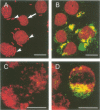
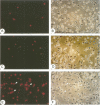
Abstract
Shigella, the etiological agent of dysentery, kills macrophages by inducing apoptosis. Deletion mutants in the invasion invasion plasmid antigen B (ipaB) of Shigella flexneri are not cytotoxic. Here, we localized IpaB to the cytoplasm of macrophages infected with S. flexneri. Purified IpaB induced apoptosis when microinjected into macrophages, indicating that IpaB is sufficient to induce apoptosis. Using a GST-IpaB fusion protein as a ligand in affinity purification, we isolated four IpaB binding proteins from macrophages which were identified as the precursor and the mature polypeptides of interleukin-1beta converting enzyme (ICE) or a highly homologous protease. We found that IpaB binds directly to ICE and this enzyme is activated during S. flexneri infection. Furthermore, specific inhibitors of ICE prevented Shigella-induced apoptosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand B. S., Malhotra V., Bhattacharya S. K., Datta P., Datta D., Sen D., Bhattacharya M. K., Mukherjee P. P., Pal S. C. Rectal histology in acute bacillary dysentery. Gastroenterology. 1986 Mar;90(3):654–660. doi: 10.1016/0016-5085(86)91120-0. [DOI] [PubMed] [Google Scholar]
- Barzu S., Nato F., Rouyre S., Mazie J. C., Sansonetti P., Phalipon A. Characterization of B-cell epitopes on IpaB, an invasion-associated antigen of Shigella flexneri: identification of an immunodominant domain recognized during natural infection. Infect Immun. 1993 Sep;61(9):3825–3831. doi: 10.1128/iai.61.9.3825-3831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Kronheim S. R., Cantrell M., Deeley M. C., March C. J., Prickett K. S., Wignall J., Conlon P. J., Cosman D., Hopp T. P. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988 Jul 5;263(19):9437–9442. [PubMed] [Google Scholar]
- Clerc P. L., Ryter A., Mounier J., Sansonetti P. J. Plasmid-mediated early killing of eucaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect Immun. 1987 Mar;55(3):521–527. doi: 10.1128/iai.55.3.521-527.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M., Hug H., Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995 May 4;375(6526):78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- Faucheu C., Diu A., Chan A. W., Blanchet A. M., Miossec C., Hervé F., Collard-Dutilleul V., Gu Y., Aldape R. A., Lippke J. A. A novel human protease similar to the interleukin-1 beta converting enzyme induces apoptosis in transfected cells. EMBO J. 1995 May 1;14(9):1914–1922. doi: 10.1002/j.1460-2075.1995.tb07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994 Dec 9;269(49):30761–30764. [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. Mch2, a new member of the apoptotic Ced-3/Ice cysteine protease gene family. Cancer Res. 1995 Jul 1;55(13):2737–2742. [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Takahashi A., Armstrong R., Krebs J., Fritz L., Tomaselli K. J., Wang L., Yu Z., Croce C. M., Salveson G. Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res. 1995 Dec 15;55(24):6045–6052. [PubMed] [Google Scholar]
- Gu Y., Wu J., Faucheu C., Lalanne J. L., Diu A., Livingston D. J., Su M. S. Interleukin-1 beta converting enzyme requires oligomerization for activity of processed forms in vivo. EMBO J. 1995 May 1;14(9):1923–1931. doi: 10.1002/j.1460-2075.1995.tb07184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hermant D., Ménard R., Arricau N., Parsot C., Popoff M. Y. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol Microbiol. 1995 Aug;17(4):781–789. doi: 10.1111/j.1365-2958.1995.mmi_17040781.x. [DOI] [PubMed] [Google Scholar]
- High N., Mounier J., Prévost M. C., Sansonetti P. J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992 May;11(5):1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson S., Bergman T., Vanooteghem J. C., Cornelis G., Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993 Jan;61(1):71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniga K., Tucker S., Trollinger D., Galán J. E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995 Jul;177(14):3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostura M. J., Tocci M. J., Limjuco G., Chin J., Cameron P., Hillman A. G., Chartrain N. A., Schmidt J. A. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kinoshita M., Noda M., Copeland N. G., Jenkins N. A. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 beta-converting enzyme. Genes Dev. 1994 Jul 15;8(14):1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Green D. R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995 Aug 11;82(3):349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S314–S318. doi: 10.1093/clinids/13.supplement_4.s314. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Munday N. A., Vaillancourt J. P., Ali A., Casano F. J., Miller D. K., Molineaux S. M., Yamin T. T., Yu V. L., Nicholson D. W. Molecular cloning and pro-apoptotic activity of ICErelII and ICErelIII, members of the ICE/CED-3 family of cysteine proteases. J Biol Chem. 1995 Jun 30;270(26):15870–15876. doi: 10.1074/jbc.270.26.15870. [DOI] [PubMed] [Google Scholar]
- Ménard R., Sansonetti P. J., Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993 Sep;175(18):5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard R., Sansonetti P., Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994 Nov 15;13(22):5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard R., Sansonetti P., Parsot C., Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell. 1994 Nov 4;79(3):515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Perdomo O. J., Cavaillon J. M., Huerre M., Ohayon H., Gounon P., Sansonetti P. J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994 Oct 1;180(4):1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Dressler H. pGSTag--a versatile bacterial expression plasmid for enzymatic labeling of recombinant proteins. Biotechniques. 1992 Dec;13(6):866–869. [PubMed] [Google Scholar]
- Sansonetti P. J., Arondel J., Cavaillon J. M., Huerre M. Role of interleukin-1 in the pathogenesis of experimental shigellosis. J Clin Invest. 1995 Aug;96(2):884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Makino S., Yamada M., Okada N., Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988 Jun;170(6):2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. L., McLaughlin J., Goga A., Havlik M., Witte O. The nuclear tyrosine kinase c-Abl negatively regulates cell growth. Cell. 1994 Apr 8;77(1):121–131. doi: 10.1016/0092-8674(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Chin J., Bayne E. K., Limjuco G., Weidner J., Miller D. K., Chapman K., Kostura M. J. The interleukin-1 beta-converting enzyme (ICE) is localized on the external cell surface membranes and in the cytoplasmic ground substance of human monocytes by immuno-electron microscopy. J Exp Med. 1995 Nov 1;182(5):1447–1459. doi: 10.1084/jem.182.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Muegge K., Keller J. R., Kung H. F., Young H. A., Durum S. K. Direct evidence for an intracellular role for IFN-gamma. Microinjection of human IFN-gamma induces Ia expression on murine macrophages. J Immunol. 1990 Mar 1;144(5):1777–1782. [PubMed] [Google Scholar]
- Smith M. R., Munger W. E., Kung H. F., Takacs L., Durum S. K. Direct evidence for an intracellular role for tumor necrosis factor-alpha 1. Microinjection of tumor necrosis factor kills target cells. J Immunol. 1990 Jan 1;144(1):162–169. [PubMed] [Google Scholar]
- Tewari M., Quan L. T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D. R., Poirier G. G., Salvesen G. S., Dixit V. M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995 Jun 2;81(5):801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Wang L., Miura M., Bergeron L., Zhu H., Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994 Sep 9;78(5):739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Wassef J. S., Keren D. F., Mailloux J. L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989 Mar;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Fitting C., Cavaillon J. M., Sansonetti P. J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Invest. 1994 Sep;94(3):1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A., Kenny B., Ménard R., Prévost M. C., Holland I. B., Sansonetti P. J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994 Feb;11(4):619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992 Jul 9;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]