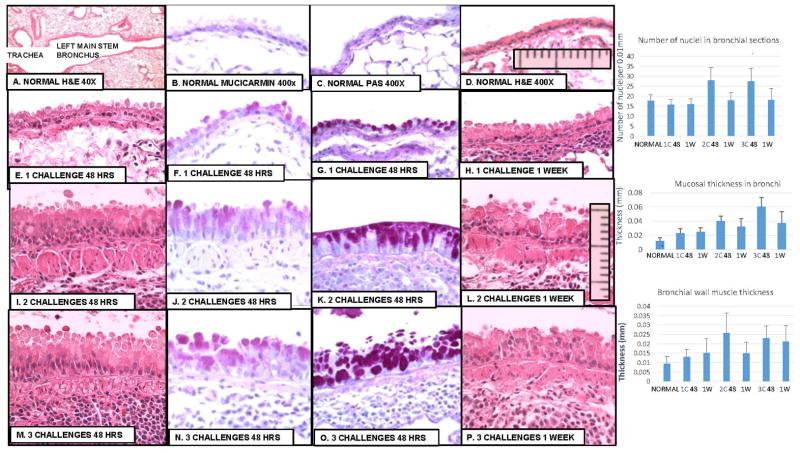
Increasing bronchial mucosal hyperplasia and hypertrophy as well as bronchial muscular hypertrophy seen after tracheal challenge are shown in Figure 2, and statistical analysis of measurements of changes in Table 5. Minimal changes occur as early as 48 hours after a single challenge (Fig. 2 E–H) but these quickly resolve if there is no further challenge. Significant submucosal muscle hypertrophy as well as bronchial mucosal hyperplasia and hypertrophy peak about 48–72 hours after the 2nd and 3rd challenges (Fig. 2. I–K; M–O). These gradually decrease so that by 2 weeks only a few focal areas remain.
Figure 2. Bronchial mucosal lesions.
A. 40X; B–H. 400X. A and B: normal lung. C, D: 1 challenge; E, F: 2 challenges; G, H: 3 challenges. A. Shows selection of tissue section including left main stem bronchus were changes can be measured accurately. B and F includes ocular micrometer scale used to count nuclei and measure thickness of mucosa and submucosal muscle. Table 5 shows the measurements and statistical analysis. Clearly by the second challenge there are lesions associated with asthma including mucosal hyperplasia and hypertrophy and hypertrophy of submucosal smooth muscle. There is a significant increase in mucosal cell nuclei at 48 hours after 2 and 3 challenges (p<01); in mucosal thickness and submucosal smooth muscle thickness after each challenge (p<01) as well as significant increases from one challenge to the next.
Table 5.
Goblet cell hyperplasia and Hypertrophy and bronchial smooth muscle hypertrophy.
| A, Number of nuclei per 0.01 mm in cross sections of bronchial epithelium in OVA-exposed mice. | |||||||
|---|---|---|---|---|---|---|---|
| Untreated | 1C, 48 hr | 1C, 1 Wk | 2C, 48 hr | 2C, 1 Wk | 3C, 48 hr | 3C, 1 Wk | |
| n | 11 | 11 | 9 | 19 | 14 | 12 | 12 |
| Mean | 17.7 | 15.7 | 16 | 27.7 | 17.8 | 27.4 | 18.1 |
| SD | 3 | 2.7 | 2.7 | 6.6 | 4 | 6.4 | 5.9 |
| ANOVA, Tukey HSD | NS | NS | P < .01* | NS | P < .01* | NS | |
| B. Mucosal thickness (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Untreated | 1 C, 48 hr | 1 C, 1 Wk | 2 C, 48 hr | 2 C, 1 Wk | 3 C, 48 hr | 3 C, 1 Wk | |
| n | 18 | 19 | 15 | 30 | 35 | 30 | 30 |
| Mean | 0.0117 | 0.023 | 0.0246 | 0.0403 | 0.032 | 0.06 | 0.0365 |
| SD | 0.0048 | 0.006 | 0.0058 | 0.0065 | 0.0112 | 0.0134 | 0.0166 |
| ANOVA, Tukey HSD | P<.01 vs UNT | P<.01 vs UNT; P<.01 vs 1C 48 hr | P<.01 vs UNT | P<.01 vs UNT; P<.05 vs 2C 48 hr | P<.01 vs UNT | P<.01 vs UNT; P<.01 vs 3C, 48 hr | |
| C. Submucosal smooth muscle thickness (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Untreated | 1 C, 48 hr | 1 C, 1 Wk | 2 C, 48 hr | 2 C, 1 Wk | 3 C, 48 hr | 3 C, 1 Wk | |
| n | 18 | 20 | 15 | 25 | 36 | 30 | 30 |
| Mean | 0.0093 | 0.013 | 0.0151 | 0.0257 | 0.0149 | 0.023 | 0.021 |
| SD | 0.004 | 0.0038 | 0.0075 | 0.0107 | 0.0058 | 0.0064 | 0.0087 |
| ANOVA, Tukey HSD | P<.05 vs UNT; | P<.05 vs UNT; P<.05 vs 1C, 48 hr | P<.01 vs UNT; | P<.05 vs UNT; P<.01 vs 2C, 48 hr | P<.01 vs UNT; | P<.05 vs UNT; P<.05 vs 3C, 48 hr | |
C, challenge; hr, hours; n, number of separate microscopic fields counted per treatment; NS, not significant; SD, standard deviation; Wk, week.
compared to untreated.
Eosinophilic vasculitis
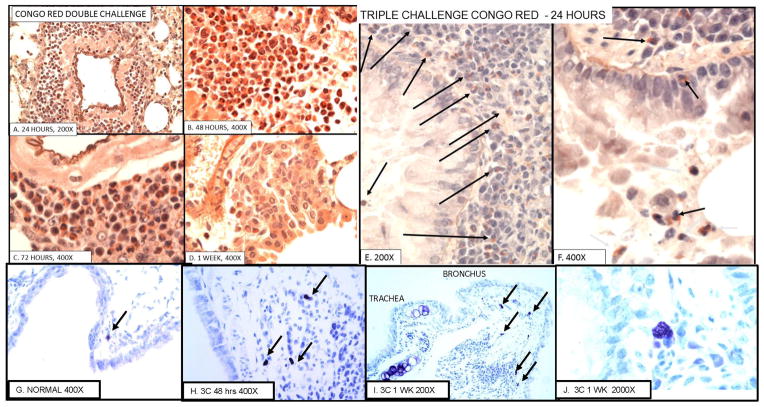
To confirm the nature of the PMNs in the acute vasculitis and peribronchial inflammation tissues from each challenge and time point were stained with Congo Red. Selected examples of the results are shown in Fig. 3 A–F. Twenty-four to 48 hours after a single (not shown) or double challenge most of the perivascular infiltrate is eosinophilic PMNs (Fig 3A, B). By 72 hours (Fig. 3C), there is an increasing number of mononuclear cells and by 1 week (Fig. 3D) most of the perivascular cells are mononuclear. However, even after 3 challenges, when most of the perivascular and peribronchial inflammation is mononuclear (see Fig. 4,) eosinophils are seen in the submucosa and lumen of the bronchi (Fig. 3. E, F).
Figure 3. Eosinophilic inflammation and mast cells.
A–D. Demonstration of eosinophilic vasculitis by Congo Red stain after double challenge and E, F: bronchial eosinophilia 24 hours after triple challenge. G–J. Mast cells in normal and challenged mice. A–D. Double challenge A. 24 hrs.; B. 48 hrs.; C. 72 hrs.; D. 1 week. E, F. Bronchi 24 hrs. after triple challenge. B shows high percentage of Congo Red positive eosinophils at day 2 and C shows lesser numbers of eosinophils and more mononuclear cells. These are replaced by round cells by 1 week (D). Only a few eosinophils remain by day 14 (not shown). E and F show eosinophils in bronchi after 3 challenges. There are few perivascular eosinophils after 3 challenges (see Fig 3). G. a mast cell in normal lung. H 3 mast cells 48 hours after triple challenge. I Low magnification showing 5 mast cells in proximal left main stem bronchus. J. High magnification of mast cell. Mast cells are only found in the most proximal main stem bronchus and not in lung periphery in both normal, sensitized, as well as sensitized and challenged mice.
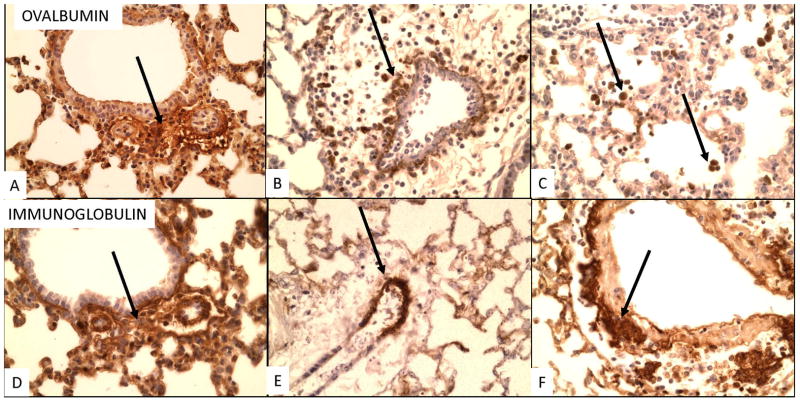
Figure 4. Immunoperoxidase labeling for OVA (A–D) and Ig (E–L) after pulmonary challenge.
200X or 400X. A and E show faint perivascular labeling around small vessels (arrows) at 6 hours after a single challenge. This is seen more clearly in enlargements in supplemental Fig. 2. At 24 hours most of the OVA labeling is in macrophages both in zones of perivascular inflammation (B, arrow) and scattered in alveoli (C, arrows). This is also seen 6 hrs after 3 challenges (D). At 24 hours Ig is seen as deposits in walls of vessels with inflammation. (F, G), but not in macrophages. 6 hrs after 3 challenges Ig is seen in dense deposits around vessels. D and H are pictures of serial sections showing perivascular localization of Ig and OVA in alveolar macrophages. We did not see OVA labeling at 72 hrs or later. After 72 hours labeling of Ig is mostly cellular. I, J: 1 challenge, 72 hrs and 1 week; K, L: 3 challenges, 72 hrs and 1 week.
Mast cells
Mast cells in the lung are limited to the first high power microscopic field between the trachea and the main stem bronchus (Fig. 3 G–J). Mast cells are not seen in either the remaining main stem bronchus or the peripheral lung of normal or sensitized and challenged mice. The number of mast cells per section ranged from 0 to 4 in normal mice to 0 to 3 after one challenge, 1 to 5 after 2 challenges and 0 to 6 after 3 challenges. Thus, mast cells may be focally increased at the tracheobronchial junction after challenge but the differences are not statistically significant.
Immunostaining for immunoglobulin (Ig) and OVA (Fig. 4)
Six hours after 1 challenge faint deposits of Ig and OVA are visible next to small vessels (see Supplemental Fig. 2). By 24 hours Ig deposits increase around small vessels and OVA is seen next to inflamed vessels, but OVA is mostly in alveolar macrophages (Fig. 4B, C). At later time points Ig is localized in cells around inflamed vessels (Fig. 4 I–L) and staining for OVA is negative. Staining for complement is not seen so that although the deposition if Ig and OVA is consistent with immune complexes, this cannot be proven at this time. Later cellular staining may be to uptake of Ig by inflammatory cells or presence of immunoglobulin containing plasma cells. Staining for complement was not possible in the formalin fixed paraffin embedded sections.
iBALT formation
The evolution of iBALT formation is shown in Fig. 5. The peribronchial collections of mononuclear cells first seen at 72 hours after a single challenge are made up mostly of Pax5+ B-cells (Fig 5B) with a scattering of CD3+ T-cells (Fig. 5C) and few eosinophils (Fig. 5D). Similar peribronchial B-cell collections are seen 6 hours after a second challenge, which is not surprising, since the second challenge is given 3 days after the first. By 72 hours after the second injection the peribronchial B-cell form ball-like masses (Fig. 5I–J) that are distinct from adjacent inflammation which is still esoinophilic (Fig. 5L). After the third challenge (Fig. 5M–Q), well-formed follicles containing Pax5+, Ig- B-cells zones and Pax5-, Ig+ (plasma cell) zones along with adjacent alveolar mixed mononuclear cell infiltrates predominate. In fact, most of the inflammatory change is composed of dense collections of B-cells that appear to arise in vascular areas next to bronchi. After 3 challenges focal collections of iBALT are still seen at 2 weeks, but in general all changes are substantially reduced from the peak at 24 hours (Fig. 1; bottom row, Table 3).
Figure 5. iBALT formation after single (A–D), double (E–L) and triple challenge (M–Q).
A–D. Single challenge, 72 hrs. E–H. Double challenge, 6 hrs. I–L Double challenge, 72 hrs. M–Q, Triple challenge, 24 hrs. The first evidence of iBALT is a peribronchial cuff of B-cells 72 hours after a single challenge (A–D). This is also present 6 hours after double challenge (E–H) and expands into larger zones of B-cells (Pax5+) at 72 hrs. (I–L). Peribronchial iBALT contains few, if any, eosinophils (N, L), although eosinophils are seen in adjacent Pax5- zones (compare J with L). From 24 to 72 hours after triple challenge, well-formed B-cell follicles appear around vessels next to bronchi (M–Q). These consist of zones of Pax5+ B-cells (white outline in M, Pax 5 stain in N) and Ig+, Pax 5- cells (black outline in M, Ig stain in 0), with less frequent T-cells (CD3, P), and macrophages (F4/80, Q). The two top arrows in M point from Pax5- zones to Ig+ zones in O. The three lower arrows in M point from a Pax5+ zone to corresponding Ig- zone in O; CD3+ zone in P and F4/80 zone in Q. Note mucous hyperplasia in M.
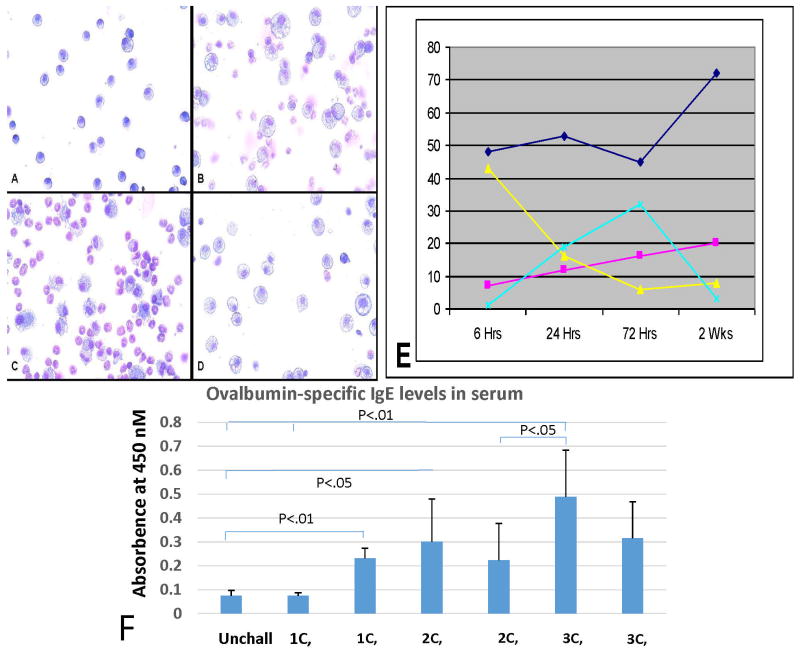
BAL fluid cells
Staining and percentage of cell types in BAL is shown in Fig. 6 A–D. The total cell counts rose from 3 × 104 on day 0 to 4.5 × 105 on day 1, then declined gradually to 9 × 104 at 2 weeks. One day following intranasal challenge there is a marked increase in cells with a corresponding increase in the percentage of neutrophils and eosinophils with a decrease in monocytes. By day 3 the percentage of eosinophils increases to over 50% while the percentage of monocytes drops (Fig. 6E). In addition, there is a marked increase in the number and size of monocyte cytoplasmic vacuoles. The percentage of eosinophils remains high for 1 week and then drops to pre-challenge levels at 2 weeks after challenge. Similar changes were recorded after triple challenge, but fewer time points are available.
Figure 6. Cells in bronchial lavage fluid (BAL) and IgE antibody to OVA in sera.
A–D Cells in BAL at 0, 24, 72 hours and 2 weeks. E. Graph of percentage of cells: eosinophils-light blue; monocytes-dark blue; lymphocytes-red; neutrophils-yellow. The points in E represent the average of 3 mice. F. shows development of IgE antibody to OVA at 48 hours and 1 week after 1, 2 or 3 challenges. Significant increases first occur 1 week after the first challenge or 48 hours after the second challenge which is about the same time as 48 hours after the second challenge (5 days after the first challenge).
Peripheral blood cells
The percentage of various white blood cells is shown in Supplemental Table 1. The only significant changes from normal or unchallenged mice is slightly but significantly elevated eosinophils in method 2 after 2 or 3 challenges and in method 3 after 1 challenge.
IgE antibody to OVA
The titers of IgE antibody to OVA in 3 to 6 mice 48 hours and 1 week after 1, 2 or 3 challenges are shown in Fig 6 F. There is no elevation over normal in sensitized, but unchallenged mice. There is a significant difference after each challenge, with levels reaching 5 times those of unchallenged mice after 3 challenges.
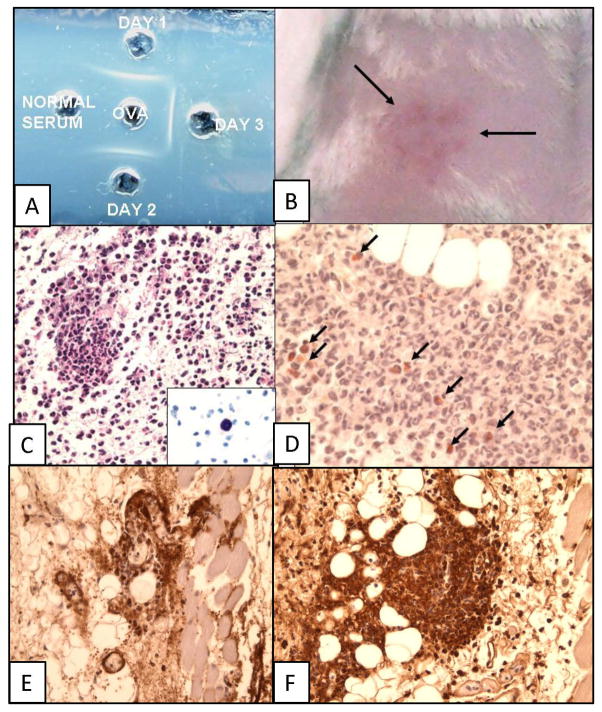
Precipitating Antibody and Arthus Reaction
The serum of each mouse tested positive for precipitating antibody by double diffusion in agar (Fig. 7A). Six hours after an injection of 40 μg of OVA into the shaved skin of each of the sensitized mice there is a grossly indurated and erythematous skin lesion (Fig. 7B. associated with focal PMNs around small subdermal vessels (Fig. 7 C–D), as well as diffuse PMN infiltration beneath the dermal muscle layer. No microscopic inflammation is seen in non-sensitized control mice or skin sites of sensitized mice injected with saline. Congo Red staining (Fig. 7D) reveals that most of the cells are neutrophilic PMNs, with only a few eosinophils; staining for T- and B-cells is essentially negative, but a few macrophages identified by F4/80 staining are seen (not shown). Mast cells are clearly seen in areas of the skin not involved in inflammation (Fig. 7C, insert) and are not increased in the sensitized mice. Both OVA (Fig. 7E) and Ig (Fig. 7F) are present in the perivascular lesions in the skin consistent with formation of immune complexes. We did not see an immediate skin reaction as described by Saloga et al. 34 that they observed after skin testing of mice sensitized by 10 consecutive inhalations of OVA.
Figure 7. Precipitating antibody (gel diffusion) and cutaneous Arthus reaction.
A. Double-diffusion-in-agar showing precipitating antibody to OVA in sensitized mice. B. Gross picture of skin showing area of inflammation after injection of OVA into skin of sensitized mouse (Arthus reaction). C. H&E and D, Congo Red staining of skin section of Arthus reaction showing predominance of neutrophils and few eosinophils (arrows in I) in dermis of skin 6 hours after injection of OVA (Arthus reaction). Insert in C shows polychromatic staining of mast cells in the skin. E. OVA and F. Ig immunoperoxidase staining.
Cytokines
There is a significant elevation of IL-5 48 hours after the first challenge and of IL-13 after all three challenges in mice sensitized according to method 2 (Supplemental Fig. 3). In addition, IL-4 is elevated 24 hours after challenge in the short model of sensitization (data not shown). The levels of the other cytokines measured (IL-12, IL-2 and INF-γ) are not elevated.
Discussion
According to the Mayo Clinic, asthma is a condition in which airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. According to the National Library of Medicine asthma is a respiratory condition marked by spasms in the bronchi of the lungs, causing difficulty in breathing. It usually results from an allergic reaction or other forms of hypersensitivity leading to reversible airway obstruction, airway hyperactivity and airway inflammation. These and other functional descriptions of asthma include a broad spectrum of conditions that may be useful clinically, but essentially ignore various possible differences in pathologic mechanisms and tissue pathology. Human acute asthma, for example, is included in the immunopathologic category of atopic or anaphylactic reactions unleashed when allergen reacts with and cross-links IgE receptors on mast cells35. Repeated activation of this mechanism results in the pathologic features of chronic human asthma: hyperplasia of bronchial smooth muscle, hyperplasia of bronchial mucous cells, chronic peribronchial inflammation with increased mast cells and thickening of the basement membrane of the bronchial mucosa 35. Herein we show that the ovalbumin mouse model of asthma has features of immune complex disease which cast doubt on its validity as a model for asthma. Bronchial lesions consistent with asthma (mucosal hyperplasia and hypertrophy and hyperplasia of bronchial smooth muscle) are preceded by immune complex vasculitis and iBALT formation. It is not clear why lesions typical of asthma are induced by an immune complex reaction; elevation of Th2 type cytokines that induce asthma like inflammation is seen but these are not usually associated with Th1 type immune complex reactions. Elevations of serum IgE suggest that some atopic-like Th2 mechanism may become active after the initial Th1 reaction, but the lack of mast cells in most of the major bronchus and lung periphery rule against this.
Immune complex eosinophilic vasculitis
The early lesions induced by a single injection of antigen into the lung of mice sensitized to produce the mouse model of asthma are fibrinoid necrosis of arterial walls and infiltration of eosinophilic PMNs. There is no evidence of acute bronchial constriction or mucous hyperplasia characteristic of acute human asthma at this time, although hyperplasia and hypertrophy of mucous cells begins about 3 days after primary challenge. We consider the acute vasculitis to be immune complex mediated except that the inflammatory cells are up to 70% eosinophilic PMNs rather than neutrophilic. Mice sensitized by multiple OVA injections or a single injection In alum demonstrate Arthus skin reactions 36 when skin tested by injection of OVA. The sera of these mice contain high titers of precipitating antibody as detected by double-diffusion-in-agar and both Ig and OVA are present in the vascular lesions of the skin as well as the vascular lesions of the lung. Immune complex vasculitis has been repeatedly demonstrated when soluble protein antigen is injected into the trachea of experimental animals either actively of passively immunized with antibody to the antigen.
Active pulmonary Arthus reactions
Active Arthus reactions in the lung were employed from 1912 to 1972 to determine the effect of immune complex induced injury37–44. This involved active immunization followed by pulmonary challenge with antigen. For example, Schlecht and Schwenker 37 saw pulmonary hemorrhage and acute alveolitis in immunized guinea pigs after inhalation of horse serum. Opie 38, repeated the approach in the lung originally described by Arthus in 1903 for the skin. In rabbits that had been immunized by repeated injections of horse serum, injection of horse serum into the lung produced “an acute localized consolidation with leucocytes and edema surrounding a central focus of necrosis.” Most of Opie’s studies were done on skin reactivity where he found that the reaction depends on the titer of precipitating antibody. In rabbits immunized to ovalbumin, Cannon and Walsh 40 describe alveolitis and bronchitis featuring vasculitis, but the histologic findings are not described. Fried 39 observed an influx of “granulocytes” 12 to 15 hours after intratracheal injection of horse serum into immunized rabbits. Eosinophils were also seen, but “on rare occasions”. The reaction peaked at 24 to 36 hours and then gradually subsided. This is essentially what we describe in OVA sensitized mice following a single intranasal injection of OVA, except for the predominance of eosinophils seen in our study. The suggestion that active Arthus reactions in the lung might have similarities to asthma was made by Kallos and Kallos, 1984 45, on the basis of an influx on eosinophils in the lungs of sensitized and challenged guinea pigs (see also Kung et al. 16). However, Sanjar et al., 46 noted that there is no evidence for a causal relationship between increased airway reactivity and eosinophilia in this model. The application of this model has extended to two divergent approaches: i, using passive reverse Arthus reactions in the lung to study the mechanisms involved in immune complex inflammation and ii, in mice as a model for asthma.
Reverse passive Arthus reactions in the lung
A number of laboratories have used a reverse passive Arthus reaction in the lung introduced by Ward and collaborators 47–51 to identify components of the early immune complex initiated inflammatory reaction. The standard protocol for this model is to inject antibody into the trachea followed within minutes by injection of antigen (bovine serum albumin or OVA) intravenously. Within 4 to 6 hours a neutrophil-rich exudate develops in alveolar and interstitial areas and then gradually fades. Deposits of antigen and antibody may be identified in the involved areas. Antibody, complement (C3), neutrophils and release of oxygen radicals are required 47. The inflammatory lesions are not localized perivascularlly as in the model described in the asthma models in this paper, since the antibody-antigen reaction takes place in the airway and not the vasculature. This inflammation is blocked by superoxide dismutase 48, but enhanced by tumor necrosis factor (TNF) released from resident macrophages 49. It requires interaction between neutrophils and endothelial cells mediated by endothelial-leukocyte adhesion molecule1 50, β2-integrins51, and endothelins, C3 and mast cells52; urokinase/urokinase receptors 53, FcγR- mediated TNF-α and CXCR2 ligand (CXCR2L) production 54, and Gαi2 protein-coupled C5a receptor which regulates IgG FcR function and enhances transmigration of neutrophils into inflammatory lesions 55. The key is the early C5aR-Gi-dependent signal for FcγRIII activation, local synthesis of TNF-α and CXCR2L leading to recruitment of PMNs. The factors responsible for eosinophic infiltration in our studies are under investigation.
Why eosinophilic vasculitis?
Most of the studies using the mouse models of asthma describe a marked increase in eosinophils, in bronchial lavage fluid 28, but not a significant increase in the peripheral blood (0 in controls to up to 0.1% in blood after challenge) whereas there is a marked increase in neutrophils in the blood (from 16% in controls to 28% in sensitized and three times challenged mice 56. This is more consistent with a Th1 type response (immmune complex) than a Th2 type response (atopic). The precise role of eosinophils in asthma is not clear. They may only be bystander cells or may contribute toxicity to the bronchial epithelium and to bronchoconstriction 57. A role for eosinophils is supported by the finding that the inflammation produced by the OVA challenge model is greatly reduced in mice depleted of eosinophils 58, 59. However, the striking eosinophilic vasculitis has not been described previously because previous studies were done after 3 OVA challenges. We show that eosinophilic vasculitis is readily seen after 1 or 2 OVA challenges, but is essentially replaced by mononuclear vasculitis after 3 challenges (Figs. 1 and 3). Although there is early fibrinoid necrosis, there appears to be little damage to bronchial epithelium or surrounding tissue and the lesions resolve quickly. Eosinophils contain at least 5 preformed enzymatic and non-enzymatic granular proteins that contribute to airway damage, inflammation and tissue remodeling 60. However, degranulation and peroxidase-mediated oxidation of airway proteins do not appear to play a significant role in OVA-induced inflammation in the mouse as mice deficient for eosinophil peroxidase display the same AHR and pathologies as wild-type mice 61.
The accumulation of eosinophils in the lung instead of neutrophils that would be expected in an immune complex vasculitis is most likely mediated by CCL11 (chemokine C-C motif ligand 11, also called eotaxin), by Th2 cytokines (e.g. IL-5) and by IL-23. CCL11 is increased in the OVA mouse model of asthma associated with AHR and pulmonary eosinophilia 62–64. Levels of CCL11 are consistently higher in asthmatic patients versus controls 65. IL-23 is produced by activated macrophages and dendritic cells and stimulates IL-17 and IL-22 secretion from innate immune cells 66. Eosinophilic infiltrate is increased in transgenic mice overexpressing the IL-23 receptor 67, 68, expression of eosinophilic peroxidase is greatly decreased in OVA challenged IL-23 knockout mice compared to WT mice 69, and inhibition of IL-23 release inhibits airway inflammation 70 reduces AHR and the number of eosinophils in BAL after OVA challenge 71. We propose that activation of IL-23 may be enhanced by uptake by and activation of alveolar macrophages by OVA in sensitized mice. Activation of CCL11, IL-5 and IL-23 in the lung may explain why the acute inflammatory response to OVA challenge in the lung is eosinophilic, whereas that in the skin reaction is predominantly neutrophilic 35, 36. IL-23 is most likely not activated in previously unchallenged skin and CCL11 is secreted by epithelial cells in the lung that are not present in the dermis of the skin.
Goblet cell hypertrophy and hyperplasia (GCHTH), a prominent feature in OVA challenged mice, is first seen 3 days after the first challenge Fig. 2). GCHTH is most likely stimulated by IL-17 72 and other Th2 cytokines, such as IL-13 and IL-33 73 as well as NF-κB 74. GCHT and increased intrabronchial mucous most likely affects changes in air exchange but it is not clear how this affects airway hypersensitivity. In most reports of the mouse models goblet cell hyperplasia is described. Our histologic analysis indicates an increase in size and mucous content of goblet cells (hypertrophy) and hyperplasia (increase in cell number) as well as smooth muscle hypertrophy.
Where are the mast cells?
In human allergic reactions, binding of the allergen to IgE on mast cells with subsequent release of mediators is the classic mechanism of the acute asthmatic response and increased numbers of mast cells are present in the peribronchial inflammation seen in chronic asthma. Mast cells are not increased in OVA challenged mice 24, and, are very rarely seen in normal mouse lung 75. However, mast cells have been described if sensitized mice are re-challenged repeatedly with OVA for 1 to 6 months 76. In the present study, mast cells were easily identified in the skin. However upon careful scanning sections selected to include the trachea and main stem bronchus of normal and inflamed lungs, 0 to 6 mast cells were seen only in the most proximal part of the main stem bronchus (Fig. 3 G–J). This is consistent with the findings of Haile et al. 77 that mast cells could be found in the connective tissue between the large vessels and the bronchi, but not in the smaller bronchi or alveolar tissue. They also did not see an increase in mast cells after challenge. Mast cells have been identified in the lungs and BAL of C57BL/6 mice by Fuchs et al. 78, but the role of these in AHR is not definitive. They found that AHR was not different in wild type and mast cell-deficient mice after OVA challenge, but reactivity in mast cell-deficient mice was increased after engraftment of bone marrow derived mast cells. This interesting result suggests that putative resident mast cells in the lung, if present, do not contribute to AHR, but that transferred mast cells from the bone marrow do.
iBALT formation
BALT is an adaptive immune response in the lung and is a site where local immune responses can occur rapidly after exposure to inhaled antigens. BALT is a constitutive mucosal lymphoid tissue in rats and rabbits, but is not present normally in humans or mice. However, BALT is inducible in humans and mice after inflammation in the lung 79, 80, so-called inducible BALT (iBALT). iBALT is seen in chronic lung infections in humans 81, as well as in mice with repeated virus infections of the lung 82. iBALT has also been described in humans with asthma 83. Mice lacking spleen, lymph nodes and Peyer’s patches develop iBALT in response to influenza challenge, clear influenza infection and, when BALT is present, survive higher doses of the virus than do normal mice 84. The presence of iBALT in response to one viral infection enhances the immune response to subsequent infection with unrelated viruses (heterologous immunity 82). Thus, BALT may be considered a local site for immune memory that can respond quickly to a second pulmonary infection. IL-5 and IL-17, both elevated in OVA sensitized mice 24, 67, 85–88, may act synergistically to stimulate iBALT formation. IL-5 increases B-cell proliferation 89. IL-17 increases expression of the chemokines CXCL12 and CXCL13 90 which are critical for follicle formation 91 with or without the participation of dendritic cells 92. Thus, the late histologic changes in the OVA mouse lung inflammation model appear to be mediated by Th2 and Th17 cytokines and are rapidly reversed when these cytokines are no longer secreted.
Comparison to human pulmonary vasculitis
It is not clear how this experimental induction of chronic vascular and peribronchial inflammation in the lung in mice compares to naturally occurring pulmonary vasculitis in humans 93, 94. The lesions produced involve what would be considered medium-sized vessels in humans. This makes the small-vessel vasculitides associated with anti-neutrophil cytoplasmic antibody (ANCA) and the large vessels lesions such as Giant cell arteritis and Takayasu’s arteritis, unlikely candidates. Churg-Strauss syndrome is a likely candidate 95. Also known as eosinophilic granulomatosis with polyangitis or allergic granulomatosis, this syndrome features high numbers of eosinophils and eosinophilic vasculitis in the lung associated with asthma and/or allergic rhinitis 96. The lesions of adult respiratory distress syndrome (ARDS) involve small vessels and result in damage to the alveolar-capillary membrane 97, which is not seen in this model. The most likely associations for the acute lesions would be with primary immune complex-mediated vasculitis, such as Goodpasture’s syndrome involving the lung, polyarteritis nodosa or serum sickness. However, there may be no naturally occurring lesions in the lung that correspond to those produced by the massive and multiple doses of soluble antigen to sensitized mice.
Final comments
Of what use is the study of the physiologic changes or pharmacologic alterations of the mouse model of asthma for understanding human asthma? Clearly the sensitization and bronchial challenge of mice with OVA produces histologic changes in the bronchi consistent with human asthma. Thus, it could be argued that, even though the basic pathologic mechanism (immune complex vasculitis) is different from that of human asthma (atopic or anaphylactic), identifying physiologic mechanisms or efficacious treatment might be possible using this model. The role of an atopic mechanisms in producing the bronchial lesions is unclear. Although IgE antibody becomes elevated after the first challenge, it is not elevated in sensitized, unchallenged mice and mast cells are only seen in the most proximal main stem bronchi and not in the remaining main stem bronchi or more distal bronchi where the asthma-like hypertrophy and hyperplasia of mucosa cells and smooth muscle hypertrophy is seen.
On the other hand, intervention directed against the underlying pathogenic mechanism of human asthma would not be possible in the mouse model because the reaction responsible for the lesions of the mouse model of asthma is not the same as for human asthma. For example, one of the possible mechanisms thought to mediate desensitization of allergic reactions by immunizing with the allergen is an attempt to shift the immune response from IgE antibody to IgG antibody. In this approach, the IgG antibody prevents the allergen from reacting with IgE on mast cells and thus inhibits the atopic reaction. However, in the mouse model it appears that IgG antibody actually initiates the pathologic response to OVA challenge; precipitating antibody is present in sensitized, unchallenged mice at a time when IgE antibody is not yet elevated.
Supplementary Material
A–C. CD3, 20X A. spleen, B. thymus, C. pulmonary lymph node; D–F. Pax5 20X D. spleen, E. thymus, F. pulmonary lymph node; G–H. F4/80, G. spleen, 20X; H. pulmonary lymph node, 10X; I. liver, 20X. Arrows in A, D and G point to central arteriole of white pulp. Arrow in H points to labeled cells in medulla of lymph node. J–L. Protein C (surfactant) Lung. J. 20X, K. 20X, L. 40X, arrows in L point to small cuboidal cells in alveolar walls. c-cortex, m-medulla. CD3 labels T-cell zones of spleen (periarteriolar sheath), thymus and lymph node; Pax5 labels B-cell zones: mantle and marginal zone of splenic white pulp, cortex of lymph node and very few subcortical cells in thymus. F4/80 labels cells in red pulp of spleen, a few cells in medulla of lymph node and Kupffer cells in the liver, but does not label cells in lymph node cortex. Protein C is marker for Type II pneumocytes.
The arrows point to putative immunoperoxidase labeling of OVA (A) and Ig (B) in perivascular zones 6 hours after the first challenge with OVA.
Cytokine measurements at 48 hours and 1 week after 1, 2 and 3 OVA challenges of sensitized mice. Blue, IL-5; Green, IL-13. Controls are a mean of 4 mice; experimental the average of 2–4 mice. The bars indicate standard errors.
Acknowledgments
Thanks to Helen Johnson at the Wadsworth Core Laboratory for helping to cut tissue sections, to Nancy Andersen for assistance with the Luminex assay, and to Kathleen Curran for preforming differential counts on peripheral blood smears. Dr. Guest carried out the experiments, obtained tissues and did all histological and immunostaining. Dr. Sell evaluated all slides, took photomicrographs and prepared figures. Both authors contributed to manuscript preparation.
Abbreviations
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar
- BALT
bronchus-associated lymphoid tissue
- C3
complement
- CCL
chemokine C-C motif ligand
- CXCR
chemokine receptor
- GCHTH
goblet cell hypertrophy and hyperplasia
- iBALT
induced BALT
- Ig
immunoglobulin
- IL
interleukin
- i.p
intraperitoneal
- i.v
intravenous
- OVA
ovalbumin
- PFA
paraformaldehyde
- PMN
polymorphonuclear
- PPC
prosurfactant protein C
- TNF
tumor necrosis factor
Footnotes
Disclosures
The authors declare that they have no conflicts of interest.
References
- 1.Nakajima H, Iwamoto I, Tomoe S, et al. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. The American review of respiratory disease. 1992;146(2):374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- 2.Cohn L. Food for thought: can immunological tolerance be induced to treat asthma? Am J Respir Cell Mol Biol. 2001;24(5):509–512. doi: 10.1165/ajrcmb.24.5.f207. [DOI] [PubMed] [Google Scholar]
- 3.Zosky GR, Sly PD. Animal models of asthma. Clin Exp Allergy. 2007;37(7):973–988. doi: 10.1111/j.1365-2222.2007.02740.x. [DOI] [PubMed] [Google Scholar]
- 4.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Disease models & mechanisms. 2008;1(4–5):213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar RK, Foster PS. Are mouse models of asthma appropriate for investigating the pathogenesis of airway hyper-responsiveness? Frontiers in physiology. 2012;3:312. doi: 10.3389/fphys.2012.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullane K, Williams M. Animal models of asthma: reprise or reboot? Biochem Pharmacol. 2014;87(1):131–139. doi: 10.1016/j.bcp.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Kumar RK, Herbert C, Foster PS. The “classical” ovalbumin challenge model of asthma in mice. Curr Drug Targets. 2008;9(6):485–494. doi: 10.2174/138945008784533561. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Hamelmann E, Joetham A, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186(3):449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116(6):1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizutani N, Nabe T, Yoshino S. IL-17A promotes the exacerbation of IL-33-induced airway hyperresponsiveness by enhancing neutrophilic inflammation via CXCR2 signaling in mice. J Immunol. 2014;192(4):1372–1384. doi: 10.4049/jimmunol.1301538. [DOI] [PubMed] [Google Scholar]
- 11.Chvatchko Y, Kosco-Vilbois MH, Herren S, Lefort J, Bonnefoy JY. Germinal center formation and local immunoglobulin E (IgE) production in the lung after an airway antigenic challenge. J Exp Med. 1996;184(6):2353–2360. doi: 10.1084/jem.184.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabe T, Ikedo A, Hosokawa F, et al. Regulatory role of antigen-induced interleukin-10, produced by CD4(+) T cells, in airway neutrophilia in a murine model for asthma. Eur J Pharmacol. 2012;677(1–3):154–162. doi: 10.1016/j.ejphar.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Brusselle GG, Kips JC, Tavernier JH, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24(1):73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 14.Ma W, Bryce PJ, Humbles AA, et al. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest. 2002;109(5):621–628. doi: 10.1172/JCI14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessel EM, Van Oosterhout AJM, Garsen J, Van Loveren H, Savelkoul HFJ, Nijkamp RP. Immediate asthmatic reactions and changes in airway responsiveness after single versus chronic ovalbumin inhalation in sensitized mice. Allergy: Eur J Allergy Clin Immunol. 1993;48(Supplement 16):101. [Google Scholar]
- 16.Kung TT, Jones H, Adams GK, 3rd, et al. Characterization of a murine model of allergic pulmonary inflammation. International archives of allergy and immunology. 1994;105(1):83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalo JA, Lloyd CM, Kremer L, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J Clin Invest. 1996;98(10):2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol. 1996;14(5):425–438. doi: 10.1165/ajrcmb.14.5.8624247. [DOI] [PubMed] [Google Scholar]
- 19.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101(8):1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans KL, Bond RA, Corry DB, Shardonofsky FR. Frequency dependence of respiratory system mechanics during induced constriction in a murine model of asthma. J Appl Physiol. 2003;94(1):245–252. doi: 10.1152/japplphysiol.00593.2002. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen LP, Omoluabi O, Parra S, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38(3):256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen LP, Lin R, Parra S, et al. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci U S A. 2009;106(7):2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Locke NR, Royce SG, Wainewright JS, Samuel CS, Tang ML. Comparison of airway remodeling in acute, subacute, and chronic models of allergic airways disease. Am J Respir Cell Mol Biol. 2007;36(5):625–632. doi: 10.1165/rcmb.2006-0083OC. [DOI] [PubMed] [Google Scholar]
- 24.Durrant DM, Gaffen SL, Riesenfeld EP, Irvin CG, Metzger DW. Development of allergen-induced airway inflammation in the absence of T-bet regulation is dependent on IL-17. J Immunol. 2009;183(8):5293–5300. doi: 10.4049/jimmunol.0803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying L, Fu Z, Luo J, et al. Cytotoxic T lymphocyte antigen 4 immunoglobulin modified dendritic cells attenuate allergic airway inflammation and hyperresponsiveness by regulating the development of T helper type 1 (Th1)/Th2 and Th2/regulatory T cell subsets in a murine model of asthma. Clin Exp Immunol. 2011;165(1):130–139. doi: 10.1111/j.1365-2249.2011.04405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwak HJ, Nam JY, Song JS, No Z, Yang SD, Cheon HG. Discovery of a novel orally active PDE-4 inhibitor effective in an ovalbumin-induced asthma murine model. Eur J Pharmacol. 2012;685(1–3):141–148. doi: 10.1016/j.ejphar.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Kashiwakura JC, Ando T, Matsumoto K, et al. Histamine-releasing factor has a proinflammatory role in mouse models of asthma and allergy. J Clin Invest. 2012;122(1):218–228. doi: 10.1172/JCI59072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson SJ, Harmer MJ, Lee RL, et al. Recurring BALB/c mouse lung inflammatory responses to episodic allergen exposure. Journal of toxicology and environmental health Part A. 2013;76(3):176–191. doi: 10.1080/15287394.2013.752323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw OM, Harper JL. An efficient single prime protocol for the induction of antigen-induced airways inflammation. J Immunol Methods. 2013;395(1–2):79–82. doi: 10.1016/j.jim.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Albert EJ, Duplisea J, Dawicki W, Haidl ID, Marshall JS. Tissue eosinophilia in a mouse model of colitis is highly dependent on TLR2 and independent of mast cells. Am J Pathol. 2011;178(1):150–160. doi: 10.1016/j.ajpath.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sell S, Guest I, McKinstry KK, et al. Immunopathology of influenza: Pulmonary viral exanthema, BALT and immune sensitive carcinoma in experimental mouse models. Viral Immunology. 2014 [Google Scholar]
- 32.Kerstens HM, Poddighe PJ, Hanselaar AG. A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem. 1995;43(4):347–352. doi: 10.1177/43.4.7897179. [DOI] [PubMed] [Google Scholar]
- 33.Hornbek P. Double-immunodiffusion assay for detecting specific antibodies. Curr Prot Immunol. 2001;(Unit 2.3):2.3.1–2.3.4. doi: 10.1002/0471142735.im0203s00. [DOI] [PubMed] [Google Scholar]
- 34.Saloga J, Renz H, Lack G, et al. Development and transfer of immediate cutaneous hypersensitivity in mice exposed to aerosolized antigen. J Clin Invest. 1993;91(1):133–140. doi: 10.1172/JCI116162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sell S. Immunology, Immunopathology and Immunity. 6. Washington, DC: ASM Press; 2001. p. 753. [Google Scholar]
- 36.Arthus M. Injections repetees de serum de cheval chez le lapin. C R Soc Biol (Paris) 1903;55:817. [Google Scholar]
- 37.Schlecht J, Schwenker Ubar die Beziehunger der Eosinophilie zur Anaphylaxie. Deutsches Arch J Klin Med. 1912;108:405–428. [Google Scholar]
- 38.Opie EL. The Fate of Antigen (Protein) in an Animal Immunized against It. J Exp Med. 1924;39(5):659–675. doi: 10.1084/jem.39.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried BM. Allergic Lobar Pneumonia: Experimental Study. J Exp Med. 1933;57(1):111–119. doi: 10.1084/jem.57.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cannon PR, Walsh TE, Marshall CE. Acute local anaphylactic inflammation of the lungs. Am J Pathol. 1941;17(5):777–784. [PMC free article] [PubMed] [Google Scholar]
- 41.McKinnon GE, Andrews EC, Jr, Heptinstall RH, Germuth FG., Jr An immunohistochemical study of the occurrence of intravascular antigen-antibody precipitation and its role in anaphylaxis in the rabbit. Bulletin of the Johns Hopkins Hospital. 1957;101(5):258–280. [PubMed] [Google Scholar]
- 42.Eskensay A. Immunomorphology of experimental Arthus phenomenon of the lung. Rev Immunol (Paris) 1971;35:85–101. [PubMed] [Google Scholar]
- 43.Richerson HB, Cheng FH, Bauserman SC. Acute experimental hypersensitivity pneumonitis in rabbits. The American review of respiratory disease. 1971;104(4):568–575. doi: 10.1164/arrd.1971.104.4.568. [DOI] [PubMed] [Google Scholar]
- 44.Eastham WN, Muller HK. Changes in guinea-pig lungs following the inhalation of powdered egg albumen. Pathology. 1972;4(3):235–241. doi: 10.3109/00313027209068946. [DOI] [PubMed] [Google Scholar]
- 45.Kallos P, Kallos L. Experimental asthma in guinea pigs revisited. Int Arch Allergy Appl Immunol. 1984;73(1):77–85. doi: 10.1159/000233441. [DOI] [PubMed] [Google Scholar]
- 46.Sanjar S, Aoki S, Kristersson A, Smith D, Morley J. Antigen challenge induces pulmonary airway eosinophil accumulation and airway hyperreactivity in sensitized guinea-pigs: the effect of anti-asthma drugs. Br J Pharmacol. 1990;99(4):679–686. doi: 10.1111/j.1476-5381.1990.tb12989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson KJ, Ward PA. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormick JR, Harkin MM, Johnson KJ, Ward PA. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 49.Warren JS, Yabroff KR, Remick DG, et al. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989;84(6):1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulligan MS, Varani J, Dame MK, et al. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991;88(4):1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulligan MS, Varani J, Warren JS, et al. Roles of beta 2 integrins of rat neutrophils in complement- and oxygen radical-mediated acute inflammatory injury. J Immunol. 1992;148(6):1847–1857. [PubMed] [Google Scholar]
- 52.Baumann U, Chouchakova N, Gewecke B, et al. Distinct tissue site-specific requirements of mast cells and complement components C3/C5a receptor in IgG immune complex-induced injury of skin and lung. J Immunol. 2001;167(2):1022–1027. doi: 10.4049/jimmunol.167.2.1022. [DOI] [PubMed] [Google Scholar]
- 53.Shushakova N, Eden G, Dangers M, et al. The urokinase/urokinase receptor system mediates the IgG immune complex-induced inflammation in lung. J Immunol. 2005;175(6):4060–4068. doi: 10.4049/jimmunol.175.6.4060. [DOI] [PubMed] [Google Scholar]
- 54.Skokowa J, Ali SR, Felda O, et al. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J Immunol. 2005;174(5):3041–3050. doi: 10.4049/jimmunol.174.5.3041. [DOI] [PubMed] [Google Scholar]
- 55.Wiege K, Ali SR, Gewecke B, et al. Galphai2 is the essential Galphai protein in immune complex-induced lung disease. J Immunol. 2013;190(1):324–333. doi: 10.4049/jimmunol.1201398. [DOI] [PubMed] [Google Scholar]
- 56.Sutherland MA, Shome GP, Hulbert LE, Krebs N, Wachtel M, McGlone JJ. Acute stress affects the physiology and behavior of allergic mice. Physiol Behav. 2009;98(3):281–287. doi: 10.1016/j.physbeh.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56(4):985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 58.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 59.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305(5691):1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 60.Acharya KR, Ackerman SJ. Eosinophil Granule Proteins: Form and Function. J Biol Chem. 2014;289(25):17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denzler KL, Borchers MT, Crosby JR, et al. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167(3):1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 62.Scheerens J, van Gessel SB, Nijkamp FP, Folkerts G. Eotaxin protein levels and airway pathology in a mouse model for allergic asthma. Eur J Pharmacol. 2002;453(1):111–117. doi: 10.1016/s0014-2999(02)02364-6. [DOI] [PubMed] [Google Scholar]
- 63.Eum SY, Maghni K, Hamid Q, et al. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol. 2003;111(5):1049–1061. doi: 10.1067/mai.2003.1416. [DOI] [PubMed] [Google Scholar]
- 64.Baek KJ, Cho JY, Rosenthal P, Alexander LE, Nizet V, Broide DH. Hypoxia potentiates allergen induction of HIF-1alpha, chemokines, airway inflammation, TGF-beta1, and airway remodeling in a mouse model. Clinical immunology. 2013;147(1):27–37. doi: 10.1016/j.clim.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu D, Zhou J, Bi H, et al. CCL11 as a potential diagnostic marker for asthma? The Journal of asthma: official journal of the Association for the Care of Asthma. 2014:1–8. doi: 10.3109/02770903.2014.917659. [DOI] [PubMed] [Google Scholar]
- 66.McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev. 2014;260(1):129–144. doi: 10.1111/imr.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Hua S. Mechanisms of pathogenesis in allergic asthma: Role of interleukin-23. Respirology. 2014;19(5):663–669. doi: 10.1111/resp.12299. [DOI] [PubMed] [Google Scholar]
- 68.Peng J, Yang XO, Chang SH, Yang J, Dong C. IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation. Cell research. 2010;20(1):62–71. doi: 10.1038/cr.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciprandi G, Cuppari C, Salpietro C. Serum IL-23: a surrogate biomarker for asthma? Clin Exp Allergy. 2012;42(9):1416–1417. doi: 10.1111/j.1365-2222.2012.04068.x. author reply 1418. [DOI] [PubMed] [Google Scholar]
- 70.Ishizuka T, Hisada T, Aoki H, Mori M. Resolvin E1: a novel lipid mediator in the resolution of allergic airway inflammation. Expert review of clinical immunology. 2008;4(6):669–672. doi: 10.1586/1744666X.4.6.669. [DOI] [PubMed] [Google Scholar]
- 71.Masaki K, Suzuki Y, Kagawa S, et al. Dual role of interleukin-23 in epicutaneously-sensitized asthma in mice. Allergology international: official journal of the Japanese Society of Allergology. 2014;63 (Suppl 1):13–22. doi: 10.2332/allergolint.13-OA-0632. [DOI] [PubMed] [Google Scholar]
- 72.Xia W, Bai J, Wu X, et al. Interleukin-17A promotes MUC5AC expression and goblet cell hyperplasia in nasal polyps via the Act1-mediated pathway. PLoS One. 2014;9(6):e98915. doi: 10.1371/journal.pone.0098915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanabe T, Shimokawaji T, Kanoh S, Rubin BK. IL-33 stimulates CXCL8/IL-8 secretion in goblet cells but not normally differentiated airway cells. Clin Exp Allergy. 2014;44(4):540–552. doi: 10.1111/cea.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou E, Fu Y, Wei Z, Yang Z. Inhibition of allergic airway inflammation through the blockage of NF-kappaB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 2014 doi: 10.1039/c4fo00384e. [DOI] [PubMed] [Google Scholar]
- 75.Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118(1):41–49. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- 76.Ikeda RK, Miller M, Nayar J, et al. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171(9):4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 77.Haile S, Lefort J, Joseph D, Gounon P, Huerre M, Vargaftig BB. Mucous-cell metaplasia and inflammatory-cell recruitment are dissociated in allergic mice after antibody- and drug-dependent cell depletion in a murine model of asthma. Am J Respir Cell Mol Biol. 1999;20(5):891–902. doi: 10.1165/ajrcmb.20.5.3446. [DOI] [PubMed] [Google Scholar]
- 78.Fuchs B, Sjoberg L, Moller Westerberg C, et al. Mast cell engraftment of the peripheral lung enhances airway hyperresponsiveness in a mouse asthma model. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1027–1036. doi: 10.1152/ajplung.00227.2012. [DOI] [PubMed] [Google Scholar]
- 79.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Advances in immunology. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shilling RA, Williams JW, Perera J, et al. Autoreactive T and B cells induce the development of bronchus-associated lymphoid tissue in the lung. Am J Respir Cell Mol Biol. 2013;48(4):406–414. doi: 10.1165/rcmb.2012-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68(1):1–8. doi: 10.1159/000028109. [DOI] [PubMed] [Google Scholar]
- 82.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol. 2003;163(4):1341–1355. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elliot JG, Jensen CM, Mutavdzic S, Lamb JP, Carroll NG, James AL. Aggregations of lymphoid cells in the airways of nonsmokers, smokers, and subjects with asthma. Am J Respir Crit Care Med. 2004;169(6):712–718. doi: 10.1164/rccm.200308-1167OC. [DOI] [PubMed] [Google Scholar]
- 84.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 85.McKinley L, Alcorn JF, Peterson A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishioka T, Yamada Y, Kimura H, et al. Elevated macrophage inflammatory protein 1alpha and interleukin-17 production in an experimental asthma model infected with respiratory syncytial virus. International archives of allergy and immunology. 2013;161 (Suppl 2):129–137. doi: 10.1159/000350427. [DOI] [PubMed] [Google Scholar]
- 87.Kim MS, Cho KA, Cho YJ, Woo SY. Effects of interleukin-9 blockade on chronic airway inflammation in murine asthma models. Allergy, asthma & immunology research. 2013;5(4):197–206. doi: 10.4168/aair.2013.5.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang JY, Lee SY, Rhee CK, Kim SJ, Kwon SS, Kim YK. Effect of aging on airway remodeling and muscarinic receptors in a murine acute asthma model. Clin Interv Aging. 2013;8:1393–1403. doi: 10.2147/CIA.S50496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 90.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 91.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fleige H, Ravens S, Moschovakis GL, et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med. 2014;211(4):643–651. doi: 10.1084/jem.20131737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 94.Brown KK. Pulmonary vasculitis. Proc Am Thorac Soc. 2006;3(1):48–57. doi: 10.1513/pats.200511-120JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951;27(2):277–301. [PMC free article] [PubMed] [Google Scholar]
- 96.Hellmich B, Ehlers S, Csernok E, Gross WL. Update on the pathogenesis of Churg-Strauss syndrome. Clinical and experimental rheumatology. 2003;21(6 Suppl 32):S69–77. [PubMed] [Google Scholar]
- 97.Thommasen HV. The role of the polymorphonuclear leukocyte in the pathogenesis of the adult respiratory distress syndrome. Clinical and investigative medicine Medecine clinique et experimentale. 1985;8(2):185–194. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A–C. CD3, 20X A. spleen, B. thymus, C. pulmonary lymph node; D–F. Pax5 20X D. spleen, E. thymus, F. pulmonary lymph node; G–H. F4/80, G. spleen, 20X; H. pulmonary lymph node, 10X; I. liver, 20X. Arrows in A, D and G point to central arteriole of white pulp. Arrow in H points to labeled cells in medulla of lymph node. J–L. Protein C (surfactant) Lung. J. 20X, K. 20X, L. 40X, arrows in L point to small cuboidal cells in alveolar walls. c-cortex, m-medulla. CD3 labels T-cell zones of spleen (periarteriolar sheath), thymus and lymph node; Pax5 labels B-cell zones: mantle and marginal zone of splenic white pulp, cortex of lymph node and very few subcortical cells in thymus. F4/80 labels cells in red pulp of spleen, a few cells in medulla of lymph node and Kupffer cells in the liver, but does not label cells in lymph node cortex. Protein C is marker for Type II pneumocytes.
The arrows point to putative immunoperoxidase labeling of OVA (A) and Ig (B) in perivascular zones 6 hours after the first challenge with OVA.
Cytokine measurements at 48 hours and 1 week after 1, 2 and 3 OVA challenges of sensitized mice. Blue, IL-5; Green, IL-13. Controls are a mean of 4 mice; experimental the average of 2–4 mice. The bars indicate standard errors.