Abstract
Objective
Neonatal abstinence syndrome (NAS), a postnatal opioid withdrawal syndrome, increased 3-fold from 2000 to 2009. Since 2009, opioid pain reliever prescriptions and complications increased markedly throughout the US. Understanding recent changes in NAS and its geographic variability would inform state and local governments in targeting public health responses.
Study Design
We utilized diagnostic and demographic data for hospital discharges from 2009 to 2012 from the Kids’ Inpatient Database and the Nationwide Inpatient Sample. NAS-associated diagnoses were identified utilizing ICD-9-CM codes. All analyses were conducted with nationally weighted data. Expenditure data were adjusted to 2012 US dollars. Between-year differences were determined utilizing least squares regression.
Results
From 2009 to 2012, NAS incidence increased nationally from 3.4 (95%CI: 3.2-3.6) to 5.8 (95%CI 5.5-6.1) per 1,000 hospital births, reaching a total of 21,732 infants with the diagnosis. Aggregate hospital charges for NAS increased from $732M to $1.5B (p<0.001), with 81% attributed to state Medicaid programs in 2012. NAS incidence varied by geographic Census division, with the highest incidence rate (per 1000 hospital births) of 16.2 (95%CI 12.4-18.9) in the East South Central Division (KY, TN, MS, AL) and the lowest in West South Central Division 2.6 (95%CI 2.3-2.9; [OK, TX, AR, LA]).
Conclusion
NAS incidence and hospital charges grew substantially during our study period. This costly public health problem merits a public health approach to alleviate harm to women and children. States, particularly in areas of the country most affected by the syndrome, must continue to pursue primary prevention strategies to limit the effects of opioid pain reliever misuse.
Keywords: Neonatal abstinence syndrome, neonatal drug withdrawal syndrome, neonatal opioid withdrawal syndrome, opioid pain reliever
Introduction
Neonatal abstinence syndrome (NAS) is a withdrawal syndrome that occurs in opioid-exposed infants shortly after birth.(1-3) Infants with NAS have longer, more complicated postnatal hospitalizations characterized by a myriad of clinical signs ranging from feeding difficulty to seizures.(1, 4, 5) Recently, NAS emerged as a significant public health problem, increasing in number and healthcare expenditures.(5) By 2009, one infant was born per hour with the syndrome accounting for an estimated $720 million in hospital charges.(5) The increase in NAS occurred temporally with an increase in opioid pain reliever (OPR) use(6) among several populations, including pregnant women.(7, 8)
Data from the Centers for Disease Control and Prevention suggest that since 2009, when the most recent national estimates of NAS were reported, OPR use continued to increase. In 2012, the total number of OPR prescriptions rose to 259 million, enough for every American adult to have one bottle.(9, 10) Recent data also highlight substantial variation in OPR use across different US geographic regions.(9) To date, however, there are no national studies describing geographic variation in NAS. Understanding recent changes in NAS, including its variability in geographic regions would inform state and local governments in targeting public health responses.
We sought to determine if the incidence of NAS increased since 2009 in parallel with the marked increase in OPR use nationally and if the incidence varied across the US. Further, we aimed to determine if healthcare utilization patterns of infants with NAS changed over time.
Methods
Study Design and Setting
For this retrospective serial cross-sectional analysis, we used data from the Kids’ Inpatient Database (KID) for 2009 and 2012 and from the Nationwide Inpatient Sample (NIS) for 2010 and 2011. Both datasets are compiled by the Agency for Healthcare Research and Quality as part of the Healthcare Utilization Project (HCUP). The KID is the largest publicly available all-payer database for hospitalized children in the US. The KID contains 2 to 3 million pediatric inpatient records per year from 2,500 to 4,100 hospitals and is created through systematic random sampling to select 10% of uncomplicated term births and 80% of other pediatric discharges. This sampling strategy gives the KID statistical power to evaluate rare conditions and provide more precise point estimates for all pediatric conditions.(11) The NIS is the largest publicly available all-payer inpatient database in the US, containing more than 8 million hospital stays sampled from a 20% stratified sample of 1,000 community hospitals.(12) Both the KID and NIS have been used broadly in national studies of pediatric(5, 13, 14) and adult(5, 15, 16) conditions. As the study used de-identified data, it was considered exempt from human subjects review by the Vanderbilt University School of Medicine.
Identification of Sample
Infants with NAS were identified if the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 779.5 (drug withdrawal syndrome in a newborn) appeared in any 1 of 25 diagnostic fields.(17) Infants with presumed iatrogenic NAS from medical treatment were excluded using strategies described previously.(5) KID and NIS provide data for hospital births using ICD-9-CM codes (V3000 to V3901 with the last two digits of “00” or “01”) if the patient is not transferred from another acute care hospital or health care facility. Uncomplicated births are identified using the diagnosis related group code for “Normal Newborn” (391, version 24).(11, 12)
Descriptive Variables
Infants with NAS are more likely to have neonatal respiratory complications, feeding difficulty, seizures, and low birthweight.(1) Clinical characteristics of infants were obtained using the following ICD-9-CM codes in any one of the diagnostic fields during the birth hospitalization: transient tachypnea of the newborn (770.6), meconium aspiration syndrome (770.11, 770.12), respiratory distress syndrome (769.x), other neonatal respiratory diagnoses (770.x excluding above codes and 770.7), feeding difficulty (779.3x), concern for sepsis (771.81), jaundice (774.x) and seizure (779.0, 780.3). Additional descriptive variables, including primary payer (private, Medicaid, uninsured, and other) and sex were provided in the KID and NIS.
Outcome Variables
National incidence rates of NAS were estimated by dividing the total number of infants with NAS by the total number of hospital births and expressed as incidence per 1,000 births. Beginning in 2012, the KID and NIS samples increased, providing sufficient reliability to create estimates by US Census Bureau geographic division. Length of stay (LOS) data were obtained from the KID and NIS; as infants not receiving pharmacotherapy for NAS are unlikely to have LOS >6 days,(1) we evaluated LOS for all infants with NAS and then for infants with NAS who had a LOS >6 days (presumed pharmacologically treated). Throughout the manuscript we will refer to infants presumed to be pharmacologically treated as “pharmacologically treated.” Hospital charges were obtained from the KID and NIS and adjusted to 2012 US$.(18) Missing charges (<3%) were imputed using a regression approach using the command “impute” with diagnosis related groups, LOS, age and NAS as predictors. Mean charges before and after imputation were compared and were not significantly different; data with imputed values are presented.
Data Analysis
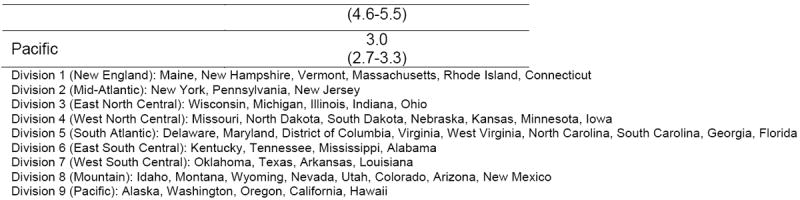
Statistical analyses were conducted using Stata Version 13.1 (StataCorp, College Station, TX). For all analyses, survey weights provided by HCUP were applied to facilitate nationally representative estimates. For 2012, differences in clinical characteristics and primary payer for infants with NAS versus all other hospital births were assessed. Trends for LOS and hospital charges were evaluated using variance weighted least squared regression.(5) NAS incidence rates were calculated by division (9 overall: New England, Mid-Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, Pacific) for 2012. Maps were generated to evaluate geographic variation of NAS using the spmap command(19) in Stata, with map data obtained from the National Oceanic and Atmospheric Administration.(20) Throughout our analysis, all testing was 2-sided, with data reported with standard errors or 95% confidence intervals.
Results
In 2012, there were an estimated 21,732 (95% CI 20,052-23,413) infants diagnosed with NAS and 3,716,916 (95%CI 3,607,375-3,826,456) other hospital births. Infants with NAS were more likely than other hospital births to have complications, including low birthweight (24.4% vs. 7.2%), transient tachypnea of the newborn (11.7% vs. 3.1%), meconium aspiration syndrome (2.8% vs. 0.4%), respiratory distress syndrome (4.5% vs. 2.0%), jaundice (32.8% vs. 19.1%), feeding difficulty (17.3% vs. 3.0%), seizures (1.4% vs. 0.1%) and possible sepsis (14.8% vs. 2.2%; p<0.001). Infants with NAS were also more likely than other hospital births to be insured by Medicaid (81.5% vs. 46.4%; p<0.001; Table 1).
Table 1.
Characteristics of infants with neonatal abstinence syndrome vs. all other hospital births, 2012.
| Infants with Neonatal Abstinence Syndrome | All Other Hospital Births | p-value | |||
|---|---|---|---|---|---|
| N = 21,732 | N = 3,716,916 | ||||
| N | % | N | % | ||
| Female | 9,902 | 45.6% | 1,817,513 | 48.9% | <0.001 |
| Clinical Characteristics | |||||
| Low Birthweight | 5,308 | 24.4% | 267,885 | 7.2% | <0.001 |
| Respiratory Diagnoses | |||||
| Transient Tachypnea | 2,552 | 11.7% | 113,483 | 3.1% | <0.001 |
| Meconium Aspiration Syndrome | 613 | 2.8% | 13,235 | 0.4% | <0.001 |
| Respiratory Distress Syndrome | 977 | 4.5% | 74,001 | 2.0% | <0.001 |
| Jaundice | 7,134 | 32.8% | 708,872 | 19.1% | <0.001 |
| Feeding Difficulty | 3,765 | 17.3% | 111,288 | 3.0% | <0.001 |
| Seizures | 309 | 1.4% | 4,208 | 0.1% | <0.001 |
| Sepsis | 3,218 | 14.8% | 81,845 | 2.2% | <0.001 |
| Insurance | <0.001 | ||||
| Private | 2,688 | 12.4% | 1,717,308 | 46.2% | |
| Medicaid | 17,717 | 81.5% | 1,726,432 | 46.4% | |
| Uninsured | 853 | 3.9% | 144,137 | 3.9% | |
| Other | 405 | 1.9% | 118,918 | 3.2% | |
Point estimate (standard error) N for NAS = 21,732 (857); unweighted sample n = 16,254
Point estimate (standard error) N for all other hospital births = 3,716,916 (55,864); unweighted sample n = 1,094,748
From 2009 to 2012, incidence (95%CI) of NAS increased from 3.4 (3.2-3.6) to 5.8 (5.5-6.1) per 1,000 hospital births overall (Figure 1). There was significant geographic variation in NAS diagnoses. In the most recent study year, the East South Central division (Kentucky, Tennessee, Mississippi, Alabama) had the highest incidence of NAS at 16.2 (12.4-18.9) per 1,000 hospital births, compared with the West South Central division (Oklahoma, Texas, Arkansas, Louisiana) with the lowest national incidence rate of 2.6 (2.3-2.9) per 1,000 hospital births (Figure 2).
Figure 1. Incidence of neonatal abstinence syndrome per 1,000 hospital births in the United States, 2009-2012.

* Data obtained from the Kids’ Inpatient Database for 2009 and 2012 and from the Nationwide Inpatient Sample in 2010 at 2011.
** 2009: 3.4 (95%CI 3.2-3.6); 2010: 4.8 (95%CI 4.3-5.2); 2011: 5.0 (95%CI 4.4-5.4); 2012: 5.8 (95%CI 5.5-6.1)
Figure 2. Incidence of neonatal abstinence syndrome per 1,000 hospital births by US Census Bureau geographic division, 2012.

From 2009 to 2012, there was no significant change in overall mean LOS for all NAS infants, pharmacologically treated NAS infants, or for uncomplicated term infants with mean LOS in 2012 of 16.9 (16.0-17.7), 23.0 (22.2-23.8), and 2.1 (2.1-2.1) days, respectively. Inflation-adjusted mean hospital charges increased for all groups and in 2012 reached $66,700 ($61,800-$71,600) for infants with NAS, $93,400 ($86,900-$100,000) for pharmacologically treated NAS infants, and $3,500 ($3,400-$3,600) for uncomplicated term infants (Table 2).
Table 2.
Mean length of stay and inflation-adjusted hospital charges for all infants with neonatal abstinence syndrome, infants with neonatal abstinence syndrome with a length of hospital stay > 6 days and uncomplicated term infants, 2009-2012.
| Year | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|
| N (95% CI) | N (95% CI) | N (95% CI) | N (95% CI) | |
| Neonatal Abstinence Syndrome
| ||||
| Mean Length of Stay (days) |
16.5 (15.9-17.2) |
17.2 (15.8-18.5) |
16.6 (15.1-18.1) |
16.9 (16.0-17.7) |
| Mean Hospital Charges (2012 US$) |
$53,800 ($49,400-$58,300) |
$59,000 ($49,600-$68,400) |
$62,300 ($52,900-$71,700) |
$66,700 ($61,800-$71,600) |
|
| ||||
| Pharmacologically Treated Neonatal Abstinence Syndrome
| ||||
| Mean Length of Stay (days) |
22.7 21.9-23.4 |
22.9 21.6-24.1 |
22.8 21.5-24.2 |
23.0 22.2-23.8 |
| Mean Hospital Charges (2012 US$) |
$75,700 ($69,500-$82,000) |
$80,500 ($68,000-$93,100) |
$87,700 ($76,300-$99,100) |
$93,400 ($86,900-$100,000) |
|
| ||||
| Uncomplicated Term Infant
| ||||
| Mean Length of Stay (days) |
2.1 (2.1-2.1) |
2.1 (2.1-2.1) |
2.1 (2.1-2.1) |
2.1 (2.1-2.1) |
| Mean Hospital Charges (2012 US$) |
$2,800 ($2,700-$2,900) |
$3,500 ($3,300-$3,800) |
$3,700 ($3,400-$3,900) |
$3,500 ($3,400-$3,600) |
During the study period aggregate hospital charges for NAS nearly doubled, from an estimated total of $731,841,300 in 2009 to $1,449,389,600 in 2012. Through all study years the majority of hospital charges were attributed to state Medicaid programs, growing from $563,809,300 to $1,170,206,600 (Table 3, p<0.001).
Table 3.
Aggregate Hospital Charges by Primary Payer for Neonatal Abstinence Syndrome, 2009-2012.
| Year | 2009 | 2010 | 2011 | 2012 | p-for-trend | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total Charges | SE | Total Charges | SE | Total Charges | SE | Total Charges | SE | ||
| Private | $133,553,300 | $11,176,700 | $167,466,500 | $24,810,000 | $208,363,300 | $30,929,400 | $202,233,600 | $12,054,400 | <0.001 |
| Medicaid | $563,809,300 | $33,650,300 | $865,649,700 | $79,181,000 | $903,654,700 | $94,344,100 | $1,170,206,600 | $68,789,500 | <0.001 |
| Uninsured | $20,079,300 | $1,603,200 | $35,995,700 | $4,906,100 | $30,842,700 | $4,735,100 | $40,370,800 | $3,004,500 | <0.001 |
| Other | $14,248,300 | $2,628,000 | $29,379,400 | $6,807,800 | $30,117,700 | $8,011,000 | $33,395,300 | $4,890,800 | <0.001 |
|
| |||||||||
| Total | $731,841,300 | $40,290,000 | $1,098,996,200 | $98,050,800 | $1,174,848,900 | $117,316,500 | $1,449,389,600 | $76,698,100 | <0.001 |
Discussion
The incidence of NAS in the United States nearly doubled during our study period and has grown nearly 5-fold since 2000.(5) NAS results in longer, more costly, and complicated hospital stays when compared to other hospital births. The rapid rise in NAS parallels the increase in OPR use in the US, suggesting that preventing opioid overuse and misuse, especially before pregnancy, may prevent NAS. NAS is a rapidly increasing public health problem that merits a focused public health approach to mitigate its now far-reaching impact.
We found significant geographic variation in NAS that parallels variations in OPR prescribing.(9) We found high rates of NAS in New England (Maine, New Hampshire, Vermont, Massachusetts, Rhode Island, Connecticut; 13.7, 95%CI 12.5-14.5) and the East South Central (Kentucky, Tennessee, Mississippi, Alabama; 16.2, 95%CI 12.4-18.9) divisions. The New England division contains 2 of the top 5 prescribing states of long-acting OPR (Maine, New Hampshire) and the East South Central division contains 3 of the top 5 prescribing states of short-acting OPR (Alabama, Tennessee, Kentucky),(9) further supporting the association between increased OPR prescribing and NAS.
As expected, we found that infants with NAS were more likely to have low birthweight, significant respiratory complications including meconium aspiration and respiratory distress syndrome, feeding difficulties, possible sepsis and seizures - all of which may have contributed to longer LOS compared to other hospital births. More difficult to measure are the associated costs to families affected by the syndrome. Hospitalization for NAS most commonly involves an admission to a neonatal intensive care unit that disrupts maternal and infant bonding. Preventing NAS will prevent the clinical complications of the syndrome and potentially improve outcomes more difficult to measure, including maternal attachment.(21)
Infants with NAS had an overall mean LOS of 16 days and those requiring pharmacologic treatment had a mean LOS of 23 days. We hypothesize that overall mean LOS is positively skewed by some infants who are non-pharmacologically treated or show minimal signs of withdrawal. Interestingly, LOS did not change significantly for either group during the study period. Care for NAS is variable,(4, 22) and research suggests that LOS may be able to be decreased with protocol adherence,(23) use of clonidine as an adjunct,(24) breastfeeding when appropriate (e.g., when the mother is enrolled in treatment),(25-27) rooming in,(28, 29) and a site of care outside of the neonatal intensive care unit environment.(30)
Notably, some cases of NAS in our cohort likely occurred in the setting of medication-assisted treatment (MAT) with methadone or buprenorphine. For pregnant women with opioid dependency, current evidence suggests that enrollment in MAT improves pregnancy outcomes including preterm birth.(31, 32) However, the literature supporting MAT in pregnancy was developed in the context of heroin use; data supporting optimal management of pregnant women with OPR dependency are limited.(31) With increasing use of OPR in pregnancy,(7) there is an urgent need for research to guide appropriate management of OPR dependency in pregnancy.
Nationally, over 80% of infants with NAS are enrolled in state Medicaid programs, accounting for the majority of the estimated $1.5 billion in total hospital charges for the syndrome. Given the length of NAS-related hospital care, some states incur substantial expenditures in their Medicaid programs for NAS. For example, the Tennessee Medicaid program estimates that infants with NAS accounted for 1.7% of live births but 13.0% of expenditures on births in 2012.(33) In addition to administering and partially funding Medicaid, states also regulate prescribers and pharmacists. Therefore, states are well positioned to employ public health interventions aimed at preventing OPR misuse. Prescription drug monitoring programs (PDMPs) are an intervention employed in every state except Missouri.(34) PDMPs vary in scope and structure and are a tool to prevent behaviors that increase risk of OPR-related complications (e.g. targeting doctor shopping to mitigate risk of overdose death(35)).
Limitations
Our study contains limitations that merit discussion. First, our reliance on administrative data may lead to misclassification bias. There are few studies comparing administrative to clinical data; however, one study noted that administrative data systematically underreported actual NAS.(36) Next, it is possible that the increase in NAS we observed is secondary to observer bias, as the syndrome has received significant attention recently. However, the temporal increases in NAS we observed mirror national increases in OPR use and adverse effects (e.g. overdose deaths) attributed to their use. Further, our finding of significant geographic variability in the diagnosis of NAS correlated with geographic variations in use and adverse effects in the US.(9) In addition, it is important to note that hospital charges do not equal hospital costs and do not include professional fees. In our analysis, we assumed that infants with NAS who had a LOS less than 7 days were not pharmacologically treated; however, this may not always be true.
Conclusion
NAS has grown nearly 5-fold since 2000, accounting for an estimated $1.5 billion in annual hospital expenditures across the US. This costly public health problem merits a public health approach to alleviate harm to women and children. Federal and state policymakers should be mindful of the impact the OPR epidemic continues to have on pregnant women and their infants and consider these vulnerable populations in efforts aimed at primary prevention. Lastly, efforts aimed at primary prevention and treatment improvements should be targeted at the most affected areas of the country.
Acknowledgments
The authors would like to acknowledge Kelly Patrick for her contributions to this manuscript.
Funding/Support: This study was supported by CTSA award KL2TR000446 from the National Center for Advancing Translational Sciences and by the National Institute on Drug Abuse through the award 1K23DA038720-01 (Dr. Patrick).
Role of the Sponsor: The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript or the decision to submit.
Abbreviations
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- KID
Kids’ Inpatient Database
- NIS
Nationwide Inpatient Sample
- LBW
Low Birthweight
- NAS
Neonatal Abstinence Syndrome
- OPR
Opioid Pain Reliever
Footnotes
Conflict of Interest Disclosures: No disclosures or potential conflicts of interest to report.
References
- 1.Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–60. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 2.Kellogg A, Rose CH, Harms RH, Watson WJ. Current trends in narcotic use in pregnancy and neonatal outcomes. Am J Obstet Gynecol. 2011;204(3):259 e1-4. doi: 10.1016/j.ajog.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Finnegan LP, Kron RE, Connaughton JF, E JP. Assessment and treatment of abstinence in the infant of the drug-dependent mother. Int J Clin Pharmacol Biopharm. 1975;12(1-2):19–32. [PubMed] [Google Scholar]
- 4.Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US Children’s Hospitals, 2004-2011. J Perinatol. 2014 doi: 10.1038/jp.2014.114. [DOI] [PubMed] [Google Scholar]
- 5.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934–40. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 6.Vital Signs: Overdoses of Prescription Opioid Pain Relievers — United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–81. [PubMed] [Google Scholar]
- 7.Epstein RA, Bobo WV, Martin PR, Morrow JA, Wang W, Chandrasekhar R, et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol. 2013;23(8):498–503. doi: 10.1016/j.annepidem.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: variation among States in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(26):563–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Prescription Drug Overdose in the United States: Fact Sheet. Atlanta, GA: Centers for Disease Control and Prevention; 2014. Available from: http://www.cdc.gov/homeandrecreationalsafety/overdose/facts.html. [Google Scholar]
- 11.HCUP Kids’ Inpatient Database. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2000, 2003, 2006, 2009, 2012. Available from: http://www.hcup-us.ahrq.gov/kidoverview.jsp. [Google Scholar]
- 12.HCUP Nationwide Inpatient Sample. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2009-2011. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 13.Patrick SW, Davis MM, Sedman AB, Meddings JA, Hieber S, Lee GM, et al. Accuracy of hospital administrative data in reporting central line-associated bloodstream infections in newborns. Pediatrics. 2013;131(Suppl 1):S75–80. doi: 10.1542/peds.2012-1427i. [DOI] [PubMed] [Google Scholar]
- 14.Auger KA, Patrick SW, Davis MM. Infant hospitalizations for pertussis before and after Tdap recommendations for adolescents. Pediatrics. 2013;132(5):e1149–55. doi: 10.1542/peds.2013-1747. [DOI] [PubMed] [Google Scholar]
- 15.Afana M, Brinjikji W, Cloft H, Salka S. Hospitalization Costs for Acute Myocardial Infarction Patients Treated With Percutaneous Coronary Intervention in the United States Are Substantially Higher Than Medicare Payments. Clinical cardiology. 2014 doi: 10.1002/clc.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozhimannil KB, Arcaya MC, Subramanian SV. Maternal Clinical Diagnoses and Hospital Variation in the Risk of Cesarean Delivery: Analyses of a National US Hospital Discharge Database. PLoS medicine. 2014;11(10):e1001745. doi: 10.1371/journal.pmed.1001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Classification of Diseases, 9th Revision - Clinical Modification. American Medical Association; Chicago, IL: 2012. p. 360. [Google Scholar]
- 18.US Bureau of Labor Statistics. [2014 July 15];Consumer Price Index. 2014 Available from: http://www.bls.gov/cpi/
- 19.Pisati M. Simple thematic mapping. The Stata Journal. 2004;4:361–78. [Google Scholar]
- 20.National Oceanic and Atmospheric Administration. US States and Territories Silver Spring, MD: 2014. Available from: http://www.nws.noaa.gov/geodata/catalog/national/html/us_state.htm. [Google Scholar]
- 21.Eapen V, Dadds M, Barnett B, Kohlhoff J, Khan F, Radom N, et al. Separation anxiety, attachment and inter-personal representations: disentangling the role of oxytocin in the perinatal period. PLoS One. 2014;9(9):e107745. doi: 10.1371/journal.pone.0107745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–7. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 23.Hall ES, Wexelblatt SL, Crowley M, Grow JL, Jasin LR, Klebanoff MA, et al. A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatrics. 2014;134(2):e527–34. doi: 10.1542/peds.2013-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agthe AG, Kim GR, Mathias KB, Hendrix CW, Chavez-Valdez R, Jansson L, et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123(5):e849–56. doi: 10.1542/peds.2008-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansson LM. ABM clinical protocol #21: Guidelines for breastfeeding and the drug-dependent woman. Breastfeed Med. 2009;4(4):225–8. doi: 10.1089/bfm.2009.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wachman EM, Hayes MJ, Brown MS, Paul J, Harvey-Wilkes K, Terrin N, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309(17):1821–7. doi: 10.1001/jama.2013.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansson LM, Velez M, Harrow C. Methadone maintenance and lactation: a review of the literature and current management guidelines. J Hum Lact. 2004;20(1):62–71. doi: 10.1177/0890334403261027. [DOI] [PubMed] [Google Scholar]
- 28.Abrahams RR, Kelly SA, Payne S, Thiessen PN, Mackintosh J, Janssen PA. Rooming-in compared with standard care for newborns of mothers using methadone or heroin. Canadian family physician Medecin de famille canadien. 2007;53(10):1722–30. [PMC free article] [PubMed] [Google Scholar]
- 29.Hunseler C, Bruckle M, Roth B, Kribs A. Neonatal opiate withdrawal and rooming-in: a retrospective analysis of a single center experience. Klin Padiatr. 2013;225(5):247–51. doi: 10.1055/s-0033-1347190. [DOI] [PubMed] [Google Scholar]
- 30.Saiki T, Lee S, Hannam S, Greenough A. Neonatal abstinence syndrome--postnatal ward versus neonatal unit management. Eur J Pediatr. 2010;169(1):95–8. doi: 10.1007/s00431-009-0994-0. [DOI] [PubMed] [Google Scholar]
- 31.ACOG Committee on Health Care for Underserved Women American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstetrics and gynecology (New York 1953) 2012;119(5):1070–6. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 32.Fullerton CA, Kim M, Thomas CP, Lyman DR, Montejano LB, Dougherty RH, et al. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv. 2014;65(2):146–57. doi: 10.1176/appi.ps.201300235. [DOI] [PubMed] [Google Scholar]
- 33.TennCare. [2014 October 27];Neonatal Abstinence Syndrome among TennCare enrollees Provisional 2012 data Nashville. TN2013. Available from: http://www.tn.gov/tenncare/forms/TennCareNASData2012.pdf.
- 34.PDMP Assist. [2014 June 18];PDMP Program Status 2014. Available from: http://www.pdmpassist.org/pdf/PDMPProgramStatus2014.pdf.
- 35.Peirce GL, Smith MJ, Abate MA, Halverson J. Doctor and pharmacy shopping for controlled substances. Med Care. 2012;50(6):494–500. doi: 10.1097/MLR.0b013e31824ebd81. [DOI] [PubMed] [Google Scholar]
- 36.Burns L, Mattick RP. Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug Alcohol Rev. 2007;26(5):487–92. doi: 10.1080/09595230701494416. [DOI] [PubMed] [Google Scholar]


