Abstract
During the past decade, cancer stem cells (CSCs) have been increasingly identified in many malignancies. Although the origin and plasticity of these cells remain controversial, tumour heterogeneity and the presence of small populations of cells with stem-like characteristics is established in most malignancies. CSCs display many features of embryonic or tissue stem cells, and typically demonstrate persistent activation of one or more highly conserved signal transduction pathways involved in development and tissue homeostasis, including the Notch, Hedgehog (HH), and Wnt pathways. CSCs generally have slow growth rates and are resistant to chemotherapy and/or radiotherapy. Thus, new treatment strategies targeting these pathways to control stem-cell replication, survival and differentiation are under development. Herein, we provide an update on the latest advances in the clinical development of such approaches, and discuss strategies for overcoming CSC-associated primary or acquired resistance to cancer treatment. Given the crosstalk between the different embryonic developmental signalling pathways, as well as other pathways, designing clinical trials that target CSCs with rational combinations of agents to inhibit possible compensatory escape mechanisms could be of particular importance. We also share our views on the future directions for targeting CSCs to advance the clinical development of these classes of agents.
Introduction
The theory that malignancies arise from a small subset of stem-cell-like cancer cells has received increasing attention during the past decade. These cells, referred to as cancer stem cells (CSCs) or cancer-initiating cells (CICs), have been identified in many malignancies and are hypothesized to form the clonogenic core of tumour tissues.1 The origin of CSCs in human tumours is, however, not fully understood. Such cells could potentially originate from a more-differentiated cancer cell that acquires self-renewal properties, perhaps as a result of epithelial-to-mesenchymal transition (EMT).2 Alternatively, CSCs might derive from a normal tissue stem cell that undergoes transformation as a result of oncogenic somatic mutations, under the influence of extrinsic microenvironmental factors.3,4 Although the co-occurrence of subpopulations of cancer cells with different tumorigenic properties within individual tumours is no longer in question,5 the CSC hypothesis remains controversial. This controversy arises as a consequence of the technical and logistical challenges in isolating and identifying CSCs from human solid tumours that contain heterogeneous cell populations, and the limited number of validated surrogate assays currently available to substantively confirm stem-cell-like properties.6 These cells tend to comprise a small fraction of total tumour mass and are, therefore, difficult to unequivocally identify histologically. Moreover, tumour dissociation from normal tissues and subsequent flow cytometric analysis of tumour cells is not always possible with human biospecimens. Furthermore, markers that identify CSCs vary across different tumour types, and no clear-cut and clinically validated assay is currently available to quantify such cells in human tumours.7 Nevertheless, some promising candidate biomarkers have been identified,8 and surrogate assays for CSCs include the formation of secondary ‘spheroids’ in suspension culture, the generation of 3D organoids, and in vivo ‘limiting dilution’ tumorigenicity in immunocompromised mice.9 Importantly, spheroid or organoid assays might be adaptable for clinical purposes; rigorous studies are needed to establish whether these assays can be used as surrogate biomarkers in a clinical setting. From a biological standpoint, the CSC hypothesis is supported by evidence from genetically engineered mouse models, which have elucidated the contribution of CSCs to the pool of proliferating tumour cells, as well as their potential as therapeutic targets in certain tumour types.10–12
In experimental models, CSCs seem to be more resistant to chemotherapy and radiotherapy than ‘differentiated’ tumour cells.13–15 Indeed, CSCs residing in fibrotic tissue and other microenvironmental niches can escape from the effects of conventional cytotoxic treatments.16 Expansion of the remaining highly tumorigenic CSCs can resume after treatment cessation, driving tumour growth that presents as clinically relapsed or recurrent disease. On the basis of these theories and observations, numerous researchers hypothesize that treatments targeting the CSC population could be more effective than existing therapies, and could dramatically transform treatment outcomes in oncology.
CSCs have been shown to have one or more aberrations in various signalling pathways; however, abnormal activity of pathways that control stem-cell self-renewal, and have important roles in embryonic development and differentiation, which include Notch, Hedgehog (HH), and Wnt, are probably most crucial to the tumorigenicity of CSCs. Increasing evidence demonstrates that these embryonic pathways can interact with other cellular signalling pathways, such as those involving NFκB, MAPK, PI3K, and EGF. Therefore, these developmental pathways might be important therapeutic targets for blockade of CSC self-renewal and proliferation, and tumour progression.17
Many new agents targeting the Notch, HH, and Wnt pathways have entered clinical trials since our previous Review article was published in this journal in 2011.18 Thus, an update on the approval status and progress of these investigational agents towards routine clinical practice is warranted and is provided herein. In addition, we discuss strategies that hold the potential to further increase the effectiveness of such treatments, in particular, inhibition of the crosstalk between embryonic and other signalling pathways.
Targeting the Notch pathway
Notch signalling
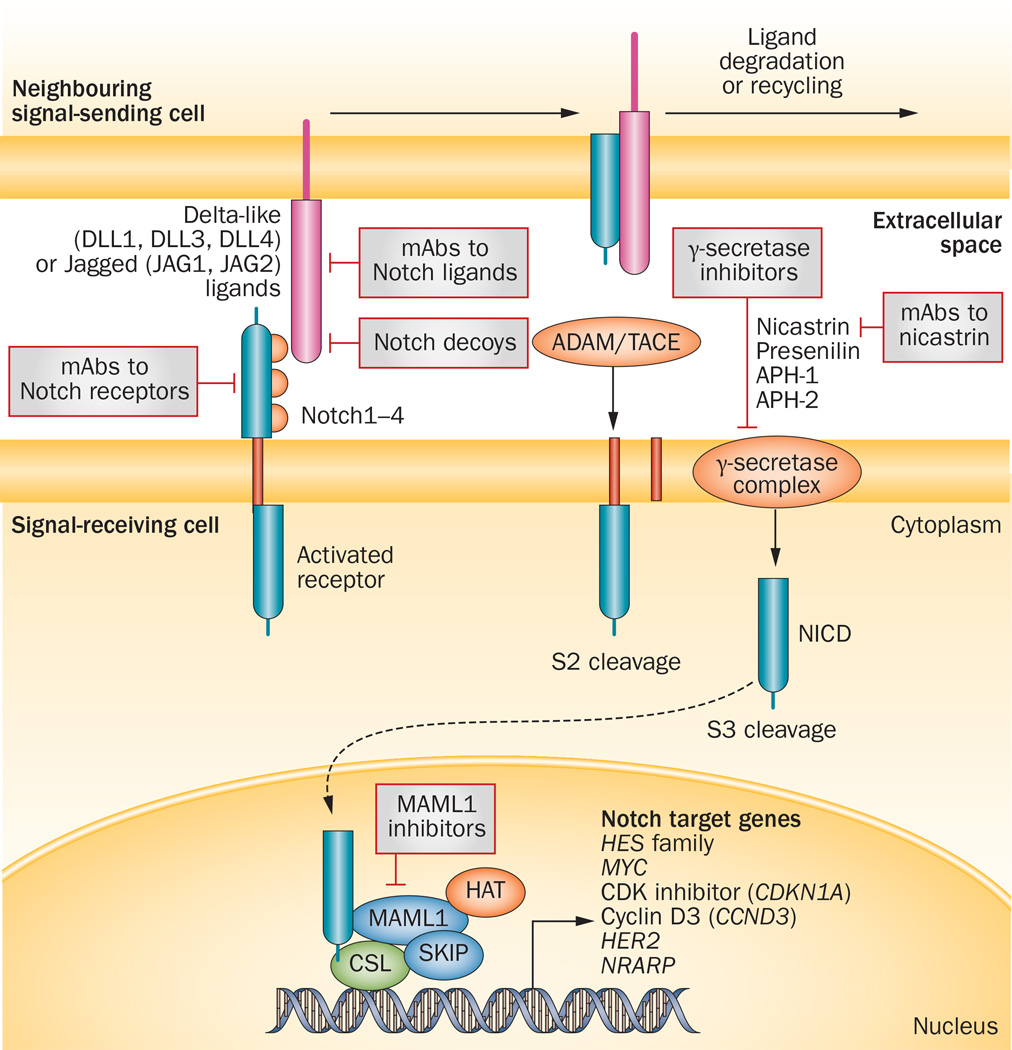
Notch signalling, similar to the Wnt and HH pathways, is a primordial, evolutionarily conserved cell-fate-determination pathway that has great relevance to multiple aspects of cancer biology, from CSCs to angiogenesis to tumour immunity.19 Notch signalling via transmembrane ligands and receptors is primarily involved in the communication between contiguous cells.20,21 That is, interaction between a transmembrane ligand on one cell and a transmembrane receptor on a neighbouring cell triggers a two-step proteolytic cleavage of the receptor; the first cleavage is mediated by a disintegrin and metallo-proteinase (ADAM) enzymes—either ADAM 10 or ADAM17, also known as tumour necrosis factor-α converting enzyme (TACE)—and the second by γ-secretase, which releases an intracellular fragment that can interact with nuclear factors to regulate target-gene expression (Figure 1). The Notch signalling pathway is complex and multifaceted, reflecting its roles in diverse functional activities. The pathway comprises five canonical Notch ligands (Delta-like ligand 1 [DLL1], DLL3 and DLL4, and Jagged1 and Jagged2) and four Notch receptor paralogues (Notch1–4).22 Different tumours and tumour subtypes can express different Notch receptors and ligands. Furthermore, post-translational modifications of Notch receptors can change their affinity for ligands and their intracellular half-lives.19,23 In addition, noncanonical Notch signalling pathways are beginning to be delineated, and some of these have relevance to cancer.24–29 Crosstalk with the Wnt and/or HH pathways might also determine the overall effect of Notch signalling, adding an additional layer of complexity30 The significance of the diversity in Notch signalling outputs in the context of clinical oncology is twofold: on the one hand, targeting Notch signalling has the potential to simultaneously affect multiple cell types within a tumour, from CSCs to immune cells, vascular endothelial cells and tumour cells; on the other hand, the successful development of agents targeting the Notch pathway will require a mechanistic understanding of the role of Notch signalling in specific cancers, and ideally, the development and use of mechanism-based combination regimens. The main issues in the development of agents targeting Notch signalling in oncology include: choice of the most appropriate inhibitor for each patient; identification of pharmacodynamic biomarkers as surrogate end points for pathway inhibition; selection of mechanism-based combination regimens; and patient stratification according to recognized efficacy biomarkers.
Figure 1.
The canonical Notch signalling pathway and relevant pharmacological inhibitors under development in cancer. DLL1, DLL3 and DLL4, and Jagged ligands (JAG1 and JAG2) expressed on the cell surface can induce signalling in adjacent cells expressing their cognate receptors Notch1–4. Ligand binding promotes sequential cleavage of the Notch receptors by ADAM/TACE enzymes (S2 cleavage) and then γ-secretase (S3 cleavage), resulting in release the NICD, which interacts with transcriptional regulators in the nucleus to instigate a Notch gene-expression profile. Notch target genes, in turn, regulate pivotal cell-fate choices, including differentiation, cell-cycle progression and survival. The final phenotypic effect is dependent on the specific signalling context, paralogue, ligand and dosage. Under many conditions, and in several types of cancer stem-like cells, Notch signalling can delay differentiation, and maintain proliferative and survival potential. Potential therapeutic inhibitors of targets involved in the Notch signalling include soluble decoy receptors, mAbs targeting the Notch ligands or receptors in the extracellular space, and small-molecules or mAb inhibitors targeting the γ-secretase complex. Abbreviations: ADAM, a disintegrin and metalloproteinase; APH-1/2, anterior pharynx-defective-1/2; CSL, CBF1/Su(H)/Lag-1; DLL, delta-like ligand; HAT, histone acetyltransferase; HES, hairy and enhancer of split-1; JAG1, Jagged-1; JAG2, Jagged-2; mAb, monoclonal antibody; MAML1, Mastermind-like 1; NICD, Notch intracellular domain; NRARP, Notch-regulated ankyrin-repeated protein; SKIP, ski-interacting protein; TACE, TNF-α-converting enzyme (also known as ADAM17).
Investigational Notch-signalling inhibitors
γ-secretase inhibitors
At present, several classes of Notch-pathway inhibitors are in clinical development, with significant differences in the targets, mechanism of action, and drug class (Table 1). A major class of agents targeting the Notch pathway is the γ-secretase inhibitors (GSIs), which prevent the final proteolytic cleavage of Notch receptors that releases the active intracellular fragment (Figure 1); GSI were the first class of Notch inhibitors to reach clinical development in oncology.31 These agents have been shown to have strong antineoplastic activity in numerous preclinical models, especially in combination with either chemotherapy or targeted agents. For example, in a mouse model of HER2-positive breast cancer, GSIs in combination with trastuzumab achieved complete cures and abrogated recurrence.32 GSIs also have demonstrated anti-CSC activity in ex vivo patient-derived tumour specimens and breast-cancer-derived secondary mammospheres.33–36 Clinical benefit from GSIs has also been observed; for example, the oral GSI PF-0308414 was found to have promising clinical activity in a phase I dose-finding study in patients with advanced-stage solid tumours.37 Among a total of 64 patients who received PF-0308414, one patient with advanced thyroid cancer had a complete response, and five of seven patients with desmoid tumours (71.4%) achieved a partial response.37
Table 1.
Investigational agents that target the Notch signalling pathway in clinical development*
| Compound and combination or intervention | Phase | Tumour type |
Clinicaltrials.gov identifier |
Status‡ |
|---|---|---|---|---|
| R04929097 (GSI; Roche) | ||||
| Single agent | I | Paediatric relapsed/refractory solid or CNS tumours, lymphoma or T-cell leukaemia | NCT01236586 | Withdrawn before enrolment |
| Plus vismodegib | I | Breast cancer (HER2, metastatic or unresectable) | NCT01071564 | Active, not recruiting |
| Plus letrozole | I | Breast cancer (postmenopausal ER+ stage II–III) | NCT01208441 | Terminated |
| Plus carboplatin and paclitaxel before surgery | I | Stage II–III TNBC | NCT01238133 | Active, not recruiting |
| Plus cetuximab | I | Metastatic colorectal cancer | NCT01198535 | Terminated |
| Plus radiation and temozolomide | I | Glioma (malignant, newly diagnosed) | NCT01119599 | Active, not recruiting |
| Plus vismodegib | I | Sarcoma (metastatic) | NCT01154452 | Recruiting |
| Plus capecitabine | I | Refractory solid tumours | NCT01158274 | Active, not recruiting |
| Plus gemcitabine | Ib | Advanced-stage solid tumours | NCT01145456 | Completed |
| Plus cediranib | I | Advanced-stage solid tumours | NCT01131234 | Active, not recruiting |
| Plus dexamethasone | I | Relapsed/refractory solid tumours, CNS tumours, lymphoma or T-cell leukaemia | NCT01088763 | Terminated |
| Administration schedules | I | Metastatic/unresectable solid malignancies | NCT01096355 | Completed |
| Plus exemestane | I/II | Breast cancer (pre/postmenopausal, advanced- stage or metastatic) | NCT01149356 | Terminated |
| Plus WBRT or stereotactic radiosurgery | I/randomized II | Brain metastases (from breast cancer and other tumours) | NCT01217411 | Terminated |
| Bevacizumab ± RO4929097 | I/II | Malignant glioma and anaplastic astrocytoma (progressive or recurrent) | NCT01189240 | Active, not recruiting |
| Single agent | II | TNBC (advanced-stage, metastatic or recurrent) | NCT01151449 | Active, not recruiting |
| Single agent | II | Colorectal cancer (metastatic) | NCT01116687 | Completed |
| FOLFOX6 + bevacizumab ± RO4929097 | Randomized II | Colorectal cancer (metastatic) | NCT01270438 | Withdrawn before enrolment |
| Plus temsirolimus | II | Endometrial (advanced-stage solid tumours) | NCT01198184 | Completed |
| In platinum-resistant disease | II | Epithelial ovarian, fallopian tube, or primary peritoneal cancer (recurrent and/or metastatic) | NCT01175343 | Active, not recruiting |
| Single agent | II | Glioblastoma (recurrent/progressive) | NCT01122901 | Active, not recruiting |
| Single agent | II | Glioma (recurrent invasive) | NCT01269411 | Terminated |
| After recently completed frontline chemotherapy | II | NSCLC (advanced-stage) | NCT01193868 | Terminated |
| Plus erlotinib (dose escalation) | II | NSCLC (stage IV or recurrent) | NCT01193881 | Active, not recruiting |
| Surgery (with neoadjuvant RO4929097) | II | Melanoma (stage IIIB, IIIC, or IV that can be removed by surgery) | NCT01216787 | Withdrawn before enrolment |
| After autologous HSCT | II | Multiple myeloma | NCT01251172 | Withdrawn before enrolment |
| Single agent | II | Pancreatic carcinoma (stage IV or metastatic) | NCT01232829 | Active, not recruiting |
| Single agent | Partially randomized II | Recurrent or stage IV prostate cancer (previously treated) | NCT01200810 | Active, not recruiting |
| After VEGF inhibitor failure | II | Renal-cell carcinoma (advanced-stage) | NCT01218620 | Active |
| LY900009 (GSI; Eli Lilly) | ||||
| Single agent | I | Advanced-stage cancers | NCT01158404 | Completed |
| PF-03084014 (GSI; Pfizer) | ||||
| Single agent | I | Advanced-stage cancers, T-ALL, or lymphoblastic lymphoma | NCT00878189 | Active |
| Single agent | II | Desmoid tumours or aggressive fibromatosis | NCT01981551 | Recruiting |
| BMS-906024 (intravenous GSI; Bristol–Myers Squibb) | ||||
| With FOLFIRI, weekly paclitaxel, or carboplatin and paclitaxel | Ib | Advanced-stage solid tumours | NCT01653470 | Recruiting |
| With dexamethasone after dose-escalation phase | I | T-ALL or T-cell lymphoblastic lymphoma | NCT01363817 | Recruiting |
| Single agent | I | Advanced-stage solid tumours | NCT01292655 | Recruiting |
| BMS-986115 (oral GSI; Bristol-Myers Squibb) | ||||
| Single agent | I | Advanced-stage solid tumours | NCT01986218 | Recruiting |
| MEDI0639 (anti-DLL4 antibody; Medimmune) | ||||
| Single agent | I | Advanced-stage solid tumours | NCT01577745 | Active |
| OMP-59R5 (anti-Notch2/3 antibody; OncoMed/Cellgene) | ||||
| With nab-paclitaxel and gemcitabine | Ib/II | Untreated stage IV pancreatic cancer | NCT01647828 | Recruiting |
| Plus etoposide and cisplatin | Ib/II | Extensive-stage SCLC | NCT01859741 | Recruiting |
| Single agent | I | Dose-escalation study in solid tumours | NCT01277146 | Active, not recruiting |
| OMP-52M51 (anti-Notch1 antibody; OncoMed/Cellgene) | ||||
| Single agent | I | Dose-escalation study in lymphoid malignancy | NCT01703572 | Recruiting |
| Single agent | I | Dose-escalation study in solid tumours | NCT01778439 | Recruiting |
| Demcizumab (aka OMP-21M18; anti-DLL4 antibody; OncoMed/ Cellgene) | ||||
| With FOLFIRI | I | Metastatic colorectal cancer | NCT01189942 | Unknown |
| Single agent | I | Dose-escalation study in solid tumours | NCT01189942 | Completed |
| Plus carboplatin and pemetrexate | I | NSCLC | NCT01189968 | Recruiting |
| With gemcitabine ± abraxane | Ib (non-randomized) | Locally advanced or metastatic pancreatic cancer | NCT01189929 | Recruiting |
| Enoticumab (aka REGN421/SAR153192; anti-DLL4 antibody; Sanof) | ||||
| Single agent | I | Advanced-stage solid tumours | NCT00871559 | Completed |
Trials investigating drug interaction, topical treatment, organ dysfunction, expanded access, comparing two different doses, and nontherapeutic studies are excluded.
Data are from ClinicalTrials.gov as of June 6, 2014; default status is recruiting or active but not recruiting. Abbreviations: aka, also known as; CNS, central nervous system; ER, oestrogen receptor; FOLFIRI, 5-fluorouracil plus folinic acid and irinotecan; FOLFOX6, 5-fluorouracil plus folinic acid and oxaliplatin regimen for six cycles; GSI, γ-secretase inhibitor; HSCT, haematopoietic stem-cell transplantation; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; T-ALL, T-cell acute lymphoblastic leukaemia; TNBC, triple-negative breast cancer; WBRT, whole-brain radiotherapy.
In humans, the dose-limiting toxicity of GSIs is secretory diarrhoea.38–40 Preclinical studies have shown that this effect is due to goblet-cell metaplasia of the small-intestinal epithelium—a target-mediated effect resulting from inhibition of Notch1 and Notch2, which abolishes the proliferative potential of crypt progenitors and causes them to differentiate into post-mitotic goblet cells.41 Patients have also been observed to develop a cutaneous rash after treatment with GSIs in several phase I clinical trials.37,42 This adverse event might be attributable to production of thymic stromal lymphopoietin (TSLP), a cytokine that mediates the release of T-cell-attracting chemokines and results in the development of atopic dermatitis in the skin: TSLP is expressed in the epidermis upon loss of Notch function in mice.43 Despite the adverse effects associated with production of this cytokine, TSLP1 might nevertheless be a useful biomarker of systemic Notch-pathway inhibition. GSIs also have the potential to modulate T-cell responses via other mechanisms, and this activity has been used therapeutically in experimental models of graft-versus-host disease (GVHD) and aplastic anaemia.44 To limit toxicity, most investigators use intermittent GSI administration regimens, from 3-days-on-4-days-off to once weekly, depending on the pharmacokinetics of individual agents. In addition, combining GSIs with glucocorticoids45 or antioestrogen agents46 decreased intestinal toxicity in animal models, and intermittent administration of two different GSIs in combination with tamoxifen, letrozole or exemestane was well tolerated in pilot clinical studies.47, 48 Thus, the advantages of GSIs include generally favourable tissue penetration, low cost, ease of administration and potential pan-Notch inhibitory activity.19,23 Potential disadvantages are systemic toxicity and off-target effects, as γ-secretase has >90 substrates in addition to the Notch receptors; GSIs potentially inhibit the cleavage of all substrates, which might contribute to their toxicity and/or effectiveness in ways that are not currently understood. Importantly, whether intermittent administration of GSIs—which largely spares intestinal epithelial stem cells and, therefore, reduces toxicity—has suboptimal therapeutic effects on CSCs and tumours remains unclear.
Different chemical classes of GSIs are not pharmacologically equivalent and are not interchangeable. Indeed, the pharmacokinetics, activity against different Notch paralogues, and off-target effects of different GSIs vary significantly. For example, RO4929097—the agent tested in most of the GSI clinical trials to date—induces its own hepatic metabolism via cytochrome P450 family 3 subfamily A polypeptide 4 (CYP3A4), which limits the achievable area under the curve (AUC) of drug exposure (that is, bioavailability).49 One agent in development, BMS-906024, is an intravenous compound, whereas other GSIs are formulated for oral administration (Table 1). Furthermore, evidence indicates that cleavage of Notch4 is inhibited by some GSIs, but not by others.50 At present, whether a specific chemical class of GSI is preferable in terms of safety and/or efficacy is not clear, and the results of ongoing clinical and preclinical studies will provide valuable information on this topic.
Anti-DLL4 antibodies
Targeting DLL4 with monoclonal antibodies (mAbs) is another strategy to block Notch signalling that is being developed in the clinic (Figure 1). Productive tumour angiogenesis requires cooperation between VEGF-A, which induces proliferation of endothelial ‘tip’ cells and expression of DLL4 in ‘stalk’ cells.51 In this context, DLL4 inhibits endothelial proliferation and promotes branching morphogenesis, and the balance between proliferation and branching is key to the formation of a functional capillary network. As such, treatment with anti-DLL4 mAbs results in disorganized angiogenesis, characterized by endothelial proliferation without formation of functional capillaries.22 In a recent clinical trial, the anti-DLL4 mAb enoticumab (also known as REGN421 and SAR153192) had a reasonable safety profile and demonstrated preliminary efficacy signals.52 The most-common severe (grades 3–4) adverse events were fatigue, headache, hypertension and nausea;52 reversible severe adverse events included increased levels of the cardiac proteins natriuretic peptides B and troponin I, and right and left ventricular dysfunctions.52 Two partial responses and 16 stable disease responses (three prolonged for >6 months) were observed among the 53 patients with ovarian cancer and other solid tumours who were treated with enoticumab.52 However, caution is needed as, in animal models, chronic treatment with anti-DLL4 mAbs caused haemangiomas due to unopposed VEGF-mediated endothelial proliferative activity, and liver toxicity due to sinusoidal endothelial toxicity.53
Interestingly, high expression of the Notch ligand DLL4 has been shown to predict resistance to sunitinib—targets of which include VEGF receptors—in metastatic renal cell carcinoma (mRCC).54 Thus, whether DLL4 and VEGF inhibitors can be safely combined in the treatment of cancer is of interest, as this approach might deliver superior antiangiogenic activity compared with either class of agent alone. A phase I trial (NCT01131234)55 is examining the combination of the GSI RO4929097 and the VEGF-targeting agent cediranib in patients with advanced-stage solid tumours. Preliminary data from this trial suggest that the combination was well tolerated at the dose levels used, with diarrhoea, hypertension, fatigue and nausea representing the most-common treatment-related adverse events.56
Other approaches to targeting Notch signalling
Other Notch inhibitors in the clinical pipeline include mAbs targeting various Notch receptors, mAbs to the γ-secretase complex component nicastrin, and soluble decoy Notch receptors that interfere with ligand–receptor interactions (Table 1; Figure 1).19 A recent addition to this drug superfamily is a novel class of disease-specific protease-activated mAbs (termed ‘probodies’) to Notch ligands; currently an anti-Jagged1/2 probody is in preclinical development.57 The context-specific activation of these agents against Notch targets offers the possibility of tumour-selective inhibition of Notch signalling, potentially improving the therapeutic window and overcoming the need for intermittent inhibition.
Notch1 has been shown to act as a tumour suppressor in neuroendocrine tumours, and inhibitors of histone deacetylases (HDACs), which are enzymes that can protect cells from apoptosis and that might therefore reduce the clinical activity of anticancer therapies, have been demonstrated to upregulate Notch1 expression.58 Thus, a phase II trial in patients with neuroendocrine tumours (NCT00985946) tested the hypothesis that, by relieving the antiapoptotic effects of HDACs, HDAC inhibition could suppress tumour growth and induce tumour-cell apoptosis via upregulation of Notch1;59 however, this study was stopped early as no clinical responses were observed. Another trial studied the effects of the same HDAC inhibitor in metastatic medullary or radioiodine-resistant differentiated thyroid cancers (NCT01013597).60 This study has completed accrual, but results have not been published. Additionally, natural phytochemicals, such as resveratrol, have been reported to inhibit Notch signalling.19 At least one clinical trial is currently investigating possible inhibitory effects of resveratrol on Notch signalling in gastrointestinal neuroendocrine tumours.61
Further progress in the development of Notch inhibitors for CSC targeting will require an unbiased comparison of the many pharmacological strategies developed thus far, and possibly the development of next-generation agents. The agents used in the greatest number of early clinical trials to date are not necessarily the most promising ones, for the reasons discussed. Moreover, developmental pathways, including Notch, function in concert with other-pathways to dictate cell fate, rather than as a simple on-off switch. Ideally, the most scientifically sound approach to targeting this pathway would leverage tumour-specific or target-specific agents used in mechanism-based combinations, in tumour types in which the roles of particular Notch family members in CSC can be documented. Indirect strategies targeting amenable nodes in Notch signalling other than ligands or receptors are also an attractive possibility.
Biomarkers of Notch signalling
Predictive biomarkers
Patient stratification is a key issue for the clinical development of Notch inhibitors. Ideally, biomarkers indicative of Notch-pathway activity with functional relevance in a specific tumour would guide patient stratification (that is, biomarkers predictive of response), but attempts to identify candidates for Notch-targeted therapy based on this premise have not proved straightforward. Expression levels of canonical Notch target genes is correlated with Notch-activating mutations and is a good indicator of Notch-pathway activity in T-cell acute lymphoblastic leukaemia (T-ALL);62,63 however, this relationship does necessarily imply that expression of the same target genes is indicative of Notch activity in all malignancies owing to a number of factors—not least the notorious context-dependence of Notch signalling cascade and outcomes, which are dynamic and influenced by other chromatin cofactors.64 Moreover, expression of different Notch paralogues varies across tumours.23 Although, in theory, all Notch receptors signal through the same canonical pathway, their target genes can differ. For example, in preclinical models of endocrine-resistant breast cancer, both Notch1 and Notch4 are oncogenic;46 however, they modulate vastly different and minimally overlapping sets of target genes,46 which has complicated biomarker identification. Detection of nuclear accumulation of the cleaved Notch1 intracellular domain is a good predictor of canonical Notch1 activity.65 However, reliable antibodies that specifically detect cleaved Notch receptor peptides in clinical samples are presently limited to Notch1.65 In addition, consideration of the importance of emerging noncanonical Notch signalling pathways, through which Notch can function independently of target-gene transcription mediated by conventional ligand binding,66 is required. Gain-of-function mutations in Notch receptors or Notch-pathway regulatory proteins have been detected in more than 50% of T-ALL cases and in a much smaller proportion of solid tumours.23 In many cases, these mutations produce truncated proteins that lack functional extracellular domains and are, therefore, ligand-independent. However, whether these mutations can serve as efficacy biomarkers for monotherapy with Notch-pathway inhibitors remains unclear in light of the lack of clinical activity observed in patients with Notch1 mutations.65 In the future, investigators who plan to develop Notch inhibitors for a specific indication should simultaneously investigate biomarker expression, especially those indicative of the pathway activity, such as Notch cleavage fragments and potential Notch target genes that correlate with sensitivity to inhibition of this pathway in relevant preclinical models. At present, expression of the cleaved Notch1 intracellular domain and Notch target gene HES4 represent the most-promising predictive biomarkers of response to Notch-targeted therapies in triple-negative breast cancer (TNBC) and salivary adenoid cystic carcinoma.67
Biomarkers of target modulation
The pharmacodynamic biomarkers that are tightly linked to Notch-pathway inhibition are also underdeveloped, especially for solid tumours.68 Of note, monotherapy Notch-pathway inhibition will not necessarily result in radiologically detectable tumour-volume effects in all indications, as they predominantly target the rare clono-genic CSC population. Nevertheless, such effects have been seen with some GSIs in patients with central nervous system (CNS) tumours,42 and for metastatic oestrogen receptor (ER)-positive breast cancers after treatment with GSI in combination with exemestane.47
Ex vivo spheroid (‘tumoursphere’) formation assays from patient-derived samples might provide indirect information on the effects of treatment on CSCs, especially in pre-surgical (neoadjuvant) studies in which post-treatment tumour samples are likely to be available for analysis. Tumoursphere-formation assays have been used especially in studies of neurological malignancies and breast cancer.69,70 Although a clinically standardized assay is not available, promising approaches have been developed, such as the ‘sphere limiting dilution assay’, which can enable more-precise quantification compared with non-limiting dilution assay.71
Molecular biomarkers of systemic Notch inhibition (such as inhibition of HES1 expression in hair follicles, or TSLP production) might have utility as biomarkers of target engagement, but do not necessarily prove or reflect Notch inhibition in tumour tissue. Neoadjuvant studies or studies including post-treatment biopsies will be necessary to guide our understanding of how molecular biomarkers correlate with target engagement, as well as clinical response to and effectiveness of Notch-pathway inhibition.
Combination regimens
Mechanism-based drug combinations incorporating Notch-pathway inhibitors deserve thorough investigation. Such approaches will require that all cross-signalling networks in CSCs are considered in a context-dependent manner and specific disease settings. Notch signalling can interface with and influence a large number of cancer-relevant pathways—controlling EMT, DNA repair, the cell cycle, and apoptosis.2,72 For example, cooperation between β-catenin (Wnt) and Notch signalling has been documented in colorectal cancer73,74 and myelodysplastic syndrome (MDS) or acute myeloid leukaemia (AML);75 Notch and transforming growth factor-β (TGF-β) are known to have cooperative tumorigenic effects in RCC76 and TNBC.77 As described, Notch-signalling inhibitors, similar to inhibitors that target other developmental pathways relevant to CSCs, will not necessarily produce singleagent, short-term tumour volume responses in early phase clinical trials, unless they have considerable cytostatic, cytotoxic or antiangiogenic activity separate from their effect on CSCs. Nevertheless, these agents show promise with regards to survival, through prevention of disease recurrence or relapse that might be mediated by CSCs that persist after exposure to other therapies. An instructive example is provided by the GSI MRK003, a compound only used in preclinical models to date. This agent had a marked single-agent tumour-volume effect in models of endocrine-resistant breast cancer.46 By contrast, in a mouse xenograft model of HER2-positive breast cancer, the same agent had no single-agent tumour-volume effect, nor did it increase the remarkable tumour-volume effect of trastuzumab.32 Nonetheless, the GSI–trastuzumab combination completely abolished tumour recurrence, presumably by interfering with CSCs, whereas treatment with trastuzumab alone resulted in a 50% recurrence rate.32 Similar results were seen in the same study with a chemically different GSI: LY411575.32 Thus, as with other targeted agents, it is possible that Notch-pathway inhibitors will show optimal efficacy in the context of combination regimens. These investigational agents should, therefore, be tested in combinations with established drugs (and if warranted, in combinations with other novel agents), on the basis of the available preclinical mechanistic rationale. Examples include combinations of Notch-pathway inhibitors with endocrine therapy in ER-positive breast cancer,46,47,78 with HER2 inhibitors in HER2-amplified breast cancer,32,79 and with taxanes and MET inhibitors in TNBC.80,81 In fact, combinations of inhibitors of Notch signalling with endocrine therapy have been translated to the clinic with promising results in terms of safety and preliminary efficacy signals.55,62 For instance, in a phase Ib dose-escalation study, the oral compound RO4929097 in combination with exemestane showed one partial response and six cases of stable disease among 15 patients with recurrent ER-positive breast cancer.47 In addition, phase I/II trials with GSIs and chemotherapy or targeted therapies are being conducted. For example, a phase I trial of combined RO4929097 and cediranib treatment reported one partial response and 11 cases of stable disease in 20 patients with advanced-stage solid tumours.56
Current clinical relevance
Despite accumulating evidence supporting the importance of Notch signalling in the regulation of CSCs in numerous malignancies, clinical experience with Notch-pathway inhibitors remains relatively limited. Most of the completed clinical trials have been performed with one particular GSI, RO4929097, which had a potential pharmacokinetic liability—auto-induction of RO4929097 metabolism that resulted in marked reduction of steady-state drug levels.82 Several other structurally and pharmacologically distinct GSIs are being investigated clinically, and a number of biological agents directed at specific components of the Notch pathway have recently entered preclinical and clinical testing (Table 1). It remains to be determined which of these classes of agents hold the most promise in the treatment of cancer. Some Notch-pathway components (such as Notch4) are not targets of current biological agents, and might be one of the resistance mechanisms to some GSIs; further research on the effects of inhibiting these components is needed. In addition, novel Notch mutations and biomarkers of Notch activity that predict sensitivity to GSIs in some tumours have only recently been discovered, and further efforts in this area are required. At this time, the most-promising avenue for therapeutic targeting of this pathway seems to be mechanism-based, biomarker-driven combinations, but such approaches also need further development.
Targeting the Hedgehog pathway
Hedgehog signalling
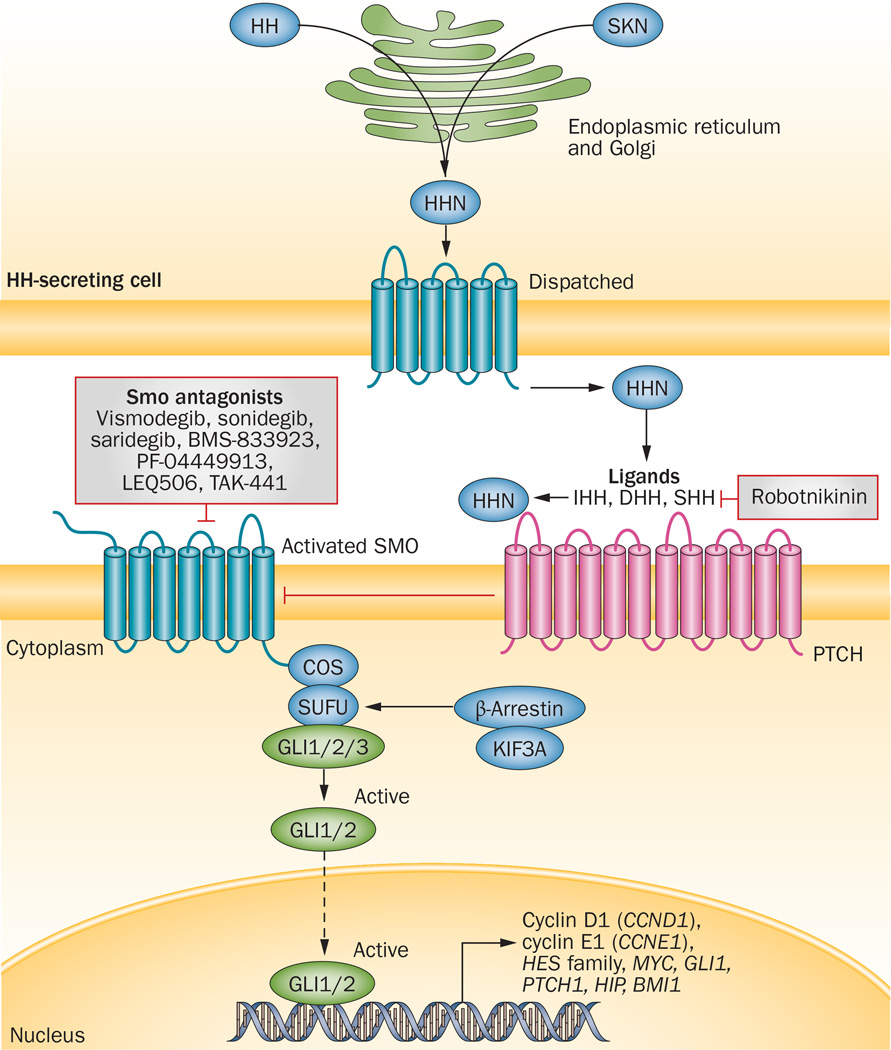
The HH signalling pathway is implicated in tissue-patterning during embryonic development and the repair of normal tissues, and epithelial-to-mesenchymal transition.83 Binding of HH ligands—Sonic hedgehog (SHH), Indian hedgehog (IHH), or Desert hedgehog (DHH)—relieves the inhibitory effect of their Patched (PTCH) transmembrane receptors on Smoothened (SMO), which is also located in the cell membrane (Figure 2).84 Subsequently, the signalling cascade initiated by SMO leads to activation and nuclear localization of GLI transcription factors, which drive expression of HH target genes; most of the target genes are involved in proliferation, survival, and angiogenesis.85 This cascade represents a novel target for cancer therapy, as aberrations in the HH pathway contribute to tumorigenesis and tumour growth through several mechanisms. These mechanisms include mutations in the key members of the pathway, such as loss-of-function mutations in PTCH1 gene encoding Patched 1 and gain-of-function mutation in the SMO gene, that result in ligand-independent activation of the HH pathway, as well as ligand-dependent signalling by either autocrine or paracrine routes.85 Mutation-driven mechanisms of HH-pathway activation have been demonstrated in basal-cell carcinoma (BCC) of the skin, medulloblastoma, and rarely rhabdomyosarcoma.86 Indeed, PTCH1 mutations are associated with HH-pathway hyperactivation in >90% of BCCs and 30% of adult medulloblastomas.87 Furthermore, patients with Gorlin syndrome (basal-cell nevus syndrome), an autosomal dominant condition associated with germline loss of one copy of the PTCH1 gene, have a predisposition to development of BCC and medulloblastoma.88 The aetiology of rhabdomyosarcomas, although thought to originate by a similar mechanism, is controversial.89
Figure 2.
The canonical HH-signalling pathway and pharmacological inhibitors targeting this pathway that are under ongoing development as anticancer therapies. The HH-processing pathway involves HHC autocatalysis, and SKN and Dispatched proteins, which mediate the release of HHN ligands (IHH, DHH and SHH). In the absence of HHN binding, PTCH interacts with and inhibits the activity of SMO; HHN binding to PTCH releases its inhibitory effects on SMO, resulting in SMO accumulation and sequestration of COS and SUFU proteins in cilia, which releases the GLI transcription factors to exert their effects in the nucleus. KIF3A and β-arrestin are required for localization of SMO to cilia. GLI1/2 promote a gene-expression pattern relevant to tumorigenesis. Development of investigational anticancer agents that inhibit SMO activation is of great interest. In addition, other potential targets, such as extracellular HHN ligands, GLI1/2 activation, or inhibition of gene transcription are under preclinical investigation. Besides the FDA-approved agent vismodegib, a number of small-molecule inhibitors of SMO are currently under clinical investigation. Abbreviations: COS, Costal; DHH, Desert hedgehog; HH, Hedgehog; HHC, Hedgehog C-terminal domain; HHN, Hedgehog N-terminal domain; HIP, Hedgehog interacting protein; IHH, Indian hedgehog; Ptch, Patched; SHH, Sonic hedgehog; SKN, Skinny hedgehog; SMO, Smoothened; SUFU, suppressor of fused.
Similarly to the Notch cascade, HH signalling can involve canonical and noncanonical pathways. Canonical signalling follows the PTCH1–SMO–GLI axis (Figure 2), whereas noncanonical pathways can be SMO-independent.85 The noncanonical signals are largely attributed to various tumour-associated signalling pathways integrating with HH signalling, in part by influencing the activity of GLI transcription factors.85 As such, HH-pathway activation can be triggered by many other intracellular signals, including those mediated by TGF-β, KRAS–MAPK/ERK, PI3K–AKT, IGF, TNF-α induced mTOR/S6K1 activation, and inactivation of hSNF5 (a regulator of chromatin remodelling, also known as SMARCB1).90–94 Determining the role of these pathways in cancer, and the molecular crosstalk between them, is an important consideration for the development of HH-targeting agents, and the appropriate selection of a class of inhibitors for therapeutic intervention.
Clinical drug development
Table 2 summarizes the agents targeting the HH-signalling pathway—by either autocrine or paracrine mechanisms—that are currently in clinical development. The most clinically advanced agent targeting the HH pathway is vismodegib, which was approved by the US FDA in 2012 and the European Medicines Agency (EMA) in 2013 for the treatment of metastatic BCC, or locally advanced BCC in patients who are not candidates for surgery or radiotherapy.95,96 Vismodegib is a direct, cyclopamine-competitive antagonist of SMO. A recent analysis of pooled data from patients with advanced-stage BCC included in the pivotal phase II that led to FDA approval and phase I studies indicates that a median overall survival duration of 2.8 years was achieved in patients with metastatic BCC who received vismodegib monotherapy, compared with 2.0 years estimated from the literature for standard treatments.97 These data indicate that the SMO inhibitor might improve overall survival in patients with BCC and distant metastases. Currently, vismodegib is being evaluated in the neoadjuvant setting for locally advanced BCC,98 and in various settings in other advanced malignancies.
Table 2.
Investigational agent targeting the HH signalling pathway (SMO antagonists) in clinical development*
| Compound and combination or intervention | Phase | Tumour type |
Clinicaltrials.gov identifier |
Status‡ |
|---|---|---|---|---|
| Vismodegib (Genentech) | ||||
| Single agent | I | Medulloblastoma (paediatric) | NCT00822458 | Completed |
| Single agent | I | Solid tumours | NCT00607724; NCT00968981 | Completed |
| With sirolimus | I | Pancreatic cancer, solid tumours | NCT01537107 | Recruiting |
| With RO4929097 (Notch inhibitor) | I | Breast cancer | NCT01071564 | Active, not recruiting |
| Following autologous HSCT | I | Multiple myeloma (in high-risk 1st remission or relapsed) | NCT01330173 | Active, not recruiting |
| With erlotinib ± gemcitabine (2 cohorts) | I | Solid tumours | NCT00878163 | Active, not recruiting |
| With temozolomide ± vismodegib | I/randomized II | Medulloblastoma (SHH molecular subtype) | NCT01601184 | Recruiting |
| Androgen ablation ± preoperative vismodegib (before radical prostatectomy) | I/randomized II | Prostate cancer | NCT01163084 | Active, not recruiting |
| With RO4929097 (Notch inhibitor) | I/II | Sarcoma | NCT01154452 | Recruiting |
| Single agent | II | BCC | NCT00833417 | Completed |
| Single agent | II | BCC (operable) | NCT01201915 | Completed |
| Single agent | II | BCC | NCT01367665 | Recruiting |
| Single agent | II | Basal cell nevus syndrome | NCT00957229 | Active, not recruiting |
| Intermittent vismodegib vs photodynamic therapy after 7 months of vismodegib treatment | Randomized II | BCC | NCT01556009 | Active, not recruiting |
| Plus radiotherapy | II | BCC; head and neck cancer | NCT01835626 | Recruiting |
| Neoadjuvant vismodegib vs placebo preceding excision by Mohs micrographic surgery | Randomized II | BCC | NCT01543581 | Unknown |
| Off-label use of vismodegib as an adjuvant to surgery | II | BCC | NCT01631331 | Active, not recruiting |
| With cytarabine | II | AML and high-risk MDS | NCT01880437 | Recruiting |
| Single agent | II | B-cell lymphoma or CLL | NCT01944943 | Recruiting |
| Single agent | II | Chondrosarcomas | NCT01267955 | Active, not recruiting |
| With FOLFOX or FOLFIRI | Randomized II | Colorectal cancer | NCT00636610 | Completed |
| FOLFOX ± vismodegib | Randomized II | Gastric and GEJ cancers | NCT00982592 | Active, not recruiting |
| Single agent | II | Glioblastoma multiforme | NCT00980343 | Completed |
| Single agent | II | Pontine glioma (paediatric) | NCT01774253 | Recruiting |
| Single agent | II | Medulloblastoma (recurrent/refractory) | NCT00939484 | Active, not recruiting |
| Single agent | II | Medulloblastoma (paediatric) | NCT01239316 | Active, not recruiting |
| Molecular risk-directed therapy with radiation and chemotherapy | II | Medulloblastoma (paediatric) | NCT01878617 | Recruiting |
| Maintenance therapy after 2nd or 3rd complete remission | Randomized II | Ovarian cancer | NCT00739661 | Completed |
| With gemcitabine and nab-paclitaxel | II | Pancreatic cancer | NCT01088815 | Recruiting |
| Gemcitabine ± vismodegib | Randomized II | Pancreatic cancer | NCT01064622 | Active, not recruiting |
| Cisplatin and etoposide ± vismodegib or cixutumumab (3 arms) | Randomized II | SCLC (extensive stage) | NCT00887159 | Active, not recruiting |
| BMS-833923 (aka XL 139; Bristol–Myers Squibb/Exelixis) | ||||
| Single agent | I | Solid tumours (Japan) | NCT01413906 | Completed |
| Single agent | I | Solid tumours (USA) | NCT00670189 | Active, not recruiting |
| With cisplatin and capecitabine | I | Gastric, GEJ, or oesophageal cancers | NCT00909402 | Completed |
| Alone or with lenalidomide, dexamethasone, or with bortezomib (3 arms) | I | Multiple myeloma | NCT00884546 | Completed |
| With carboplatin/etoposide followed by BMS-833923 alone | I | SCLC (extensive stage) | NCT00927875 | Completed |
| With dasatinib | I, II | CML (chronic phase) | NCT01218477 | Completed |
| Dasatinib ± BMS-833923 | Randomized II | CML (chronic phase) | NCT01357655 | Active, not recruiting |
| Sonidegib (aka erismodegib and LDE225; Novartis) | ||||
| Single agent | 0 | Pancreatic cancer (resectable) | NCT01694589 | Recruiting |
| Single agent | I | BCC, solid cancers, or medulloblastoma | NCT00880308 | Completed |
| With nilotinib | I | CML (chronic or accelerated phase) | NCT01456676 | Completed |
| Single agent | I | Solid tumours (East Asia) | NCT01208831 | Active, not recruiting |
| With FOLFIRINOX | I | Pancreatic cancer | NCT01485744 | Unknown |
| With gemcitabine | I | Pancreatic cancer | NCT01487785 | Active, not recruiting |
| With etoposide and cisplatin | I | SCLC (extensive stage) | NCT01579929 | Recruiting |
| With paclitaxel | I | Solid tumours | NCT01954355 | Recruiting |
| With buparlisib (PI3K inhibitor) | I | Solid tumours | NCT01576666 | Recruiting |
| With gemcitabine plus nab-paclitaxel in neoadjuvant setting | I/II | Pancreatic cancer | NCT01431794 | Recruiting |
| Single agent | I/II | Medulloblastoma (paediatric) | NCT01125800 | Active, not recruiting |
| Plus ruxolitinib | Ib/II | Myelofibrosis | NCT01787552 | Recruiting |
| Single agent | II | Acute leukaemias | NCT01826214 | Recruiting |
| Two dose levels of sonidegib | Randomized II | BCC | NCT01327053 | Active, not recruiting |
| Single agent | II | BCC previously treated with other SMO inhibitors | NCT01529450 | Terminated |
| Single agent | II | Nevoid BCC syndrome | NCT01350115 | Completed |
| Single agent | Randomized II | Breast cancer (stage II–III, ER, HER2) | NCT01757327 | Recruiting |
| Sonidegib vs temozolomide | Randomized III | Medulloblastoma (SHH molecular subtype) | NCT01708174 | Recruiting |
| Saridegib (aka IPI-926; Infinity) | ||||
| Single agent | I | Solid tumours | NCT00761696 | Completed |
| With cetuximab | I | Head and neck cancer | NCT01255800 | Completed |
| With FOLFIRINOX | I | Pancreatic cancer | NCT01383538 | Active, not recruiting |
| With gemcitabine | I/II | Pancreatic cancer | NCT01130142 | Completed |
| Single agent | II | Chondrosarcoma | NCT01310816 | Completed |
| Single agent | II | Myelofibrosis | NCT01371617 | Completed |
| Glasdegib (aka PF-04449913; Pfizer) | ||||
| Single agent | I | Solid tumours | NCT01286467 | Completed |
| Single agent | I | Selected haematological malignancies | NCT00953758 | Completed |
| With low-dose cytarabine or decitabine, or with daunorubicin plus cytarabine | I/II | AML and MDS | NCT01546038 | Recruiting |
| Single agent | II | High-risk acute leukaemia after allogeneic HSCT | NCT01841333 | Recruiting |
| Single agent | II | MDS and CMML | NCT01842646 | Recruiting |
| LEQ506 (Novartis) | ||||
| Single agent | I | Solid tumours, BCC, and medulloblastoma | NCT01106508 | Active, not recruiting |
| TAK-441 (Millennium) | ||||
| Single agent | I | Solid tumours | NCT01204073 | Completed |
Trials investigating drug interaction, topical treatment, organ dysfunction, expanded access, comparing two different doses, and nontherapeutic studies are excluded.
Data are from ClinicalTrials.gov as of June 6, 2014; default status is recruiting or active but not recruiting. Abbreviations: aka, also known as; AML, acute myeloid leukaemia; BCC, basal cell carcinoma; CLL, chronic lymphoblastic leukaemia; CML, chronic myeloid leukaemia; CMML, chronic myelomonocytic leukaemia; ER, oestrogen receptor; FOLFIRI, 5-fluorouracil plus folinic acid and irinotecan; FOLFIRINOX, 5-fluorouracil plus folinic acid, irinotecan and oxaliplatin; FOLFOX, 5-fluorouracil plus folinic acid and oxaliplatin; GEJ, gastroesophageal junction; HH, Hedgehog; HSCT, haematopoietic stem-cell transplantation; MDS, myelodysplastic syndrome; SCLC, small-cell lung cancer; SHH, Sonic hedgehog; SMO, Smoothened.
A survival benefit for vismodegib monotherapy might be expected in BCC, considering that around 95% of such tumours are associated with activation of the HH pathway. Vismodegib monotherapy has also been explored in glioblastoma, in particular, in a unique pilot phase II study in patients with relapsed or refractory glioblastoma who might benefit from debulking surgery.99 A total of 40 patients were randomly assigned to neoadjuvant treatment with daily vismodegib for 1 week versus no treatment, and all patients subsequently underwent surgery and received adjuvant vismodegib until disease progression.99 Although survival differences were not observed, compared with adjuvant treatment only, neoadjuvant and adjuvant vismodegib treatment did substantially decrease CSC content and/or viability in tumour tissues, based on reduced capacity of tumour-derived CD133+ cells to form neurospheres.99 However, in contrast to studies in BCC, this study did not show compelling clinical efficacy of vismodegib as a single agent, because the median PFS and overall survival durations of the patients were comparable to the control groups of historical studies: 1.8 months and 8.3 months, respectively.99
Currently, several phase II trials are investigating the efficacy of various SMO inhibitors in different tumour types, and in combination with a range of chemotherapy regimens. However, the results of the studies performed to date that combined vismodegib with chemotherapy in unselected patient populations have been disappointing. For example, three randomized, phase II studies investigating the addition of vismodegib to a chemotherapy backbone in patients with gastric or gastroesophageal junction tumours (n = 124), pancreatic cancer (n = 106) and extensive-stage small-cell lung cancer (ES-SCLC; n = 155) showed no statistically significant improvement in PFS or overall survival.100–102 In addition, a phase II single-arm study in which patients with pancreatic cancer were treated with gemcitabine, nab-paclitaxel and vismodegib showed PFS of 5.5 months and overall survival of 10 months;103 however, a PFS duration of 5.5 months is numerically similar to that observed in a phase III study of gemcitabine and nab-paclitaxel,104 suggesting limited or minimal contribution of vismodefib to the treatment effect. Similarly, in a phase II, randomized, placebo-controlled clinical trial in 199 patients with previously untreated metastatic colorectal cancer (mCRC),105 the addition of vismodegib to combination treatment with FOLFOX (5-fluorouracil [5-FU], folinic acid and oxaliplatin) or FOLFIRI (5-FU, folinic acid and irinotecan) chemotherapy plus bevacizumab did not increase PFS or the overall response rate (ORR). This trial failed to validate the hypothesis that inhibition of HH-signalling networks between tumour cells and stromal cells might have clinical antitumour activity in combination with standard-of-care chemotherapy.105 In addition, expression of HH ligands, or SMO or PTCH1 mRNA in tumour tissue did not predict clinical benefit in exploratory analyses.105
Vismodegib has also been investigated as a potential maintenance therapy. In a phase II, randomized, placebo-controlled trial,106 among 104 patients with ovarian cancer who were in second or third complete remission after chemotherapy, maintenance therapy with this agent did not result in a statistically significant improvement in PFS. Furthermore, a higher incidence of treatment discontinuation occurred in the vismodegib group versus the placebo cohort.106 The frequency of HH-ligand expression was lower than expected in archival tumour tissue from the study participants, being detected in only 13.5% of samples;106 thus, correlation between tumour expression of HH ligands and clinical benefit was problematic. Moreover, exploratory analyses of the relationship between PFS and expression levels of SMO or GLI1 also did not suggest any correlation.106
The negative trial results of targeting the HH pathways in unselected patients are not limited to studies of vismodegib. Three randomized phase II trials of the SMO inhibitor saridegib (also known as IPI-926) in pancreatic cancer, chondrosarcoma and myelofibrosis were stopped early due to lack of clinical activity.107,108 Thus, several other trials are evaluating rational treatment with SMO inhibitors in patients with tumours in which CSC self-replication is driven by autocrine secretion of HH ligands (Table 2). For example, a phase III, multicentre, open-label, randomized study is comparing the efficacy and safety of oral sonidegib (also known as erismodegib and LDE225) versus temozolomide in the treatment of patients with relapsed HH-pathway-activated medulloblastoma (NCT01708174).109 In a randomized, double-blind phase II trial of sonidegib, a high disease control rate, including complete, partial, and stable-disease responses, was observed in patients with locally advanced and metastatic BCC at both 200 mg and 800 mg daily dose levels, with 200 mg having a more favourable risk–benefit profile.110 Considering the characteristic activation of HH signalling in this cancer type, these data lend support to the activity of sonidegib in other cancers driven by this pathway. Of note, HH signalling is implicated in tumorigenesis and progression of SCLC, and in one study, therapeutic activity with sonidegib plus etoposide and cisplatin was observed in 50% (7/14) of patients with ES-SCLC;111 however, a randomized trial will be necessary to evaluate the effect of sonidegib in this combination therapy approach. Of note, a phase II, randomized trial investigating cisplatin and etoposide therapy with and without vismodegib in patients with SCLC found no statistically significant improvement in PFS or overall survival with addition of vismodegib.100
The HH-pathway downstream transcription factor GLI1 has been found to have an important role in resistance to therapy in leukaemia cells by inducing expression of UDP glucuronosyltransferase enzymes that glucuronidate and inactivate drugs used to treat this disease, such as ribavirin and cytarabine.112 Subsequently, several clinical trials of agents targeting SMO have been initiated in patients with relapsed or refractory high-risk AML (NCT02073838, NCT01880437 and NCT02129101).113–115
Biomarkers of HH signalling
Predictive biomarkers to guide patient selection might be critical for the successful clinical evaluation of HH-pathway inhibitors against cancer types other than BCC or medulloblastomas that are specifically associated with a high frequency of driver mutations in this pathway. Even in medulloblastoma, the frequency of identification of actionable genetic mutations ranges from only 15–30%, depending on the age distribution.86,87 These data underscore the need for identification of biomarkers to design rational combination therapies according to the output of the active signalling pathways identified.87
Sequencing of PTCH1 and SMO can be challenging, as whole-locus sequencing is required, especially for PTCH1—owing to the lack of a mutational hot-spot in this tumour suppressor gene. Presently, mRNA expression signatures are used as biomarkers of the activity of the HH pathway, as no antibodies specific for HH target-gene products that work reliably in immunohistochemistry (IHC) assays are available.116 However, Ellison et al.117 published an elegant paper describing the classification of medulloblastomas into SHH, Wnt, or non-SHH/Wnt subtypes based on IHC with antibodies targeting four different proteins. In addition, Shou and colleagues118 have developed a proprietary reverse-transcription PCR (RT-PCR)-based HH-signature assay as a patient pre-selection tool for HH-inhibition therapy; this 5-gene HH signature was selected from 32 differentially expressed candidate genes associated with SHH-subgroup classification of medulloblastoma, and includes the following genes: GLI1; SHROOM2; SPHK1; PDLIM3; and OTX2.118 A predictive model was generated, and the predictive value of this assay was analysed using pretreatment medulloblastoma samples derived from 50 patients enrolled in three phase I studies of sonidegib.118 Of these patients, 41 were predicted to have HH-inactive tumours. These 41 patients all had either disease progression, stable disease, or were not evaluable for tumour response.118 Among the remaining nine patients, who had tumours with HH activation, six had objective responses—three complete responses and three partial responses.118 Nevertheless, the value of this assay for predicting response to HH-pathway inhibition in patients with recurrent medulloblastoma is under evaluation in an ongoing phase III trial of sonidegib. Developing assays to evaluate expression of effectors of the HH pathway, such as GLI1 and GLI2, might be important to identify patients with tumours that are likely to respond to inhibitors of HH signalling—particularly for tumours other than BCC or SHH-subtype medulloblastomas; consequently, development of specific antibodies is warranted.
Resistance mechanisms
To date, clinical efficacy has not been demonstrated in trials of SMO inhibitors, except in patients with tumours driven by mutations in components of the HH-signalling cascade, such as SMO and PTCH1.119 Possible reasons for primary resistance to HH-inhibitor monotherapy and the lack of additional efficacy or benefit in combination with chemotherapy, compared with the outcomes of chemotherapy alone, include insufficient drug concentrations in the stroma. This resistance mechanism has been discussed in detail by Graham and colleagues,120 who described an unusual pharmacokinetic profile for vismodegib, owing to high-affinity, reversible binding to plasma proteins, solubility-limited absorption, and slow metabolic elimination. Compensatory upregulation of other signalling represents another potential resistance mechanism; for example, primary resistance to SMO inhibitors can be due to noncanonical activation of GLI transcription factors through pathways that bypass SMO and, therefore, the effects of SMO inhibitors.121
A mutation in SMO (D473H) in a tumour that progressed after 3 months of vismodegib treatment was detected in a patient with medulloblastoma who harboured a PTCH1 mutation in both primary and metastatic lesion biopsies taken before therapy.122 The SMO D473H mutation was only present after tumour progression and caused disruption of the vismodegib-binding site on SMO, and was, therefore, considered as the cause of acquired resistance.122 This type of mutation was also found in a mouse model of vismodegib-resistant medulloblastoma.122 Other mechanisms of acquired resistance, such as amplification of GLI2 or cyclin D1, noncanonical GLI activation, and noncanonical GLI-independent signalling downstream of SMO have also been described in preclinical models, including both xenograft and genetically engineered mouse models.121,123,124 These models successfully demonstrated the new hypotheses that parallel, interacting, and compensatory pathways are intertwined with the HH signalling pathway and can be upregulated in cancer. However, these preclinical models aimed at discovering new scientific hypotheses are not necessarily the best model to predict clinical outcome. Thus, to maximize predictive capability, preclinical experiments should be carefully designed and results should be evaluated statistically, before considering clinical trials to test the efficacy and toxicity of novel combinations of therapies.125
At present, the molecules and pathways that are implicated in resistance to SMO inhibitors include the PI3K pathway, small GTPases, Src-family kinases and arachidonate metabolites.126 Interestingly, in preclinical medulloblastoma models, resistance to SMO inhibitors could be prevented through combination therapy with SMO and PI3K–AKT inhibitors.123 Furthermore, vismodegib was not effective in oesophageal cancer cells with overactivation of mTOR–S6K1 signalling (a downstream target of PI3K–AKT), owing to SMO-independent, mTOR/S6K1-mediated GLI1 activation; combination treatment with the mTOR inhibitor everolimus and vismodegib showed better inhibitory effects on the growth of these cells in a xenograft model than either drug alone.94 Other preclinical studies suggest that this principle could be applied to other cancers. For example, in a model of tamoxifen-resistant breast cancer, the PI3K inhibitor LY294002 decreased SMO and GLI1 protein levels by 50%, suggesting a possible rationale for combined therapy to overcome endocrine resistance through targeting residual HH activity with SMO inhibitors.121 In addition, simultaneous activation of HH and PI3K signalling was seen in a PTEN-deficient glioblastoma model, and combined inhibition of the PI3K/mTOR and HH pathways induced synthetic lethality in PTEN-deficient cells in vitro and in vivo.127 On the basis of these models, two phase I combination therapy trials are currently investigating simultaneous targeting of the HH and PI3K–mTOR pathways with vismodegib plus sirolimus,128 or sonidegib plus buparlisib (also known as BKM120; Table 2).129
Whether resistance to one SMO inhibitor can be overcome by another SMO inhibitor remains unclear. Most SMO inhibitors currently in clinical trials compete with cyclopamine binding to SMO, and therefore all presumably target the same—or an overlapping—binding site.85,130 A possible exception is itraconazole, an antifungal agent with pleiotropic effects including direct inhibition of SMO by binding at a site distinct from the one targeted by vismodegib, thereby preventing SMO accumulation in cilia, an event that is necessary for activation of HH signalling.131 Thus, itraconazole might be effective as a second-line therapy, because it inhibits SMO with less potency than vismodegib and is associated with a different spectrum of adverse events than other drugs in this class, including vismodegib.130 However, whether itraconazole can overcome resistance to vismodegib mediated by SMO missense mutations remains to be established; in one study itraconazole did not induce a statistically significant decrease in GLI1-mRNA expression or tumour shrinkage in patients previously treated with vismodegib.130 Nevertheless, itraconazole could potentially serve as a lead compound for the development of more-potent second-generation SMO inhibitors.
An alternate strategy to overcome resistance to SMO inhibitors is to inhibit a different pathway target. Strong evidence of SMO-independent GLI activation suggests the development of direct GLI inhibitors could be warranted. Arsenic, another well-known pleiotropic agent, is a ‘direct’ inhibitor of GLI transcription factors and inhibits HH activity even downstream of vismodegib-resistant, mutant SMO.132 A combination of arsenic and itraconazole was effective in vitro and in vivo in models with either wild-type or mutant SMO. This combination was also active in cells harbouring all of the known SMO resistant mutations and in cells overexpressing GLI2.133
In summary, the development of HH-pathway inhibitors will benefit from advances in several research areas: the identification of more-informative biomarkers; rational, mechanism-based therapeutic combinations capable of addressing parallel or compensatory noncanonical signalling; and the development of more-effective next-generation agents, encompassing the repurposing of known drugs and compounds that inhibit the pathway through mechanisms distinct from that of cyclopamine to new indications.
Targeting the Wnt pathway
Wnt signalling
The Wnt-signalling cascade comprises three major pathways: the canonical Wnt pathway, which involves activation of β-catenin-T-cell-specific transcription factor (TCF)–lymphoid enhancer-binding factor (LEF) transactivation complex and is implicated in tumorigenesis; the noncanonical planar-cell polarity pathway, which regulates the cytoskeleton; and the noncanonical Wnt–calcium pathway, which regulates intracellular calcium levels.134 Of these pathways, canonical Wnt signalling is the best understood and its inhibition has been the focus of intensive research in cancer and other diseases. Indeed, along with the Notch and HH pathways, suppression of Wnt signalling has led to the development of agents that hold promise to interfere with carcinogenesis, tumour invasiveness and metastasis (Figure 3).
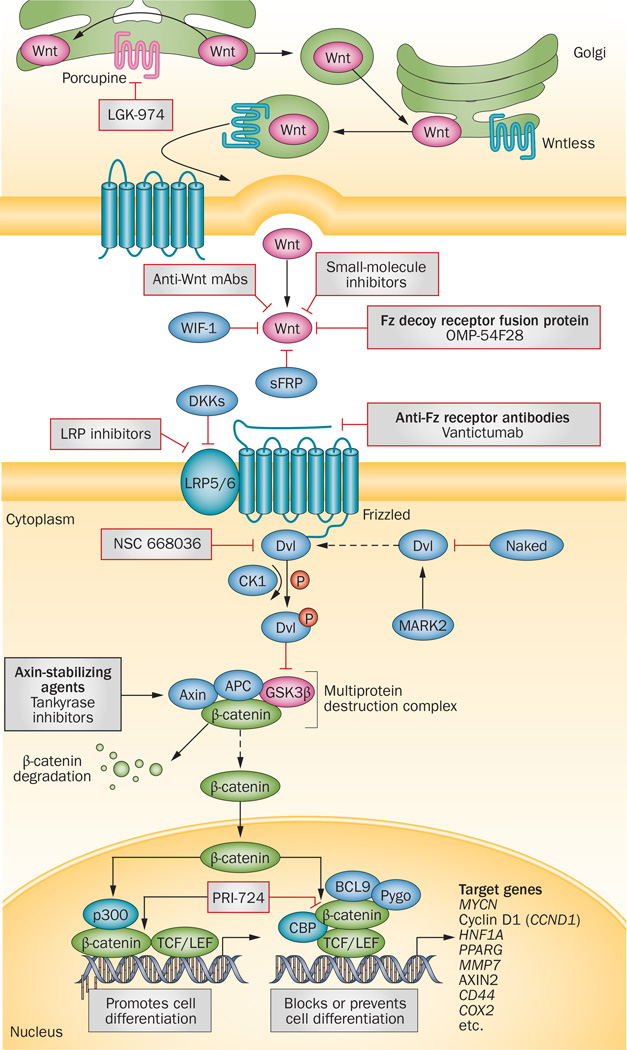
Figure 3.
The canonical Wnt signalling pathway and pharmacological inhibitors under investigation in cancer. Various proteins, including Porcupine and Wntless, regulate the secretion on Wnt proteins. LGK974 is an investigational agent that targets Porcupine to inhibit Wnt-ligand secretion from the endoplasmic reticulum. Once released, Wnt protein binding to Fz-family receptors on neighbouring cells results in intracellular signal transduction and gene expression with diverse consequences of relevance to cancer. Small molecules and mAbs targeting either the Wnt proteins or Fz receptor complexes have been developed to inhibit ligand–receptor interactions. The anti-Fz mAb vantictumab and the Wnt decoy receptor OMP-54F28 are currently being tested in clinical trials. Activation of the canonical Wnt signalling pathway leads to β-catenin accumulation as a result of disruption of a multiprotein destruction complex (dashed arrow), mediated by phospho-Dvl, which enables β-catenin to influence gene-expression patterns that determine cell differentiation. Small molecules that stabilize the multiprotein destruction complex and, thus, promote β-catenin degradation, such as tankyrase inhibitors, are in preclinical development. PRI-724 is an investigational agent to disrupt β-catenin–CBP complex, which might shift the balance from β-catenin-mediated gene-expression patterns that block differentiation (and thus promote cell ‘stemness’) to those that promote differentiation, is currently undergoing testing in clinical trials. Abbreviations: APC, adenomatous polyposis coli protein; BCL9, B-cell lymphoma 9; CBP, cyclic AMP response element-binding protein; CK1, casein kinase 1; DKKs, dickkopfs; Dvl, Dishevelled; Fz, Frizzled; GSK3β, glycogen synthase kinase 3β; LRP5/6, low-density lipoprotein receptor-related protein 5/6; mAbs, monoclonal antibodies; PPARG, peroxisome proliferator-activated receptor γ; Pygo, Pygopus; sFRP, secreted Frizzled-related protein; TCF/LEF, T-cell-specific transcription factor/lymphoid enhancer-binding factor; WIF-1, Wnt inhibitory factor 1.
The prototype Wnt ligand is a lipid-modified secretory glycoprotein of 350–400 amino acids; to date, at least 19 Wnt ligands have been identified in humans. Wnt proteins undergo two types of lipid post-translational modifications that are necessary for secretion: the addition of palmitate moiety to cysteine residues,135 and serine palmitoylation by Porcupine in the endoplasmic reticulum (Figure 3).136,137 After they are secreted from the cell, Wnt ligands bind to a receptor complex consisting of the primary receptor Frizzled (Fz), a member of the G-protein-coupled receptor family, and a co-receptor, low-density lipoprotein receptor-related protein 5/6 (LRP5/6). The interaction of Wnt proteins with their receptors can be inhibited by binding of the ligands to endogenous antagonists, including the secreted Frizzled-related-proteins (sFRPs) and Wnt inhibitory factor-1 (WIF-1).138, 139 Wnt signalling is also regulated by inhibition of the LRP co-receptors by Dickkopf-related proteins (DKK).140 When ligand binding and receptor activation is achieved, a signal is propagated via the segment polarity protein dishevelled homologue (Dvl) phosphoprotein, which is located in the cytoplasm.141 Activated Dvl inhibits Axin-mediated β-catenin phosphorylation, resulting in accumulation of cytoplasmic β-catenin;142 in the absence of Wnt signalling, a multiprotein destruction complex composed the of the scaffold protein axin, adenomatous polyposis coli (APC), and glycogen synthase kinase 3β (GSK3β) targets cytoplasmic β-catenin for ubiquitination and proteasomal degradation.143 In the presence of active Wnt signalling, the accumulation of β-catenin enables its translocation to the nucleus, where it induces cellular responses via transactivation of target genes in conjunction with TCF–LEF transcription factors.144
In several types of malignancy, preclinical data suggests that Wnt signalling contributes to the maintenance of the CSC population.145 One example is non-melanoma cutaneous tumour stem cells, which are maintained by Wnt–β-catenin signalling in murine models, and potentially in humans.146 Additionally, Wnt–β-catenin signalling seems to have a role in EMT.147 In turn, EMT might promote the induction of a CSC phenotype.2 Thus, targeting Wnt signalling in various cancers might represent a beneficial therapeutic approach.
Agents targeting the Wnt pathway in cancer
Several types of Wnt-signalling inhibitors are under ongoing development as anticancer therapies. For convenience, we divide these agents into three categories: agents approved by the FDA for other indications before their recognition as the Wnt-pathway inhibitors; agents in preclinical development, and investigational agents in clinical studies.
Approved agents
Two nonsteroidal anti-inflammatory drugs (NSAIDs) have been found to inhibit Wnt signalling: sulindac targets Dvl,148 and is currently being investigated in phase II trials in oncology; celecoxib inhibits β-catenin signalling by cyclo-oxygenase (COX)-dependent and COX-independent mechanisms,149 and has demonstrated antineoplastic activity in CRC cells.150 In addition, thiazolidinedione antidiabetic agents (glitazones) cause reverse β-catenin translocation to the plasma membrane,151 although further investigation of their potential anticancer activity is necessary.
Preclinical investigational agents
XAV-939, JW 55, G007-LK, G244-LM, WIKI4 and IWR-1 have been shown to stabilize axin by inhibiting tankyrase, a poly(ADP-ribose) transferase that targets axin for proteosomal degradation.152–154 This stabilization of axin, and thus the APC–axin–GSK3β destruction complex, results in degradation of β-catenin (Figure 3). Multiple non-NSAID inhibitors of Dvl, have also been developed; for example, NSC668036, 3289–8625, PCN-N3 and FJ9.155,156 Specifically, these agents decrease the interaction between Fz and Dvl at the membrane, and this inhibition of Dvl activation stabilizes the destruction complex, promoting β-catenin degradation. In addition, AV65 and artificial F-box are agents that have preclinical antineoplastic activity via a similar mechanism.157,158
Agents under clinical investigation
ICG-001 and the second-generation compound PRI-724, suppress the interaction of β-catenin with co-activator cyclic AMP response binding protein (CREB) binding protein (CBP or CREBBP) decreasing CBP-dependent gene expression.159, 160 In a phase Ia study,159 18 patients were treated with PRI-724 (dose escalation from 40–1,280 mg/m2 per day) via continuous infusion for 7 days. The compound had an acceptable toxicity profile, with only one grade 3 dose-limiting toxicity event: reversible hyperbilirubinaemia.159 In this trial, downregulation of survivin (BIRC5) expression in circulating tumour cells was correlated with increasing plasma drug concentrations, implicating this factor as a potential pharmacodynamic biomarker. PRI-724 is currently being evaluated in combination with a modified FOLFOX6 regimen for patients with refractory CRC, with gemcitabine in a phase Ib trial in patients with refractory pancreatic cancer, and a phase Ib/IIa trial for haematological malignancies (Table 3).161–163 In addition to these agents targeting β-catenin, a drug has been developed that inhibits Porcupine and thereby reduces the processing and secretion of Wnt proteins. Currently, this agent, known as LGK-974, is being tested in phase I trials in Wnt-ligand-dependent tumours—melanoma, breast cancer, and pancreatic cancer (Table 3).164
Table 3.
Investigational agents that target the Wnt signalling pathway in clinical development*
| Compound and combination or intervention | Phase | Tumour type | Clinicaltrials.gov identifier | Status‡ |
|---|---|---|---|---|
| PRI-724 (CBP/βcatenin antagonist; Prism BioLab/Eisai) | ||||
| Single agent | Ia | Solid tumours | NCT01302405 | Completed |
| With FOLFOX 6 | Ib | Colorectal cancer (metastatic) | NCT0132405 | Recruiting |
| With gemcitabine | Ib | Pancreatic cancer (advanced metastatic) | NCT01764477 | Recruiting |
| With dasatinib in CML, and with cytarabine in AML | I/II | Myeloid leukaemia | NCT01606579 | Recruiting |
| LGK-974 (Porcupine inhibitor; Novartis) | ||||
| Single agent | I | Melanoma, breast neoplasms, lobular carcinoma, TNBC, and pancreatic cancer | NCT01351103 | Recruiting |
| Vantictumab (aka OMP-18R5; anti-Frizzled-1/2/5/7/8 antibody; OncoMed/Cellgene) | ||||
| Single agent | Ib | Solid tumours | NCT01345201 | Completed |
| With paclitaxel | I | Breast cancer | NCT01973309 | Recruiting |
| With docetaxel | I | NSCLC | NCT01957007 | Recruiting |
| With nab-paclitaxel and gemcitabine | I | Pancreatic cancer | NCT02005315 | Recruiting |
| OMP-54F28 (Frizzled-8-Fc decoy fusion protein; OncoMed/Cellgene) | ||||
| Single agent | I | Solid tumours | NCT01608867 | Active, not recruiting |
| Plus sorafenib | Ib | Dose-escalation study in hepatocellular carcinoma | NCT02069145 | Recruiting |
| With paclitaxel and carboplatin | Ib | Dose-escalation study in platinum-sensitive ovarian cancer | NCT02092363 | Recruiting |
| With nab-paclitaxel and gemcitabine | Ib | Dose-escalation study in previously untreated stage IV pancreatic cancer | NCT02050178 | Recruiting |
| OTSA101 (Radiolabelled anti-Frizzled-10 antibody; OncoTherapy) | ||||
| Radiolabelled antibody | I | First-in-human study in synovial sarcoma | NCT01469975 | Recruiting |
Trials investigating drug interaction, topical treatment, organ dysfunction, expanded access, comparing two different doses, and nontherapeutic studies are excluded.
Data are from ClinicalTrials.gov as of June 6, 2014; default status is recruiting or active but not recruiting. Abbreviations: aka, also known as; AML, acute myeloid leukaemia; CML, chronic myeloid leukaemia; FOLFOX6, 5-fluorouracil plus folinic acid and oxaliplatin regimen for six cycles; NSCLC, non-small-cell lung cancer; TNBC, triple negative breast cancer.
Two major types of mAbs directed at inhibiting Wnt signalling are also under clinical investigation: those that neutralize Wnt ligands, and those that inhibit the Wnt receptors Fz and LRP. An example of the first drug class is a Wnt3A-neutralizing mAb that had antiproliferative and pro-apoptotic effects in a mouse model of prostate cancer.165 Furthermore, an anti-Fz10 radiolabelled mAb is being evaluated in a phase I trial for the treatment of synovial sarcoma (Table 3). Vantictumab (OMP-18R5),166–171 a mAb that blocks five Fz receptors (Fz1, Fz2, Fz5, Fz7 and Fz8), and a fusion protein decoy receptor (truncated Fz8), OMP-54F28,172–174 are also under investigation in phase I studies in advanced-stage solid tumours (Table 3). It has been shown that OMP-54F28 inhibits patient-derived xenograft tumour growth and, in particular, decreases CSC numbers.175 In a phase I dose-escalation clinical trial,176 OMP-54F28 was well tolerated and six of 25 patients experienced stable disease responses; two patients with desmoid tumours had prolonged stable disease for >6 months.176
In summary, investigational agents targeting the Wnt-signalling pathway are all currently in early clinical trials.166,167 As more targets within the Wnt pathway are discovered and the existing targets become better understood, novel agents will undoubtedly be developed for clinical testing. Given the critical role of Wnt signalling in CSCs, agents targeting Wnt could potentially achieve antitumour effects that conventional chemotherapy has been unable to reach.
Crosstalk between signalling pathways
During embryogenesis, developmental pathways operate in coordination. Unsurprisingly, therefore, instances of crosstalk among Notch, HH, Wnt, and other signalling pathways have been reported in a variety of cell types.177 Crosstalk between signalling pathways has the potential to profoundly add to the complexity of cellular responses to external stimuli and poses challenges for investigational drugs.
Interacting developmental signalling pathways
The crosstalk between developmental signalling pathways active in CSCs might offer the opportunity to inhibit multiple cascades by directly targeting only one. For example, preclinical studies have indicated that the GSI Notch-pathway inhibitor PF-03084014 can also inhibit the Wnt pathway by decreasing active β-catenin levels post-translationally. Specifically, Kwon et al.178 demonstrated a direct interaction between membrane-associated Notch and β-catenin, which promoted Numb-mediated lysosomal degradation of both Notch and β-catenin; thus, by promoting accumulation of uncleaved Notch in the cell membrane, PF-03084014 indirectly promoted β-catenin degradation. Also, β-catenin can drive activation of Notch signalling by increasing expression of the JAG1 gene, which encodes the Notch ligand Jagged1.75,179 These findings suggest that Notch inhibitors, including GSIs, could block β-catenin mediated tumorigenesis, and vice versa. Interestingly, preclinical data shows that PF-03084014-responsive tumours have elevated baseline expression levels of Notch and Wnt pathway genes,180 supporting the importance of crosstalk between these pathways in tumorigenesis. Furthermore, PF-03084014 inhibited both Notch and Wnt pathways, resulting in a reduction in tumour growth in a CRC xenograft model.180 Crosstalk between Notch and Wnt signalling might also explain why desmoid tumours often respond to Notch-pathway inhibition, irrespective of whether β-catenin accumulation occurs by CTNNB1 somatic gain-of-function mutation, or by APC loss-of-function in a setting of familial adenomatous polyposis.181 Of note, a phase I trial of PF-03084014 reported that four of nine evaluable patients with desmoid tumours experienced partial responses and the remaining five patients had some evidence of tumour shrinkage with prolonged disease stabilization.182 Moreover, the Notch and HH pathways are simultaneously activated in desmoid tumours, as well as desmoid-derived mesencyhmal cell lines.183 Thus, desmoid tumours are an example of neoplasms in which all three ‘CSC pathways’ are simultaneously activated, opening the possibility that these tumours could respond to inhibitors of these pathways alone or in combination.
An important consideration regarding the effect of inhibiting one (or more) of these CSC pathways is the potential therapeutic escape through compensatory upregulation of an interconnected pathway. For instance, prolonged, systemic inhibition of Notch signalling can result in HH-pathway activation in the skin as a compensatory phenomenon.184 This effect might explain why loss of Notch function has been associated with an increased risk of non-melanoma skin cancer development, as reported in patients with Alzheimer disease who were treated with a GSI to block β-amyloid production; in a phase III trial of the GSI semagacestat (GSI450139) in patients with Alzheimer disease, the incidence of non-melanoma skin cancers was 2% in the placebo cohort versus 10–11% in the GSI group.184
Crosstalk can also occur between the Wnt and HH pathways, through a mechanism involving the endogenous Wnt-inhibitory factor sFRP-1, which is induced by the HH pathway transcription factor GLI1 in gastric cancer cell lines.185 Specifically, HH signalling was found to inhibit Wnt signalling via upregulation of sFRP-1,185 and to suppress β-catenin transcriptional activity.186 Furthermore, HH signalling can induce the Notch ligand Jagged2, at least in some models.187 These findings suggests that HH-pathway inhibitors might, at least in some cases, inhibit Jagged2-mediated Notch signalling, but may also trigger a compensatory increase in Wnt signalling.
To reduce the potential for CSCs to escape inhibition of individual stem-cell signalling pathways involved in embryonic development that are frequently activated in cancer cells, efforts have been made to target multiple pathways through combination therapy approaches. Indeed, phase I and phase Ib/II trials combining vismodegib to target the HH pathway and the GSI RO4929097 to inhibit Notch signalling have been conducted in patients with breast cancer and sarcoma, respectively (Table 1 and Table 2).69,188 This combination approach is based on the following rationales: first, the HH and Notch pathways are often active concomitantly in BCCs and medulloblastomas that arise in Ptch-deficient mice and human soft-tissue sarcomas;189 second, HH signalling leads to transactivation of the Notch-target gene HES1 in a Notch-independent manner,190 and a negative regulator of Notch signalling, Numb, also suppresses the HH transcription factor GLI1 by targeting it for degradation;191 third, preclinical investigations in a prostate cancer model showed that combined inhibition of Notch and HH signalling was necessary to deplete the CSC subpopulation of tumour cells.192 Unfortunately, these combination trials in sarcoma and breast cancer were halted due to pharmacokinetic issues encountered during the development of RO4929097. At present, correlative studies on CSC inhibition are underway to confirm mechanisms of action and/or to identify downstream-target effects by molecular analysis.
Broader signalling crosstalk
Crosstalk between BCR–ABL kinase and Wnt signalling has been recognized in chronic myeloid leukaemia (CML): BCR–ABL kinase stabilizes β-catenin via tyro-sine phosphorylation, which enables β-catenin to escape from binding to the axin–APC–GSK3β complex and, therefore, from degradation.193 Owing to this crosstalk, imatinib is able to decrease β-catenin accumulation by inhibiting BCR–ABL kinase,193 which might at least partially explain the activity of imatinib in desmoid tumours. Interaction between KIT and β-catenin has also been reported. In particular, β-catenin levels in CSCs isolated from ovarian cancers decreased when KIT was inhibited, indicating that KIT regulates β-catenin.194 Furthermore, ABC transporters, such as ABCG2, are known transcriptional targets of β-catenin and are involved in efflux of xenobiotics from cells, and concurrent upregulation of KIT, β-catenin and ABCG2 levels was noted.194 These findings led to the hypothesis that KIT and/or β-catenin mediate chemoresistance by increasing expression of ABC transporters.194
GSK3β, a core component of Wnt signalling through its role in the β-catenin destruction complex, is a serine/ threonine kinase that mediates indirect Wnt–HH crosstalk: GSK3β phosphorylates SMO and GLI1 proteins, leading to their proteasomal degradation.121 The PI3K–AKT axis inhibits GSK3β and, thus, can concomitantly activate HH and Wnt–β-catenin signalling.121 In a study of tamoxifen-resistant breast cancer cells, an inhibitor of PI3K–AKT pathway also blocked activation of both HH and Wnt signalling.121 In addition, GSK3β has been reported to modulate Notch signalling, either positively or negatively depending on the context. GSK3β was demonstrated to phosphorylate and negatively regulate Notch2195 and the Notch co-activator MAML1.196 Conversely, GSK3β has been shown to phosphorylate and positively regulate Notch1 in different models.197 These observations imply that pharmacological inhibition of Wnt or PI3K–AKT has the potential to also modulate Notch and HH, resulting in desirable or undesirable effects. For example, an AKT inhibitor could potentially cause a compensatory increase in Notch activity via GSKβ.
Oestrogen and Notch signalling interact in ER-positive breast cancer cells: oestrogen decreases the activity of Notch1, whereas oestrogen deprivation and tamoxifen cause re-activation of Notch1.198 Notch1, in turn, can activate ER-dependent transcription in the absence of oestrogen through nuclear inhibitor of nuclear factor κB kinase α (IKKα),199 thereby promoting endocrine resistance. More recently, Notch4 has been shown to cause endocrine resistance in PKCα-positive, ER-positive breast cancer cells.46 As might be expected based on these interactions, combinations of anti-oestrogens and Notch inhibitors were highly effective in preclinical models of ER-positive breast cancer,46,198 and have shown promise in pilot clinical trials in this disease.47,48
Conclusions
Targeting CSC via modification of the Wnt, HH and Notch embryonic developmental signalling pathways characteristic of these cells holds the promise of preventing disease relapses. However, developing such agents is fraught with challenges. In particular, more-accurate preclinical models for testing CSC-targeted agents must be developed as the current approaches are not ideal for identifying therapeutics that are likely to be clinically effective. It is now clear that all signalling pathways, including those used by the embryonic ‘stem cell’, do not operate in isolation, but function as a coordinated network. The phenotype of tumour cells or CSCs is an output of the entire signalling network. Thus, the development of CSC inhibitors will require a working understanding of key nodes in the stem-cell signalling network. This knowledge, in turn, is propelling the design of mechanism-based combination regimens. Furthermore, informative molecular biomarkers that interrogate pathway activity and predict efficacy are necessary for patient identification and stratification, and as biological correlatives of activity. Moreover, single-agent tumour volume reduction might not be an appropriate end point for CSC agents, owing to therapeutic effects based on interference with rare tumour cell subpopulations; recurrence, progression or survival-based end points, and/or surrogate end points that predict such outcomes, will be more informative. A ‘one-size-fits-all’ treatment regimen is unlikely to be identified. Rather, systems biology and personalized medicine will enable the design of a range of regimens, which might be useful in different subgroups of patients, or even sequentially in the same patient, adapting treatment to clonal evolution within the tumour. This field remains in its infancy, and like other emerging fields in cancer therapeutics (such as immunotherapy), considerable research effort will be required to yield mature products. However, for the first time in medical history we have the tools to understand cell fate determination in human tumours and target it therapeutically.
Key points.
-
▪
Preclinical models provide evidence of cancer stem cells (CSCs) contributing to cancer proliferation, relapse and metastasis; this theory is being examined and validated in the clinical setting, currently in advanced malignancies
-
▪
Over the past few years, new investigational agents have been developed to block the Notch, Hedgehog (HH) or Wnt signalling pathways for targeting CSCs
-
▪
To date, robust antitumour activity has not been observed by targeting CSCs using Notch, HH or Wnt inhibitors, either as single agents or in combination with standard chemotherapy, in clinical trials
-
▪
Combination approaches to overcome the crosstalk among Notch, HH and Wnt pathways, as well as other signalling pathways, has been examined mostly in preclinical models, with promising results
-
▪
The success of the combination therapy in clinical trials might depend on CSC-tumour microenvironment interactions, perhaps in the context of the genotypes and phenotypes of the bulk tumour, CSCs, and the tumour microenvironment
-
▪
A number of clinical trials have incorporated surrogate CSC assays to measure the effects of an investigational agent on CSCs, but further technological improvements in assays are needed
Review criteria.
Data for this Review were identified by searching PubMed, Google Scholar, ClinicalTrials.gov, and the ASCO and American Association for Cancer Research online abstract databases. The search terms included “Notch”, “Wnt”, “Hedgehog”, “cancer stem cells”, “embryonic signalling pathway”, “crosstalk”, and “γ-secretase inhibitor”. In general, full-text articles published in English were selected when assessing papers to cite in this Review.
Acknowledgements
We gratefully acknowledge Dr Richard Swerdlow of PSI INTERNATIONAL, Inc., Rockville, MD, USA, for his assistance in organizing the references and formatting the manuscript, and Melissa Maher at Technical Resources International, Inc., Bethesda, MD, USA, for drafting the Figures.
Footnotes
Competing interests
L.M. has participated in collaborative research on Notch inhibitors with CytoMX and Merck Oncology. M.K. is an equity holder in Prism Pharma. The other authors declare no competing interests.
Author contributions
All authors contributed substantially to researching the data for the article, discussions of content, writing the article, and review/editing of the manuscript before submission.
Contributor Information
Naoko Takebe, Email: Takeben@mail.nih.gov.
S. Percy Ivy, Email: ivyp@ctep.nci.nih.gov.
References
- 1.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells—old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 2.Espinoza I, Miele L. Deadly crosstalk: notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013;341:41–45. doi: 10.1016/j.canlet.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells—what challenges do they pose? Nat. Rev. Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tirino V, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13–24. doi: 10.1096/fj.12-218222. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann C, Jobs G, Thomas M, Burtscher H, Kubbies M. Established breast cancer stem cell markers do not correlate with in vivo tumorigenicity of tumor-initiating cells. Int. J. Oncol. 2012;41:1932–1942. doi: 10.3892/ijo.2012.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karsten U, Goletz S. What makes cancer stem cell markers different? Springerplus. 2013;2:301. doi: 10.1186/2193-1801-2-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr. Opin. Genet. Dev. 2014;24:68–73. doi: 10.1016/j.gde.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schepers AG, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 13.Hoey T, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Varnat F, Siegl-Cachedenier I, Malerba M, Gervaz P, Ruiz i Altaba A. Loss of WNT–TCF addiction and enhancement of HH–GLI1 signalling define the metastatic transition of human colon carcinomas. EMBO Mol. Med. 2010;2:440–457. doi: 10.1002/emmm.201000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermeulen L, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 16.LaBarge MA. The difficulty of targeting cancer stem cell niches. Clin. Cancer Res. 2010;16:3121–3129. doi: 10.1158/1078-0432.CCR-09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merchant AA, Matsui W. Targeting Hedgehog—a cancer stem cell pathway. Clin. Cancer Res. 2010;16:3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 19.Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol. Ther. 2013;139:95–110. doi: 10.1016/j.pharmthera.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-del Arco P, et al. Alternative promoter usage at the Notch1 locus supports ligand-independent signaling in T cell development and leukemogenesis. Immunity. 2010;33:685–698. doi: 10.1016/j.immuni.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy A, et al. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity. 2012;36:105–119. doi: 10.1016/j.immuni.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Gu JW, et al. Notch signals in the endothelium and cancer “stem-like” cells: opportunities for cancer therapy. Vasc. Cell. 2012;4:7. doi: 10.1186/2045-824X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling—are we there yet? Nat. Rev. Drug Discov. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 24.Perumalsamy LR, Marcel N, Kulkarni S, Radtke F, Sarin A. Distinct spatial and molecular features of notch pathway assembly in regulatory T cells. Sci. Signal. 2012;5:ra53. doi: 10.1126/scisignal.2002859. [DOI] [PubMed] [Google Scholar]
- 25.Perumalsamy LR, Nagala M, Banerjee P, Sarin A. A hierarchical cascade activated by non-canonical Notch signaling and the mTOR–Rictor complex regulates neglect-induced death in mammalian cells. Cell Death Differ. 2009;16:879–889. doi: 10.1038/cdd.2009.20. [DOI] [PubMed] [Google Scholar]
- 26.Perumalsamy LR, Nagala M, Sarin A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc. Natl Acad. Sci. USA. 2010;107:6882–6887. doi: 10.1073/pnas.0910060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raafat A, et al. Rbpj conditional knockout reveals distinct functions of Notch4/Int3 in mammary gland development and tumorigenesis. Oncogene. 2009;28:219–230. doi: 10.1038/onc.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sade H, Krishna S, Sarin A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J. Biol. Chem. 2004;279:2937–2944. doi: 10.1074/jbc.M309924200. [DOI] [PubMed] [Google Scholar]
- 29.Lee KS, et al. Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes Dev. 2013;27:2642–2647. doi: 10.1101/gad.225169.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011;13:211. doi: 10.1186/bcr2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deangelo DJ, et al. A phase I clinical trial of the notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias [abstract] J. Clin. Oncol. 2006;24(Suppl.):a6585. [Google Scholar]
- 32.Pandya K, et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br. J. Cancer. 2011;105:796–806. doi: 10.1038/bjc.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abel EV, et al. The Notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer. PLoS ONE. 2014;9:e91983. doi: 10.1371/journal.pone.0091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grudzien P, et al. Inhibition of Notch signaling reduces the stem-like population of breast cancer cells and prevents mammosphere formation. Anticancer Res. 2010;30:3853–3867. [PubMed] [Google Scholar]
- 35.Hassan KA, et al. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin. Cancer Res. 2013;19:1972–1980. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito N, et al. A high Notch pathway activation predicts response to γ-secretase inhibitors in proneural subtype of glioma tumor-initiating cells. Stem Cells. 2014;32:301–312. doi: 10.1002/stem.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messersmith WA, et al. A phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin. Cancer Res. 2015;21:60–67. doi: 10.1158/1078-0432.CCR-14-0607. [DOI] [PubMed] [Google Scholar]
- 38.Milano J, et al. Modulation of Notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 39.Searfoss GH, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J. Biol. Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 40.Wong GT, et al. Chronic treatment with the γ-secretase inhibitor LY-411575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 41.van Es JH, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 42.Krop I, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 43.Dumortier A, et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS ONE. 2010;5:e9258. doi: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roderick JE, et al. Therapeutic targeting of NOTCH signaling ameliorates immune-mediated bone marrow failure of aplastic anemia. J. Exp. Med. 2013;210:1311–1329. doi: 10.1084/jem.20112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Real PJ, Ferrando AA. NOTCH inhibition and glucocorticoid therapy in T-cell acute lymphoblastic leukemia. Leukemia. 2009;23:1374–1377. doi: 10.1038/leu.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun J, et al. Crosstalk between PKCa and Notch-4 in endocrine-resistant breast cancer cells. Oncogenesis. 2013;2:e60. doi: 10.1038/oncsis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Means-Powell J, et al. A phase Ib dose escalation trial of RO4929097 (a γ-secretase inhibitor) in combination with exemestane in patients with ER+ metastatic breast cancer [abstract P2-14-04] Cancer Res. 2012;72(Suppl.):280s. doi: 10.1016/j.clbc.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albain K. Combination of Notch inhibitor MK-0752 and endocrine therapy for early stage ERa breast cancer in a pre-surgical window study. Cancer Res. 2010;70(Suppl.):113s-114s. [Google Scholar]
- 49.Tolcher AW, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J. Clin. Oncol. 2012;30:2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison H, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hellstrom M, et al. DLL4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 52.Jimeno A, et al. Phase I study of REGN421/ SAR153192, a fully-human delta-like ligand 4 (DLL4) monoclonal antibody (mAb), in patients with advanced solid tumors [abstract] J. Clin. Oncol. 2013;31(Suppl.):a2502. [Google Scholar]
- 53.Yan M. Therapeutic promise and challenges of targeting DLL4/NOTCH1. Vasc. Cell. 2011;3:17. doi: 10.1186/2045-824X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie M, He CS, He Z, Lin Q. Clinical and prognostic implications of delta-like ligand 4 and hypoxia-inducible factors in metastatic renal cell carcinoma (mRCC) patients treated with sunitinib as first-line therapy [abstract] J. Clin. Oncol. 2013;31(Suppl.):e15567. [Google Scholar]
- 55.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01131234. [DOI] [PubMed]
- 56.Sahebjam S, et al. A phase I study of the combination of RO4929097 and cediranib in patients with advanced solid tumours (PJC-004/ NCI 8503) Br. J. Cancer. 2013;109:943–949. doi: 10.1038/bjc.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sagert J, et al. Tumor-specific inhibition of Jagged-dependent Notch signaling using a Probody™ therapeutic [abstract] Mol. Cancer Ther. 2013;12(Suppl. 11):C158. [Google Scholar]
- 58.Mohammed TA, et al. A pilot phase II study of valproic acid for treatment of low-grade neuroendocrine carcinoma. Oncologist. 2011;16:835–843. doi: 10.1634/theoncologist.2011-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rajguru S, et al. A phase II study of the histone deacetylase inhibitor panobinostat (LBH589) in low-grade neuroendocrine tumors [abstract] J. Clin. Oncol. 2012;30(Suppl.):e14554. [Google Scholar]
- 60.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01013597. [DOI] [PubMed]
- 61.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/ct2/show/NCT01476592. [DOI] [PubMed]
- 62.Paryan M, et al. Investigation of deregulated genes of Notch signaling pathway in human T cell acute lymphoblastic leukemia cell lines and clinical samples. Mol. Biol. Rep. 2013;40:5531–5540. doi: 10.1007/s11033-013-2654-8. [DOI] [PubMed] [Google Scholar]
- 63.Fogelstrand L, et al. Prognostic implications of mutations in NOTCH1 and FBXW7 in childhood T-ALL treated according to the NOPHO ALL-1992 and ALL-2000 protocols. Pediatr. Blood Cancer. 2014;61:424–430. doi: 10.1002/pbc.24803. [DOI] [PubMed] [Google Scholar]
- 64.Wang H, et al. NOTCH1-RBPJ complexes drive target gene expression through dynamic interactions with superenhancers. Proc. Natl Acad. Sci. USA. 2014;111:705–710. doi: 10.1073/pnas.1315023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takebe N, Nguyen D, Yang SX. Targeting Notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol. Ther. 2014;141:140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersen P, Uosaki H, Shenje LT, Kwon C. Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol. 2012;22:257–265. doi: 10.1016/j.tcb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoeck A, et al. Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov. 2014;4:1154–1167. doi: 10.1158/2159-8290.CD-13-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen D, et al. Notch1 phenotype and clinical stage progression in non-small cell lung cancer. J. Hematol. Oncol. 2015;8:9. doi: 10.1186/s13045-014-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01071564. [DOI] [PubMed]
- 70.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/ct2/show/NCT01119599. [DOI] [PubMed]
- 71.Rota LM, Lazzarino DA, Ziegler AN, LeRoith D, Wood TL. Determining mammosphere-forming potential: application of the limiting dilution analysis. J. Mammary Gland Biol. Neoplasia. 2012;17:119–123. doi: 10.1007/s10911-012-9258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Espinoza I, Pochampally R, Xing F, Watabe K, Miele L. Notch signaling: targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther. 2013;6:1249–1259. doi: 10.2147/OTT.S36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez-Majada V, et al. Nuclear IKK activity leads to dysregulated notch-dependent gene expression in colorectal cancer. Proc. Natl Acad. Sci. USA. 2007;104:276–281. doi: 10.1073/pnas.0606476104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fre S, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc. Natl Acad. Sci. USA. 2009;106:6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kode A, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sjolund J, et al. The Notch and TGF-β signaling pathways contribute to the aggressiveness of clear cell renal cell carcinoma. PLoS ONE. 2011;6:e23057. doi: 10.1371/journal.pone.0023057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Izrailit J, Berman HK, Datti A, Wrana JL, Reedijk M. High throughput kinase inhibitor screens reveal TRB3 and MAPK–ERK/TGFβ pathways as fundamental Notch regulators in breast cancer. Proc. Natl Acad. Sci. USA. 2013;110:1714–1719. doi: 10.1073/pnas.1214014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albain K, et al. Modulation of cancer stem cell biomarkers by the Notch inhibitor MK0752 added to endocrine therapy for early stage ER+ breast cancer [abstract S1-5] Cancer Res. 2011;71(Suppl.) [Google Scholar]
- 79.Osipo C, et al. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a γ-secretase inhibitor. Oncogene. 2008;27:5019–5032. doi: 10.1038/onc.2008.149. [DOI] [PubMed] [Google Scholar]
- 80.Zhang CC, et al. Synergistic effect of the γ-secretase inhibitor PF-03084014 and docetaxel in breast cancer models. Stem Cells Transl. Med. 2013;2:233–242. doi: 10.5966/sctm.2012-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang S, Chung WC, Miele L, Xu K. Targeting Met and Notch in the Lfng-deficient, Met-amplified triple-negative breast cancer. Cancer Biol. Ther. 2014;15:633–642. doi: 10.4161/cbt.28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strosberg JR, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur. J. Cancer. 2012;48:997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Odoux C, et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68:6932–6941. doi: 10.1158/0008-5472.CAN-07-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 86.Ng JM, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nat. Rev. Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kool M, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to Smoothened inhibition. Cancer Cell. 2014;25:393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson RL, et al. Human homolog of Patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 89.Roma J, Almazan-Moga A, Sanchez de Toledo J, Gallego S. Notch, Wnt, and Hedgehog pathways in rhabdomyosarcoma: from single pathways to an integrated network. Sarcoma. 2012;2012:695603. doi: 10.1155/2012/695603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dennler S, et al. Induction of Sonic hedgehog mediators by transforming growth factor-β: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo . Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 91.Jagani Z, et al. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog–Gli pathway. Nat. Med. 2010;16:1429–1433. doi: 10.1038/nm.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji Z, Mei FC, Xie J, Cheng X. Oncogenic KRAS activates Hedgehog signaling pathway in pancreatic cancer cells. J. Biol. Chem. 2007;282:14048–14055. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 93.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc. Natl Acad. Sci. USA. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–87. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dirix L. Discovery and exploitation of novel targets by approved drugs. J. Clin. Oncol. 2014;32:720–721. doi: 10.1200/JCO.2013.53.7118. [DOI] [PubMed] [Google Scholar]
- 96.Sekulic A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis KD, et al. Vismodegib in the treatment of patients with metastatic basal cell carcinoma (mBCC) and distant metastases: survival in the pivotal phase II and phase I studies [abstract] J. Clin. Oncol. 2014;32(5s Suppl.):a9012. [Google Scholar]
- 98.Mortier L, et al. A phase II study to assess vismodegib in the neoadjuvant treatment of locally advanced basal cell carcinoma (laBCC): the Vismodegib Neoadjuvant (VISMONEO) study [abstract] J. Clin. Oncol. 2014;32(5s Suppl.):TPS9014. [Google Scholar]
- 99.Sloan AE, et al. Targeting glioma-initiating cells in GBM: ABTC-0904, a randomized phase 0/II study targeting the Sonic Hedgehog-signaling pathway [abstract] J. Clin. Oncol. 2014;32(5s Suppl.):a2026. [Google Scholar]
- 100.Belani CP, et al. Three-arm randomized phase II study of cisplatin and etoposide (CE) versus CE with either vismodegib (V) or cixutumumab (Cx) for patients with extensive stage-small cell lung cancer (ES-SCLC) (ECOG 1508) [abstract] J. Clin. Oncol. 2013;31(Suppl.):a7508. [Google Scholar]
- 101.Catenacci DVT, et al. Final analysis of a phase IB/randomized phase II study of gemcitabine (G.) plus placebo (P) or vismodegib (V), a hedgehog (Hh) pathway inhibitor, in patients (pts) with metastatic pancreatic cancer (PC): a University of Chicago phase II consortium study [abstract] J. Clin. Oncol. 2013;31(Suppl.):a4012. [Google Scholar]
- 102.Cohen DJ, et al. Vismodegib (V), a Hedgehog (HH) pathway inhibitor, combined with FOLFOX for first-line therapy of patients (pts) with advanced gastric and gastroesophageal junction (GEJ) carcinoma: a New York Cancer Consortium led phase II randomized study [abstract] J. Clin. Oncol. 2013;31(Suppl.):a4011. [Google Scholar]
- 103.De Jesus-Acosta A, et al. A phase II study of vismodegib, a Hedgehog (Hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA) [abstract] J. Clin. Oncol. 2014;32(Suppl. 3):a257. [Google Scholar]
- 104.Von Hoff DE, et al. Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT) [abstract] J. Clin. Oncol. 2012;30(Suppl. 34):LBA148. [Google Scholar]
- 105.Berlin J, et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin. Cancer Res. 2013;19:258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 106.Kaye SB, et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin. Cancer Res. 2012;18:6509–6518. doi: 10.1158/1078-0432.CCR-12-1796. [DOI] [PubMed] [Google Scholar]
- 107.Madden JI. Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic. Cancer. Infinity Pharmaceuticals. 2012 [online], http://phx.corporate-ir.net/phoenix.zhtml?c=121941&p=irol-newsArticle&ID=1653550&highlight.
- 108.Madden JI. Infinity Stops Phase 2 Trials of Saridegib in Chondrosarcoma and Myelofibrosis. FierceBiotech. 2012 [online], http://www.fiercebiotech.com/press-releases/infinity-stops-phase-2-trials-saridegib-chondrosarcoma-and-myelofibrosis.
- 109.US National Library of Medicine. ClinicalTrials.gov. 2015 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01708174. [DOI] [PubMed]
- 110.Migden MR, et al. Randomized, double-blind study of sonidegib (LDE225) in patients (pts) with locally advanced (La) or metastatic (m) basal-cell carcinoma (BCC) [abstract] J. Clin. Oncol. 2014;32(5s Suppl.):a9009a. [Google Scholar]
- 111.Pietanza MC, et al. Phase I trial of the Hedgehog (Hh) inhibitor, LDE225, in combination with etoposide and cisplatin (EP) for initial treatment of extensive stage small cell lung cancer (ES-SCLC) [abstract] J. Clin. Oncol. 2014;32(5s Suppl.):a7602. doi: 10.1016/j.lungcan.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zahreddine HA, et al. The Sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511:90–93. doi: 10.1038/nature13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.US National Library of Medicine. ClinicalTrials.gov. 2015 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01880437. [DOI] [PubMed]
- 114.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT02073838. [DOI] [PubMed]
- 115.US National Library of Medicine. ClinicalTrials.gov. 2015 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT02129101. [DOI] [PubMed]
- 116.Yauch RL, et al. A paracrine requirement for Hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 117.Ellison DW, et al. Medulloblastoma: clinicopathological correlates of, SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shou Y, et al. A five-gene Hedgehog signature developed as a patient preselection tool for hedgehog inhibitor therapy in medulloblastoma. Clin. Cancer Res. 2015;21:585–593. doi: 10.1158/1078-0432.CCR-13-1711. [DOI] [PubMed] [Google Scholar]
- 119.Von Hoff DD, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. N. Engl. J. Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 120.Graham RA, et al. Pharmacokinetics of Hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: the role of alpha-1-acid glycoprotein binding. Clin. Cancer Res. 2011;17:2512–2520. doi: 10.1158/1078-0432.CCR-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramaswamy B, et al. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012;72:5048–5059. doi: 10.1158/0008-5472.CAN-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yauch RL, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buonamici S, et al. Interfering with resistance to Smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoon JW, et al. Noncanonical regulation of the Hedgehog mediator GLI1 by c-MYC in Burkitt lymphoma. Mol. Cancer Res. 2013;11:604–615. doi: 10.1158/1541-7786.MCR-12-0441. [DOI] [PubMed] [Google Scholar]
- 125.Hollingshead MG. Antitumor efficacy testing in rodents. J. Natl Cancer Inst. 2008;100:1500–1510. doi: 10.1093/jnci/djn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical Hedgehog signaling. Vitam. Horm. 2012;88:55–72. doi: 10.1016/B978-0-12-394622-5.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gruber Filbin M, et al. Coordinate activation of SHH and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities. Nat. Med. 2013;19:1518–1523. doi: 10.1038/nm.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/ct2/show/NCT01537107. [DOI] [PubMed]
- 129.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/ct2/show/NCT01576666. [DOI] [PubMed]
- 130.Kim DJ, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J. Clin. Oncol. 2014;32:745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 131.Kim J, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–399. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim J, Lee JJ, Kim J, Gardner D, Beachy PA. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc. Natl Acad. Sci. USA. 2010;107:13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim J, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to Smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J. Invest. Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 136.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 137.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 138.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/ β-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 139.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 140.Itasaki N, et al. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 141.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 142.Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J. Biol. 2005;4:2. doi: 10.1186/jbiol22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Behrens J. Control of β-catenin signaling in tumor development. Ann. N. Y. Acad. Sci. 2000;910:21–33. doi: 10.1111/j.1749-6632.2000.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 144.Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- 145.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat. Rev. Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 146.Malanchi I, et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 147.Vincan E, Barker N. The upstream components of the Wnt signalling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clin. Exp. Metastasis. 2008;25:657–663. doi: 10.1007/s10585-008-9156-4. [DOI] [PubMed] [Google Scholar]
- 148.Lee HJ, Wang NX, Shi DL, Zheng JJ. Sulindac inhibits canonical Wnt signaling by blocking the PDZ domain of the protein Dishevelled. Angew. Chem. Int. Ed. Engl. 2009;48:6448–6452. doi: 10.1002/anie.200902981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 150.Smith ML, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur. J. Cancer. 2000;36:664–674. doi: 10.1016/s0959-8049(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 151.Yoshizumi T, et al. Thiazolidinedione, a peroxisome proliferator-activated receptor-γ ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int. J. Oncol. 2004;25:631–639. [PubMed] [Google Scholar]
- 152.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 153.Waaler J, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72:2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- 154.Waaler J, et al. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71:197–205. doi: 10.1158/0008-5472.CAN-10-1282. [DOI] [PubMed] [Google Scholar]
- 155.Fujii N, et al. An antagonist of dishevelled protein–protein interaction suppresses β-catenin-dependent tumor cell growth. Cancer Res. 2007;67:573–579. doi: 10.1158/0008-5472.CAN-06-2726. [DOI] [PubMed] [Google Scholar]
- 156.Grandy D, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J. Biol. Chem. 2009;284:16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Su Y, Ishikawa S, Kojima M, Liu B. Eradication of pathogenic β-catenin by Skp1/ Cullin/F box ubiquitination machinery. Proc. Natl Acad. Sci. USA. 2003;100:12729–12734. doi: 10.1073/pnas.2133261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yao H, et al. AV-65, a novel Wnt/β-catenin signal inhibitor, successfully suppresses progression of multiple myeloma in a mouse model. Blood Cancer J. 2011;1:e43. doi: 10.1038/bcj.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.El-Khoueiry AB, et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors [abstract] J. Clin. Oncol. 2013;31(Suppl.):a2501. [Google Scholar]
- 160.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/β-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 161.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01302405. [DOI] [PubMed]
- 162.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/ct2/show/NCT01764477. [DOI] [PubMed]
- 163.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01606579. [DOI] [PubMed]
- 164.US National Library of Medicine. ClinicalTrials.gov. 2015 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01351103. [DOI] [PubMed]
- 165.Li X, et al. Prostate tumor progression is mediated by a paracrine TGF-β/Wnt3a signaling axis. Oncogene. 2008;27:7118–7130. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith DC, et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the Wnt pathway in a phase I study for patients with advanced solid tumors [abstract] J. Clin. Oncol. 2013;31(Suppl.):a2540. [Google Scholar]
- 168.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01345201. [DOI] [PubMed]
- 169.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT02005315. [DOI] [PubMed]
- 170.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01957007. [DOI] [PubMed]
- 171.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01973309. [DOI] [PubMed]
- 172.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01608867. [DOI] [PubMed]
- 173.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT02069145. [DOI] [PubMed]
- 174.US National Library of Medicine. ClinicalTrials.gov. 2015 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT02092363. [DOI] [PubMed]
- 175.Smith DC, et al. A first-in-human phase I study of anti-cancer stem cell (CSC) agent OMP-54F28 (FZD8-Fc) targeting the Wnt pathway in patients with advanced solid tumors [abstract] Mol. Cancer Ther. 2013;12(11 Suppl.):B79. [Google Scholar]
- 176.Jimeno A, et al. A first-in-human phase I study of anticancer stem cell agent OMP-54F28 (FZD8-Fc), decoy receptor for Wnt ligands, in patients with advanced solid tumors [abstract] J. Clin. Oncol. 2014;32(5s Suppl.):a2505. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 177.Katoh M. Networking of Wnt, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30–38. doi: 10.1007/s12015-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 178.Kwon C, et al. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat. Cell Biol. 2011;13:1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rodilla V, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl Acad. Sci. USA. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arcaroli JJ, et al. Tumours with elevated levels of the Notch and Wnt pathways exhibit efficacy to PF-03084014, a γ-secretase inhibitor, in a preclinical colorectal explant model. Br. J. Cancer. 2013;109:667–675. doi: 10.1038/bjc.2013.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann. Oncol. 2012;23:562–569. doi: 10.1093/annonc/mdr386. [DOI] [PubMed] [Google Scholar]
- 182.Messersmith WA, et al. First-in-patient phase I study of the novel gamma secretase inhibitor PF-03084014 in patients with advanced solid tumor malignancies [abstract 588] Eur. J. Cancer. 2012;48180(Suppl. 6) [Google Scholar]
- 183.Carothers AM, et al. Mesenchymal stromal cell mutations and wound healing contribute to the etiology of desmoid tumors. Cancer Res. 2012;72:346–355. doi: 10.1158/0008-5472.CAN-11-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Doody RS, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 185.He J, et al. Suppressing Wnt signaling by the Hedgehog pathway through sFRP-1. J. Biol. Chem. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 186.Schneider FT, et al. Sonic hedgehog acts as a negative regulator of β-catenin signaling in the adult tongue epithelium. Am. J. Pathol. 2010;177:404–414. doi: 10.2353/ajpath.2010.091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rabadan MA, et al. Jagged2 controls the generation of motor neuron and oligodendrocyte progenitors in the ventral spinal cord. Cell Death Differ. 2012;19:209–219. doi: 10.1038/cdd.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.US National Library of Medicine. ClinicalTrials.gov. 2015 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/show/NCT01154452. [DOI] [PubMed]
- 189.Dakubo GD, Mazerolle CJ, Wallace VA. Expression of Notch and Wnt pathway components and activation of Notch signaling in medulloblastomas from heterozygous patched mice. J. Neurooncol. 2006;79:221–227. doi: 10.1007/s11060-006-9132-2. [DOI] [PubMed] [Google Scholar]
- 190.Ingram WJ, McCue KI, Tran TH, Hallahan AR, Wainwright BJ. Sonic hedgehog regulates Hes1 through a novel mechanism that is independent of canonical Notch pathway signalling. Oncogene. 2008;27:1489–1500. doi: 10.1038/sj.onc.1210767. [DOI] [PubMed] [Google Scholar]
- 191.Di Marcotullio L, et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat. Cell Biol. 2006;8:1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- 192.Domingo-Domenech J, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of Notch-and Hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cilloni D, Saglio G. Molecular pathways: BCR–ABL. Clin. Cancer Res. 2012;18:930–937. doi: 10.1158/1078-0432.CCR-10-1613. [DOI] [PubMed] [Google Scholar]
- 194.Chau WK, Ip CK, Mak AS, Lai HC, Wong AS. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32:2767–2781. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
- 195.Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3β down-regulates Notch activity, a link for Notch and Wnt pathways. J. Biol. Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 196.Saint Just Ribeiro M, Hansson ML, Lindberg MJ, Popko-Scibor AE, Wallberg AE. GSK3β is a negative regulator of the transcriptional coactivator MAML1. Nucleic Acids Res. 2009;37:6691–6700. doi: 10.1093/nar/gkp724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Foltz DR, Santiago MC, Berechid BE, Nye JS. Glycogen synthase kinase-3β modulates Notch signaling and stability. Curr. Biol. 2002;12:1006–1011. doi: 10.1016/s0960-9822(02)00888-6. [DOI] [PubMed] [Google Scholar]
- 198.Rizzo P, et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68:5226–5235. doi: 10.1158/0008-5472.CAN-07-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hao L, et al. Notch-1 activates estrogen receptor-α-dependent transcription via IKKα in breast cancer cells. Oncogene. 2010;29:201–213. doi: 10.1038/onc.2009.323. [DOI] [PMC free article] [PubMed] [Google Scholar]