Abstract
Objective
To examine the longitudinal association between levels of lower extremity performance (LEP) and health-related quality of life (HRQoL) in older Mexican Americans aged 72 years or older participating in the Hispanic Established Population for the Epidemiological Study of the Elderly (2000-2006).
Method
LEP was measured in 621 non-institutionalized participants with the Short Physical Performance Battery (SPPB). Participants were divided into high (SPPB score 10-12), intermediate (SPPB score 7-9), and low (SPPB score 0-6) groups based on LEP. HRQoL was assessed using the Medical Outcomes Study Short Form (SF-36), which includes a Physical Composite Scale (PCS) and a Mental Composite Scale (MCS).
Results
Participants in the high LEP group had slower rates of decline in the PCS, and those in the intermediate LEP group had slower rates of decline in the MCS score over time.
Discussion
Increased LEP was associated with slower rates of decline in physical and mental HRQoL in older Mexican Americans.
Keywords: elderly, quality of life, lower extremity, Mexican Americans
The elderly Hispanic population in the United States is increasing at a rate double that of the non-Hispanic White population and is projected to reach 17 million by 2050 (Federal Interagency Forum on Aging-Related Statistics, 2010). Approximately three quarters of the Hispanic population are Mexican Americans (Ennis, Ríos-Vargas, & Albert, 2011). Older Mexican Americans are characterized by low income, few years of formal education, low physical activity, high rates of obesity, diabetes and disability, and longer life expectancy (Markides et al., 1999). With this population growth of aging Mexican Americans, there is an increase in the prevalence of morbidity and disability.
Lower extremity performance (LEP) is a good predictor of hospitalization, falls, disability, and mortality compared with upper extremity performance, especially among the elderly (Guralnik, Ferrucci, Simonsick, Salive, & Wallace, 1995; Marsh et al., 2006; Penninx et al., 2000; Puthoff & Nielsen, 2007). However, few studies have examined whether LEP is associated with quality of life in older adults (Davis et al., 2012; Fagerstrom & Borglin, 2010; Roh, Kim, Paik, Kim, & Gong, 2012). For example, findings from the Lifestyle Interventions and Independence for Elders-Pilot Study (LIFE-P) showed mobility strongly correlated with health-related quality of life (HRQoL; Groessl et al., 2007). Similarly, ability to walk was strongly associated with better HRQoL in a Swedish cohort of 1,128 older adults (Fagerstrom & Borglin, 2010). A recent cross-sectional study also reported a strong association between limitation in LEP and HRQoL in a sample of 215 older adults who attended the Vancouver Falls Prevention Clinic (Davis et al., 2012).
Previous reports from the Hispanic Established Populations Epidemiological Study of the Elderly (EPESE) indicated that diabetes (Graham et al., 2007) and arthritis (Bindawas et al., 2011) are associated with poor physical and mental HRQoL. No previous studies have examined the impact of LEP on physical and mental HRQoL in this population. The purpose of this study was to examine whether LEP is associated with HRQoL over time in elderly Mexican Americans, participating in the Hispanic Established Population for the Epidemiological Study of the Elderly (2000-2006). We hypothesized that higher LEP would be associated with a higher level of physical and mental health over time, after controlling for demographics and health-related covariates.
Method
Participants are from the Hispanic EPESE, an ongoing population-based longitudinal study of Mexican Americans aged 65 years or older, residing in Texas, New Mexico, Colorado, Arizona, and California. The original probability-based sample was representative of approximately 500,000 older Mexicans Americans living in the Southwest in the mid-1990s. The demographics and characteristics of the sample have been described elsewhere (Markides et al., 2001; Markides et al., 1999; Markides et al., 1996; Rudkin, Markides, & Espino, 1997). In the current study, we included 621 participants from the Hispanic EPESE cohort who were enrolled in a sub-study of disability in 2000/2001 in which the respondents completed the SF-36 and had the ability to independently complete the Short Physical Performance Battery (SPPB; Bindawas et al., 2011).
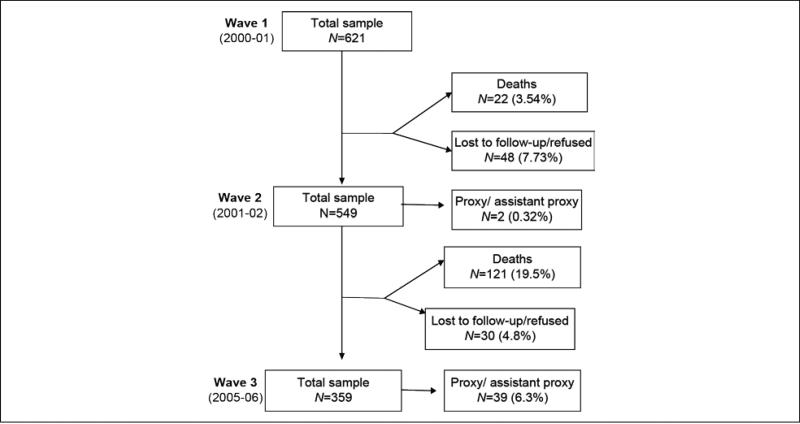
At baseline (2000/2001—Wave 1 for this subsample), 621 participants were interviewed at home in either Spanish or English depending on their preference. In Wave 2 (2001/2002), 549 participants were re-interviewed in person and 2 by proxy. Forty-eight refused to be interviewed or were lost in follow-up, and 22 were confirmed dead through either the National Death Index (NDI) or reports from relatives. In Wave 3 (2005/2006), 359 participants were re-interviewed in person and 39 by proxy. Thirty refused to be interviewed or were lost in the follow-up, and 121 were confirmed dead (Figure 1). Proxy interviews were excluded from the present analysis because they were not able to complete the physical performance tests involving the upper and lower extremities. Participants with missing information at any of the follow-ups were significantly more likely to be older; be unmarried; report heart attack, hip fracture, high depressive symptoms; and have lower scores on the Mini-Mental State Exam, SPPB, total body muscle strength, and the physical and mental components of the SF-36 compared with those participants without missing information (p< .05).
Figure 1.
Status of the sample at baseline and follow-up.
Measurements
Lower extremity performance
For the present study, the SPPB was used to measure LEP (Guralnik et al., 1994). The SPPB includes three lower body tests (Guralnik et al., 1995). Standing balance was assessed by asking participants to try to stand with their feet side-by-side, in semi-tandem (with the heel of one foot next to the big toe of the other foot) and in tandem (with the heel of one foot directly in front of the other foot), for 10 s each. Standing balance scores ranged from 0 to 4, with 0 indicating no standing balance and 4 indicating full-tandem standing for 10 s. Walking speed was assessed by measuring the time (nearest second) for the participants to walk 8 ft. Scores ranged from 0 to 4, with 0 reflecting an incomplete walk and 1 to 4 indicating quartiles by completion times (higher scores indicating faster completion). The time for repeated chair stands (5 in total) was estimated to the nearest 10th of a second based on the ability to rise from a sitting position with arms folded across the chest. These scores also ranged from 0 (reflecting no chair stands completed) to 1 to 4, reflecting quartiles related to completion time (higher scores indicating faster completion).
The final SPPB score was calculated by combining the results of the three tests (with a possible range of 0-12), with higher scores indicating greater LEP functioning. The SPPB has excellent reliability and sensitivity (Ostir, Kuo, Berges, Markides, & Ottenbacher, 2007; Ostir, Volpato, Fried, Chaves, &Guralnik, 2002). For the present study, participants were divided into three groups based on LEP performance: low (SPPB score 0-6), intermediate (SPPB score 7-9), and high (SPPB score 10-12), as recommended by previous studies (Ostir et al., 2007; Ostir et al., 2002).
Health-related quality of life
The SF-36 is the most commonly used scale to assess HRQoL in the elderly (Kosinski, Keller, Ware, Hatoum, & Kong, 1999). It allowed us to determine HRQoL by measuring participants’ perceived physical and mental health (Centers for Disease Control and Prevention, 2000) rather than broader components of quality of life such as income, satisfaction, and quality of the environment (Schalock, Bonham, & Verdugo, 2008). The SF-36 has been translated into many languages, including Spanish. The Spanish version of the SF-36 is a valid measure of self-reported health status for Mexican Americans as well as for other Hispanic groups (Graham et al., 2007; Peek, Ray, Patel, Stoebner-May, & Ottenbacher, 2004).
The SF-36 consists of 36 items and 8 domains relating to an individual's physical and mental status (Hart, 1999; Keller, Majkut, Kosinski, & Ware, 1999; Peek et al., 2004; Vetter, 2007). The Physical Composite Scale (PCS) is a summary of physical functioning (PF), role limitation because of physical function (RP), bodily pain (BP), and general health (GH) ratings. The Mental Composite Scale (MCS) is a summary of general mental health (MH), role limitations because of emotional problems (RE), social functioning (SF), and vitality (VT) ratings (Peek et al., 2004; Schalock et al., 2008). The PCS and MCS are global scores that range from 0 to 100, with higher values reflecting better HRQoL (Khanna &Tsevat, 2007; Kosinski et al., 1999; Peek et al., 2004). The scales demonstrate good discriminate validity (Ware, Kosinski, & Keller, 1994). The Cronbach's alpha for the SF-36 in the Hispanic EPESE cohort ranged from 0.76 to 0.96 (Peek et al., 2004). The minimum clinically important difference (MCID) for various geriatric conditions ranges between 5 and 10 points for each SF-36 domain score and between 2.5 and 5 points for the PCS and MCS scores (Khanna &Tsevat, 2007).
Covariate Variables
Socio-demographic variables included age, sex, marital status, years of formal education, nativity (foreign born vs. U.S. born), and language of interview (English or Spanish). Height was measured using a tape measure placed against the wall, and weight was measured using a Metro 9,800 scale. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (Barrera et al., 2004).
Depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). This instrument assesses depression symptoms using scores ranging from 0 to 60. Participants with a CES-D score of ≥16 were considered to have high depressive symptoms (Angel &Guarnaccia, 1989; Radloff, 1977). Cognitive status was measured using the 30-item Mini-Mental State Exam (MMSE) with scores ranging from 0 to 30. Lower scores indicate poorer cognitive ability (Folstein, Folstein, & McHugh, 1975). The MMSE score was analyzed as a dichotomized variable (<21 vs. ≥21), using a cut-point frequently used for populations with lower levels of education (Uhlmann & Larson, 1991). Participants with MMSE scores <21 were classified as having low cognitive ability.
The presence of various medical conditions was assessed with a series of questions asking participants if they had ever been told by a physician that they had diabetes, heart attack, stroke, hypertension, arthritis, or cancer. Total body muscle strength was measured using two instruments: the Nicholas Manual Muscle Tester (NMMT; Lafayette Instruments; Ottenbacher et al., 2002) and the Jamar Hydraulic Dynamometer (Lafayette Instruments; Alfaro-Acha et al., 2006; Al Snih, Markides, Ottenbacher, &Raji, 2004; Al Snih, Markides, Ray, Ostir, & Goodwin, 2002; Peolsson, Hedlund, & Oberg, 2001). The total body muscle strength measure was a sum of the strength of the following muscle groups: hip abductors, hip flexors, knee extensors, shoulder abductors, and grip strength. In a previous study, we found that the test–retest reliability of the Nicholas Manual Muscle Tester in measuring upper- and lower-extremity muscle strength, in this sample, were greater than 0.90 (Ottenbacher et al., 2002).
Statistical Analyses
Chi-square and analysis of variance (ANOVA) were used to examine the distribution of participant characteristics and covariates according to LEP groups at baseline. General linear mixed models using the MIXED procedure in SASc were used to examine the association between SPPB and both physical and mental component scales of the SF-36 over time (2000/2001, 2001/2002, and 2005/2006), controlling for age, sex, marital status, education, nativity, language of interview, BMI, high depressive symptoms, low cognitive status, total body muscle strength, and medical conditions (diabetes, heart attack, stroke, hypertension, arthritis, and cancer). Time was analyzed as a categorical variable with baseline (Wave 1) as a reference.
General linear mixed models provide an outline for the analysis of repeated measures. In the presence of missing repeated measures for a subject, the MIXED procedure does not exclude this subject from the analysis; instead, it uses all the available data. This method leads to a valid analysis when the missing data can be assumed missing at random (Littell, Milliken, Stroup, Wolfinger, &Schabenberger, 2006; Patetta, 2002). All variables were analyzed as time-dependent covariates except for age, gender, education, nativity, and SPPB. The mixed-model approach was chosen for several reasons. First, it is the model that best accounts for missing or incomplete observations. Second, it allows for modeling of time-dependent changes in variables as well as time-dependent changes in the magnitude of association between variables. Finally, mixed models allow flexibility in modeling the effect of time on the outcome (Zeger & Liang, 1986).
Two mixed models were constructed to test the relationship between LEP levels and both PCS and MCS over a 6-year period. Model 1 included socio-demographic variables (age, sex, marital status, education, nativity, and language of interview), LEP, time, and the interaction between LEP and time. In Model 2, BMI, high depressive symptoms, low cognitive status, total body muscle strength, and medical conditions (diabetes, heart attack, stroke, hypertension, arthritis, and cancer) were added to the variables included in Model 1 to examine whether these variables modify the association between LEP and HRQoL. All analyses were from unweighted data and performed using the SAS System for Windows, Version 9.1.3. All analyses were from unweighted data and performed using the SAS System for Windows, Version 9.1.3.
Results
The baseline sample consisted of 60% women and 40% men, with a mean age of 78 years. Two hundred twenty-two (35.8%) participants had low LEP, 230 (37%) had intermediate LEP, and 169 (27.2%) had high LEP (Table 1). Participants in the lower LEP group were significantly more likely to be older, female, married, have lower levels of education, higher depressive symptoms, lower cognitive status, more medical (comorbid) conditions, lower total body muscle strength, and poorer physical and mental HRQoL than those in the intermediate or high LEP groups (Table 1). The average mean of the SPPB at each wave was 7.0 (SD = 3.4), 6.7 (SD = 3.7), and 5.7 (SD = 3.7), respectively. The average mean of the 8-foot walk test at each wave was 5.5 (SD = 3.1), 6.7 (SD = 3.3), and 8.2(SD = 6.1), respectively. The average mean of the chair-repeated stand test at each wave was 13.9 (SD = 4.7), 12.2 (SD = 4.4), and 14.9 (SD = 6.7), respectively.
Table 1.
Descriptive Characteristics of Participants by LEP Groups at Baseline (n = 621).
| Lower extremity performance levels |
||||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| (SPPB score 0-6) | (SPPB score 7-9) | (SPPB score 10-12) | ||
| Explanatory variables | n = 222 | n = 230 | n = 169 | p value |
| Age (years) | 79.7 ± 6.0 | 77.1 ± 4.3 | 77.2 ± 4.5 | <.001 |
| Sex (% Men) | 35.1 | 36.1 | 52.1 | .002 |
| Married (%) | 52.3 | 51.7 | 40.8 | .040 |
| Nativity (% U.S. born) | 41.9 | 37.8 | 37.2 | .570 |
| Education (years) | 4.8 ± 3.6 | 5.2 ± 3.9 | 5.6 ± 3.8 | .001 |
| No. English interview (%) | 13.51 | 20 | 18.93 | .150 |
| BMI (kg/m2) | 28 ± 6 | 28.5 ± 5 | 27.7 ± 5.4 | .030 |
| High depressive symptoms (CES-D ≥ 16) (%) | 16.2 | 12.6 | 4.73 | <.001 |
| Low cognitive status (MMSE < 21) (%) | 48.6 | 32.6 | 20.1 | <.001 |
| Number of medical conditions | 1.3 ± 0.9 | 1.2 ± 1 | 0.8 ± 0.8 | <.001 |
| TBMS (Kg) | 56.9 ± 18.5 | 70.3 ± 22.6 | 77.3 ± 21.5 | <.001 |
| SF-36 physical functioning | 38.4 ± 32.1 | 67.6 ± 29 | 81.2 ± 20.9 | <.001 |
| SF-36 role of physical | 46.8 ± 44.6 | 73.6 ± 39.9 | 85.7 ± 31.4 | <.001 |
| SF-36 bodily pain | 57.8 ± 25.1 | 73.9 ± 24.9 | 78.8 ± 22.1 | <.001 |
| SF-36 general health | 48.7 ± 19.6 | 62.6 ± 21.2 | 72.5 ± 18.2 | <.001 |
| SF-36 vitality | 54.7 ± 18.4 | 67.5 ± 19.2 | 75.5 ± 17.1 | <.001 |
| SF-36 social functioning | 70.3 ± 26.9 | 84.8 ± 21.5 | 92 ± 15 | <.001 |
| SF-36 role of emotion | 71.7 ± 41.1 | 81.1 ± 35.7 | 93.9 ± 21.1 | <.001 |
| SF-36 mental health | 75.4 ± 16.9 | 80.9 ± 16.5 | 87.9 ± 13.3 | <.001 |
| PCS | 33.1 ± 12.1 | 44 ± 11.2 | 48.2 ± 9 | <.001 |
| MCS | 53.5 ± 8.9 | 54.6 ± 9.2 | 57.9 ± 6.1 | <.001 |
Note. LEP = lower extremity performance; SPPB = Short Physical Performance Battery; BMI = body mass index; CES-D = Center for Epidemiological Studies Depression; MMSE = Mini-Mental State Examination; TBMS = total body muscle strength; SF = short form; PCS = SF-36 Physical Component Summary; MCS = SF-36 Mental Component Summary.
Table 2 presents the results of the general linear mixed-model estimates of physical HRQoL as a function of baseline LEP groups over a 6-year period. The association between LEP groups and PCS score at baseline (intercept of PCS score) was statistically significant for the intermediate and high LEP groups after adjusting for time and socio-demographic variables (Estimate = 7.99, SE = 0.95, p< .0001; and Estimate = 10.66, SE = 1.04, p< .0001, respectively). The association decreased but remained statistically significant after adjusting for BMI, high depressive symptoms, low cognitive status, total body muscle strength, and medical conditions (Estimate = 5.97, SE = 1.00, p≤ .0001; and Estimate = 6.20, SE = 1.10, p≤ .0001, respectively). The interaction term between LEP groups and time of follow-up (slope of total PCS score over 6 years) was statistically significant for the intermediate and high LEP groups. Adjusting for socio-demographic variables (Model 1), both groups had slower rates of decline in the PCS score at Wave 2 (Estimate = 2.48, SE = 1.22, p = .0429; and Estimate = 4.31, SE = 1.26, p = .0006, respectively) compared with Wave 1. The association remained statistically significant after adjusting for all covariates only for the high LEP group (Estimate = 5.11, SE = 1.46, p = .0005) compared with Wave 1. High scores in muscle strength were associated with slower rates of decline in the PCS score (Estimate = 0.14, SE = 0.02, p< .0001) while high BMI (Estimate = −0.29, SE = 0.07, p = .0002) and high number of medical conditions (Estimate = −2.45, SE = 0.45, p< .0001) were associated with greater decline in the PCS score over time.
Table 2.
General Linear Mixed Models Estimates for Physical Health-Related Quality of Life as a Function of Baseline LEP Groups Over a 6-Year Period (2000-2006).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Explanatory variables | β (SE) | p value | β (SE) | p value |
| Intercept | 51.82 (7.37) | <.0001 | 50.19 (8.28) | <.0001 |
| Time 0 | Reference | Reference | ||
| Time 1 | –8.94 (7.69) | .2454 | –10.51 (9.61) | .2749 |
| Time 2 | –6.26 (11.01) | .5699 | 1.63 (14.81) | .9125 |
| LEP groups | ||||
| Low | Reference | Reference | ||
| Intermediate | 7.99 (0.95) | <.0001 | 5.97 (1.00) | <.0001 |
| High | 10.66 (1.04) | <.0001 | 6.20 (1.10) | <.0001 |
| Intermediate × Time 0 | Reference | Reference | ||
| Intermediate × Time 1 | 2.48 (1.22) | .0429 | 1.51 (1.39) | .2764 |
| Intermediate × Time 2 | –0.58 (1.52) | .7052 | –1.52 (1.71) | .3732 |
| High × Time 0 | Reference | Reference | ||
| High × Time 1 | 4.31 (1.26) | .0006 | 5.11 (1.46) | .0005 |
| High × Time 2 | 2.60 (1.56) | .0972 | 3.42 (1.83) | .0624 |
| Age | –0.24 (0.091) | .0091 | –0.13 (0.092) | .1578 |
| Sex | 2.89 (0.98) | .0035 | –1.40 (1.14) | .2184 |
| Marital status | 0.059 (0.94) | .9502 | 1.08 (0.92) | .2395 |
| Education in years | 0.13 (0.097) | .1704 | 0.088 (0.097) | .3636 |
| Nativity (foreign born vs. U.S. born) | 1.01 (0.74) | .1738 | 1.44 (0.71) | .045 |
| English or Spanish interview | –2.18 (1.10) | .0478 | –1.24 (1.12) | .2700 |
| BMI | –0.29 (0.079) | .0002 | ||
| High depressive symptoms | –1.66 (1.37) | .2224 | ||
| Low cognitive status | –1.72 (0.89) | .054 | ||
| Medical conditions | –2.45 (0.45) | <.0001 | ||
| Total body muscle strength | 0.14 (0.024) | <.0001 | ||
Note. Model 1 included age, gender, marital status, education, nativity, and language of interview.
Model 2 included BMI, high depressive symptoms, low cognitive status, total body muscle strength, and medical conditions (diabetes, heart attack, stroke, hypertension, arthritis, and cancer) along with variables in Model 1. LEP = lower extremity performance; B = coefficient estimates; SE = standard error; BMI = body mass index.
Table 3 presents the results of the general linear mixed-model estimates of mental HRQoL as a function of baseline LEP groups over a 6-year period. The association between LEP groups and MCS score at baseline (intercept of MCS score) was statistically significant for the high LEP group (Estimate = 3.95, SE = 0.85, p≤ .0001), than those in the low or intermediate LEP groups adjusting for time and socio-demographic variables.
Table 3.
General Linear Mixed Models Estimates for Mental Health-Related Quality of Life as a Function of Baseline LEP Groups Over a 6-Year Period (2000-2006).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Explanatory variables | β (SE) | p value | β (SE) | p value |
| Intercept | 56.60 (5.63) | <.0001 | 45.50 (6.45) | <.0001 |
| Time 0 | Reference | Reference | ||
| Time 1 | –6.17 (6.95) | .375 | 4.25 (8.31) | .6095 |
| Time 2 | –12.95 (10.97) | .2387 | –3.89 (10.87) | .7205 |
| LEP groups | ||||
| Low | Reference | Reference | ||
| Intermediate | 1.25 (0.78) | .1101 | 1.18 (0.83) | .1526 |
| High | 3.95 (0.85) | <.0001 | 3.41 (0.91) | .0002 |
| Intermediate × Time0 | Reference | Reference | ||
| Intermediate × Time 1 | 2.26 (1.07) | .0353 | –0.10 (1.13) | .9303 |
| Intermediate × Time 2 | 4.61 (1.45) | .0016 | 2.64 (1.25) | .0355 |
| High × Time 0 | Reference | Reference | ||
| High × Time 1 | 1.26 (1.11) | .2575 | –0.77 (1.21) | .525 |
| High × Time 2 | 4.24 (1.51) | .0051 | 1.15 (1.36) | .3985 |
| Age | –0.052 (0.069) | .4565 | 0.075 (0.071) | .2918 |
| Sex | –0.74 (0.75) | .3202 | –2.89 0.89 | .0014 |
| Marital status | 2.20 (0.73) | .0028 | 1.45 (0.72) | .0444 |
| Education in years | –0.008 (0.071) | .8987 | –0.032 (0.064) | .6141 |
| Nativity (foreign born vs. U.S. born) | 1.20 (0.54) | .0264 | 0.77 (0.47) | .1008 |
| English or Spanish interview | –3.17 (0.89) | .0004 | –2.01 (0.90) | .0263 |
| BMI | 0.034 (0.062) | .5819 | ||
| High depressive symptoms | –10.4 (1.12) | <.0001 | ||
| Low cognitive status | 0.40 (0.73) | .5796 | ||
| Medical conditions | –0.23 (0.36) | .5304 | ||
| Total body muscle strength | 0.043 (0.02) | .0284 | ||
Note. Model 1 included age, gender, marital status, education, nativity, and language of interview.
Model 2 included BMI, high depressive symptoms, low cognitive status, total body muscle strength, and medical conditions (diabetes, heart attack, stroke, hypertension, arthritis, and cancer) along with variables in Model 1. LEP = lower extremity performance; B = coefficient estimates; SE = standard error; BMI = body mass index.
When BMI, high depressive symptoms, low cognitive status, total body muscle strength, and medical conditions were added to Model 1, the association in Model 2 decreased but remained statistically significant (Estimate = 3.41, SE = 0.91, p = .0002). The interaction term between LEP groups and time of follow-up (slope of total MCS score over 6 years) was statistically significant for the intermediate and high LEP groups. Participants in the intermediate LEP group had slower rates of decline in the MCS score at Waves 2 and 3 (Estimate = 2.26, SE = 1.07, p = .0353; and Estimate = 4.61, SE = 1.45, p = .0016, respectively) compared with Wave 1 (Model 1). The association remained statistically significant only at Wave 3 (Estimate = 2.64, SE = 1.25, p = .0355) after adjusting for all covariates (Model 2). Participants in the high LEP group had slower rates of decline in the MCS score at Wave 3 (Estimate = 4.24, SE = 1.51, p = .0051) compared with Wave 1 (Model 1), but the association was no longer statistically significant when all covariates were added in the equation (Model 2). Being female (Estimate = 2.89, SE = 0.89, p = .0014), married (Estimate = 1.45, SE = 0.72, p = .0444), and those who performed high in muscle strength (Estimate = 0.04, SE = 0.01, p = .0284) were associated with slower rates of decline in MCS score, while those interviewed in Spanish (Estimate = −2.01, SE = 0.90, p = .0263) and who reported high depressive symptoms (Estimate = −10.38, SE = 1.12, p< .0001) were associated with greater decline in MCS score over time
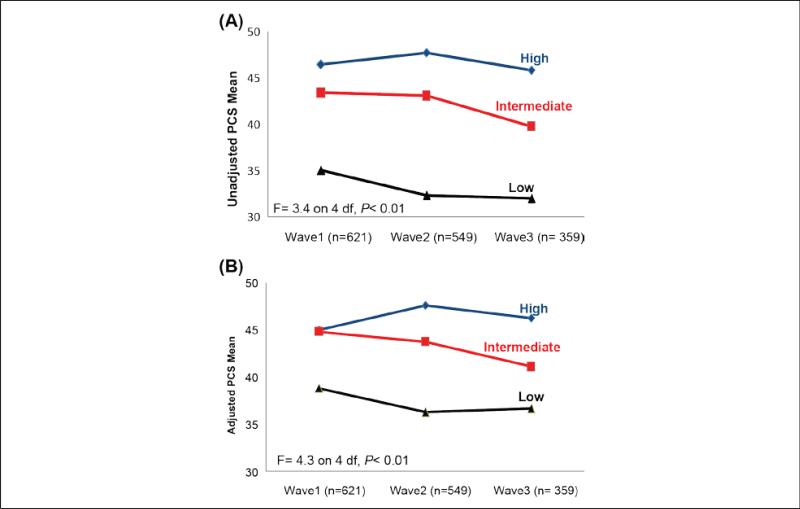
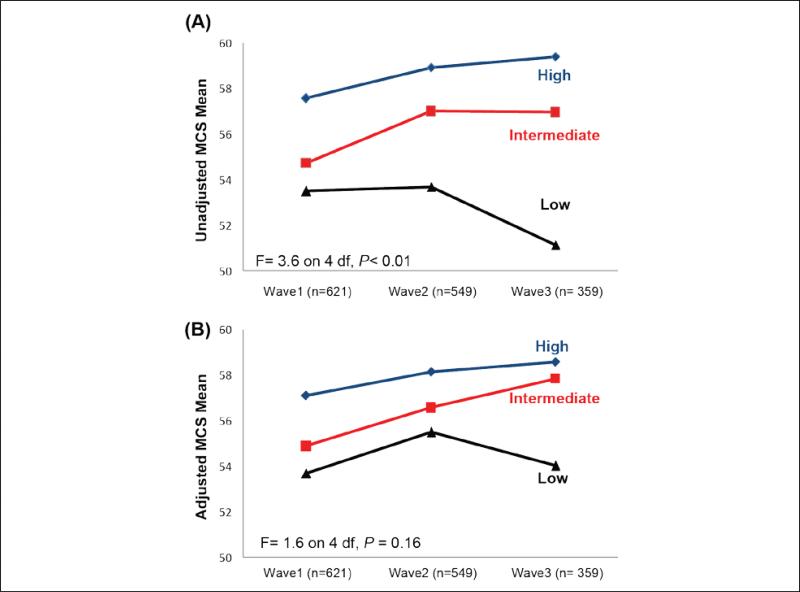
The unadjusted and adjusted mean distributions of the PCS and MCS scores over the three time points according to LEP status are shown in Figures 2 and 3. The Figures indicate that participants in the low LEP group had the lowest PCS and MCS scores at each time point. Moreover, participants in the high LEP group had stable PCS and MCS scores over the 6 years.
Figure 2.
(A) Unadjusted and (B) Adjusted mean PCS scores (range = 0-100) over time according to low, intermediate, and high lower extremity performance (adjusted for all covariates with main and interaction effects). Note. PCS = Physical Composite Scale.
Figure 3.
(A) Unadjusted and (B) Adjusted mean MCS scores (range = 0-100) over time according to low, intermediate, and high lower extremity performance (adjusted for all covariates with main and interaction effects). Note. MCS = Mental Composite Scale.
Discussion
This study examined the association between LEP and health-related quality of life (HRQoL) among older Mexican Americans over 6 years. Participants in the high LEP group had slower rates of decline in the PCS over time compared with participants in the low or intermediate LEP groups. Participants in the intermediate LEP group had slower rates of decline in the MCS over time than those in the low or high LEP group. In this sample, LEP was associated with HRQoL independent of demographics factors, medical conditions, BMI, high depressive symptoms, cognitive status, and muscle strength. We also found high muscle strength was associated with slower rates of decline in both the physical and MCS scores over time. Being a woman or being married were factors associated with slower rates of decline in the MCS over time.
Our findings are similar to those reported in the literature. For example, Groessl et al. (2007) examining 424 older adults from the LIFE-P study found that high performance in balance, chair stands, and 400-m walk time tests were associated with better HRQoL. Ekstrom et al., examining 155 older adults from the “Good Aging in Skane” study, found slower gait speed and timed get-upand-go (TUG) scores associated with poorer physical and mental HRQoL (Ekstrom, Dahlin-Ivanoff, &Elmstahl, 2011). Findings from the Boston Area Community Health (BACH)/Bone Survey showed poor lower extremity physical function measured with timed walk and chair stand test associated with lower physical HRQoL (Hall, Chiu, Williams, Clark, & Araujo, 2011).
Our findings regarding muscle strength are similar to those reported in the literature. For example, Samuel et al. found among 84 older adults that high performance in muscle strength (upper and lower extremities) was associated with high PCS scores in the SF-36 (Samuel, Rowe, Hood, &Nicol, 2012). Sayer et al. found lower performance in hand grip strength associated with reduced HRQoL among 2,987 older adults (Sayer et al., 2006).
High BMI, depressive symptoms, and a high number of medical conditions have also been reported in the literature associated with decreased HRQoL. For example, Giuli et al. (2014) found overweight and obesity associated with lower PCS scores among 205 older adults. Gallegos-Carrillo et al. (2009) found in a random sample of 1,085 beneficiaries of the Mexican Institute of Social Security in Mexico City aged 60 years and older that high depressive symptoms and comorbidities were associated with decreased HRQoL (Gallegos-Carrillo et al., 2009).
It is widely understood that LEP declines significantly with age (Forrest, Zmuda, &Cauley, 2006). Studies are needed, however, to determine whether interventions to maintain or increase LEP can improve both physical and mental components of quality of life in older adults. Interventions designed to improve LEP may be particularly effective in the management of chronic conditions such as diabetes, obesity, and arthritis. These conditions are prevalent in older Mexican Americans. A randomized controlled trial of older adults with hip fracture found that extended outpatient rehabilitation, including whole-body progressive resistance training improved quality of life as measured by SF-36 (Binder et al., 2004). Similar findings have been reported regarding the effectiveness of walking programs, strengthening exercise, or hydrotherapy in elderly patients with lower extremity limitations due to fractures and osteoarthritis (Fransen, Nairn, Winstanley, Lam, & Edmonds, 2007; Hale, Waters, &Herbison, 2012; Loew et al., 2012; Sylliaas, Brovold, Wyller, &Bergland, 2011)
The current investigation has a number of limitations. First, using older participants makes follow-up studies inherently biased, as unhealthy participants are less likely to be included over time. However, we used mixed models, an analytic approach that allows the use of available data and evaluates time-dependent effects (Littell et al., 2006; Patetta, 2002). Second, as this sample was not randomly selected from 7-year survivors of the original Hispanic EPESE, the findings may not be generalizable to the larger population of older Mexican Americans. This study also has several strengths including the prospective design and its large well-defined community sample of older Mexican Americans in the United States (Markides, Rudkin, Angel, & Espino, 1997). To our knowledge, this is the first investigation to examine the longitudinal association of LEP and HRQoL in older Mexican Americans.
Implications
Our findings suggest that health care professionals should measure HRQoL to better understand how physical limitations, particularly those involving the lower extremities, might affect broader areas of physical and cognitive functions (Tulsky& Rosenthal, 2002). At the public health level, it would also be valuable to emphasize the importance of developing prevention programs targeting older populations with poor LEP to maximize HRQoL. HRQoL has been identified as an important topic area in Healthy People 2020 (Koh, 2010; U.S. Department of Health and Human Services).
Conclusion
Our results suggest that higher levels of LEP were associated with better physical and mental HRQoL in older Mexican Americans over time. Future research is required to determine whether interventions to improve LEP will lead to enhanced physical function and better HRQoL in high-risk and underserved older adults.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institutes of Health (R01AG10939, Kyriakos S. Markides, PI; R01AG17638, R24HD065702, Kenneth J. Ottenbacher, PI) and by the UTMB Claude D. Pepper Older Americans Independence Center (P30 AG024832). Saad M. Bindawas was supported by a research grant from King Abdulaziz City for Science and Technology (AT-34-343).
The sponsors of this study had no role in the design, method, participant recruitment, data collection, analysis, or preparation of the manuscript.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2006;61:859–865. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clinical and Experimental Research. 2004;16:481–486. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. Journal of the American Geriatrics Society. 2002;50:1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- Angel R, Guarnaccia PJ. Mind, body, and culture: Somatization among Hispanics. Social Science & Medicine. 1989;28:1229–1238. doi: 10.1016/0277-9536(89)90341-9. [DOI] [PubMed] [Google Scholar]
- Barrera G, Bunout D, Gattas V, de la Maza MP, Leiva L, Hirsch S. A high body mass index protects against femoral neck osteoporosis in healthy elderly subjects. Nutrition. 2004;20:769–771. doi: 10.1016/j.nut.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Bindawas SM, Snih SA, Grady JJ, Protas EJ, Graham JE, Markides KS, Ottenbacher KJ. Evidence of reduced health-related quality of life in older Mexican Americans with arthritis. Ethnicity & Disease. 2011;21:230–236. [PMC free article] [PubMed] [Google Scholar]
- Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: A randomized controlled trial. Journal of the American Medical Association. 2004;292:837–846. doi: 10.1001/jama.292.7.837. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Measuring healthy days monograph. Author; Atlanta, GA: 2000. [Google Scholar]
- Davis JC, Bryan S, McLeod R, Rogers J, Khan K, Liu-Ambrose T. Exploration of the association between quality of life, assessed by the EQ-5D and ICECAP-O, and falls risk, cognitive function and daily function, in older adults with mobility impairments. BMC Geriatrics. 2012;12 doi: 10.1186/1471-2318-12-65. Article 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom H, Dahlin-Ivanoff S, Elmstahl S. Effects of walking speed and results of timed get-up-and-go tests on quality of life and social participation in elderly individuals with a history of osteoporosis-related fractures. Journal of Aging and Health. 2011;23:1379–1399. doi: 10.1177/0898264311418504. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Ríos-Vargas M, Albert NG. The Hispanic population: 2010 (No. C2010BR-04). U.S. Census Bureau, Department of Commerce, Economics and Statistics Administration; Washington, DC: 2011. [Google Scholar]
- Fagerstrom C, Borglin G. Mobility, functional ability and health-related quality of life among people of 60 years or older. Aging Clinical and Experimental Research. 2010;22:387–394. doi: 10.1007/BF03324941. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics . Older Americans 2010: Key indicators of well-being (Older Americans 2010) Vol. 2010. U.S. Government Printing Office; Washington, DC: 2010. [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forrest KY, Zmuda JM, Cauley JA. Correlates of decline in lower extremity performance in older women: A 10-year follow-up study. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2006;61:1194–1200. doi: 10.1093/gerona/61.11.1194. [DOI] [PubMed] [Google Scholar]
- Fransen M, Nairn L, Winstanley J, Lam P, Edmonds J. Physical activity for osteoarthritis management: A randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis & Rheumatology. 2007;57:407–414. doi: 10.1002/art.22621. [DOI] [PubMed] [Google Scholar]
- Gallegos-Carrillo K, Garcia-Pena C, Mudgal J, Romero X, Duran-Arenas L, Salmeron J. Role of depressive symptoms and comorbid chronic disease on health-related quality of life among community-dwelling older adults. Journal of Psychosomatic Research. 2009;66:127–135. doi: 10.1016/j.jpsychores.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Giuli C, Papa R, Bevilacqua R, Felici E, Gagliardi C, Marcellini F, Tirabassi G. Correlates of perceived health related quality of life in obese, overweight and normal weight older adults: An observational study. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-35. Article 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Stoebner-May DG, Ostir GV, Al Snih S, Peek MK, Markides K, Ottenbacher KJ. Health related quality of life in older Mexican Americans with diabetes: A cross-sectional study. Health and Quality of Life Outcomes. 2007;5 doi: 10.1186/1477-7525-5-39. Article 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groessl EJ, Kaplan RM, Rejeski WJ, Katula JA, King AC, Frierson G, Pahor M. Health-related quality of life in older adults at risk for disability. American Journal of Preventive Medicine. 2007;33:214–218. doi: 10.1016/j.amepre.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. The New England Journal of Medicine. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Wallace RB. A Short Physical Performance Battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Hale LA, Waters D, Herbison P. A randomized controlled trial to investigate the effects of water-based exercise to improve falls risk and physical function in older adults with lower-extremity osteoarthritis. Archives of Physical Medicine and Rehabilitation. 2012;93:27–34. doi: 10.1016/j.apmr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Hall SA, Chiu GR, Williams RE, Clark RV, Araujo AB. Physical function and health-related quality-of-life in a population-based sample. Aging Male. 2011;14:119–126. doi: 10.3109/13685538.2010.502267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DL. Orthotics and prosthetics National Office Outcomes Tool (OPOT): Initial reliability and validity assessment for lower extremity prosthetics. Journal of Prosthetics and Orthotics. 1999;11:101–111. [Google Scholar]
- Keller SD, Majkut TC, Kosinski M, Ware JE., Jr. Monitoring health outcomes among patients with arthritis using the SF-36 Health Survey: Overview. Medical Care. 1999;37(5 Suppl.):MS1–MS9. doi: 10.1097/00005650-199905001-00001. [DOI] [PubMed] [Google Scholar]
- Khanna D, Tsevat J. Health-related quality of life—An introduction. American Journal of Managed Care. 2007;13(Suppl. 9):S218–S223. [PubMed] [Google Scholar]
- Koh HK. A 2020 vision for healthy people. The New England Journal of Medicine. 2010;362:1653–1656. doi: 10.1056/NEJMp1001601. [DOI] [PubMed] [Google Scholar]
- Kosinski M, Keller S, Ware JJ, Hatoum H, Kong S. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: Relative validity of scales in relation to clinical measures of arthritis severity. Medical Care. 1999;37(5 Suppl.):MS23–MS39. doi: 10.1097/00005650-199905001-00003. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger R, Schabenberger O. SAS for mixed models. 2nd ed. SAS Institute; Cary, NC: 2006. [Google Scholar]
- Loew L, Brosseau L, Wells GA, Tugwell P, Kenny GP, Reid R, Panel O. Ottawa panel evidence-based clinical practice guidelines for aerobic walking programs in the management of osteoarthritis. Archives of physical medicine and rehabilitation. 2012;93(7):1269–1285. doi: 10.1016/j.apmr.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Markides KS, Black SA, Ostir GV, Angel RJ, Guralnik JM, Lichtenstein M. Lower body function and mortality in Mexican American elderly people. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2001;56(4):M243–M247. doi: 10.1093/gerona/56.4.m243. [DOI] [PubMed] [Google Scholar]
- Markides KS, Rudkin L, Angel R, Espino D. Health status of Hispanic elderly. In: Martin LG, Soldo BJ, editors. Racial and ethnic differences in the health of older Americans. National Academy Press; Washington, DC: 1997. pp. 285–300. [PubMed] [Google Scholar]
- Markides KS, Stroup-Benham CA, Goodwin JS, Perkowski LC, Lichtenstein M, Ray LA. The effect of medical conditions on the functional limitations of Mexican-American elderly. Annals of Epidemiology. 1996;6:386–391. doi: 10.1016/s1047-2797(96)00061-0. [DOI] [PubMed] [Google Scholar]
- Markides KS, Stroup-Benham C, Black S, Satish S, Perkowski L, Ostir G. The health of Mexican American elderly selected findings from the Hispanic EPESE. In: Wykle M, Ford A, editors. Serving minority elders in the 21st century. Springer; New York, NY: 1999. pp. 72–90. [Google Scholar]
- Marsh AP, Miller ME, Saikin AM, Rejeski WJ, Hu N, Lauretani F, Ferrucci L. Lower extremity strength and power are associated with 400-meter walk time in older adults: The InCHIANTI study. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2006;61:1186–1193. doi: 10.1093/gerona/61.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. American Journal of Epidemiology. 2007;166:599–605. doi: 10.1093/aje/kwm121. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: Results from the Women's Health and Aging Study. Journal of Clinical Epidemiology. 2002;55:916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Branch LG, Ray L, Gonzales VA, Peek MK, Hinman MR. The reliability of upper- and lower-extremity strength testing in a community survey of older adults. Archives of Physical Medicine and Rehabilitation. 2002;83:1423–1427. doi: 10.1053/apmr.2002.34619. [DOI] [PubMed] [Google Scholar]
- Patetta M. Longitudinal data analysis with discrete and continuous responses: Course notes. SAS Institute; Cary, NC: 2002. [Google Scholar]
- Peek MK, Ray L, Patel K, Stoebner-May D, Ottenbacher KJ. Reliability and validity of the SF-36 among older Mexican Americans. The Gerontologist. 2004;44:418–425. doi: 10.1093/geront/44.3.418. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2000;55(11):M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- Peolsson A, Hedlund R, Oberg B. Intra-and inter-tester reliability and reference values for hand strength. Journal of Rehabilitation Medicine. 2001;33:36–41. doi: 10.1080/165019701300006524. [DOI] [PubMed] [Google Scholar]
- Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Physical Therapy. 2007;87:1334–1347. doi: 10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D. Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roh YH, Kim KW, Paik NJ, Kim TK, Gong HS. How much are upper or lower extremity disabilities associated with general health status in the elderly? Clinical Orthopaedics and Related Research. 2012;470:3246–3252. doi: 10.1007/s11999-012-2417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin L, Markides K, Espino D. Functional disability in older Mexican Americans. Topics in Geriatric Rehabilitation. 1997;12:38–46. [Google Scholar]
- Samuel D, Rowe P, Hood V, Nicol A. The relationships between muscle strength, biomechanical functional moments and health-related quality of life in non-elite older adults. Age Ageing. 2012;41:224–230. doi: 10.1093/ageing/afr156. [DOI] [PubMed] [Google Scholar]
- Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35:409–415. doi: 10.1093/ageing/afl024. [DOI] [PubMed] [Google Scholar]
- Schalock RL, Bonham GS, Verdugo MA. The conceptualization and measurement of quality of life: Implications for program planning and evaluation in the field of intellectual disabilities. Evaluation and Program Planning. 2008;31:181–190. doi: 10.1016/j.evalprogplan.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Sylliaas H, Brovold T, Wyller TB, Bergland A. Progressive strength training in older patients after hip fracture: A randomised controlled trial. Age Ageing. 2011;40:221–227. doi: 10.1093/ageing/afq167. [DOI] [PubMed] [Google Scholar]
- Tulsky DS, Rosenthal M. Quality of life measurement in rehabilitation medicine: Building an agenda for the future. Archives of Physical Medicine and Rehabilitation. 2002;83(12 Suppl. 2):S1–S3. doi: 10.1053/apmr.2002.36954. [DOI] [PubMed] [Google Scholar]
- Uhlmann R, Larson E. Effect of education on the Mini-Mental State Examination as a screening test for dementia. Journal of the American Geriatrics Society. 1991;39:876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Health-related quality of life & well-being: Healthy people 2020. Retrieved from http://healthypeople.gov/2020/about/QoLWBabout.aspx.
- Vetter T. A primer on health-related quality of life in chronic pain medicine. Anesthesia & Analgesia. 2007;104:703–718. doi: 10.1213/01.ane.0000255290.64837.61. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User's Manual. The Health Institute; Boston, MA: 1994. [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]